Abstract
Bone, a calcified tissue composed of 60% inorganic component (hydroxyapatite), 10% water and 30% organic component (proteins), has three functions: providing mechanical support for locomotion, protecting vital organs, and regulating mineral homeostasis. A lifelong execution of these functions depends on a healthy skeleton, which is maintained by constant bone remodeling in which old bone is removed by the bone-resorbing cell, osteoclasts, and then replaced by new bone formed by the bone-forming cell, osteoblasts. This remodeling process requires a physical interaction of bone with these bone cells. Moreover, numerous cancers including breast and prostate have a high tendency to metastasize to bone, which is in part attributable to the capacity of the tumor cells to attach to bone. The intensive investigation in the past two decades has led to the notion that the cell-bone interaction involves integrins on cell surface and bone matrix proteins. However, the biochemical composition of bone and emerging evidence are inconsistent with this belief. In this review, I will discuss the current understanding of the molecular mechanism underlying the cell-bone interaction. I will also highlight the facts and new findings supporting that the inorganic, rather than the organic, component of bone is likely responsible for cellular attachment.
Keywords: Bone, hydroxyapatite, Osteoclast, Osteoblast, Breast Cancer, Prostate Cancer
INTRODUCTION
The skeleton represents a unique organ system in the body since, unlike most other organs, the skeletal organ system is composed of a calcified tissue (namely, bone), which consists of about 60% inorganic component (hydroxyapatite), 10% water and 30% organic component (bone matrix proteins) [1-2]. This chemical and biochemical composition of bone renders the skeleton distinct mechanical and biological features which are crucial for its three major functions: providing mechanical support for stature and locomotion, protecting vital organs such as brain and bone marrow, and maintaining mineral homeostasis.
Bone is a dynamic tissue that undergoes constant remodeling (termed bone remodeling) [1,3]. Bone remodeling is a physiological process in which old bone is degraded by osteoclasts, the bone-resorbing cell, and subsequently replaced by new bone formed by osteoblasts, the bone-forming cell [4-7]. The remodeling process plays a crucial role in maintaining a healthy skeleton, which is a prerequisite for a proper execution of the important skeletal functions throughout life [3]. In bone remodeling, differentiation and function of both types of bone cells depend on attachment onto bone [4-7]. Thus, this calcified tissue is not isolated in the body and its maintenance and function requires a close interaction with the cellular components of the body including osteoblasts and osteoclasts.
While the interaction between bone and bone cells is vital for physiologic functions, aberrant interactions between bone and some other cells have been shown to occur in certain pathological conditions. In particular, a number of cancer cells including those of breast and prostate are capable of interacting with bone and the abnormal interactions are implicated in the metastasis of these tumors to bone [8-9].
Given that the cell-bone interaction is not only involved in the regulation of physiologic processes but also implicated in the pathogenesis of various disorders including breast and prostate tumor bone metastases, enormous efforts have been undertaken to elucidate the molecular mechanism underlying the cell-bone interaction in the past several decades. Most previous studies have been focused on adhesion molecules belonging to the integrin family since integrins play an important role in cell-extracellular matrix (ECM) protein interaction and numerous bone matrix proteins contain binding motifs for integrins [2,10-11]. As a result, the current notion is that the cell-bone interaction is primarily mediated by integrins. However, emerging evidence does not support this view. Moreover, the chemical and biochemical composition of bone is also inconsistent with this belief.
In this review, I will first provide an overview of the composition of bone as well as the known adhesion molecules. I will then discuss the current understanding of the molecular mechanisms underlying the interaction of bone with osteoclasts, osteoblasts, breast cancer cells and prostate cancer cells. Based on recent findings and the unique chemical and biochemical composition of bone, I will also provide my perspectives that the inorganic, rather than the organic, component of bone is likely to play a predominant role in mediating the cell-bone interaction. I hope that these discussions will facilitate future studies aimed at elucidating the precise molecular basis of the cell-bone interaction, which will not only provide a better understanding of bone biology but, more importantly, may also reveal new therapeutic targets for various bone disorders including osteoporosis and tumor bone metastasis.
COMPOSITION OF BONE
In order to effectively delineate the molecular mechanism of the cell-bone interaction, it is essential to carefully examine the composition of bone to identify the biochemical and structural features capable of supporting the cellular attachment. Bone is a composite material consisting of both inorganic and organic components [1,2,12]. The inorganic component is primarily crystalline hydroxyapatite: [Ca3(PO4)2]3Ca(OH)2. The organic component of bone comprises more than 30 proteins with type I collagen being the most abundant (>90%) [1,2,12]. By weight, the inorganic constituent accounts for about 60% of the tissue while the organic component makes up approximately 30%. The remaining 10% is water. By volume, the inorganic component, the organic component and water are roughly 40%, 35% and 25%, respectively [1,2,12].
The inorganic component, made of the small plate-shaped (20-50 nm long, 15 nm wide and 2-5 nm thick) hydroxyapatite crystals, contains impurities [1]. The common impurity is carbonate in place of the phosphate groups [13]. Other known substitutions include potassium, magnesium, strontium and sodium in place of the calcium ions, and chloride and fluoride in place of the hydroxyl groups [14-16]. These impurities are believed to decrease the crystallinity of the bone mineral and thus may change certain mineral properties such as solubility, which is important for mineral homeostasis and bone adaptation [1,17].
The organic component of bone largely consists of type I collagen (~90%) and the remaining ~10% noncollagenous proteins [1-2]. Type I collagen is a triple-helical molecule containing three polypeptide chains, each of which is composed of approximately 1000 amino acids. Two of the three polypeptides are identical α1(I) chain and the third one is a structurally similar, but genetically different, α2(I) chain. These polypeptide chains are cross-linked by hydrogen bonding between hydroxylproline and other charged residues to form a very linear molecule of around 300nm in length. These linear molecules are aligned together in a parallel fashion to form collagen fibrils, which are then grouped in bundles to produce the collagen fiber.
While type I collagen represents the major structural component of the bone matrix, noncollagenous proteins, though present in very small quantities in bone, significantly contribute to its biologic function [1-2]. Almost 30 noncollagenous proteins including numerous ECM proteins, growth factors and cytokines have been identified from bone. The physiologic roles for most of the noncollagenous proteins have not fully been elucidated, but they are believed to play an important role in bone matrix mineralization as well as in the regulation of osteoclast and osteoblast function. Specifically, many of the noncollagenous proteins have been shown to control the differentiation and function of the bone cells by supporting their attachment onto bone.
Based on the basic constituents and biochemical organization of bone, the calcified tissue may employ the following major potential mechanisms to interact with cells: 1) as a major component of bone, hydroxyapatite may possess certain biochemical moieties capable of interacting with cell surface proteins; 2) as a major constituent of the organic component, type I collagen represents another important anchor mediating the cellular attachment; and 3) some noncollagenous proteins may be involved in the bone-cell interaction. Importantly, whereas one of these different mechanisms may play a predominant role in the interaction of bone and certain cell types, it is also possible that some cellular interactions with bone involve more than one mechanism.
INTEGRINS IN CELL-BONE INTERACTION
There are four major known classes of cell adhesion molecules: the cadherin family, the immunoglobulin superfamily, the selectin family and the integrin family [18-19]. Cadherins are characterized by the presence of five characteristic cadherin repeats in the extracellular domain and are primarily involved in homotypic cell-cell adhesion. Members of the immunoglobulin superfamily contain varying numbers of immunoglobulin-related domains and play an important role in both homotypic and heterotypic cell-cell adhesions. Selectins are lectin-like adhesion molecules and mediate heterotypic cell-cell interactions via recognition of carbohydrate moieties in the counter-receptors on other cells. Selectins mainly play a role in the adhesion of leukocytes to endothelium. In contrast, integrins are the major known cell surface molecules that have been shown to mediate the interaction between cells and ECM proteins [10-11]. Therefore, most previous studies on the cell-bone interaction have been focused on this class of cell adhesion proteins.
Integrins consist of two transmembrane glycoprotein subunits, the α chain and the β chain [10-11]. The α and β subunits non-covalently interact with each other, forming a functional heterodimer. Many combinations of subunits exist and each combination may bind one or more ligands. Conversely, many ECM proteins can act as ligands for more than one integrin. Integrins interact with ECM proteins through their extracellular domains. Upon binding ligands, integrins are activated, leading to the interaction with components of the cytoskeleton and also intracellular signaling molecules through their intracellular domains [20-21]. Through these interactions, integrins may regulate diverse cellular functions, including cell adhesion, motility, spreading, growth, and differentiation [11].
Bone contains many ECM proteins that have capacity to interact with integrins [2,22]. Type I collagen, the most abundant protein in bone, is a ligand for integrins α1β1, α2β1 and α3β1. Moreover, a number of noncollagenous bone matrix proteins such as osteopontin, bone sialoprotein, bone acidic glycoprotein, thrombospondin and fibronectin contain arginine-glycine-aspartic acid (RGD) sequence, which recognizes integrin αvβ3. Fibronectin can also interact with many other integrins including α3β1, α4β1, α5β1, α8β1, αvβ1, α4β7, αvβ6 and αvβ8 [2,22]. As discussed below, the intensive investigation in the past two decades has led to the current view that several integrins, in particular integrin αvβ3, play a key role in the cell-bone interaction.
CELL-BONE MATRIX INTERACTION IN PHYSIOLOGIC PROCESSES
The cell-bone interaction plays a pivotal role in physiologic bone remodeling which is a lifelong process of bone renewal in which old bone is resorbed by osteoclasts and then replaced by new bone formed by osteoblasts [1,23]. The actions of osteoclasts and osteoblasts in bone remodeling depend on their physical interaction with bone matrix. The constant bone remodeling is important in three ways [3]: 1) bone remodeling is needed to repair fatigue damage. Bone, like other structural materials, is subject to fatigue. After a number of loading cycles, tiny cracks may form. Bone remodeling functions to replace bone containing cracks and prevent structural failure; 2) bone remodeling is required to adapt bone material properties to the mechanical demands that are placed on bones; and 3) finally, bone remodeling plays a critical role in regulating calcium homeostasis. Bone is a major reservoir for calcium. When serum calcium becomes low and there is no intake calcium, calcium needs to be released from bone by osteoclasts to meet the demand [3].
Osteoclast-Bone Interaction
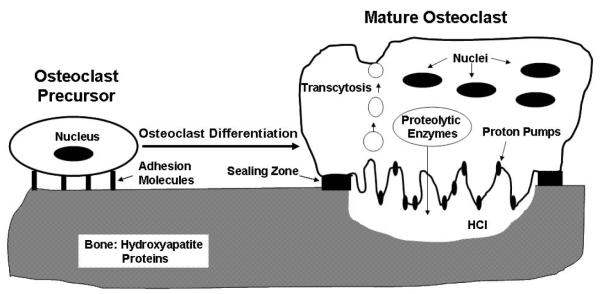
Osteoclasts are multinucleated giant cells derived from mononuclear cells of the monocyte/macrophage lineage [4,24]. Attachment of osteoclast precursors onto bone is a critical prerequisite for osteoclast differentiation [25,26] (Fig. 1). In addition, fully differentiated mature osteoclasts also need to remain attached to resorb bone [4,27-28]. As depicted in Fig. 1, osteoclast function depends on the establishment of a functional resorption compartment, which involves the tight osteoclast attachment onto bone through a special structure called the sealing zone and the formation of the ruffled border membrane. The ruffled border membrane is highly rich in proton pumps which transport protons into the resorption compartment to maintain a low-pH environment that is critical for the dissolution of the inorganic component of bone matrix. Dissolution of the inorganic component of bone is then followed by the degradation of the organic component of bone, which is carried out by various proteolytic enzymes also released through the ruffled border membrane. The sealing zone seals the resorption compartment from its surroundings to prevent the leakage of the protons and proteolytic enzymes. Degraded products are removed outside of the resorption compartment by a process called transcytosis in which degraded products are endocytosed at the ruffled membrane, transported along the transcytotic vesicular pathway, and then released at anti-resorptive side of cells (Fig. 1).
Fig. (1).
Osteoclast Differentiation and Function Depend on Attachment on Bone Matrix
Most previous studies on osteoclast-bone interaction have been mainly focused on integrins, in particular integrin αvβ3, primarily for two reasons: 1) osteoclasts express a variety of integrins, including αvβ3, α2β1 and αvβ1 [29-31] and 2) various bone matrix proteins such as osteopontin, vitronectin, and bone sialoprotein contain the RGD sequence, which is recognized by integrin αvβ3 [25-26]. Consistent with this notion, early in vitro and in vivo assays demonstrated that anti-β3 blocking antibody and RGD-containing peptides blocked osteoclastic bone resorption [32-35], suggesting that integrin αvβ3 may play a functional role in osteoclast attachment on bone. Subsequent studies with β3 knockout (KO) mice indeed confirmed a role for integrin β3 in osteoclast function: the KO mice exhibited an osteosclerotic phenotype due to a functional defect in osteoclasts [36]. Unexpectedly, the KO mice studies revealed that β3 plays a role in osteoclast function by regulating cytoskeleton reorganization, rather than by acting as an anchoring molecule since the β3-/- osteoclasts did not lose the capacity to attach to bone [36-37]. This in vivo data is supported by an independent finding that peptidomimetic antagonists of integrin αvβ3 inhibit osteoclastic bone resorption by impairing osteoclast activity, not by blocking osteoclast attachment onto bone [38].
Osteoclasts also express integrin α2β1 [29-31], which recognizes type I collagen [10,20], suggesting that this integrin may be involved in mediating osteoclast attachment onto bone. It is widely known that osteoclasts can efficiently attach onto dentin/bone slices to resorb bone in in vitro bone resorption assays [37,39-40]. In these dentin/bone slices, bone matrix is not de-mineralized and thus type I collagens are highly packed with minerals. Therefore, it is unlikely that integrin α2β1 and type I collagen play a role in mediating osteoclast attachment onto bone. Thus, the adhesion molecule(s) mediating the interaction between osteoclasts and bone have still not been fully elucidated.
The adhesion molecule(s) involved in regulating the attachment of osteoclast precursors on bone has also not been functionally identified and characterized. It was previously thought that integrin αvβ5 may play a functional role in this adhesion process because integrin β5 subunit is highly homologous to integrin β3 and integrin αvβ5 also recognizes RGD sequence and more importantly it is abundantly expressed by osteoclast precursors [25,41]. More interestingly, integrin β3 and β5 are reciprocally expressed during osteoclast differentiation [42-44]. However, despite the numerous in vitro studies suggesting a role for integrin β5 in the attachment, integrin β5 knockout failed to show bone phenotype and exhibited normal osteoclast differentiation [45], suggesting that other unidentified adhesion molecules may play a role in attachment of osteoclast precursor on bone.
In many pathologic conditions, osteoclast formation and/or function are elevated, leading to excessive destruction of bone. The most prevalent metabolic bone disease, postmenopausal osteoporosis, is caused in part by increased osteoclast activity due to the decline in estrogen levels resulting from the cessation of ovary function in postmenopausal women [46]. Furthermore, osteoclasts are also implicated in bone erosion in rheumatoid arthritis [47]. Finally, osteoclasts have been shown to play an essential role in osteolysis associated with the bone metastases of several tumors including breast and prostate cancers [9]. The future identification and characterization of the adhesion molecules mediating the interaction between osteoclast precursors/mature osteoclasts and bone will not only advance the understanding of osteoclast biology but, more importantly, will also elucidate novel therapeutic targets for various bone diseases such as postmenopausal osteoporosis, bone erosion in rheumatoid arthritis and tumor-induced osteolysis.
Osteoblast-Bone Interaction
Osteoblasts are derived from mesenchymal stem cells (MSC) through a multi-step differentiation pathway [6,48]. MSC give rise to osteoprogenitors, which differentiate into pre-osteoblasts and then mature osteoblasts. In bone remodeling, the replacement of resorbed bone is achieved by osteoblasts derived from MSC and/or osteoprogenitors which are recruited from the marrow [48]. It has been well established that adhesive interaction of MSC and/or osteoprogenitors with extracellular matrix components plays critical roles in osteoblast differentiation, survival, proliferation, and matrix mineralization [49-51], thus indicating that the cell-bone interaction is critical for bone formation in vivo. As depicted in Fig 2, as a result of bone resorption, chemokines embedded in bone are released and activated to recruit mesenchymal progenitors while type I collagen and noncollagenous proteins are de-mineralized and become available to interact with the recruited cells.
Fig. (2).
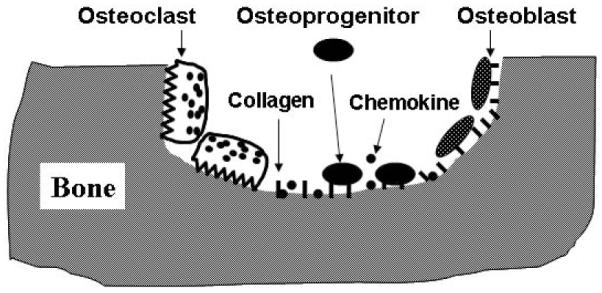
Osteoblast Differentiation and Function in Bone Remodeling
Osteoprogenitor cells and osteoblasts have been shown to express multiple integrins, including α1β1, α2β1, α3β1, α4β1, α5β1, α6β1, α8β1, αvβ3, and αvβ5 [29,52-53]. Among these integrins, α2β1 and α5β1-mediated interactions with ECM proteins have been shown to play an important role in osteoblast differentiation and function. In vitro assays demonstrated that type I collagen promotes the development and maintenance of the osteoblast phenotype [54]. Using type I collagen-specific peptides or antibodies, several groups showed that the disruption of the α2β1-mediated interaction with collagen inhibits the activity of the transcription factor Runx2/Cbfa1, the expression of osteoblast-specific genes, and matrix mineralization [55-58]. Moreover, it was also demonstrated that bioadhesive surface containing GFOGER adhesion motif form type I collagen supports osteoblast attachment and promotes osteoblastic differentiation [59].
Using fibronectin-specific peptides and blocking antibodies against fibronectin and integrins, it was demonstrated that the α5β1-mediated interaction with fibronectin is important for osteoblast attachment, survival, proliferation, osteoblast-specific gene expression, and matrix mineralization [60-63]. These studies support that integrin-ECM protein interactions, particularly the α5β1-fibronectin and α2β1-collagen type I interactions, play a critical role in mediating the osteoblast-bone interaction in bone remodeling. Since type I collagen is abundantly present in bone matrix whereas fibronectin is very scarce, the integrin interaction with type I collagen is likely to play a predominant role in the interaction between osteoblasts and bone.
While the elucidation of the molecular mechanism underlying the osteoclast-bone interaction may reveal novel therapeutic targets for various bone diseases involving elevated osteoclast formation and function, a better understanding of the molecular mechanism of the osteoblast-bone interaction may facilitate the development of biologically active implants and grafting materials for more efficient osteointegration and enhanced bone repair.
CELL-BONE MATRIX INTERACTION IN PATHOLOGIC PROCESSES
Bone metastases are a frequent complication of cancer, occurring in almost 70% of patients with advanced breast and prostate carcinomas and in 15-30% of patients with other types of cancers such as the carcinoma of the lung, bladder, colon/rectum, stomach, uterus and kidney [64]. It is estimated that around 350,000 people die with bone metastases each year in the US [9]. Bone metastases often lead to serious clinical consequences such as life-threatening hypercalcemia, spinal core compression, pathologic fractures and extreme bone pain. More devastatingly, patients with bone metastases have poor prognosis: only 20% of breast cancer patients live for more than five years after the initial discovery of bone metastasis [64] and about half of prostate cancer patients with bone metastases after radical prostatectomy do not survive for more than 5 years [65]. Thus, bone metastases are a prevalent and grave complication of many cancers.
However, the precise molecular mechanism underlying the preferential metastasis of these cancers to bone has not been fully elucidated [9,66]. Over 100 years ago, to explain the high preference of breast cancer to metastasize to bone, Stephen Paget proposed the “Seed and Soil” hypothesis writing that “the evidence seems to be irresistible that in cancer of the breast, the bones suffer in a special way, which cannot be explained by any theory of embolism alone.” Paget’s hypothesis emphasizes an essential point: the preferential metastasis of breast cancer to bone is mediated primarily by events after extravasation of breast cancer cells from the bloodstream - recruitment of breast cancer cells to bone via chemotaxis and/or the unique ability of breast cancer cells to preferentially attach onto bone via adhesion molecules expressed on the surface of the tumor cells. Thus, cell-bone interaction may be implicated in the pathogenesis and development of bone metastases of various cancers including breast, prostate, lung, kidney, thyroid and skin.
Currently, we still lack effective and safe drugs for preventing and treating cancer bone metastases [67-68]. Given that the cancer cell-bone interaction is an essential event in cancer bone metastases, an effective interruption of the cancer cell-bone interaction has long been recognized as an attractive therapeutic strategy for preventing and treating breast and prostate cancer bone metastases. Identification and functional characterization of the key adhesion molecule(s) mediating the adhesive event can greatly facilitate the development of better therapeutics for preventing and treating cancer bone metastases.
Breast Cancer-Bone Interaction
Normal and cancerous breast cells express many integrins including α2β1, α3β1, α5β1, α6β4, α6β1, αvβ3, αvβ1 and αvβ5 [69-78]. Moreover, while integrins α2β1, α5β1, αvβ3 are expressed at lower levels in breast cancers compared to the normal cells, the expression of other integrins such as α6β4, α6β1, αvβ1 and αvβ5 has been shown to be unchanged or increased in the tumor cells compared to the normal cells [70,73-77]. The altered expression of these integrins is implicated in the development and progression of the breast carcinoma [79-81].
While bone biologists were actively searching for the adhesion molecule(s) mediating osteoclast-bone interaction, cancer biologists were also highly interested in elucidating the molecular mechanism by which breast cancer cells attach onto bone. Given that the numerous in vitro assays supporting a role for integrin αvβ3 in anchoring osteoclasts onto bone, cancer biologists quickly turned to focus on investigating whether integrin αvβ3 is also involved in the breast cancer cell-bone interaction. Subsequently, it was shown that a peptidomimetic antagonist of integrin αvβ3 inhibits breast cancer cell line MDA-MB-435 bone metastasis [82-84]. As a result, partially due to its perceived role in the osteoclast-bone interaction and its expression in breast cancer cells, currently integrin αvβ3 has also been considered to be a major molecule mediating breast cancer cell-bone interaction [85]. However, since β3 KO mice studies have ruled out a role for β3 in attachment of osteoclasts onto bone, integrin αvβ3 is likely involved in breast cancer bone metastasis by regulating metastatic processes other than the attachment of breast cancer cells onto bone.
Prostate Cancer-Bone Interaction
Normal and cancerous prostate cells express numerous integrin subunits including α2, α3, α4, α5, α6, α7, αv, αIIb, β1, β3, β4 and β6 [86-99]. These subunits may form many different combinations of functional integrin heterodimers with the binding specificity for various ECM ligands. Moreover, the expression of these α and β subunits has been shown to be down-regulated or unaltered in cancer cells compared to normal tissues. Thus, the integrin-mediated interactions with ECM proteins have been shown to play important roles in prostate cancer cell survival, proliferation, migration and tissue invasion, which are all important to the tumor development and progression [100-103].
Much work has been performed to specifically explore the role of these integrins in prostate cancer cell-bone interaction, leading to the current view that integrin αvβ3 plays a major role in modulating the interaction between prostate cancer cells and bone [103-104]. Consistent with this notion, the expression of αvβ3 has been shown to be up-regulated during tumor progression into a metastatic stage, and LM609, a blocking αvβ3 antibody, inhibits DU145 prostate cancer cell adhesion to ECM proteins such as osteopontin and vitronectin [99,105]. Several in vivo studies also support the view. For instance, intratumoral administration of liposome-encapsulated human αv-siRNA significantly inhibits the growth of PC-3 prostate tumor cells in skeleton, which was associated with decreased integrin αv subunit expression and increased apoptosis in tumor cells [106]. Another recent mouse model study demonstrated that the expression of fully functional αvβ3 promoted tumor growth in bone, whereas inactive mutants failed to do so [104], further suggesting that integrin αvβ3 may play a role in prostate cancer cell-bone interaction.
However, since β3 KO mice studies have ruled out a role for β3 in attachment of osteoclasts onto bone, a similar concern applies to the notion that integrin αvβ3 mediates the interaction between prostate cancer cells and bone. The two mouse models discussed above may still support a role for integrin αvβ3 in prostate cancer bone metastasis, but the integrin is likely to regulate metastatic events other than the attachment of prostate cancer cells onto bone. It is worthwhile to point out that the focus of the investigation on integrin αvβ3 was probably influenced by the findings supporting a role for this integrin in osteoclast-bone interaction and further by subsequent studies establishing a role of the integrin in breast cancer cell-bone interaction.
Since prostate cancer cells express integrin α2β1 and type I collagen accounts for 90% of the bone matrix proteins, integrin α2β1 may play a role in mediating attachment of the tumor cells onto bone. Supporting this possibility, PC-3 prostate cancer cells exhibited greater proliferation rates on type I collagen compared to plastic or fibronectin substrates [107]. Recently, it was reported that only bone metastatic prostate cancer cells attach to type I collagen, while tumor cells from visceral metastases failed to do so [108]. Moreover, a collagen-binding variant of LNCaP cells, but not the parental LNCaP cells, exhibited the capacity to develop tumors in bone when injected into the tibia of nude mice [108].
CONCLUSIONS AND PERSPECTIVES
Despite the intensive investigation on the adhesion molecule(s) mediating the cell-bone interaction, we have still not fully elucidated the molecular basis of the cell-bone interaction. One possible reason is arguably that the previous studies have been focused too much on integrins. Based on the known roles of integrins in cell-ECM protein interaction and the presence of many ECM proteins in bone, it is very logically expected that this family of adhesion molecules plays a role in the cell-bone interaction. However, the quantity and accessibility of these ECM proteins in bone matrix were not adequately considered in many of the previous studies aimed at understanding the biochemical basis of the cell-bone interaction.
As described above, by volume, bone consists of 40% inorganic component (hydroxyapatite), 25% water and 35% organic component (proteins) [1,2,12]. 90% of the organic component are collagen type I and the remaining 10% noncollagenous proteins. Only some of noncollagenous proteins such as osteopontin, vitronectin, bone sialoprotein and dentin matrix protein-1 contain a RGD. Osteopontin, vitronectin, bone sialoprotein and dentin matrix protein-1 are 330-a.a., 476-a.a., 324-a.a. and 513-a.a. long, respectively. Let’s assume that all the noncollagenous proteins contain a RGD and the average length of non-collagen proteins is 300-a.a (i.e.: RGD represent 1% of non-collagen proteins). This would lead to the estimation that only 0.03% of bone contents (35% × 10% × 1%) are actually RGD sequences. This also means that only 0.035% of the bone surface area contains RGD sequences. It is unlikely that this scarce amount of RGD on the bone surface is sufficient for integrin-dependent cellular attachment.
Moreover, the presence of a certain ECM protein in bone matrix may not necessarily mean that it is available for interaction with cell surface proteins. For instance, type I collagen is abundantly present in bone matrix, but it is highly packed in its triple-helical form and tightly surrounded by hydroxyapatite minerals in compact bone, preventing it from interacting with other proteins. Type I collagen becomes accessible only after it has been de-mineralized as a result of bone resorption by osteoclasts. Many of the previous studies were performed to determine whether cells of interest are capable of attaching onto ECM proteins coated on tissue culture dish. One should be cautious to deduce a conclusion regarding cell-bone interaction from the studies which merely examine the interaction between cells and ECM proteins coated on tissue dishes.
Based on the basic constituents and biochemical organization of bone described above, there are three major possible mechanisms by which the calcified tissue may interact with cells: 1) as a major component of bone, hydroxyapatite may interact with cells; 2) as a major constituent of the organic component of bone, type I collagen represents another important anchor mediating cellular attachment; 3) some noncollagenous proteins may be involved in bone-cell interaction. As reviewed above, most previous studies have primarily been focused on exploring the potential role of the noncollagenous proteins in cell-bone interaction. However, the scarcity of these noncollagenous proteins undermines its significance in the adhesive process. Although type I collagen is abundant in bone and is a nature ligand for integrin α1β1, α2β1 and α3β1, its biochemical form and organization in compact bone prevents it from serving as a anchoring molecule. For instance, it is unlikely that type I collagen is involved in mediating osteoclast-bone interaction even though osteoclasts express integrin α2β1 since osteoclasts are able to attach onto mineralized bone surface to initiate bone resorption. Nonetheless, type I collagen may become available to interact with integrin α1β1 and α2β1 on cell surface after minerals are dissolved and the compact triple-helical organization is dismantled as a result of osteoclastic bone resorption. As demonstrated by some studies, the osteoblast-bone interaction involves type I collagen and integrin α2β1.
However, as a major component of bone, hydroxyapatite has not received enough attention as a potential molecular moiety responsible for cellular attachment. Moreover, the limited success in the previous search for functional molecules mediating the cell-bone interaction encourages us to redirect our focus in the future efforts in identifying the adhesive molecule(s). In particular, we may need to embark on paradigm shifts from the organic component to the inorganic component as the principal substratum supporting cell anchoring, and from integrins to a different/novel class of proteins possessing hydroxyapatite-binding properties as the major adhesion molecules mediating the cell-bone interaction. Indeed, many known proteins including those found in bone matrix such as bone sialoprotein exhibit a high binding affinity for hydroxyapatite [2,109]. Bone sialoprotein binds to hydroxyapatite through polyglutamic acid regions [109]. It would not be totally a surprise if some cell surface proteins have hydroxyapatite-binding properties. Moreover, it may be further speculated that some of the impurities found in the inorganic component of bone may also play a role in supporting cell anchoring.
ACKNOWLEDGEMENTS
The work in the author’s laboratory is supported by Grant Number AR47830 from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and a pilot grant from the Breast Cancer SPORE (Specialized Programs of Research Excellence) at the University of Alabama at Birmingham. The author would like to thank Jason Ashley and Erin McCoy for their critical reading of this article.
REFERENCES
- [1].Morgan EF, Barnes GL, Einhorn TA. In: Osteoporosis. Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Academic Press; San Diego: 2008. pp. 3–26. [Google Scholar]
- [2].Zhu W, Robey PG, Boskey AL. In: Osteoporosis. Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Academic Press; San Diego: 2008. pp. 191–240. [Google Scholar]
- [3].Parfitt AM. In: Osteoporosis. Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Academic Press; San Diego: 2008. pp. 71–92. [Google Scholar]
- [4].Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- [5].Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nature Reviews Genetics. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- [6].Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- [7].Lian J, Stein GS. In: Osteoporosis. Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Academic Press; San Diego: 2008. pp. 93–150. [Google Scholar]
- [8].Orr FW, Sanchez-Sweatman OH, Kostenuik P, Singh G. Tumor-bone interactions in skeletal metastasis. Clin. Orthop. Relat Res. 1995;312:19–33. [PubMed] [Google Scholar]
- [9].Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Reviews Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- [10].Sonnenberg A. Integrins and their ligands. Curr. Top. Microbiol. Immunol. 1993;184:7–35. doi: 10.1007/978-3-642-78253-4_2. [DOI] [PubMed] [Google Scholar]
- [11].Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- [12].Baron R. In: Primer on the metabolic bone diseases and disorders of mineral metabolism. Favus MJ, editor. ASBMR; Washington DC: 2003. pp. 1–8. [Google Scholar]
- [13].Doi Y, Aoba T, Okazaki M, Takahashi J, Moriwaki Y. Analysis of paramagnetic centers in X-ray-irradiated enamel, bone, and carbonate-containing hydroxyapatite by electron spin resonance spectroscopy. Calcif. Tissue Int. 1979;28:107–112. doi: 10.1007/BF02441228. [DOI] [PubMed] [Google Scholar]
- [14].Brommage R, Neuman WF. Passive accumulation of magnesium, sodium, and potassium by chick calvaria. Calcif. Tissue Int. 1979;28:57–63. doi: 10.1007/BF02441218. [DOI] [PubMed] [Google Scholar]
- [15].McConnell D, Foreman DW, Jr., Drew I, Perkins D, Jr., Daly P. Texture and composition of bone. Science. 1971;172:971–973. doi: 10.1126/science.172.3986.971. [DOI] [PubMed] [Google Scholar]
- [16].McConnell D. Bone Mineral. Science. 1964;145:1336. doi: 10.1126/science.145.3638.1336-b. [DOI] [PubMed] [Google Scholar]
- [17].Ou-Yang H, Paschalis EP, Mayo WE, Boskey AL, Mendelsohn R. Infrared microscopic imaging of bone: spatial distribution of CO3(2-) J. Bone Miner. Res. 2001;16:893–900. doi: 10.1359/jbmr.2001.16.5.893. [DOI] [PubMed] [Google Scholar]
- [18].Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- [19].Hynes RO. Cell adhesion: old and new questions. Trends Cell Biol. 1999;9:M33–M37. [PubMed] [Google Scholar]
- [20].Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- [21].Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annual Review of Cell & Developmental Biology. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- [22].Siebers MC, ter Brugge PJ, Walboomers XF, Jansen JA. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials. 2005;26:137–146. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- [23].Martin TJ, Rodan GA. In: Osteoporosis. Marcus R, Feldman D, Kelsey J, editors. Academic Press; San Diego: 2001. pp. 361–372. [Google Scholar]
- [24].Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Revs. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- [25].Teitelbaum SL. Osteoclasts, integrins, and osteoporosis. Journal of Bone & Mineral Metabolism. 2000;18:344–349. doi: 10.1007/s007740070007. [DOI] [PubMed] [Google Scholar]
- [26].Kadoya Y, Kobayashi A, Ohashi H. Wear and osteolysis in total joint replacements. Acta Orthop Scand. 1998;69:1–16. [PubMed] [Google Scholar]
- [27].Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J. Biol. Chem. 1999;274:7724–7731. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- [28].Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J. Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- [29].Clover J, Dodds RA, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992;92:267–271. doi: 10.1242/jcs.103.1.267. [DOI] [PubMed] [Google Scholar]
- [30].Shinar DM, Schmidt A, Halperin D, Rodan GA, Weinreb M. Expression of αvand β3 integrin subunits in rat osteoclasts in situ. J. Bone Miner. Res. 1993;8:403–414. doi: 10.1002/jbmr.5650080404. [DOI] [PubMed] [Google Scholar]
- [31].Nesbitt S, Nesbit A, Helfrich M, Horton M. Biochemical characterization of human osteoclast integrins. Osteoclasts express αvβ3, α2β1 and αvβ1 integrins. J. Cell Biol. 1993;268:16737–16745. [PubMed] [Google Scholar]
- [32].Ihde DC. Chemotherapy of lung cancer. New England Journal of Medicine. 1992;327:1434–41. doi: 10.1056/NEJM199211123272006. [DOI] [PubMed] [Google Scholar]
- [33].Sato M, Sardana MK, Grasser WA, Garsky VM, Murray JM, Gould RJ. Echistatin is a potent inhibitor of bone resorption in culture. J. Cell Biol. 1990;111:1713–1723. doi: 10.1083/jcb.111.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fisher JE, Caulfield MP, Sato M, et al. Inhibition of osteoclastic bone resorption in vivo by echistatin, an “arginyl-glycyl-aspartyl” (RGD)-containing protein. Endocrinol. 1993;132:1411–1413. doi: 10.1210/endo.132.3.8440195. [DOI] [PubMed] [Google Scholar]
- [35].Engleman VW, Nickols GA, Ross FP, et al. A peptidomimetic antagonist of the αvβ3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J. Clin. Invest. 1997;99:2284–2292. doi: 10.1172/JCI119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McHugh KP, Hodivala-Dilke K, Zheng MH, et al. Mice lacking β3 integrins are osteosclerotic due to dysfunctional osteoclasts. J. Clin. Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Feng X, Novack DV, Faccio R, et al. A Glanzmann’s mutation in β3 integrin specifically impairs osteoclast function. J. Clin. Invest. 2001;107:1137–1144. doi: 10.1172/JCI12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Carron CP, Meyer DM, Engleman VW, et al. Peptidomimetic antagonists of αvβ3 inhibit bone resorption by inhibiting osteoclast bone resorptive activity, not osteoclast adhesion to bone. J. Endocrinol. 2000;165:587–598. doi: 10.1677/joe.0.1650587. [DOI] [PubMed] [Google Scholar]
- [39].Liu W, Xu D, Yang H, et al. Functional identification of three RANK cytoplasmic motifs mediating osteoclast differentiation and function. J. Biol. Chem. 2004;279:54759–54769. doi: 10.1074/jbc.M404687200. [DOI] [PubMed] [Google Scholar]
- [40].Xu D, Wang S, Liu W, Liu J, Feng X. A novel RANK cytoplasmic motif plays an essential role in osteoclastogenesis by committing macrophages to the osteoclast lineage. J. Biol. Chem. 2006;281:4678–4690. doi: 10.1074/jbc.M510383200. [DOI] [PubMed] [Google Scholar]
- [41].Sago K, Teitelbaum SL, Venstrom K, Reichardt LF, Ross FP. The integrin αvβ5 is expressed on avian osteoclast precursors and regulated by retinoic acid. J. Bone Miner. Res. 1999;14:32–38. doi: 10.1359/jbmr.1999.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Inoue M, Ross FP, Erdmann J, Abu-Amer Y, Teitelbaum SL. Tumor necrosis factor a regulates αvβ5 integrin expression by osteoclast precursors in vitro and in vivo. Endocrinol. 2000;141:284–290. doi: 10.1210/endo.141.1.7285. [DOI] [PubMed] [Google Scholar]
- [43].Inoue M, Namba N, Chappel J, Teitelbaum SL, Ross FP. Granulocyte macrophage-colony stimulating factor reciprocally regulates αv-associated integrins on murine osteoclast precursors. Mol. Endocrinol. 1998;12:1955–1962. doi: 10.1210/mend.12.12.0213. [DOI] [PubMed] [Google Scholar]
- [44].Feng X, Teitelbaum SL, Quiroz M, Towler DA, Ross FP. Cloning of the murine β5 integrin subunit promoter. Identification of a novel sequence mediating granulocyte-macrophage colony-stimulating factor-dependent repression of β5 integrin gene transcription. J. Biol. Chem. 1999;274:1366–1374. doi: 10.1074/jbc.274.3.1366. [DOI] [PubMed] [Google Scholar]
- [45].Huang X, Griffiths M, Wu J, Farese RV, Jr., Sheppard D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol. Cell. Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pacifici R. In: Osteoporosis. Marcus R, Feldman, Kelsey J, editors. Academic Press; San Diego: 2001. pp. 85–103. [Google Scholar]
- [47].Goldring SR. Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology. 2003;42(Suppl 2):ii11–ii16. doi: 10.1093/rheumatology/keg327. [DOI] [PubMed] [Google Scholar]
- [48].Myers DE, Collier FM, Minkin C, et al. Expression of functional RANK on mature rat and human osteoclasts. FEBS Letters. 1999;463:295–300. doi: 10.1016/s0014-5793(99)01650-6. [DOI] [PubMed] [Google Scholar]
- [49].Damsky CH. Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone. 1999;25:95–96. doi: 10.1016/s8756-3282(99)00106-4. [DOI] [PubMed] [Google Scholar]
- [50].Weiss RE, Reddi AH. Role of fibronectin in collagenous matrix-induced mesenchymal cell proliferation and differentiation in vivo. Exp. Cell Res. 1981;133:247–254. doi: 10.1016/0014-4827(81)90316-5. [DOI] [PubMed] [Google Scholar]
- [51].Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev. Biol. 2000;220:2–15. doi: 10.1006/dbio.2000.9633. [DOI] [PubMed] [Google Scholar]
- [52].Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J. Bone Miner. Res. 1997;12:1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- [53].Hultenby K, Reinholt FP, Heinegard D. Distribution of integrin subunits on rat metaphyseal osteoclasts and osteoblasts. Eur. J. Cell Biol. 1993;62:86–93. [PubMed] [Google Scholar]
- [54].Lynch MP, Stein JL, Stein GS, Lian JB. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp. Cell Res. 1995;216:35–45. doi: 10.1006/excr.1995.1005. [DOI] [PubMed] [Google Scholar]
- [55].Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the α2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J. Biol. Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- [56].Jikko A, Harris SE, Chen D, Mendrick DL, Damsky CH. Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. J. Bone Miner. Res. 1999;14:1075–1083. doi: 10.1359/jbmr.1999.14.7.1075. [DOI] [PubMed] [Google Scholar]
- [57].Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J. Cell Physiol. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [58].Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J. Biochem. 2001;129:133–138. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]
- [59].Reyes CD, Garcia AJ. α2β1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J. Biomed. Mater. Res. A. 2004;69:591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- [60].Puleo DA, Bizios R. RGDS tetrapeptide binds to osteoblasts and inhibits fibronectin-mediated adhesion. Bone. 1991;12:271–276. doi: 10.1016/8756-3282(91)90075-t. [DOI] [PubMed] [Google Scholar]
- [61].Moursi AM, Damsky CH, Lull J, et al. Fibronectin regulates calvarial osteoblast differentiation. J. Cell Sci. 1996;109:1369–1380. doi: 10.1242/jcs.109.6.1369. [DOI] [PubMed] [Google Scholar]
- [62].Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J. Cell Sci. 1997;110:2187–2196. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- [63].Globus RK, Doty SB, Lull JC, Holmuhamedov E, Humphries MJ, Damsky CH. Fibronectin is a survival factor for differentiated osteoblasts. J. Cell Sci. 1998;111:1385–1393. doi: 10.1242/jcs.111.10.1385. [DOI] [PubMed] [Google Scholar]
- [64].Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- [65].Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- [66].Roodman GD. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- [67].Brown J, Neville-Webbe H, Coleman RE. The role of bisphosphonates in breast and prostate cancers. Endocrine-Related Cancer. 2004;11:207–224. doi: 10.1677/erc.0.0110207. [DOI] [PubMed] [Google Scholar]
- [68].Keller ET, Dai J, Escara-Wilke J, et al. New trends in the treatment of bone metastasis. J. Cell Biochem. 2007;102:1095–1102. doi: 10.1002/jcb.21540. [DOI] [PubMed] [Google Scholar]
- [69].Koukoulis GK, Howeedy AA, Korhonen M, Virtanen I, Gould VE. Distribution of tenascin, cellular fibronectins and integrins in the normal, hyperplastic and neoplastic breast. J Submicrosc Cytol Pathol. 1993;25:285–295. [PubMed] [Google Scholar]
- [70].Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by β1 integrins in normal human breast epithelium but not in breast carcinoma. J. Cell Sci. 1995;108(Pt 5):1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- [71].Zutter MM, Mazoujian G, Santoro SA. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol. 1990;137:863–870. [PMC free article] [PubMed] [Google Scholar]
- [72].Green LJ, Mould AP, Humphries MJ. The integrin β subunit. Int. J. Biochem. Cell Biol. 1998;30:179–184. doi: 10.1016/s1357-2725(97)00107-6. [DOI] [PubMed] [Google Scholar]
- [73].Zutter MM, Krigman HR, Santoro SA. Altered integrin expression in adenocarcinoma of the breast. Analysis by in situ hybridization. Am. J. Pathol. 1993;142:1439–1448. [PMC free article] [PubMed] [Google Scholar]
- [74].Damjanovich L, Fulop B, Adany R, Nemes Z. Integrin expression on normal and neoplastic human breast epithelium. Acta Chir Hung. 1997;36:69–71. [PubMed] [Google Scholar]
- [75].Meyer T, Marshall JF, Hart IR. Expression of αv integrins and vitronectin receptor identity in breast cancer cells. Br. J. Cancer. 1998;77:530–536. doi: 10.1038/bjc.1998.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of α6β4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin. Cancer Res. 1998;4:407–410. [PubMed] [Google Scholar]
- [77].Jones JL, Royall JE, Critchley DR, Walker RA. Modulation of myoepithelial-associated α6β4 integrin in a breast cancer cell line alters invasive potential. Exp. Cell Res. 1997;235:325–333. doi: 10.1006/excr.1997.3662. [DOI] [PubMed] [Google Scholar]
- [78].Wewer UM, Shaw LM, Albrechtsen R, Mercurio AM. The integrin α6β1 promotes the survival of metastatic human breast carcinoma cells in mice. Am. J. Pathol. 1997;151:1191–1198. [PMC free article] [PubMed] [Google Scholar]
- [79].Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc. Soc. Exp. Biol. Med. 1999;222:124–138. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- [80].Berry MG, Gui GP, Wells CA, Carpenter R. Integrin expression and survival in human breast cancer. Eur. J. Surg. Oncol. 2004;30:484–489. doi: 10.1016/j.ejso.2004.01.016. [DOI] [PubMed] [Google Scholar]
- [81].White DE, Muller WJ. Multifaceted roles of integrins in breast cancer metastasis. J. Mammary. Gland. Biol. Neoplasia. 2007;12:135–142. doi: 10.1007/s10911-007-9045-5. [DOI] [PubMed] [Google Scholar]
- [82].Pecheur I, Peyruchaud O, Serre CM, et al. Integrin αvβ3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002;16:1266–1268. doi: 10.1096/fj.01-0911fje. [DOI] [PubMed] [Google Scholar]
- [83].Liapis H, Flath A, Kitazawa S. Integrin αvβ3 expression by bone-residing breast cancer metastases. Diagn. Mol. Pathol. 1996;5:127–135. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- [84].Harms JF, Welch DR, Samant RS, et al. A small molecule antagonist of the αvβ3 integrin suppresses MDA-MB-435 skeletal metastasis. Clin. Exp. Metastasis. 2004;21:119–128. doi: 10.1023/b:clin.0000024763.69809.64. [DOI] [PubMed] [Google Scholar]
- [85].Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- [86].Allen MV, Smith GJ, Juliano R, Maygarden SJ, Mohler JL. Downregulation of the β4 integrin subunit in prostatic carcinoma and prostatic intraepithelial neoplasia. Hum. Pathol. 1998;29:311–318. doi: 10.1016/s0046-8177(98)90109-5. [DOI] [PubMed] [Google Scholar]
- [87].Bonkhoff H, Stein U, Remberger K. Differential expression of α6 and α2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum. Pathol. 1993;24:243–248. doi: 10.1016/0046-8177(93)90033-d. [DOI] [PubMed] [Google Scholar]
- [88].Davis TL, Cress AE, Dalkin BL, Nagle RB. Unique expression pattern of the α6β4 integrin and laminin-5 in human prostate carcinoma. Prostate. 2001;46:240–248. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fornaro M, Manzotti M, Tallini G, et al. β1c integrin in epithelial cells correlates with a nonproliferative phenotype: forced expression of β1c inhibits prostate epithelial cell proliferation. Am. J. Pathol. 1998;153:1079–1087. doi: 10.1016/s0002-9440(10)65652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Fornaro M, Tallini G, Bofetiado CJ, Bosari S, Languino LR. Down-regulation of β1c integrin, an inhibitor of cell proliferation, in prostate carcinoma. Am. J. Pathol. 1996;149:765–773. [PMC free article] [PubMed] [Google Scholar]
- [91].Fornaro M, Tallini G, Zheng DQ, Flanagan WM, Manzotti M, Languino LR. p27(kip1) acts as a downstream effector of and is coexpressed with the β1c integrin in prostatic adenocarcinoma. J. Clin. Invest. 1999;103:321–329. doi: 10.1172/JCI4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Goel HL, Moro L, King M, et al. β1 integrins modulate cell adhesion by regulating insulin-like growth factor-II levels in the microenvironment. Cancer Res. 2006;66:331–342. doi: 10.1158/0008-5472.CAN-05-2588. [DOI] [PubMed] [Google Scholar]
- [93].Knox JD, Cress AE, Clark V, et al. Differential expression of extracellular matrix molecules and the α6-integrins in the normal and neoplastic prostate. Am. J. Pathol. 1994;145:167–174. [PMC free article] [PubMed] [Google Scholar]
- [94].Abu-Amer Y. IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-kappaB. J. Clin. Invest. 2001;107:1375–1385. doi: 10.1172/JCI10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kaneko H, Mehrotra M, Alander C, Lerner U, Pilbeam C, Raisz L. Effects of prostaglandin E(2) and lipopolysaccharide on osteoclastogenesis in RAW 264.7 cells. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77:181–186. doi: 10.1016/j.plefa.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Islam S, Hassan F, Tumurkhuu G, et al. Bacterial lipopolysaccharide induces osteoclast formation in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007;360:346–351. doi: 10.1016/j.bbrc.2007.06.023. [DOI] [PubMed] [Google Scholar]
- [97].Hayashi S, Tsuneto M, Yamada T, et al. Lipopolysaccharide-induced osteoclastogenesis in Src homology 2-domain phosphatase-1-deficient viable motheaten mice. Endocrinol. 2004;145:2721–2729. doi: 10.1210/en.2004-0172. [DOI] [PubMed] [Google Scholar]
- [98].Jiang Y, Mehta CK, Hsu TY, Alsulaimani FF. Bacteria induce osteoclastogenesis via an osteoblast-independent pathway. Infect. Immun. 2002;70:3143–3148. doi: 10.1128/IAI.70.6.3143-3148.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- [100].Sung SY, Chung LW. Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation. 2002;70:506–521. doi: 10.1046/j.1432-0436.2002.700905.x. [DOI] [PubMed] [Google Scholar]
- [101].Stewart DA, Cooper CR, Sikes RA. Changes in extracellular matrix (ECM) and ECM-associated proteins in the metastatic progression of prostate cancer. Reprod. Biol. Endocrinol. 2004;2:2. doi: 10.1186/1477-7827-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenvironments. J. Cell Biochem. 2004;91:686–705. doi: 10.1002/jcb.10702. [DOI] [PubMed] [Google Scholar]
- [103].Languino L, Goel H, Kogan S, Li J. Integrins in Prostate Cancer Progression. Endocr. Relat Cancer. 2008 doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin αvβ3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zheng DQ, Woodard AS, Tallini G, Languino LR. Substrate specificity of αvβ3 integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J. Biol. Chem. 2000;275:24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- [106].Bisanz K, Yu J, Edlund M, et al. Targeting ECM-integrin interaction with liposome-encapsulated small interfering RNAs inhibits the growth of human prostate cancer in a bone xenograft imaging model. Mol. Ther. 2005;12:634–643. doi: 10.1016/j.ymthe.2005.05.012. [DOI] [PubMed] [Google Scholar]
- [107].Kiefer JA, Farach-Carson MC. Type I collagen-mediated proliferation of PC3 prostate carcinoma cell line: implications for enhanced growth in the bone microenvironment. Matrix Biol. 2001;20:429–437. doi: 10.1016/s0945-053x(01)00159-7. [DOI] [PubMed] [Google Scholar]
- [108].Hall CL, Dai J, van Golen KL, Keller ET, Long MW. Type I collagen receptor (α2β1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Res. 2006;66:8648–8654. doi: 10.1158/0008-5472.CAN-06-1544. [DOI] [PubMed] [Google Scholar]
- [109].Tye CE, Rattray KR, Warner KJ, et al. Delineation of the hydroxyapatite-nucleating domains of bone sialoprotein. J. Biol. Chem. 2003;278:7949–7955. doi: 10.1074/jbc.M211915200. [DOI] [PubMed] [Google Scholar]