Abstract
How do people spell the thousands of words at the tips of their tongues? Are words with regular sound-to-letter correspondences (e.g., “blink”) spelled using the same neural systems as those with irregular correspondences (e.g., “yacht”)? By offering novel neuroimaging evidence, we aim to advance contemporary debate about whether people use a single lexical memory process or whether dual mechanisms of lexical memory and sublexical phonological rules work in concert. We further aim to advance understanding of how people read by taking a fresh look at the related yet distinct capacity to spell. During functional magnetic resonance imaging scanning, 12 participants heard low-frequency regular words, irregular words, and nonwords (e.g., “shelm”) and responded whether a visual presentation of the word was spelled correctly or incorrectly. While behavioral measures suggested some differences in accuracy and reaction time for the different word types, the neuroimaging results alone demonstrated robust differential processing and support a dual-route model of spelling, with implications for how spelling is taught and remediated in clinical and educational contexts.
Big and small, people hold thousands of words in memory that they can spell in a flash. How does spelling work? Spelling, like reading and writing, is a crucial component of functional literacy. Although there is a growing understanding of the cognitive representations, processes, and neural correlates underlying reading (e.g., Fiez, Balota, Raichle, & Petersen, 1999; Jobard, Crivello, & Tzourio-Mazoyer, 2003), there have been very few cognitive investigations of how spelling is represented and processed (e.g., Frith, 1980; Masterson & Apel, 2006; Treiman & Bourassa, 2000). Even fewer studies can be found on the neural foundations of spelling in the brain of literate adults; indeed, two exist to our knowledge, both with only indirect measures of spelling (Bitan et al., 2005; Booth et al., 2004). Little is also known about how the capacity to spell relates to the overall reading process, and how spelling may contribute to the acquisition and promotion of successful reading and language processes. Here, we report a novel functional magnetic resonance imaging (fMRI) study that examines the neural systems and processes underlying our human capacity to spell. We further articulate how an understanding of the neural mechanisms associated with spelling can advance research and practice across the fields of language, reading, and education.
Any complete model of the reading process must account for people’s ability to read and spell the different types of words that exist in language. In English, for example, regular words such as “blink” follow grapheme-to-phoneme conversion (GPC) (correspondence) rules. Irregular words, including “of” and “love,” do not follow GPC rules and thus must be memorized. Further, readers and spellers encounter novel nonwords, such as “surp,” which follow the letter string (orthographic) conventions of English but are meaningless and unfamiliar.
One looming question in contemporary reading research concerns the nature of both the cognitive processing and the neural systems underlying reading. Is there a single process for reading all words (regardless of their orthographic patterns and grammatical word class), or do different types of words require separate systems? The single-route model postulates that all words are read through a single process involving lexical memory (involving whole-word or word-specific memory; e.g., Harm & Seidenberg, 2004; Seidenberg & McClelland, 1989). By contrast, others have proposed that reading involves two routes: a lexical memory component and a second sub-lexical (subword, or phonological) rule –based process (e.g., Coltheart, Curtis, Atkins, & Haller, 1993; Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001; Pinker, 1997, 1999; Ullman et al., 1997). Like single-route models, dual-route models hypothesize that particular words, such as irregular words, are stored and retrieved from lexical memory. Unlike single-route models, however, dual-route models also propose that novel words and many regular words are processed using sublexical GPC rules to encode and decode words. Many iterations of the dual-route model further propose that familiar, high-frequency regular words may be stored in lexical memory or assembled based on GPC rules.
In studies of the cognitive processes underlying word reading in adults, the single-route model has received support chiefly from computational modeling, as computer models have successfully simulated the accuracy and latency effects of frequency and consistency in reading, as well as for some patterns of language impairment. Seidenberg and McClelland’s (1989) model suggested a system of reading in which a word is read based on orthographic and phonological connections to other known words. Connections are based on the consistency of words, that is, the number of words with similar orthographic patterns, even though these patterns may be considered irregular in terms of GPC rules (such as “ight”). In order to read a word, the orthography is matched to other known words and then the letters or letter strings are matched with the corresponding trained phonological units. Here, when many words with a similar pattern are known, the output will be faster and more accurate; thus, the model accurately reproduces effects of frequency and orthographic regularity that correspond to the behavioral patterns of normal readers (Kreiner & Gough, 1990). More recent proposals use sublexical processes to individuate the onset, vowel, and coda of words and match them to stored phonemes to more accurately read nonwords and replicate language impairment (Harm & Seidenberg, 2004; Plaut, McClelland, Seidenberg, & Patterson, 1996). The commonality among these single-route models is that they posit that orthography, phonology, and semantics influence reading, but that these cues combine to form a single parallel process for decoding words.
Recent neuroimaging studies of reading in adults that tested single- and dual-route models have yielded findings that are important but do not always agree. A metanalysis of 35 fMRI and positron emission topography (PET) studies of word reading found a variety of left-hemisphere areas that are commonly activated in reading tasks (Jobard et al., 2003; see also Palmer, Brown, Petersen, & Schlaggar, 2004). Results showed no brain area with greater activation for real words versus nonwords; this suggests that the initial step of the reading process is common to all word types and most likely located in the left occipitotemporal region, often referred to as the visual word form area (VWFA). The metanalysis also revealed separable, route-specific neural activation. Superior temporal areas, supramarginal gyrus (SMG), and left frontal operculum were activated in tasks requiring GPC. In tasks that required lexical and semantic word access, as with irregular words, activated areas included VWFA, basal inferior temporal cortex, posterior middle temporal gyrus (MTG), and the triangular part of the inferior frontal gyrus (IFG).
PET neuroimaging research has further supported the existence of effects of regularity, frequency, and lexicality on single-word reading (Fiez et al., 1999). Participants showed significant behavioral and neuroanatomical differences in reading high- and low-frequency regular and irregular words, as well as nonwords. Overall, neuroimaging studies of reading support both common areas that take part in reading all words and separable routes for lexical and assembled processes.
Regarding spelling, a debate has similarly focused on whether all words are spelled via a single lexical memory process or whether two mechanisms of lexical memory and sublexical phonological conversion work in concert. The handful of cognitive studies involving the development of spelling in chil dren have suggested that a dual-route model might be most appropriate to explain children ’s spelling production. Some children rely more heavily on sublexical phonological conversion rules and thus perform better on nonwords than on irregular words. Others rely more heavily on lexical memory and can spell irregular words but have more difficulty with nonwords (Treiman, 1984; Treiman & Baron, 1983; Varnhagen, McCallum, & Burstow, 1997). Overall, children tended to use GPC rules for spelling real words and nonwords and a lexical memory strategy to spell irregular words (Treiman). In addition, many children go through a period in spelling development when they demonstrate the ability to correctly spell but not to read highly regular words (Bradley & Bryant, 1985). In contrast, irregular words can often be read but not spelled correctly. These findings suggest that lexical and sublexical processes develop separately, even from the early stages of spelling acquisition.
Cognitive studies of spelling in adults have also demonstrated that word regularity has an effect on spelling and reading. Adults spell irregular words less quickly and accurately than regular words only when the words are of low frequency (Kreiner, 1992, 1996; Kreiner & Gough, 1990). This is consistent with a basic premise of the dual-route model: that high-frequency words may be stored and retrieved from lexical memory, whereas low-frequency words are either recalled from lexical memory or assembled via the sublexical route, depending on their orthographic regularity (Seymour, 1992).
From the neuropsychological literature, several adult patients with brain damage have demonstrated unique spelling abilities, such as the ability to successfully spell regular words and nonwords, but not irregular words, and vice versa (Beauvois & Dérouesné, 1981; Goodman & Caramazza, 1986; Rapcsak & Beeson, 2004; Rapp, Epstein, & Tainturier, 2002). When the lexical route is damaged, people have been observed to overregularize the spellings of common irregular words. Conversely, damage to the phonological route prevents accurate spelling of nonwords, though some regular words remain intact, suggesting that they may be stored as whole units. The fact that lexical versus phonological impairments can occur separately implies that the lexical and sublexical routes in spelling may be neurally separable. Although neuropsychological studies of brain-damaged patients have yielded important insights into the cognitive processes involved in spelling, given the variability of lesion sites, we still do not have a complete understanding of the neural systems and cognitive processes involved in normal adult spelling.
In contrast to the numerous neuroimaging studies of reading, there has been a great paucity of neuroimaging studies of spelling. To date, we identified only two related fMRI studies with implications for spelling whereupon phonological and orthographic processing was investigated using a rime-matching task (Bitan et al., 2005; Booth et al., 2004), although the authors did not explicitly set out to study spelling. The researchers hypothesized that phonological processing (a rhyming task) would activate the superior temporal gyrus (STG), orthographic processing (most closely related to spelling) would activate the fusiform gyrus, semantic processing would activate the MTG, and interactions between these processes would be modulated by parietal regions including the SMG and angular gyrus (AG). In both auditory and visual rime-matching tasks, adults showed activation in left IFG (Brodmann area [BA] 45/46) and anterior cingulate. For the visual task, adults showed additional bilateral AG and fusiform activation, with left activation stronger than right in both areas. Thus, although these studies did not directly test spelling, the findings lend support for the hypothesis that spelling involves neurally separable routes in phonological and lexical processing.
In the present study, we directly examine questions about both the cognitive and the neural processes involved in spelling. First, how is it that people successfully spell the many irregular and regular words they know and also perform well when spelling novel words and nonwords? Second, does the brain process the spelling of all words, regardless of regularity or lexicality, in the same way? Together, do the behavioral and neuroimaging findings support a single- or dual-route model of spelling?
To test these questions, participants’ behavioral and neurological responses to spelling low-frequency regular and irregular words, as well as nonwords, were measured in a block design fMRI study. We intentionally implemented three design features that would give us confidence that we would forefront the participants’ processing that underlies spelling, first and foremost, in addition to any other processing that might occur (e.g., the reading neural network, mental imagery, associated sensations/memories, and the like, which is true for all such studies). First, before entering the scanner, participants were intentionally primed for spelling by being instructed that they would be spelling (with other procedures in the spelling task explained). Second, participants in the scanner first heard a word and were asked to think of its spelling. This approximates an important feature of what people typically do when they spell in the real world. One hears a word (either in one’s own head or articulated by others) and then thinks about how to spell it (before, say, writing it in a text). Third, participants saw a visual presentation of a possible spelling of the word and were asked to judge whether it was correct or incorrect. This incorporates the other important feature of what people typically do when they spell: After thinking about how to spell a word, one often writes it down or visualizes the letters (for visual analysis/feedback) and decides whether one has achieved the right spelling or not (or, one may read a word only to be arrested by the fact that it is spelled wrong, which sets off a search for its correct spelling).
Behavioral accuracy and reaction time (RT), as well as functional neuroimaging data, were analyzed in order to determine if regular and irregular words (which differed in orthographic regularity but had equal length and frequency), as well as nonwords, were processed the same or differently. To ensure that all word types had comparable familiarity at the start, both our use of only low-frequency lexical items and our testing of nonwords were intentional experimental design features. First, the use of low-frequency regular and irregular words is an important control because of the fact that high-frequency words (regardless of their grammatical word type) may achieve a level of processing automaticity. Here, frequent exposure may cause any high-frequency word (be it regular or irregular) to be allocated to lexical memory as a whole, which, although optimal for rapid/automatic processing, may thus obscure any earlier processing by assembly of sublexical (phonological) units due to grammatical word type (regular and irregular). Second, the use of nonwords is another important control because nonwords do not exist in the lexicon and thus cannot be stored in lexical memory.
Taken together, single- and dual-route models have addressed both the acquisition of new words and the processing (including retrieval) of words from stored knowledge, with different studies focusing on one or the other while always recognizing that both processes are interrelated. In the present research, we experimentally focus on the processes that underlie the spelling of words from stored knowledge, which has implications for the types of knowledge that participate in the acquisition of spelling. To determine whether the ability to spell is made up of parts, what the relation these parts have to language and reading, and under what conditions these parts may be used, we empirically manipulated the participants’ spelling of different classes of words and their frequency. Here, the critical question is not whether phonological cues versus lexical memory exist. Nor is it whether they are used during the spelling of a new word (acquisition) versus an old word (retrieval). Instead, with the new lens afforded by neuroimaging, the key question concerns the nature and scope of the processing that underlies spelling: What parts of brain processing make up the remarkable capacity to spell and under what conditions are these parts recruited?
The single-route model of spelling suggests the hypothesis that all words are spelled using a single process that combines orthography, phonology, and semantics. Thus, according to a single-route model, we would predict that regular words, irregular words, and nonwords should be spelled with equal accuracy and RT. Predicted areas of neuroanatomical activation for all words under a single-route model include left fusiform gyrus, left temporal –occipital cortex, and left inferior frontal cortex (Fiez et al., 1999; Jobard et al., 2003; Palmer et al., 2004).
Alternatively, the dual-route model would suggest that irregular and high-frequency regular words can be stored in memory, but low-frequency regular words and nonwords are spelled using a sublexical phonological assembly process. In addition, low-frequency regular and irregular words might show different accuracy, RT, and neural activation due to their differential processing. Nonwords could show yet a different pattern than real words because they differ in lexicality and thus may demonstrate more effortful processing because they are completely novel and not related to established semantic knowledge. Neural activation for the lexical and sublexical routes is predicted to be different: Irregular words should activate areas as predicted for the single-route lexical memory model (left fusiform gyrus, left temporal –occipital cortex, left inferior frontal cortex). Regular and nonwords are predicted to activate areas associated with phonological processing (STG, insular), as well as manipulating (searching, retrieving, selecting) a phonological response, related to left inferior frontal cortex.
METHOD
Participants
Twelve adults successfully completed this study (5 women, 7 men; mean age = 21 years, range 19–22 years). All were healthy, right-handed, native speakers of English with no exposure to other languages before age 12 and no reported history of neurological, language, reading, or spelling disorders. Prior to the beginning of the study, participants were asked if they had any hearing difficulties and were tested with a sample of sound to ensure that they could reliably hear the word stimuli through the headphones irrespective of the scanner noise. Three additional participants were scanned, but their data were unusable due to equipment failure (no behavioral data recorded for two participants, and one of the headphones did not properly work for a third participant). Consequently, behavioral and fMRI data from 12 participants who successfully completed the study are included in the analyses below. Participants received a small compensation for their time and gave informed consent in full accordance with the ethical standards and protocols of the Dartmouth College Committee for the Protection of Human Subjects.
Procedure
Before entering the scanner, participants were instructed that they would be spelling real words and nonwords. Participants were told that they would first hear a word, that they should think of the correct spelling, and then respond whether the spelling on the screen was correct. They were to press the left-hand button if the word was spelled correctly (or plausibly in the case of nonwords, which can have more than one spelling) and the right-hand button if it was spelled incorrectly/implausibly. These same instructions were repeated on the screen before each experimental run. Regular and irregular words had only one correct spelling in American English, and nonword stimuli judgments were based on whether the spelling of the word was plausible or implausible in English. For example, for the auditory nonword [jem] (rhymes with aim), “jame” would be a plausible spelling, while “jym” would be implausible.
Prerecorded auditory stimuli, spoken by a monolingual English-speaking male, were played through headphones the participant wore while in the scanner. One second after the onset of the auditory presentation of a word (all words took less than 1 s to produce), participants saw a possible spelling of the word on the screen. Once the visual spelling appeared on the screen, it remained there for 2 s, during which time participants could respond. This paradigm was based on the observations that spelling in the visual and auditory modalities is largely comparable and that previous studies of lexical processing used most successfully mixed auditory and visual stimuli (Booth et al., 2002, 2004; Shaywitz et al., 2003).
The study used a block design with five runs. Each run included a block of regular words, irregular words, and non-words, with rest breaks (fixation) between each block. Order of the blocks was counterbalanced across runs. Each block consisted of six trials (3 s each) for a total of 18 words per run and a total of 30 of each type of word presented. Runs began with 24 s of fixation, a brief reminder of the instructions on the screen (e.g., “left hand = correct, right hand = incorrect”), and the three blocks of stimuli (18 s each) with intervening 25 s rest/fixation periods. Blocks were preceded by presentation of either “real words” or “nonwords” on the screen; participants were not alerted to the distinction between regular and irregular real words. Total time for each run was 2 min 16 s. Behavioral measures of response accuracy and RT were recorded online with Psyscope software and a Psyscope button box (Cohen, MacWhinney, Flatt, & Provost, 1993).
All regular and irregular words, and most nonwords, were drawn from stimuli previously standardized and used by Fiez et al. (1999) to study effects of regularity and lexicality. As noted above, very low-frequency words (fewer than 30 occurrences per million) were selected to prevent the confound of high-frequency regular words that might be stored in lexical memory as whole units. Regular and irregular words were matched for frequency, and all words were matched for number of letters and syllables (Kucera & Francis, 1967; see Table 1). Stimuli consisted of nouns (75%), verbs (15%), and adjectives (10%). Half of the visually presented spellings were correct. Each block of six words contained two to four incorrectly spelled words. All incorrect spellings of regular and irregular words were plausible grapheme combinations but not real words in English (e.g, “vace” for vase and “wrust” for rust).
Table 1.
Means and Standard Deviations (SD) for Number of Letters, Number of Syllables, and Written Frequency Across Word Types
| Letters | Syllables | Frequency | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Regular words | 4.76 | 1.04 | 1.30 | 0.53 | 7.60 | 7.48 |
| Irregular words | 4.76 | 0.94 | 1.33 | 0.48 | 7.10 | 7.14 |
| Nonwords | 4.70 | 0.92 | 1.23 | 0.43 | ||
Note. Frequencies listed are occurrences per million words (Kucera & Francis, 1967). Note that nonwords, by definition, do not have frequency.
Neuroimaging Parameters and Analyses
Neuroimaging was performed on a 1.5-T whole-body scanner (GE Medical Systems Signa, Milwaukee, WI) with a standard head coil. Stimuli were generated using an Apple G4 Laptop computer running PsyScope software (Cohen et al., 1993). Stimuli were projected to participants with an Epson (model ELP-7000) LCD projector onto a screen positioned at the head end of the bore. Participants viewed the screen through a mirror. A fiber-optic, light-sensitive key press interfaced with the PsyScope Button Box (New Micros, Dallas, TX) was used to record participants’ behavioral performance while in the scanner. In order to minimize ambient noise and present the audio stimuli, participants wore pneumatic headphones (ER-30, Etymotic Research) fitted with foam ear inserts. Cushions were used to minimize head movement.
Imaging
Anatomical images were acquired using a high-resolution, three-dimensional spoiled gradient recovery sequence (124 sagittal slices, TE (Echo Time) = 3 ms, TR (Repetition Time) = 7.6 ms, flip angle = 15°, voxel size = 1 × 1 × 1.2 mm). A brief localizer scan was conducted prior to functional scans, and a high-definition scan was conducted after the acquisition of functional images. Functional images were collected in runs using a gradient spin-echo, echo-planar sequence sensitive to blood oxygen level dependent (BOLD) contrast (T2*) (TR = 2000 ms, T2* evolution time = 20 ms, flip angle = 90°, 3.75 × 3.75 mm in-plane resolution). The acquired axial images allowed complete brain coverage (5.5-mm slice thickness, 1-mm skip between slices).
Analyses
fMRI data were analyzed using Statistical Parametric Mapping software (SPM99, Welcome Department of Cognitive Neurology, London, UK; Friston et al., 1995). For each functional run, standard preprocessing methods were used to remove sources of noise and artifact. Functional data were corrected for differences in acquisition time between slices for each whole-brain volume. To correct for any head motion, functional data were realigned within and across runs with SPM99 tools before coregistering with each participant’s anatomical data. Importantly, none of the participants were rejected because of motion. Functional data were then transformed into a standard anatomical space (2-mm isotropic voxels) based on the International Consortium for Brain Mapping (ICBM) 152 brain template (Montreal Neurological Institute; Evans et al., 1992). Normalized data were then spatially smoothed (6-mm full-width half-maximum) using a Gaussian kernel. Analyses involved formation of statistical images, regional analysis of hemodynamic responses, and random effects group analysis methods.
RESULTS
Behavioral
Accuracy
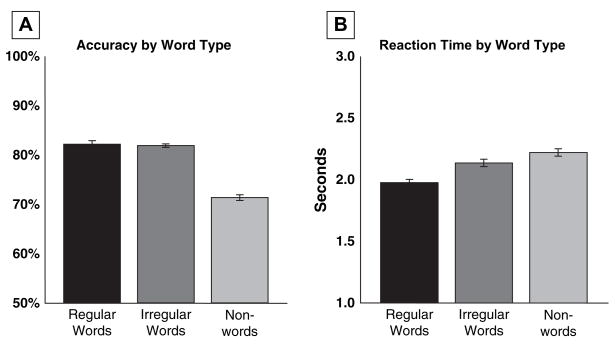
Participants spelled regular words most accurately, irregular words slightly less accurately, and nonwords least accurately, as revealed by repeated measures analysis of variance (ANOVA) (regular words, irregular words, and nonwords as repeated factors; F(2, 22) = 7.73, p = 0.003; see Figure 1 and Table 2).
Fig. 1.
Behavioral results. (A) Accuracy: regular words were spelled most accurately, irregular words slightly less accurately, and nonwords least accurately (p < 0.01). (B) Reaction time: participants performed fastest on regular words, followed by irregular words, and slowest on nonwords (p < 0.01). Error bars indicate standard error.
Table 2.
Means and Standard Deviation (SD) for Accuracy and Reaction Time (RT) Data Across Word Types
| Accuracy |
RT |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Regular words | 80.56 | 15.94 | 1.98 | 0.18 |
| Irregular words | 80.26 | 16.11 | 2.14 | 0.23 |
| Nonwords | 70.28 | 12.98 | 2.22 | 0.11 |
| All words | 77.03 | 15.43 | 2.11 | 0.23 |
Note. Accuracy is reported as percent correct and reaction times are in seconds.
RT
Participants performed fastest on regular words, followed by irregular words, and slowest on nonwords, as revealed by repeated measures ANOVA (same conditions as repeated factors; F(2, 22) = 39.39, p = 0.0001; see Figure 1 and Table 2).
Neuroimaging
Regular Versus Irregular Words
Consistent with the behavioral data in which participants were less accurate and slower to respond to irregular words, we observed overall broader recruitment of neural tissue when participants were processing irregular as opposed to regular words (irregular > regular t-contrast). Areas more active for irregular words included left IFG/orbitalis (BA 45), as well as bilateral left MTG (BA 21), right STG (BA 39), and left SMG (BA 40) (see Figure 2 and Table 3). These and further statistical contrasts of spelling conditions were examined at a significance level of p < 0.001 and an extent threshold of k > 5.
Fig. 2.
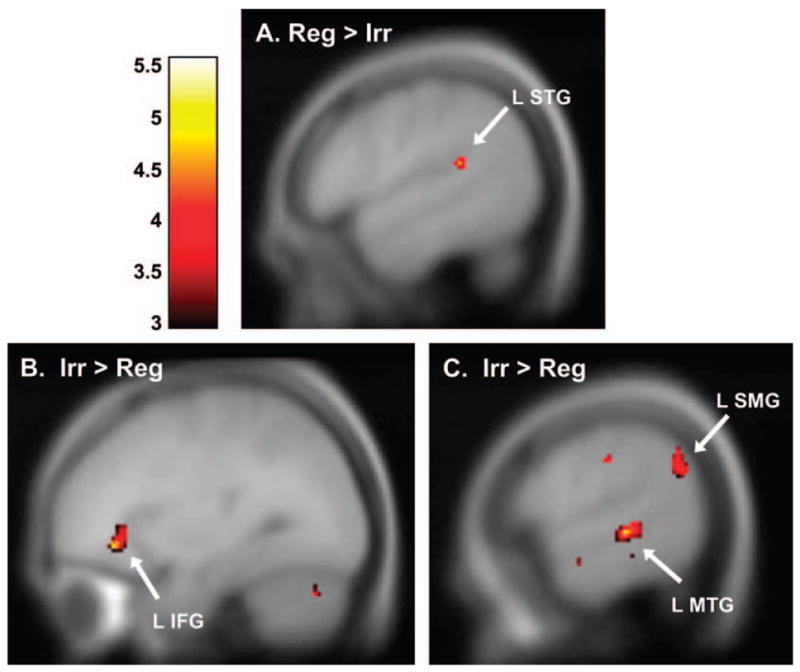
Brain images. Activations are shown on sagittal slices of a canonical composite brain. All activations were significant at p < 0.001 and k > 5, and are given in MNI coordinates (see Table 3). Color scale represents t values. (A) Participants showed greater activation in left STG for regular > irregular words (BA 22, x = −54, y = −40, z = 16, t = 5.06). (B) Participants showed greater activation in left IFG for irregular > regular (BA 45, x = −28, y = 28, z = 0, t = 4.44). (C) Participants also showed greater activation for irregular > regular in both left MTG (BA 3, x = −44, y = 0, z = 46, t = 6.27) and SMG (BA 40, x = −58, y = −58, z = 32, t =4.18). MNI, Montreal Neurological Institute; STG, superior temporal gyrus; IFG, inferior frontal gyrus; MTG, middle temporal gyrus; SMG, supramarginal gyrus. BA, Brodmann area.
Table 3.
Brain Regions Activated During All Spelling Contrasts
| BA | Left hemisphere | Right hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Condition and brain area | x | y | z | t | x | y | z | t | |
| Task > baseline | |||||||||
| Superior frontal gyrus | 8 | −22 | 52 | 24 | 3.80 | ||||
| 9 | 16 | 54 | 36 | 3.32 | |||||
| 10 | −12 | 58 | 8 | 3.40 | 6 | 60 | 16 | 3.39 | |
| Middle temporal gyrus (MTG) | 21 | −50 | −14 | −20 | 4.11 | ||||
| Posterior superior temporal gyrus (STG) | 39 | 58 | −58 | 24 | 3.35 | ||||
| Supramarginal gyrus (SMG) | 40 | −62 | −50 | 34 | 3.29 | 56 | −58 | 38 | 4.08 |
| Fusiform gyrus | 20 | −42 | −36 | −14 | 3.75 | ||||
| Caudate nucleus | — | −22 | 12 | 22 | 3.62 | ||||
| Irregular > regular words | |||||||||
| Inferior frontal gyrus (IFG) | 45 | −28 | 28 | 0 | 4.44 | ||||
| Middle frontal gyrus | 6 | 48 | 12 | 48 | 5.09 | ||||
| Superior frontal gyrus | 8 | −6 | 10 | 58 | 5.24 | ||||
| MTG | 3 | −44 | 0 | 46 | 6.27 | ||||
| 21 | −58 | −30 | −2 | 5.17 | 56 | −30 | −10 | 4.74 | |
| Anterior STG | 39 | 56 | −56 | 28 | 5.92 | ||||
| SMG | 40 | −58 | −58 | 32 | 4.18 | ||||
| Putamen | — | 20 | 2 | 10 | 5.83 | ||||
| Thalamus | — | −14 | −4 | 8 | 7.87 | ||||
| Cerebellum | — | 20 | −70 | −30 | 6.20 | ||||
| Regular > irregular words | |||||||||
| Precentral gyrus | 4 | 64 | −4 | 16 | 5.60 | ||||
| Posterior STG | 22 | −54 | −40 | 16 | 5.06 | ||||
| Fusiform gyrus | 37 | 40 | −60 | −10 | 4.89 | ||||
| Nonwords > irregular words | |||||||||
| Inferior temporal sulcus | 20 | −48 | −20 | −14 | 4.56 | ||||
| Anterior STG | 38 | 44 | 18 | −14 | 4.90 | ||||
| Insular cortex | 13 | −40 | 8 | −8 | 6.82 | ||||
| Amygdala | — | 36 | −8 | −24 | 4.71 | ||||
| Thalamus | — | 6 | −6 | −10 | 9.06 | ||||
| Cerebellum | — | 20 | −34 | −18 | 5.31 | ||||
| Regular words > nonwords | |||||||||
| Precentral gyrus | 6 | −38 | −10 | 32 | 5.19 | ||||
| Nonwords > regular words | |||||||||
| IFG | 45/47 | −32 | 32 | −6 | 4.99 | 34 | 18 | −10 | 5.28 |
| −20 | 28 | −4 | 4.45 | 38 | 20 | −20 | 5.42 | ||
| 44 | −46 | 12 | −4 | 4.56 | |||||
| Middle frontal gyrus | 10 | −24 | 60 | 6 | 4.73 | ||||
| Superior frontal gyrus | 6 | −12 | 8 | 72 | 4.30 | ||||
| Medial frontal gyrus | 9 | 6 | 50 | 20 | 4.55 | ||||
| Anterior cingulate gyrus | 32 | −2 | 42 | 24 | 4.58 | ||||
| Posterior cingulate gyrus | 31 | 0 | −30 | 32 | 4.96 | ||||
| MTG | 21 | −56 | −14 | −18 | 6.32 | 62 | −32 | −4 | 4.66 |
| STG | 21 | −32 | 10 | −20 | 5.16 | 58 | −4 | −10 | 4.98 |
| 21 | −64 | −30 | 2 | 4.84 | |||||
| 22 | −56 | −18 | −2 | 5.75 | |||||
| Insular cortex | 13 | −34 | 14 | −4 | 5.76 | ||||
| Parahippocampal gyrus | 36 | 40 | −26 | −12 | 5.51 | ||||
| Amygdala | — | −34 | −18 | −18 | 5.32 | ||||
| Precuneus | 31 | 12 | −68 | 24 | 7.28 | ||||
| Cerebellum | — | −2 | −72 | −18 | 4.52 | 6 | 68 | −24 | 5.22 |
Note. Activation foci in the tables in this article represent peaks of statistically significant increases in normalized cerebral blood flow for each contrast indicated in the table. x, medial–lateral distance relative to the midline (positive = right); y, anterior–posterior distance relative to the anterior commissure (positive = anterior); z is the superior–inferior distance relative to the anterior commissure line (posterior = superior; Klein et al., 1995). x, y, z is based on Montreal Neurological Institute (MNI) coordinate system. The anatomical region reported refers to the position of the peak based on the merged registration image of the magnetic resonance imaging and SPM99 (MNI) average brain. Significance threshold was set at p < 0.001, k > 5 for all comparisons of tasks, and p < 0.005 and k > 5 for task > baseline. There were no significant areas of activation for the contrast irregular > nonwords. BA = Brodmann area.
The opposite t-contrast of regular > irregular spelling conditions revealed greater activation only in left posterior STG (BA 22), right precentral gyrus (BA 4), and right fusiform gyrus (BA 37).
Regular Words Versus Nonwords
Only a single area, left precentral gyrus (BA 6), was signifi-cantly more activated for regular words than for nonwords. Conversely, spelling of nonwords as compared to that of regular words activated a distributed set of brain areas across both hemispheres. These areas included bilateral activation in IFG (BA 45/47), MTG (BA 21), STG (BA 22), and insular cortex (BA 13), as well as left anterior cingulate (BA 32) and left amygdala.
Irregular Words Versus Nonwords
No brain areas showed significantly greater activation for irregular words than for nonwords at p < 0.001. However, non-words elicited significantly greater activation than irregular words in several areas. Left insular cortex (BA 13), left inferior temporal gyrus (BA 20), right parahippocampal gyrus (BA 36), right MTG (BA 21), and right STG (BA 38) were more active for nonwords.
Spelling Tasks Versus Baseline
The spelling of all types of words was compared to baseline (visual fixation; task > baseline t-contrast) and examined at a significance threshold of p < 0.005 (uncorrected) and k > 5. Several brain areas classically involved in reading, language, and decision making were activated, including bilateral pre-frontal cortex (BA 9/10), left fusiform gyrus (BA 20), left MTG (BA 21), and left SMG (BA 40; see Table 3).
DISCUSSION
Here, we took a first-time look into the human brain while people processed the spelling of words in an attempt to understand how humans accomplish the remarkable feat of spelling. We sought to determine the neural basis of spelling in healthy adults using the novel lens of contemporary neuroimaging technology. Our goal was to provide new insights into the decades-old debate between single- and dual-route models of spelling hitherto impossible to resolve with behavioral evidence alone. Beyond our fascination with spelling was another deeper goal: to understand how humans’ capacity to spell relates to the overall ability to read.
On the one hand, we were encouraged that our behavioral results corroborated earlier behavioral research on both spelling and reading that showed differences in behavioral accuracy and RT for regular words, irregular words, and nonwords (e.g., Kreiner, 1992, 1996; Treiman, 1984). On the other hand, behavioral results do not directly provide support for either a single- or dual-route model of spelling. In contrast, our neuro-anatomical results are uniquely revealing and provide a new view of how people spell regular words, irregular words, and nonwords.
To be sure, our observed neuroanatomical differences between regular and irregular word spelling are most central to addressing debate about single (lexical memory) versus dual (sublexical/phonological, and lexical memory) routes of processing. Among several neural differences found in the comparison of regular and irregular words, the most key is the greater activity in left posterior STG for regular words. The STG is quintessentially involved in phonological processing (Petitto et al., 2000; Poldrack et al., 2001; Zatorre & Belin, 2001), and its greater activation during the spelling of regular words lends strong neuroanatomical evidence that regular words involve a (sublexical) phonological assembly process, different from irregular words.
By contrast, irregular words elicited greater overall activation across a variety of brain areas as compared to regular words, especially areas associated with lexical memory and lexical decision. In particular, we observed greater recruitment of the left IFG, bilateral MTG, and the left SMG for irregular words as compared to regular words. Specifically, the left IFG is involved in the search and retrieval processes associated with accessing word meanings from memory, the MTG is involved in semantic word recognition (especially in the left hemisphere; Fiez et al., 1999; Mechelli, Josephs, Lambon Ralph, McClelland, & Price, 2006; Poldrack et al., 1999; Zatorre, Meyer, Gjedde, & Evans, 1996), and the left SMG is involved in the processing of print (Palmer et al., 2004).
Remarkably, then, we observed a neural difference during word spelling based on whether the word had regular or irregular sound-to-letter spelling patterns (orthography). This differential neural recruitment used in the processing of spelling regular versus irregular words provides robust brain-based support for the viability of dual-route models and thus provides important clarification of a controversy that has been debated in the field for decades.
We were intrigued to see that the spelling of nonwords exhibited widespread brain activity that was different from the activity exhibited by spelling of real words (be they regular or irregular). The brain areas most active in nonword spelling (as compared to both regular and irregular words) included the left STG and bilateral middle and inferior temporal gyri. These findings further corroborate studies of non-word reading, including the finding that activation in the MTG is correlated with word frequency, with nonwords (zero frequency) eliciting the strongest activation (Mechelli, Gorno-Tempini, & Price, 2003). Given this pattern of activation, it seems nonwords are processed using neither strictly lexical nor sublexical process. As suggested by some computational models, it may be the case that nonwords access both pathways, both looking for similar words that are stored as whole lexical units and attempting to assemble the correct representation from sublexical/phonological units (e.g., Warrington & Shallice, 1980).
Spelling, Reading, and Language
Our spelling tasks activated well-known reading and language areas of the brain. For all spelling tasks as compared to the rest, we found activation in left fusiform gyrus, close to the coordinates of the VWFA as defined by McCandliss, Cohen, and Dehaene (2003). In addition, left SMG, active here in spelling, is frequently implicated in phonological working memory, as well as in letter identification during the reading of both real and nonwords (Mechelli et al., 2003; Paulesu, Frith, & Frackowiak, 1993). Inferior frontal cortex activation is consistent with previous reading studies that have involved directed linguistic search and response retrieval, as well as phonological processing (Kerns, Cohen, Stenger, & Carter, 2004; Poldrack et al., 1999). Our fMRI evidence is also consistent with evidence from other technologies, such as magneto-encephalography, which find that both children and adults process letter strings within left inferior occipito-temporal cortex, and that this left lateralization of neural response is specific to letter stimuli, as compared to other types of visual displays (Parviainen, Helenius, Poskiparta, Niemi, & Salmelin, 2006; Tarkiainen, Cornelissen, & Salmelin, 2002; Tarkiainen, Helenius, Hansen, Cornelissen, & Salmelin, 1999). Our spelling findings are further consistent with previous research on reading indicating that separable lexical and sublexical processes exist and that encoding and decoding are indeed tightly linked.
Future Research
Our findings here are relevant to English orthography. However, further research is warranted on how spelling is accomplished in speakers of different languages. The world’s languages differ in their orthographies. English, along with some other world languages (such as French), have deep orthography (the spelling of a word does not always match the way it is pronounced), while other languages, such as Spanish and Italian, have a shallow or transparent orthography. To the best of our knowledge, there is only one neuroimaging study that directly compared people’s reading in languages with a shallow orthography (Italian) versus a deep orthography (English). Consistent with the idea that shallow orthographies require greater reliance on GPC rules, native Italian speakers demonstrated greater left STG recruitment and less robust left IFC recruitment as compared to native English speakers (Paulesu et al., 2000). Would this cross- linguistic pattern of brain activity for reading also be true of spelling? Moreover, would proficient early-exposed bilinguals who learn to read in both deep and shallow orthographies at the same early age develop language-specific reading and spelling strategies for each of their orthographies—both behaviorally and in the brain?
CONCLUSIONS
In this study, neuroimaging data revealed differential processing in the brain during the spelling of regular words, irregular words, and nonwords. Overall, the brain areas active during spelling confirmed that spelling is indeed an important part of the network of well-known language and reading brain areas, and that these areas subsume the phonological and lexical components that constitute successful spelling. The study further confirms that fMRI can be a valuable tool for understanding complex theories about human language and its sister capacities—to spell and to read—which have been debated for years using only behavioral evidence.
Recent research on successful readers has suggested that a combination of phonics instruction (which emphasizes sub-lexical letter-to-sound correspondences) and whole-word instruction (which emphasizes lexical or word-specific memory) is optimal for successful reading acquisition (National Reading Panel, 2000). Notably, the new understanding that the present research affords about the dual-route nature of spelling suggests that successful spellers also use both sub-lexical and lexical processes.
The new understanding of spelling afforded here also has important implications for reading and education. Although it is well understood that spelling skills are necessary for successful written communication, spelling and reading may also have a direct reciprocal influence on each other’s acquisition (Castles & Coltheart, 2004; Ehri, 2000; Wagner & Torgesen, 1987). Moreover, evidence for separable phonological and lexical processes in spelling also can advance our understanding of language difficulties such as dyslexia, especially the idea that there are separate phonological (Ziegler & Goswami, 2005) and naming (lexical–semantic) deficits in dyslexia (e.g., Wolf & Bowers, 1999). In this regard, the present findings suggest that remediation methods targeted at either the phonological or the lexical levels might be necessary to help students with different types of dyslexia. To be sure, the close link observed here across language, reading, and spelling processes in the brain suggests that these three components may warrant more integrated attention in the teaching and clinical remediation of reading and language in the classroom.
Acknowledgments
We thank the participants who graciously gave of their time to be in this study. We thank Scott Grafton, John Van Horn, and Dave Kraemer for fMRI assistance. Thanks also to Donna Coch and Rachael Degenshein, and to all members of the Petitto Laboratory. Funding was provided to L.-A. P. (PI) by National Institutes of Health R01 HD045822-01 and R21 HD50558-01A1, and by Dartmouth College. Funding was provided to E. S. N. from the Dartmouth Brain Imaging Center and Dartmouth’s David C. Hodgson Endowment. Additional information can be found at http://www.dartmouth.edu/~lpetitto/lab/.
References
- Beauvois MF, Dérouesné J. Lexical or orthographic agraphia. Brain. 1981;104:21–49. doi: 10.1093/brain/104.1.21. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. Journal of Neuroscience. 2005;5:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Functional anatomy of intra-and cross-modal lexical tasks. Neuroimage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. Journal of Cognitive Neuroscience. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley L, Bryant P. Rhyme and reason in reading and spelling. Ann Arbor, MI: University of Michigan Press; 1985. [Google Scholar]
- Castles A, Coltheart M. Is there a causal link from phonological awareness to success in learning to read? Cognition. 2004;91:77–111. doi: 10.1016/s0010-0277(03)00164-1. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments and Computers. 1993;25:257–271. [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: Dual route and parallel distributed processing approaches. Psychological Review. 1993;100:589–608. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Ehri LC. Learning to read and learning to spell: Two sides of a coin. Topics in Language Disorders. 2000;20(3):19–36. [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. NeuroImage. 1992;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps: Linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Frith U. Cognitive processes in spelling. London: Academic Press; 1980. [Google Scholar]
- Goodman RA, Caramazza A. Aspects of the spelling process: Evidence from a case of acquired dysgraphia. Language and Cognitive Processes. 1986;1:263–296. [Google Scholar]
- Harm M, Seidenberg MS. Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychological Review. 2004;111:662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neu-roimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, Stenger VA, Carter CS. Prefrontal cortex guides context-appropriate responding during language production. Neuron. 2004;43:283–291. doi: 10.1016/j.neuron.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre R, Meyer E, Evans A. The neural substrates underlying word generation: A bilingual functional-imaging study. Proceedings of the National Academy of Science, USA. 1985;92:2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner DS. Reaction time measures of spelling: Testing a two-strategy model of skilled spelling. Journal of Experimental Psychology: Learning, Memory and Cognition. 1992;18:765–776. doi: 10.1037//0278-7393.18.4.765. [DOI] [PubMed] [Google Scholar]
- Kreiner DS. Effects of word familiarity and phoneme-grapheme polygraphy on oral spelling time and accuracy. The Psychological Record. 1996;46:49–70. [Google Scholar]
- Kreiner DS, Gough PB. Two ideas about spelling: Rules and word-specific memory. Journal of Memory and Language. 1990;29:103–118. [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Masterson JJ, Apel K. Effect of modality on spelling words varying in linguistic demands. Developmental Neuropsychology. 2006;29:261–277. doi: 10.1207/s15326942dn2901_13. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Human Brain Mapping. 2006 June;9 doi: 10.1002/hbm.20272. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Reading Panel. Teaching children to read: An evidence-based assessment of the scientific research literature on reading and its implications for reading instruction. Washington, DC: National Institute of Child Health and Human Development; 2000. [Google Scholar]
- Palmer ED, Brown TT, Petersen SE, Schlaggar BL. Investigation of the functional neuroanatomy of single word reading and its development. Scientific Studies of Reading. 2004;8:203–223. [Google Scholar]
- Parviainen T, Helenius P, Poskiparta E, Niemi P, Salmelin R. Cortical sequence of word perception in beginning readers. Journal of Neuroscience. 2006;26:6052–6061. doi: 10.1523/JNEUROSCI.0673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, et al. A cultural effect on brain function. Nature Neuroscience. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Petitto LA, Zatorre RJ, Guana K, Nikelski EJ, Dostie D, Evans AC. Speech-like cerebral activity in natural signed languages in the deaf: A PET study. Proceedings of the National Academy of Sciences. 2000;97:13961–13966. doi: 10.1073/pnas.97.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinker S. Words and rules in the human brain. Nature. 1997;387:547–548. doi: 10.1038/42347. [DOI] [PubMed] [Google Scholar]
- Pinker S. Words and rules: The ingredients of language. New York: Basic Books; 1999. [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: Computational principles in quasi-regular domains. Psychological Review. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JDE. Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. Journal of Cognitive Neuroscience. 2001;13:687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior pre-frontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM. The role of left posterior inferior temporal cortex in spelling. Neurology. 2004;62:2221–2229. doi: 10.1212/01.wnl.0000130169.60752.c5. [DOI] [PubMed] [Google Scholar]
- Rapp B, Epstein C, Tainturier MJ. The integration of information across lexical and sublexical processes in spelling. Cognitive Neuropsychology. 2002;19:1–29. doi: 10.1080/0264329014300060. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychological Review. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Seymour P. Cognitive theories of spelling and implications for instruction. In: Sterling CM, Robson C, editors. Psychology, spelling, and education. Clevedon, UK: Multilingual Matters; 1992. pp. 50–70. [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher J, et al. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Cornelissen PL, Salmelin R. Dynamics of visual feature analysis and object-level processing in face versus letter-string perception. Brain. 2002;125:1125–1136. doi: 10.1093/brain/awf112. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122:2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Treiman R. Individual differences among children in spelling and reading styles. Journal of Experimental Child Psychology. 1984;37:463–477. doi: 10.1016/0022-0965(84)90071-7. [DOI] [PubMed] [Google Scholar]
- Treiman R, Baron J. Individual differences in spelling: The Phoenician-Chinese distinction. Topics in Learning and Learning Disabilities. 1983;3:33–40. [Google Scholar]
- Treiman R, Bourassa DC. Children’s written and oral spelling. Applied Psycholinguistics. 2000;21:183–204. [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz WJ, Pinker S. A neural dissociation within language: Evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience. 1997;9:266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- Varnhagen CK, McCallum M, Burstow M. Is children’s spelling naturally stage-like? Reading and Writing: An Interdisciplinary Journal. 1997;9:451–481. [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101:192–212. [Google Scholar]
- Warrington EK, Shallice T. Word-form dyslexia. Brain. 1980;103:99–112. doi: 10.1093/brain/103.1.99. [DOI] [PubMed] [Google Scholar]
- Wolf M, Bowers P. The “double-deficit hypothesis” for the developmental dyslexias. Journal of Educational Psychology. 1999;91:1–24. [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cerebral Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans AC. PET studies of phonetic processing of speech: Review, replication, and reanalysis. Cerebral Cortex. 1996;6:21–30. doi: 10.1093/cercor/6.1.21. [DOI] [PubMed] [Google Scholar]
- Ziegler J, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychological Bulletin. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]