Abstract
Autoimmune thyroiditis is among the most prevalent of all the autoimmunities. Autoimmune thyroiditis is multifactorial with contributions from genetic and environmental factors. Much information has been published about the genetic predisposition to autoimmune thyroiditis both in experimental animals and humans. There is, in contrast, very little data on environmental agents that can serve as the trigger or autoimmunity in a genetically predisposed host. The best-established environmental factor is excess dietary iodine. Increased iodine consumption is strongly implicated as a trigger for thyroiditis, but only in genetically susceptible individuals. However, excess iodine is not the only environmental agent implicated as a trigger leading to autoimmune thyroiditis. There are a wide variety of other synthetic chemicals that affect the thyroid gland or have the ability to promote immune dysfunction in the host. These chemicals are released into the environment by design, such as in pesticides, or as a by-product of industry. Candidate pollutants include polyaromatic hydrocarbons, polybrominated biphenols, and polychlorinated biphenols, among others. Infections are also reputed to trigger autoimmunity and may act alone or in concert with environmental chemicals. We have utilized a unique animal model, the NOD.H2h4 mouse to explore the influence of iodine and other environmental factors on autoimmune thyroiditis.
Introduction
Autoimmune thyroiditis is among the most prevalent of all the autoimmunities, with an estimated number of new cases for 1996 to be over 40,000 [1]. The current incidence rate for thyroiditis/hypothyroidism in the United States is estimated at 21.8/100,000, over 90% of these cases are estimated to be women [1]. Autoimmune thyroiditis ranks third among the most frequent autoimmune diseases in the United States [1] and in adults is the most frequent cause of hypothyroidism [2].
Autoimmune thyroiditis, also known as Hashimoto’s thyroiditis, is an organ-specific autoimmune disorder, characterized by infiltration of the thyroid gland by inflammatory cells, often followed by hypothyroidism due to destruction of the thyroid follicles and eventual fibrous replacement of the parenchymal tissue. Autoantibodies to thyroid-specific antigens also develop. The two primary antigens in autoimmune thyroiditis are thyroglobulin (Tg) and thyroperoxidase (TPO). Tg is a glycoprotein with a molecular weight of about 660 kDA that constitutes the storage form of thyroid hormones within the thyroid follicle. TPO is the enzyme located at the apical border of the thyroid cell that is responsible for iodinating Tg and producing the thyroid hormones. The clinical diagnosis of autoimmune thyroiditis depends on both physical and biochemical abnormalities as well as serological demonstration of autoantibodies to these major thyroid antigens [3].
Autoimmune disease is multifactorial in that a genetic predisposition combines with environmental risk factors to promote disease. Autoimmune thyroiditis is clearly such a multifactorial disease. Early evidence that thyroiditis has a hereditary component stems from studies of familial aggregation (reviewed in [4]. Our own studies of juveniles with autoimmune thyroiditis showed a definite genetic propensity for thyroid autoimmunity to run in families [5]. Further evidence for the genetic control of autoimmune thyroiditis stems from observations of twins. Monozygotic twins showed a higher concordance rate of disease than did dizygotic twins [6]. However, even with identical twins the concordance rate was only about 50%, emphasizing that other important factors such as the environment play a role in disease pathogenesis.
Considerable information has been published about the genetic predisposition to autoimmune thyroiditis in both experimental animals and humans. In contrast, only few reports have been published on environmental agents that can serve as the “trigger” of autoimmunity in a genetically predisposed host. Both infectious microorganisms and environmental chemicals have been implicated, based primarily on anecdotal evidence. The study of environmental agents requires the availability of a reproducible model of a genetically predisposed animal in which such putative agents can be tested.
The best-established environmental factor promoting the development of autoimmune thyroiditis is excess dietary iodine. Iodination of salt in the 1920s was introduced as a public health measure. The program was very successful and in the United States reduced the frequency of endemic goiter in school children in the Midwest from 26 – 70% to 1 – 4% [7]. This major public health victory of virtually eliminating endemic goiter in the United States, however, caused another set of entirely different problems. There is evidence that the incidence of autoimmune thyroiditis increased concomitantly with the increased iodine content in the diet [8]. The Mayo Clinic, for example, reported an increase in the number of diagnosed cases of chronic lymphocytic thyroiditis from 2/year in the 1930's to approximately 500 new cases per year in 1985 [9].
This increased iodine consumption is strongly implicated as a trigger for thyroiditis, but only in genetically susceptible individuals [10,11]. The thyroid gland in adults requires approximately 75 to 100 ug of iodine/day to maintain a steady state between uptake and secretion of hormones from the thyroid. Although the average daily requirement has been set at 70 ug (160–200 ug for adolescents) [12], the average daily iodine intake in the United States has been estimated at between 240 to 740 ug, depending on geographical location [7], indicating a continual iodine excess for most of the population of the United States. Sources of dietary iodine include food and food additives (kelp and seaweed, iodinated salt, iodine additives to bread/flour, preservatives, red coloring, therapeutics (amiodarone, vitamins, Lugol's solution, etc.), topical antiseptics, and contrast dyes, among others.
Renewed interest in the problem of excess iodine promoting autoimmune thyroiditis is reflected by several recent clinical research reports. A threefold increase in the prevalence of autoimmune thyroiditis among schoolchildren was noted once iodine deficiency was eliminated in an area of endemic goiter in northwestern Greece [13]. Concomitantly, iodine restriction in many patients with primary hypothyroidism restored normal thyroid function [14,15]. However, these reports were limited in scope to clinical and laboratory diagnostic findings. No mechanism for how iodine may promote or induce autoimmune thyroiditis was proposed. Work performed by our laboratory and by others has demonstrated that highly iodinated Tg is more immunogenic than poorly iodinated Tg. Our studies in humans show that both antibody and in vitro T cell responses decreased to background levels when Tg lacked iodine. Tg re-iodination restored these responses [16–18]. Differing iodine content of human Tg can both create new epitopes and render others inaccessible, as recognized by monoclonal antibodies [16]. Clearly, excess iodine is an important factor in certain individuals that compromise thyroid function leading to autoimmune thyroiditis. However, excess iodine is not the only environmental agent implicated as a trigger leading to autoimmune thyroiditis.
In humans the thyroid gland can be compromised by dietary factors other than iodine. These may include naturally occurring goitrogens found in legumes and plants, and certain drugs such as amiodarone and lithium. Furthermore, there is a wide variety of synthetic chemicals that affect the thyroid gland or have the ability to promote immune dysfunction in the host. These chemicals are released into the environment by design, such as in pesticides, or as a by-product of industry. Candidate pollutants include polyaromatic hydrocarbons (PAH), polybrominated biphenols (PBBs), and polychlorinated biphenols (PCBs), among others. Infections are also reputed to trigger autoimmunity and may act alone or in concert with environmental chemicals. [19].
An inherent problem is how to test these compounds in a controlled situation. Furthermore, the effect on the thyroid may be different depending on the genetics. Studies in man are not always informative as Nice studies often do not differentiate between individuals with or without an autoimmune genetic predisposition. Subsequently, most of the basic work has been performed using animal models [20] [21–26]. We have utilized a unique animal model, the NOD.H2h4 mouse to explore the influence of iodine and other environmental factors on autoimmune thyroiditis.
NOD.H2h4 mice and iodine
Investigators at Merck Laboratories in connection with their diabetes genetics program originally developed this animal model by crossing the non-obese diabetic (NOD) mouse with the B10.A(4R) mouse strain, and extensively backcrossing to the NOD. This new mouse strain was designated as NOD.H2h4. The NOD.H2h4 has a MHC II background that is permissive for thyroiditis, the IAk [27]. None of the mice developed diabetes. However, a high proportion showed evidence of thyroiditis in older animals (50% in NOD.H2h4 vs 5% in the NOD strain) not found in either of the parental strains. Furthermore, the incidence of thyroiditis in NOD.H2h4 rose to 90% when excess iodine was added to the drinking water [28].
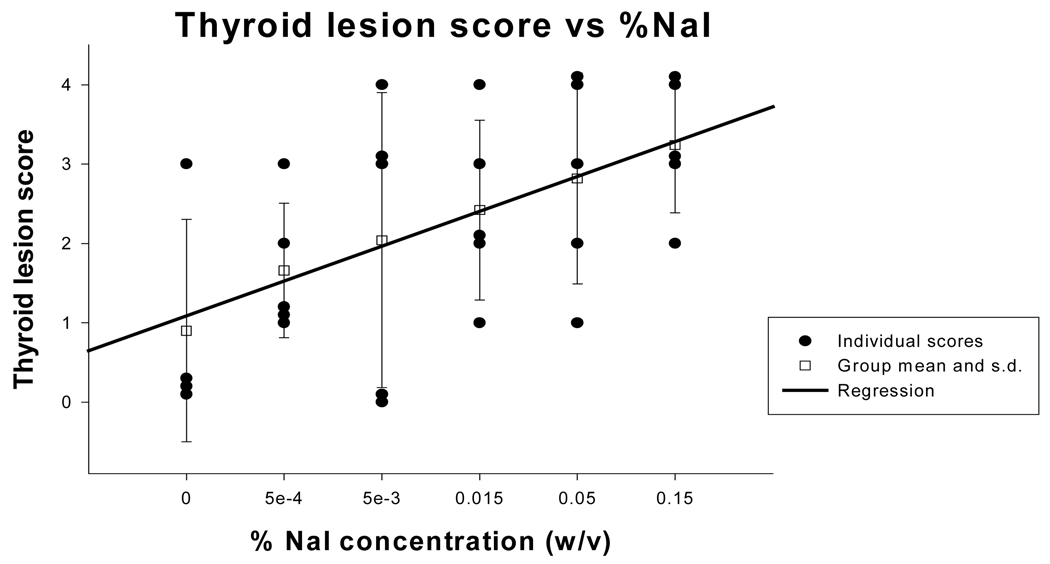
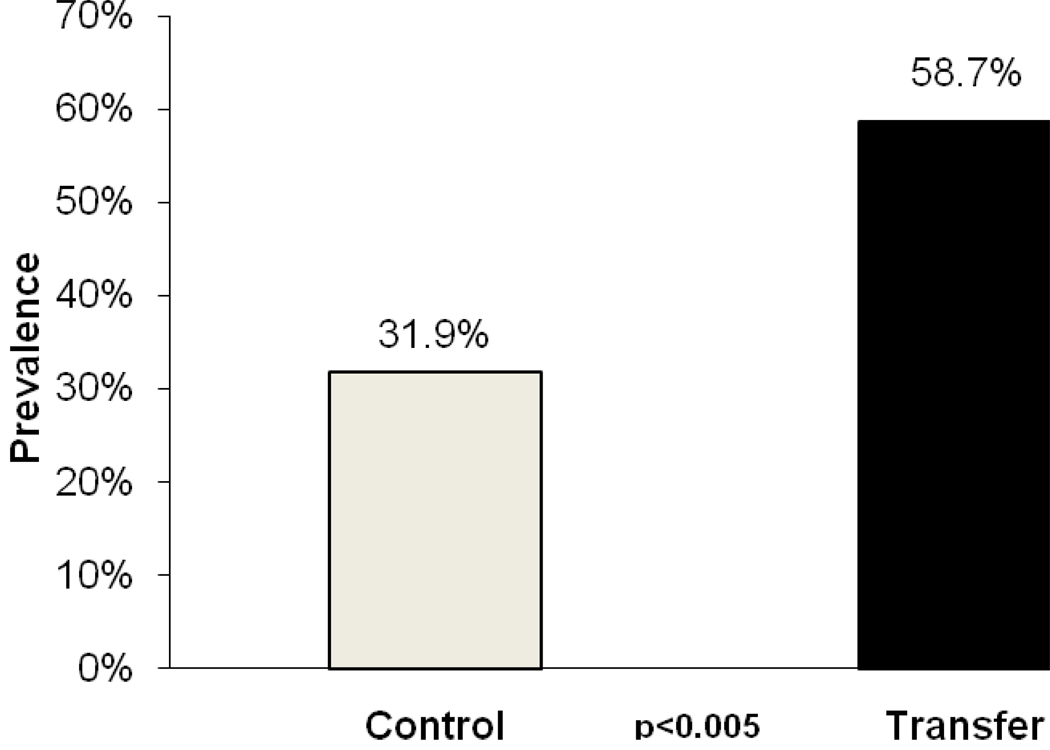
In this NOD.H2h4 mouse model, we validated that the thyroiditis is autoimmune in origin [29]. We showed that there is a mononuclear infiltration in the thyroid gland (Figure 1) with a correlation between the dose of iodine and the severity of disease [30], shown in Figure 2. We further showed that disease could be transferred with spleen cells from NOD.H2h4 mice fed excess iodine in their drinking water into young, non-diseased animals ((Mann-Whitney rank sum test p<.005), results shown in Figure 3.
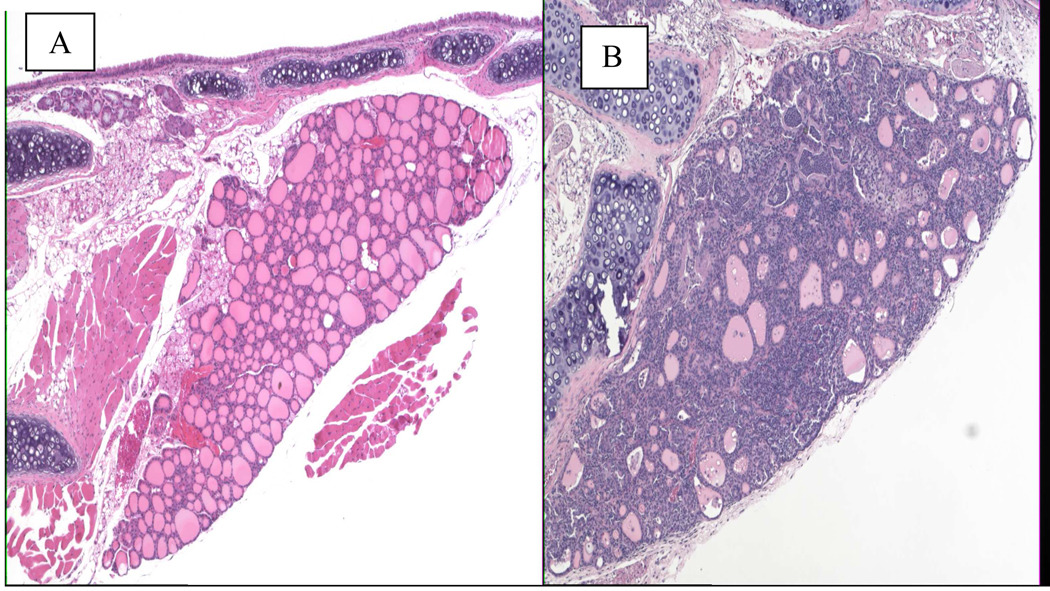
Figure 1.
Thyroid glands from NOD.H2h4 mice. A. normal unaffected gland from an untreated mouse. B. Infiltrated gland (+4) after 8 weeks of treatment with excess iodine in the drinking water.
Figure 2.
- 0 = no infiltration,
- 1+ = infiltration in <20% of the gland,
- 2+ = infiltration from 20–30% of the gland,
- 3+ = infiltration from 30–50% of gland,
- 4+ >50% infiltration of gland
Figure 3.
Combined results of three transfer experiments. All donors received 0.15% NaI water for at least 8 weeks. Spleen cells were cultured for 3 days with 25 ug/ml of mouse thyroglobulin. Transfer group recipients (n=63) received low dose iodine (0.005%w/v) two weeks prior to transfer of spleen cells given i.v. Control mice (n=69) similarly on low dose iodine received PBS instead of cells. Thyroids were assessed for lymphocytic infiltration 14 days after transfer. Conclusions: Thyroiditis can be transferred in this model using spleen cells from mice fed with iodine.
The NOD.H2h4 mouse is a spontaneous model of autoimmune thyroiditis that closely resembles human disease. The histology is similar to that of autoimmune thyroiditis in humans, and is characterized by chronic infiltration of mononuclear cells, including CD4, CD8, B cells, macrophages, and dendritic cells [31–35]. Further, the severity of disease correlates with antibody titers to Tg [32–34]. While excess iodine is not necessary for induction of thyroiditis, the ingestion of excess iodine works to exacerbate autoimmune thyroiditis in this genetically predisposed population.
Role of iodine
One of the striking observations we made in our ongoing studies of the NOD.H2h4 mouse is the constitutive expression of intracellular adhesion molecule-1 adhesion (ICAM-1) on thyrocytes [36]. Bonita et al. found that this expression of ICAM-1 was upregulated upon excess iodine ingestion, both in areas of cellular infiltration but also areas of the thyroid gland full killer cells without areas of cellular infiltration [36]. This suggests that iodine by itself could foster increased expression of ICAM-1. Subsequent work indeed suggested that iodine by itself increased ICAM-1 expression in the thyrocyte [37]. This led us to study the effect of iodide on the thyrocyte itself. Therefore, one mechanism to explain the accelerated mononuclear cell infiltration into the thyroid gland after iodine supplementation involves the local upregulation of ICAM-1. Subsequently, we learned that in the NOD.H2h4 mouse increased iodine consumption leads to an elevated reactive oxygen species (ROS) expression in the thyroid, which may be responsible for the elevated ICAM-1 expression in the thyrocyte [38]. Clearly, excess iodine intake has many roles, one is to increase thyroglobulin immunogenicity while another is to increase adhesion molecules on the thyrocyte itself.
The ICAM-1 promotor has multiple transcription binding sites with at least three different transcriptional initiation sites[39]. Multiple stimuli promote ICAM-1 expression besides cytokines including viruses, radiation, retinoic acid, and oxidants. The major intracellular signal transduction pathways involved in the regulation of ICAM-1 expression include protein kinase C (PKC), mitogen-activated protein (MAP) kinases, and the NF-kB signaling pathway [39]. The nuclear transcription factors important for the activation of ICAM-1 include AP-1, NF-kB, C/EBP, Ets, STAT and Sp1 [39]. Reports indicate that the induction of ICAM-1 transcription occurs rapidly, being detected as early as 30 minutes after stimulation [39]. This finding is in line with the observation that increased ICAM-1 expression is a very early step in the initiation of inflammation. Further, many other stimuli have been shown to facilitate ICAM-1 gene transcription [39].
How then might iodine upregulate ICAM-1 expression? Iodine is taken up by the thyrocyte, organified, and stored on the thyroglobulin molecule through the enzymatic reaction of thyroperoxidase. In doing so, there is the potential generation of ROS such as free radicals and an increase in the hydrogen peroxide generated during the enzymatic reaction. Both of these compounds are known to signal transcription of ICAM-1 [39].
Furthermore, other molecules such as interferon-gamma (IFN-g) act to upregulate ICAM-1 through the IFN-g responsive element (IRE) 100 bp upstream of the translation start site. IFN-g binds to its membrane receptor with subsequent phosphorylation of the Janus kinases, JAK-1 and 2 with subsequent phosphorylation of STAT1/3. The STATs then translocate to the nucleus, bind the IRE promotor sites leading to increased ICAM-1 expression. TNF-a, known to synergistically upregulate ICAM-1 along with IFN-g, apparently works through an independent pathway. This pathway utilizes the activation of NF-kB. Each pathway then works independently, resulting in synergy. In our studies we noted synergy in ICAM-1 expression on thyrocytes from NOD.H2h4 mice transgenic for thyroidal IFN-g [40]. This occurred only when the animals were treated with excess dietary iodine. This observation suggests that iodine and IFN-g also work by different pathways. This new knowledge of how iodine affects ICAM-1 expression may lead to determining new strategies in the quest for thyroiditis prevention in susceptible individuals.
We established that NOD-H2h4 mice have the genetic propensity for thyroiditis and at least one compound, iodine, promotes the onset of thyroiditis. However, as mentioned before, a few animals develop autoimmune thyroiditis even in the absence of excess iodine. This poses the question if there are other environmental agents that promote this disease. These NOD.H2h4 mice are ideal for their use as a model sentinel animal to examine the influence of other environmental compounds on the development of autoimmune thyroiditis.
NOD.H2h4 and other environmental influences
SPF vs Conventional Housing
NOD.H2h4 mice were bred and maintained for most experimental procedures in pathogen free conditions (SPF) at the Johns Hopkins animal facility. A second colony of NODH2h4 mice were taken out of the SPF and bred and maintained in conventional housing at the same facility. Both colonies of mice (SPF and conventional) were maintained according to the guidelines from the Animal Care and Use Committee of the Johns Hopkins University. These untreated NOD.H2h4 mice were sacrificed at different ages and assessed for prevalence of infiltrating lymphocytes by H and E staining of paraffin embedded thyroid glands. The presence of lymphocytic infiltration as an indication of spontaneously developing disease was evaluated by two different examiners. Thyroid lesions were graded as previously described [41]. Mice in both housing facilities received similar care.
Results are presented in Figure 4. We observed that mice in conventional housing developed spontaneous thyroiditis at a low level as early as 20 weeks of age. By 40 weeks of age all mice in the conventional facility showed some degree of disease. The severity of thyroiditis appeared to increase as the animals aged as shown by regression analysis (R2=0.44) (Figure 4A). In contrast, NOD.H2h4 mice maintained in SPF conditions did not develop disease before 30–39 weeks of age (Figure 4B). As the SPF mice aged the number of mice showing spontaneous thyroiditis increased slowly, but only after 39 weeks of age, with an almost 10 fold less regression than their counterparts in conventional housing(R2 = 0.05). However, many mice showed no disease at all. These results show that NOD.H2h4 mice in conventional housing developed a greater frequency of spontaneous thyroiditis over those animals housed under SPF conditions. This is unlike the parental NOD strain wherein a protective environment leads to increased diabetes not decreased (reviewed in [42]). Our data indicate that prevailing intercurrent infection and micro-organisms or other elements in a non-protective environment appear to foster the induction of autoimmune thyroid disease in the NOD.H2h4 mouse.
Figure 4.
Frequency of thyroiditis in untreated NOD.H2h4 mice housed in conventional (Con) or specific pathogen free (SPF) conditions. A. Mice housed in conventional conditiions developed earlier and more severe thyroiditis ( R2= 0.45 than B. NOD.H2h4 mice born and reared under SPF conditions (R2=0.054). Both groups showed increased frequency of thyroiditis with age.
Multipary
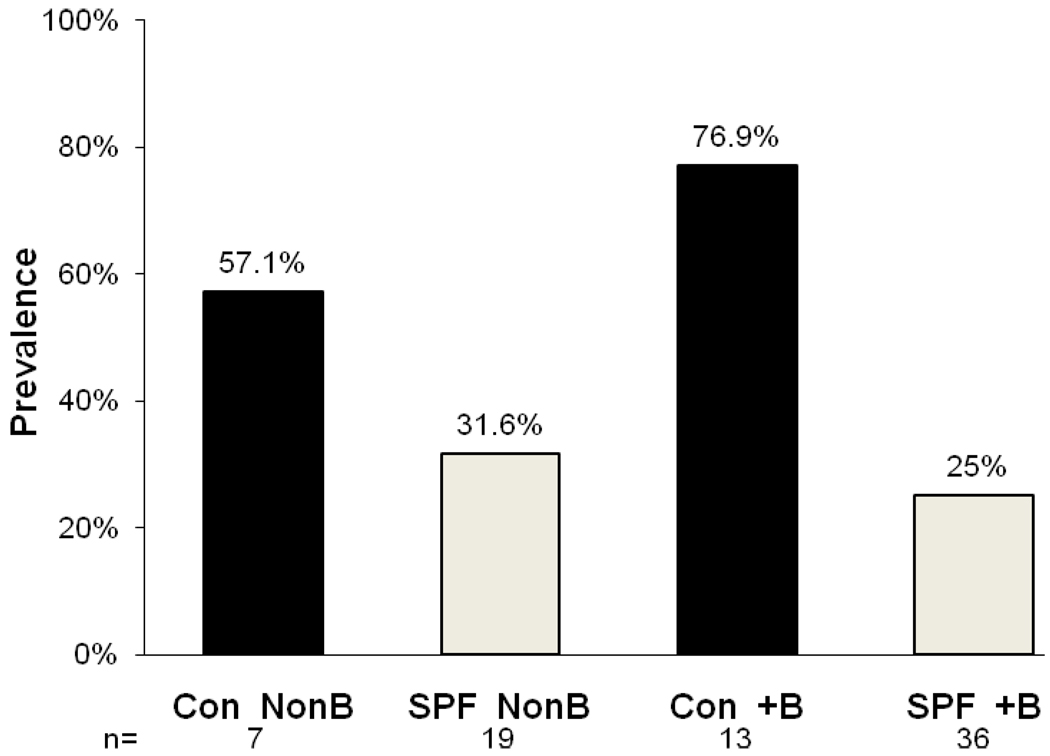
One condition in humans that associates with the onset of autoimmunity is pregnancy. The autoimmune thyroiditis that results from pregnancy is often postpartum and is frequently transient. However this happens primarily in a genetically predisposed population. For example, in women with previously-developed thyroid antibodies, the thyroiditis with resulting hypothyroidism often remains permanent [43]. Therefore, we examined the prevalence of thyroiditis in our NOD.H2h4 breeder colony compared to age matched non-parous female mice raised in either SPF or conventional housing (Figure 5).
Figure 5.
Prevalence of thyroiditis in female breeder (+B) or non-breeder (NonB) NOD.H2h4 mice born and raised in conventional (Con) or specific pathogen free (SPF) conditions. Both breeder and non-breeder groups raised in conventional conditions had a higher frequency of thyroiditis than their SPF counterparts. Conventionally raised breeder mice showed significantly greater thyroiditis over breeder mice raised under SPF conditions (p<0.03, Fisher’s exact test).
Female NOD.H2h4 breeders raised in conventional housing developed significantly more thyroiditis than those breeders raised under SPF conditions (p<0.01, Fisher’s Exact test); the frequency of thyroiditis in the conventionally-raised breeder group was also higher than the conventionally-raised non-breeder group, although the numbers were not sufficient to show significance. Female NOD.H2h4 mice raised in SPF conditions whether they were breeders or not showed similar frequencies of thyroiditis. These results imply that environmental factors when added to other unknown factors induced by pregnancy may promote thyroiditis in these mice.
Chemicals implicated in autoimmune thyroid disease
In a recent review by Brucker-Davis [44] over 90 synthetic chemicals were noted to show disruption of hormone balance or thyroid dysfunction. These chemicals arise from herbicides, insecticides, disinfectants, batteries, smoke, plasticizers, by products of combustion, petroleum, and flame retardants among others. Many of these compounds are widely distributed through the environment. However, only few environmental pollutants show evidence that they contribute to autoimmune thyroid disease. Most animals used in toxicology studies do not have a genetic predisposition for autoimmune disease. We know that in the iodine model of autoimmunity only animal strains with the correct genetic background develop an autoimmune response after excess iodine ingestion. A similar finding could also be true for testing of the other toxic compounds. Therefore, we used the NOD.H2h4 mouse as a model to test the influence of other compounds on the development of thyroiditis.
Polyaromatic Hydrocarbons (PAH)
Epidemiological work has implicated PAH in autoimmune disease in humans. Gaitan et al. have studied the effect of several organic pollutants on the thyroid function. The pollutants are organics produced from coal and found in air and water. In their study they found that these pollutants have potent anti-thyroid effects [45]. Two populations of school children from different geographical locations showed a high prevalence of goiter in equally iodine-sufficient areas [46]. However, only one group developed anti-thyroglobulin and anti-microsomal antigen autoantibodies. The investigators suggested that the PAH pollutants were triggers for the expression of autoimmune thyroiditis in a genetically predisposed population.
This group of chemicals found in smoke from combustion and as by products of petroleum has been shown to promote thyroiditis in rats. Several studies using the BUFF rat, a spontaneous model of thyroiditis, indicate that exposure by methylcholanthrene (MCA) or 7,12-methylbenz(a) anthracene, PAHs, increased the incidence of thyroiditis in younger animals [47,48]. In these studies thyroiditis was determined only by histology. Studies using MCA were continued by Silverman and Rose [49], who showed that the thyroiditis was associated with an autoantibody response to thyroglobulin and was virtually identical to the non-MCA form of the disease. Silverman and Rose pursued this model and found that there was a genetic susceptibility to the MCA-induced thyroiditis [50]. MCA serves as a prototype of a larger number of PAH to study the influence of these compounds on the promotion of thyroiditis in the genetically predisposed model, the NOD.H2h4 mouse. NOD.H2h4mice were over five times more likely to develop thyroiditis when exposed to MCA than their untreated counterparts (Table 1).
Table 1.
Increased risk of thyroiditis in NOD.H2h4 mice after different environmental exposures
| Housing | Treatment | Odds Ratio |
|---|---|---|
| SPF | Bromine | 1.5 |
| Conventional | Bromine | 3.89 |
| Conventional | LPS | 3.9 |
| Conventional | Methylcholanthrene | 5.67 |
| SPF | Iodine | 6.67 |
| Conventional | Iodine | 15.3 |
LPS 20 ug Salmonella enteriditis given twice i.p. at weekly intervals
Bromine 100 mg/l ( 0.01%)water for 16 weeks
Iodine 0.05% NaI in water for 16 weeks
Methylcholanthrene 0.003g/100 g of chow for 16 weeks
SPF Specific Pathogen Free housing
Odds Ratio over untreated controls calculated according to the formula in Sigma Stat™.
Polyhalogenated Biphenyls (PBB)
Polyhalogenated biphenyls, such as polychlorinated biphenyls (PCB) or polybrominated biphenyls (PBB) are commonly used compounds with a wide variety of industrial applications. PBB are used as flame retardants, while PCB are used as lubricants, adhesives, inks and plasticizers. One study in humans found an unexpectedly high prevalence of primary hypothyroidism (11%) in workers from a factory producing PBB and PBB oxides [51]. The hypothyroidism was associated with an elevation of anti-microsomal thyroid antibodies and, less frequently, anti-thyroglobulin antibodies. The increase was highly unusual, especially since the patients were all men. The investigators compared the prevalence to a cohort from Whickham, England [52] and found a significant increase from that group with a p<.001, using the Z test. The investigators concluded that the increase was caused by the exposure to PBB, PBB oxide, or bromine. It may be that the bromine, by itself, may be causing the thyroid dysfunction since KBr or NaBr induces morphological changes in the thyroids of normal rats [53,54].
Using KBr as a surrogate for PBB, we had the capacity to show that bromine may exacerbate autoimmune thyroiditis in an animal with a genetic predisposition, the NOD.H2h4 mouse. Treatment with KBr increased the likelihood of thyroiditis in the NOD.H2h4 mice by a modest 1.5 times in the protected SPF environment (Table 1). However, the odds ratio increased to almost 4 times if the mice were treated with KBr in conventional housing, suggesting that even small “insults” along with exposure to environmental microorganisms may be additive leading to a higher incidence of thyroiditis.
Infection
Infections are reputed to contribute to the initiation of autoimmune disease. Little firm evidence implicates specific viruses or bacteria contributing to the pathogenesis of autoimmune thyroid disease [55]. It may well be that it is the bystander effect of activated T cells and the heightened immune response, in other words the adjuvant effect, that helps trigger autoimmunity. No single organism is responsible because one of multiple microorganisms could produce the same effect. NOD-H2h4 mice were used to test the hypothesis that adjuvants alone are sufficient to trigger autoimmune thyroiditis in a genetically predisposed host. Bacterial lipopolysaccharide (LPS) is a bacterial product that is mitogenic for B cells in vitro but has also been shown to be a potent adjuvant for T cell-dependent antigens [56]. LPS treatment increased the likelihood of spontaneous thyroiditis over that present in untreated controls by almost 4 times (Table 1). While this was the only specific bacterial product tested, each time the treatment in SPF conditions was compared to the same treatment in conventional housing, the odds ratio of thyroiditis increased substantially. This indicates that potentially multiple, even non-life-threatening, normal infections may further act as triggers of thyroiditis in a genetically predisposed population.
Conclusions
There is strong evidence that environmental agents play a critical role in triggering autoimmune disease in genetically susceptible hosts. There is, however, little information about how such agents work. By using this well-defined mouse model, the NOD.H2h4 mouse, and a well-documented environmental trigger, iodine, we have determined at least one mechanism by which the fundamental issue of autoimmune thyroid disease pathogenesis can be established. Using the NOD.H2h4 mouse we have, in addition, established that this mouse model is appropriate for use in testing other environmental triggers of autoimmunity, such as chemicals and microorganisms. Further, we have shown that these triggers can be additive in nature. Even a mild-acting exposure, such as bromine administration, was potentiated by an additional exposure to microorganisms as present in conventional housing. While we have not yet identified specific organisms that promote the development of thyroiditis, we know that this phenomenon exists. Each time an environmental exposure was tested in both SPF and conventionally housed NOD.H2h4 mice, those animals exposed to the non-protected environment of conventional housing had more than a doubling of the likelihood of developing thyroiditis. This interaction between genes and environment may also very well be happening in the human population. However in humans, because of genetic heterogeneity and the diversity of environments and chemicals to which we are all exposed are so different, firmly establishing a causal relationship between environmental triggers and increased development of thyroiditis may be difficult and challenging. The importance of this unique animal model, the NOD.H2h4 mouse, in investigating, assessing, and delineating the contributions of the environmental factors acting as triggers or active contributors to the pathogenesis of autoimmune thyroid disease is of great value to medical and translational research. It is fitting that this issue be devoted to the contributions of Noel Rose and that this paper be focused on thyroiditis. The contributions of Noel Rose to thyroiditis are legion and we note that it is a subject which is continually important not only specifically for the dissection of human autoimmune thyroiditis, but also generically important for autoimmunity and in that respect we cite recent literature from this journal which focuses on this very issue [57–65]. Lastly, we note that this is an issue as part of the Journal of Autoimmunity series devoted to major figures in the field of autoimmunity and immunology and certainly Noel Rose [66–70] fits very prominently for several decades in this unique group [71–74].
Acknowledgements
This work was supported in part by NIH grant R21 ES10285
I wish to thank Noel Rose for his continual support throughout the many years that I have had the privilege to work with him.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical.Immunology &.Immunopathology. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 2.Weetman AP. Hypothyroidism: screening and subclinical disease. BMJ. 1997;314:1175–1178. doi: 10.1136/bmj.314.7088.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–340. doi: 10.1080/08916930410001705394. [DOI] [PubMed] [Google Scholar]
- 4.Burek CL, Rose NR, Najar GM, Gimelfarb A, Zmijewski CM, Polesky HF, Hoffman WH. Autoimmune Thyroid Disease. In: David C, Panayi GS, editors. Clinical Immunology, Volume 1 -Immunogenetics. Kent: Butterworth & Co., Ltd; 1984. pp. 207–233. [Google Scholar]
- 5.Burek CL, Hoffman WH, Rose NR. The presence of thyroid autoantibodies in children and adolescents with autoimmune thyroid disease and in their siblings and parents. Clin.Immunol.Immunopathol. 1982;25:395–404. doi: 10.1016/0090-1229(82)90204-5. [DOI] [PubMed] [Google Scholar]
- 6.Irvine WJ, MacGregor AG, Stuart AE, Hall GH. Hashimoto's disease in uniovular twins. Lancet. 1961;2:850–853. [Google Scholar]
- 7.Oddie TH, Fisher DA, McConahey WM, Thompson CS. Iodine intake in the United States: a reassessment. J.Clin.Endocrinol.Metab. 1970;30:659–665. doi: 10.1210/jcem-30-5-659. [DOI] [PubMed] [Google Scholar]
- 8.Beierwaltes WH. Iodine and lymphocytic thyroiditis. Bull.All.India Med.Sci. 1969;3:145. [Google Scholar]
- 9.Hay ID. Thyroiditis: a clinical update. Mayo.Clin.Proc. 1985;60:836–843. doi: 10.1016/s0025-6196(12)64789-2. [DOI] [PubMed] [Google Scholar]
- 10.Tajiri J, Higashi K, Morita M, Umeda T, Sato T. Studies of hypothyroidism in patients with high iodine intake. J.Clin.Endocrinol.Metab. 1986;63:412–417. doi: 10.1210/jcem-63-2-412. [DOI] [PubMed] [Google Scholar]
- 11.Kampe O, Jansson R, Karlsson FA. Effects of L-thyroxine and iodide on the development of autoimmune postpartum thyroiditis. J.Clin.Endocrinol.Metab. 1990;70:1014–1018. doi: 10.1210/jcem-70-4-1014. [DOI] [PubMed] [Google Scholar]
- 12.Wayne EJ, Koutras DA, Alexander WD. Clinical aspects of iodine metabolism. Blackwell: Oxford; 1964. pp. 107–160. [Google Scholar]
- 13.Zois C, Stavrou I, Kalogera C, Svarna E, Dimoliatis I, Seferiadis K, Tsatsoulis A. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Redox.Rep. 2003;13:485–489. doi: 10.1089/105072503322021151. [DOI] [PubMed] [Google Scholar]
- 14.Kasagi K, Iwata M, Misaki T, Konishi J. Effect of iodine restriction on thyroid function in patients with primary hypothyroidism. Redox.Rep. 2003;13:561–567. doi: 10.1089/105072503322238827. [DOI] [PubMed] [Google Scholar]
- 15.Yoon SJ, Choi SR, Kim DM, Kim JU, Kim KW, Ahn CW, Cha BS, Lim SK, Kim KR, Lee HC, Huh KB. The effect of iodine restriction on thyroid function in patients with hypothyroidism due to Hashimoto's thyroiditis. Yonsei Med.J. 2003;44:227–235. doi: 10.3349/ymj.2003.44.2.227. [DOI] [PubMed] [Google Scholar]
- 16.Saboori AM, Rose NR, Bresler HS, Vladut-Talor M, Burek CL. Iodination of human thyroglobulin (Tg) alters its immunoreactivity. I. Iodination alters multiple epitopes of human Tg. Clin.Exp.Immunol. 1998;113:297–302. doi: 10.1046/j.1365-2249.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saboori AM, Rose NR, Burek CL. Iodination of human thyroglobulin (Tg) alters its immunoreactivity. II. Fine specificity of a monoclonal antibody that recognizes iodinated Tg. Clin.Exp.Immunol. 1998;113:303–308. doi: 10.1046/j.1365-2249.1998.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasooly L, Rose NR, Saboori AM, Ladenson PW, Burek CL. Iodine is essential for human T cell recognition of human thyroglobulin. Autoimmunity. 1998;27:213–219. doi: 10.3109/08916939808993833. [DOI] [PubMed] [Google Scholar]
- 19.Sharma RB, Burek CL, Cihakova D, Njoku DB, Rose NR. Environmental factors in autoimmune endocrinopathies. In: Weetman AP, editor. Autoimmune Diseases in Endocrinology. Totowa, NJ: Humana Press; 2008. pp. 35–75. [Google Scholar]
- 20.Reinhardt W, Paul TL, Allen EM, Alex S, Yang YN, Appel MC, Braverman LE. Effect of L-thyroxine administration on the incidence of iodine induced and spontaneous lymphocytic thyroiditis in the BB/Wor rat. Endocrinology. 1988;122:1179–1181. doi: 10.1210/endo-122-3-1179. [DOI] [PubMed] [Google Scholar]
- 21.Allen EM, Appel MC, Braverman LE. The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology. 1986;118:1977–1981. doi: 10.1210/endo-118-5-1977. [DOI] [PubMed] [Google Scholar]
- 22.Bagchi N, Brown TR, Urdanivia E, Sundick RS. Induction of autoimmune thyroiditis in chickens by dietary iodine. Science. 1985;230:325–327. doi: 10.1126/science.4048936. [DOI] [PubMed] [Google Scholar]
- 23.Sundick RS, Herdegen DM, Brown TR, Bagchi N. The Incorporation of Dietary Iodine into Thyroglobulin Increases Its Immunogenicity. Endocrinology. 1987;120:2078–2084. doi: 10.1210/endo-120-5-2078. [DOI] [PubMed] [Google Scholar]
- 24.Cohen SB, Weetman AP. The effect of iodide depletion and supplementation in the Buffalo strain rat. J.Endocrinol.Invest. 1988;11:625–627. doi: 10.1007/BF03350197. [DOI] [PubMed] [Google Scholar]
- 25.Allen EM, Braverman LE. The effect of iodine on lymphocytic thyroiditis in the thymectomized buffalo rat. Endocrinology. 1990;127:1613–1616. doi: 10.1210/endo-127-4-1613. [DOI] [PubMed] [Google Scholar]
- 26.Ebner SA, Lueprasitsakul W, Alex S, Fang SL, Appel MC, Braverman LE. Iodine content of rat thyroglobulin affects its antigenicity in inducing lymphocytic thyroiditis in the BB/Wor rat. Autoimmunity. 1992;13:209–214. doi: 10.3109/08916939209004826. [DOI] [PubMed] [Google Scholar]
- 27.Vladutiu AO, Rose NR. Autoimmune murine thyroiditis: Relation to histocompatibility (H-2) type. Science. 1971;174:1137–1139. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- 28.Weatherall D, Sarvetnick N, Shizuru JA. Genetic control of diabetes mellitus. Diabetologia. 1992;35 Suppl 2:S1–S7. doi: 10.1007/BF00586273. [DOI] [PubMed] [Google Scholar]
- 29.Burek CL, Talor M, Hill SL, Stafford EA, Barin J, Rose NR. Adoptive transfer of iodine-induced autoimmune thyroiditis in the NOD.H2h4 mouse. FASEB J. 1999;13:A1000. [Google Scholar]
- 30.Burek CL, Talor M, Santana C, Rose NR. Thyroiditis in NOD.H2h4 mice born and reared in conventional housing and ingesting different doses of iodine. FASEB J. 2001;12:A1097. [Google Scholar]
- 31.Bonita RE, Rasooly L, Caturegli P, Rose NR, Burek CL. Characterization of cellular events associated with iodine-induced thyroiditis. Clinical Imm. 1999;90:437. [Google Scholar]
- 32.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clinical.Immunology &.Immunopathology. 1996;81:287–292. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 33.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J.Autoimmun. 1999;12:157–165. doi: 10.1006/jaut.1999.0272. [DOI] [PubMed] [Google Scholar]
- 34.Hutchings PR, Verma S, Phillips JM, Harach SZ, Howlett S, Cooke A. Both CD4(+) T cells and CD8(+) T cells are required for iodine accelerated thyroiditis in NOD mice. Cell Immunol. 1999;192:113–121. doi: 10.1006/cimm.1998.1446. [DOI] [PubMed] [Google Scholar]
- 35.Yu S, Medling B, Yagita H, Braley-Mullen H. Characteristics of inflammatory cells in spontaneous autoimmune thyroiditis of NOD.H-2h4 mice. J.Autoimmun. 2001;16:37–46. doi: 10.1006/jaut.2000.0458. [DOI] [PubMed] [Google Scholar]
- 36.Bonita RE, Rose NR, Rasooly L, Caturegli P, Burek CL. Adhesion molecules as susceptibility factors in spontaneous autoimmune thyroiditis in the NOD-H2h4 mouse. Exp.Mol.Pathol. 2002;73:155–163. doi: 10.1006/exmp.2002.2470. [DOI] [PubMed] [Google Scholar]
- 37.Sharma RB, Alegria JD, Talor MV, Rose NR, Caturegli P, Burek CL. Iodine and IFN-gamma synergistically enhance intercellular adhesion molecule 1 expression on NOD.H2h4 mouse thyrocytes. J.Immunol. 2005;174:7740–7745. doi: 10.4049/jimmunol.174.12.7740. [DOI] [PubMed] [Google Scholar]
- 38.Sharma RB, Traore K, Trush MA, Rose NR, Burek CL. Intracellular adhesion molecule-1 up-regulation on thyrocytes by iodine of NOD.H2h4 mice is reactive oxygen species (ROS) dependent. Clin.Exp.Immunol. 2008;152:13–20. doi: 10.1111/j.1365-2249.2008.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J.Leukoc.Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 40.Sharma RB, Alegria JD, Talor MV, Rose NR, Caturegli P, Burek CL. Iodine and IFN-gamma synergistically enhance intercellular adhesion molecule 1 expression on NOD.H2h4 mouse thyrocytes. J.Immunol. 2005;174:7740–7745. doi: 10.4049/jimmunol.174.12.7740. [DOI] [PubMed] [Google Scholar]
- 41.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin.Immunol.Immunopathol. 1996;81:287–292. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 42.Solomon M, Sarvetnick N. The pathogenesis of diabetes in the NOD mouse. Adv.Immunol. 2004;84:239–264. doi: 10.1016/S0065-2776(04)84007-0. 239-64. [DOI] [PubMed] [Google Scholar]
- 43.Stagnaro-Green A. Recognizing, understanding, and treating postpartum thyroiditis. Endocrinol.Metab Clin.North Am. 2000;29:417–430. doi: 10.1016/s0889-8529(05)70140-7. ix. [DOI] [PubMed] [Google Scholar]
- 44.Brucker-Davis F. Effects of environmental synthetic chemicals on thyroid function. Thyroid. 1998;8:827–856. doi: 10.1089/thy.1998.8.827. [DOI] [PubMed] [Google Scholar]
- 45.Lindsay RH, Hill JB, Gaitan E, Cooksey RC, Jolbey RL. Antithyroid effects of coal-derived pollutants. J. Toxicol. Environ. Health. 1992;37:467–481. doi: 10.1080/15287399209531686. [DOI] [PubMed] [Google Scholar]
- 46.Gaitan E, Cooksey RC, Legan J, et al. Simple goiter and autoimmune thyroiditis: Environmental and genetic factors. Clin.Ecol. 1985;3:158. [Google Scholar]
- 47.Reuber MD. 3-methylcholanthrene-induced thyroiditis in buffalo strain rats. Archives.of.Environmental.Health. 1970;21:734–739. doi: 10.1080/00039896.1970.10667327. [DOI] [PubMed] [Google Scholar]
- 48.Reuber MD, Glover EL. Thyroiditis in Buffalo strain rats ingesting 7,12-dimethylbenz(a)anthracene. Experientia. 1969;25:753. doi: 10.1007/BF01897607. [DOI] [PubMed] [Google Scholar]
- 49.Silverman DA, Rose NR. Autoimmunity in methylcholanthrene-induced and spontaneous thyroiditis in Buffalo strain rats. Proc.Soc.Exp.Biol.Med. 1971;138:579–584. doi: 10.3181/00379727-138-35945. [DOI] [PubMed] [Google Scholar]
- 50.Silverman DA, Rose NR. Spontaneous and methylcholanthrene-enhanced thyroiditis in BUF rats. I. The incidence and severity of the disease, and the genetics of susceptibility. J.Immunol. 1975;114:145–147. [PubMed] [Google Scholar]
- 51.Bahn AK, Mills JL, Synder PJ, Gann PH, Houten L, Bialik O, Hollmann L, Utiger RD. Hypothyroidism in workers exposed to polybrominated biphenyls. New England Journal.of Medicine. 1980;302:31–33. doi: 10.1056/NEJM198001033020105. [DOI] [PubMed] [Google Scholar]
- 52.Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA. The spectrum of thyroid disease in a community: The Whickham survey. Clin.Endocrinol.(Oxf) 1977;7:481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 53.Velicky J, Titlbach M, Lojda Z, Duskova J, Vobecky M, Strbak V, Raska I. Long-term action of potassium bromide on the rat thyroid gland. Acta Histochemica. 1998;100:11–23. doi: 10.1016/S0065-1281(98)80003-2. [DOI] [PubMed] [Google Scholar]
- 54.Loeber JG, Franken MA, van Leeuwen FX. Effect of sodium bromide on endocrine parameters in the rat as studied by immunohistocytochemistry and radioimmunoassay. Food Chem Toxicol. 1983;21:391–404. doi: 10.1016/0278-6915(83)90093-5. [DOI] [PubMed] [Google Scholar]
- 55.Safran M, Paul TL, Roti E, Braverman LE. Environmental factors affecting autoimmune thyroid disease. Endocrinol.Metab.Clin.North.Am. 1987;16:327–342. [PubMed] [Google Scholar]
- 56.Esquivel PS, Rose NR, Kong YC. Induction of autoimmunity in good and poor responder mice with mouse thyroglobulin and lipopolysaccharide. J.Exp.Med. 1977;145:1250–1263. doi: 10.1084/jem.145.5.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akeno N, Blackard JT, Tomer Y. HCV E2 protein binds directly to thhyroid cells and induces IL-8 production; a new mechanism for HCV induced thyroid autoimmunity. J. Autoimmun. 2008;31:339–344. doi: 10.1016/j.jaut.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J. Autoimmun. 2008;30:58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearce SH, Merriman TR. Genetics of type 1 diabetes and autoimmune thyroid disease. Endocrinol. Metab. Clin. North Am. 2009;38:289–301. doi: 10.1016/j.ecl.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Hadj-Kacem H, Rebuffat S, Mnif-Feki M, Belguith-Maalej S, Ayadi H, Peraldi-Roux S. Autoimmune thyroid diseases: genetic susceptibility of thyroid-specific genes and thyroid autoantigens contributions. Int. J. Immunogenet. 2009;36:85–96. doi: 10.1111/j.1744-313X.2009.00830.x. [DOI] [PubMed] [Google Scholar]
- 61.Tomer Y, Menconi F, Davies TF, Barbesino G, Rocchi R, Pinchera A, Concepcion E, Greenberg DA. Dissecting genetic heterogeneity in autoimmune thyroid diseases by subset analysis. J. Autoimmun. 2007;29:69–77. doi: 10.1016/j.jaut.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Ban Y, Tozaki T, Tobe T, Ban YT, Jacobson EM, Concepcion ES, Tomer Y. The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: an association analysis in Caucasian and Japanese cohorts. J. Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 63.Latif R, Morshed SA, Zaidi M, Davies TF. The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. 2009. Endocrinol. Metab. North Am. 2009;38:319–341. doi: 10.1016/j.ecl.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J. Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li HS, Jiang HY, Carayanniotis G. Modifying effects of iodine on the immunogeneicity of thyroglobulin peptides. J. Autoimmun. 2007;28:171–176. doi: 10.1016/j.jaut.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Burek CL, Rose NR. Autoimmune thyroiditis and ROS. Autoimmun Rev. 2008;7:530–537. doi: 10.1016/j.autrev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rose NR. The adjuvant effect in infection and autoimmunity. Clin Rev Allergy Immunol. 2008;34:279–282. doi: 10.1007/s12016-007-8049-7. [DOI] [PubMed] [Google Scholar]
- 69.Rose NR. Prediction and prevention of autoimmune disease: a personal perspective. Ann N Y Acad Sci. 2007;1109:117–128. doi: 10.1196/annals.1398.014. [DOI] [PubMed] [Google Scholar]
- 70.Rose NR. Life amidst the contrivances. Nat Immunol. 2006;7:1009–1011. doi: 10.1038/ni1006-1009. [DOI] [PubMed] [Google Scholar]
- 71.Whittingham S, Rowley MJ, Gershwin ME. A tribute to an outstanding immunologist - Ian Reay Mackay. J. Autoimmun. 2008;31:197–200. doi: 10.1016/j.jaut.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Gershwin ME. Bone marrow transplantation, refractory autoimmunity and the contributions of Susumu Ikehara. J. Autoimmun. 2008;30:105–107. doi: 10.1016/j.jaut.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Blank M, Gershwin ME. Autoimmunity: from the mosaic to the kaleidoscope. J. Autoimmun. 2008;30:1–4. doi: 10.1016/j.jaut.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 74.Mackay IR, Leskovsek NV, Rose NR. Cell damage and autoimmunity: a critical appraisal. J. Autoimmun. 2008;30:5–11. doi: 10.1016/j.jaut.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]