Abstract
Background:
Statin use before surgery has been associated with reduced morbidity and mortality after vascular surgery. The effect of preoperative statin use on stroke and encephalopathy after coronary artery bypass grafting (CABG) is unclear.
Methods:
A post hoc analysis was undertaken of a prospectively collected cohort of isolated CABG patients over a 10-year period at a single institution. Primary outcomes were stroke and encephalopathy. Univariable analyses identified risk factors for statin use, which were applied to a propensity score model using logistic regression and patients were divided into quintiles of propensity for statin use. Controlling for propensity score quintile, the odds ratio (OR) of combined stroke and encephalopathy (primary endpoint), cardiovascular mortality, myocardial infarction, and length of stay were compared between statin users and nonusers.
Results:
There were 5,121 CABG patients, of whom 2,788 (54%) were taking statin medications preoperatively. Stroke occurred in 166 (3.2%) and encephalopathy in 438 (8.6%), contributing to 604 patients (11.8%) who met the primary endpoint. The unadjusted OR of stroke/encephalopathy in statin users was 1.053 (95% confidence interval [CI] 0.888-1.248, p = 0.582). Adjustment based on propensity score resulted in balance of stroke risk factors among quintiles. The propensity score-adjusted OR of stroke/encephalopathy in statin users was 0.958 (95% CI 0.784-1.170, p = 0.674). There were no significant differences in cardiovascular mortality, myocardial infarction, or length of stay between statin users and otherwise similar nonusers.
Conclusions:
In this large data cohort study, preoperative statin use was not associated with a decreased incidence of stroke and encephalopathy after coronary artery bypass grafting.
GLOSSARY
- ACE
= angiotensin converting enzyme;
- ARB
= angiotensin II receptor blocker;
- CABG
= coronary artery bypass grafting;
- CI
= confidence interval;
- MI
= myocardial infarction;
- OR
= odds ratio;
- PCI
= percutaneous coronary intervention.
Coronary artery bypass grafting (CABG) may induce a spectrum of neurologic injury, including stroke, encephalopathy, and cognitive decline.1 Post-CABG stroke and encephalopathy share similar risk factors for ischemia, suggesting that they represent a continuum of injury.1,2 Patients diagnosed with post-CABG encephalopathy on clinical grounds often demonstrate unsuspected strokes on diffusion-weighted MRI.3,4
Stroke and encephalopathy add considerable morbidity and mortality after CABG, prolong the length of hospitalization, and reduce the chances of discharge home.5–7 The mechanisms of stroke and encephalopathy after CABG are unclear. The majority of patients with MRI evidence of stroke after CABG demonstrate multiple lesions in watershed territories.6,8 Major risk factors for watershed infarcts include intraoperative hypotension and atherosclerosis, suggesting that strokes result from a combination of hypoperfusion and atheroemboli.6,8–10
Preoperative use of HMG-CoA reductase inhibitors (statins) may reduce the incidence of stroke after vascular procedures like arterial bypass11,12 and carotid endarterectomy.13,14 This benefit may be conferred by both cholesterol lowering and pleiotropic effects on plaque stability, endothelial function, oxidative stress, and tissue reperfusion.15,16 In clinical trials, statins reduce the incidence of stroke among patients with risk factors for atherosclerosis,17 which includes the majority of CABG patients. In addition, aggressive cholesterol lowering after CABG has been shown to decrease the long-term incidence of stroke.18 Observational studies of acute morbidity among patients using statins prior to CABG, however, have yielded conflicting results.19–23
Based on these data, we hypothesized that statin use prior to CABG would be associated with a lower incidence of acute postoperative stroke and encephalopathy.
METHODS
Using a post hoc analysis of a prospectively collected database including a cohort of consecutive patients undergoing isolated CABG, we tested for an association between statin use and the incidence of stroke and encephalopathy (primary outcome). Secondary outcomes included postoperative myocardial infarction (MI), cardiovascular mortality, and hospital length of stay. Data collection and analyses were undertaken with the approval of The Johns Hopkins University Institutional Review Board. Between 1997 and 2007, all patients undergoing isolated CABG at the Johns Hopkins Hospital in Baltimore, MD, were followed for development of postoperative neurologic deficits during the hospitalization as part of an institutional database. Inclusion criteria were age >18 years and isolated CABG surgery. No adult patients were excluded from the database.
Data collection.
The database was designed to determine the incidence of neurologic complications after CABG and included the following prospectively collected data: demographic information, medical history and comorbidities, preadmission medication use, and intraoperative data including duration of cardiopulmonary bypass. Specific brands and dosages of preoperative medications were recorded in the database, but the duration of use prior to surgery was not available. Data were collected at the time of inclusion by direct interview or extraction from the medical record. Subjects with missing data for statin use were not included in the analysis. Postoperative outcomes included ischemic stroke, encephalopathy, MI, cardiovascular mortality, and length of stay. Cardiovascular mortality was defined as death attributable to cardiac arrhythmia, MI, ischemic stroke, cardiogenic shock, or sudden cardiac death during the hospitalization.
Neurologic consultation was requested in patients with postoperative neurologic deficits. All patients with stroke were evaluated by a board-certified neurologist with subspecialty training in vascular neurology. Stroke outcomes were diagnosed by the neurologist according to the presence of a new, focal neurologic deficit consistent with stroke. MRI or CT were considered supportive evidence and were obtained at the discretion of the primary and consulting services. Encephalopathy outcomes included seizures, decreased level of arousal, delirium, or mental status change at any point between 24 hours after CABG and hospital discharge. Encephalopathy was diagnosed by an independent research coordinator who followed patients during the hospital stay, interviewed medical providers, and reviewed records on a daily basis. Many patients with encephalopathy also had formal consultation by a board-certified neurologist. The stroke and encephalopathy outcomes were mutually exclusive.
Baseline variables were shared with an institutional quality assurance database that is submitted to the national Society of Thoracic Surgery data center. The database was routinely audited for double entries, typographic errors, and missing data at the time of submission to the national data center. In addition, the neurologic outcome data were audited at the end of every year to test for accuracy of diagnoses. Finally, all stroke outcomes were audited in preparation for this analysis.
Statistical methods.
Univariable analyses were conducted to identify risk factors for statin use with SPSS version 16 (Statistical Package for the Social Sciences, Chicago, IL). The Kolmogorov-Smirnov test was applied to evaluate normalcy of distribution. Parametric values were reported as mean ± SD and nonparametric variables were reported as median (interquartile range). Univariable analyses were performed for parametric data with Student t test and analysis of variance for continuous variables and χ2 for categorical variables. Univariable analyses of nonparametric data were performed using the Mann-Whitney U and Kruskal-Wallis tests.
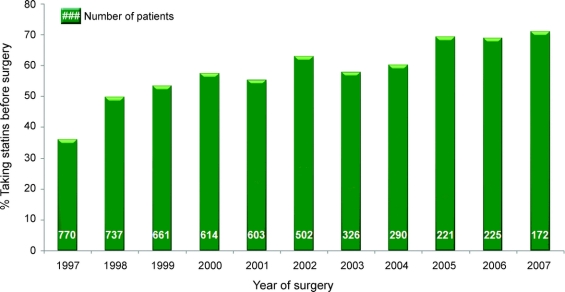
Variables that were associated with statin use (p < 0.2) were applied to construct a propensity score model for statin use with STATA version 10 (StataCorp, College Station, TX). These variables included hyperlipidemia, total preoperative cholesterol level, current tobacco use, aspirin use, angiotensin converting enzyme (ACE) inhibitor use, angiotensin II receptor blocker (ARB) use, β-blocker use, and prior percutaneous coronary intervention (PCI). The propensity score model also included known risk factors for statin use (age and year of surgery) and the number of cardiac medications (aspirin, clopidogrel, β-blockers, ACE inhibitors, and ARBs) as an indicator variable of healthcare access. The year of surgery factor included a knot at 2001, because statin use increased after 2001 in the dataset as shown the in figure.
Figure Frequency of preoperative statin medication use during the study time period
Using the propensity score, all patients were separated into quintiles of propensity for statin use. Stroke risk factors were then tested for balance between statin users and nonusers within each quintile. Based on residual imbalances in stroke risk factors, the following variables were added to the propensity score model: preoperative atrial fibrillation, hypertension, cardiopulmonary bypass use during CABG, and duration of cardiopulmonary bypass.
The odds ratio (OR) of stroke and encephalopathy (primary outcome measure) was determined for statin users compared to nonusers averaged among the quintiles of propensity for statin use. The OR was also determined using an average of quintiles 2-4 in order to eliminate potential bias from outliers in the extreme quintiles. The average ORs for stroke, encephalopathy, cardiovascular mortality, MI, and length of hospital stay were then computed as secondary outcome measures. Alpha <0.05 was selected to consider differences significant.
Sample size estimates.
In a prior study of stroke after CABG,24 the incidence of stroke among statin users was 0.88% (4/455) compared to 3.10% (11/355) among nonusers. Applying these estimates to sample size calculations, the inclusion of 854 subjects in each group (statin users vs nonusers) would provide 95% power to detect a significant difference in the incidence of stroke with an alpha value of 0.05. A similar treatment effect was found in a prior study of stroke after carotid endarterectomy.14 Given these estimates, the inclusion of >2,000 subjects in each group should be sufficient to determine a significant difference in stroke incidence even if the treatment effect is smaller than previously reported.
RESULTS
The database included 5,121 patients who underwent isolated CABG over 10 years. The baseline characteristics of the study population are shown in table 1. Statin use increased during the 10-year study period (figure). Overall, 2,788 (54%) patients were taking statin medications before surgery. Characteristics of statin users and nonusers are compared in table 2. On average, statin users were younger, used more antihypertensive and antiplatelet medications, were less likely to have had previous percutaneous coronary intervention, and had a higher prevalence of diagnosed hypercholesterolemia, atrial fibrillation, and hypertension. In addition, statin users had a lower duration of cardiopulmonary bypass and were more likely to have off-pump surgery.
Table 1 Baseline characteristics of the study population
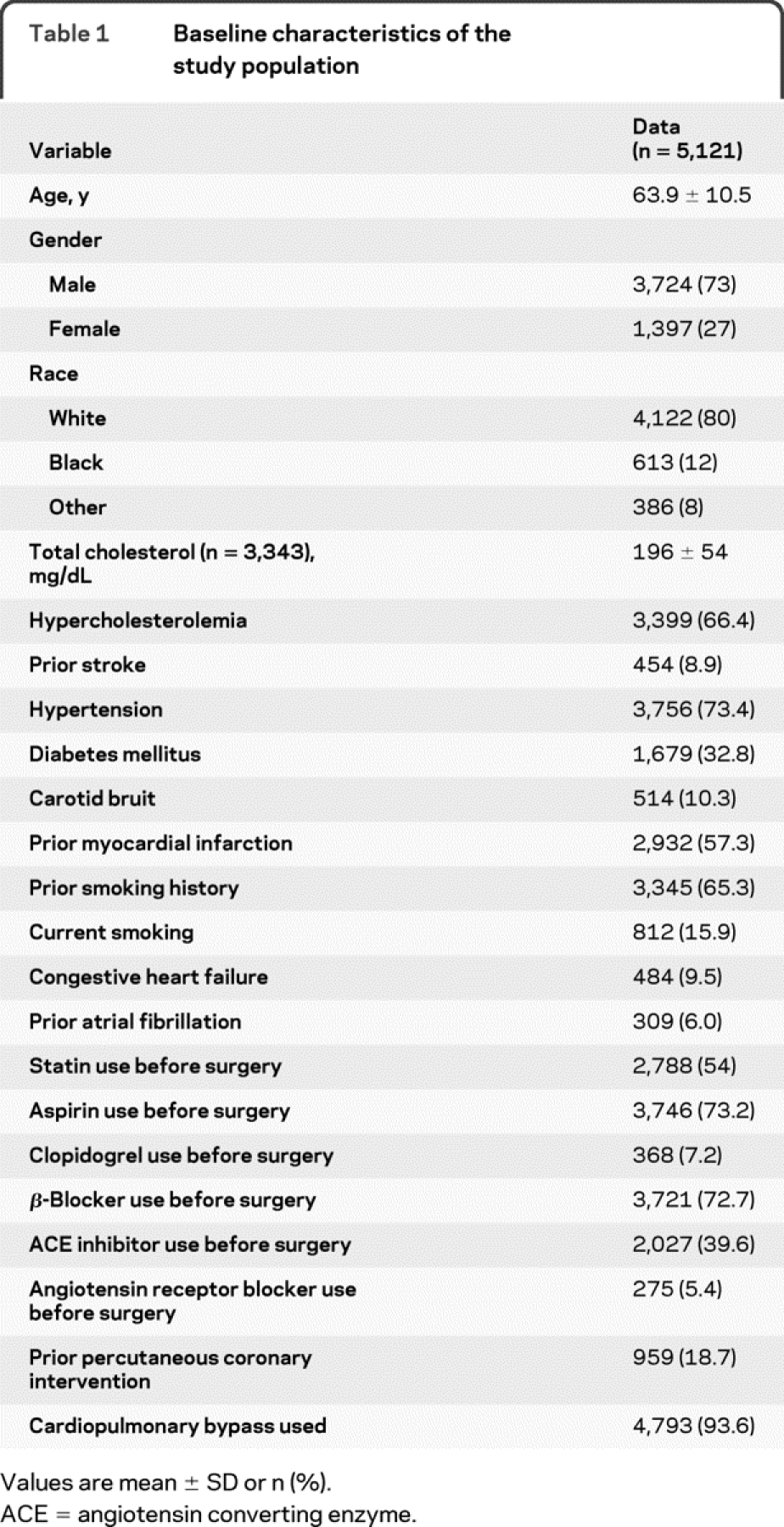
Table 2 Patient characteristics before propensity score adjustment
Stroke occurred in 166 (3.2%) and encephalopathy in 438 (8.6%) patients, contributing to 604 patients (11.8%) who achieved the primary endpoint. All-cause in-hospital death occurred in 132 patients (2.6%), of whom 83 (1.6%) died from cardiovascular causes. Postoperative MI occurred in 20 (0.4%) patients. Mean hospital length of stay was 7.8 ± 8.1 days. The unadjusted OR of combined stroke and encephalopathy (primary outcome) in statin users was 1.053 (95% confidence interval [CI] 0.888-1.248, p = 0.582) compared to statin nonusers.
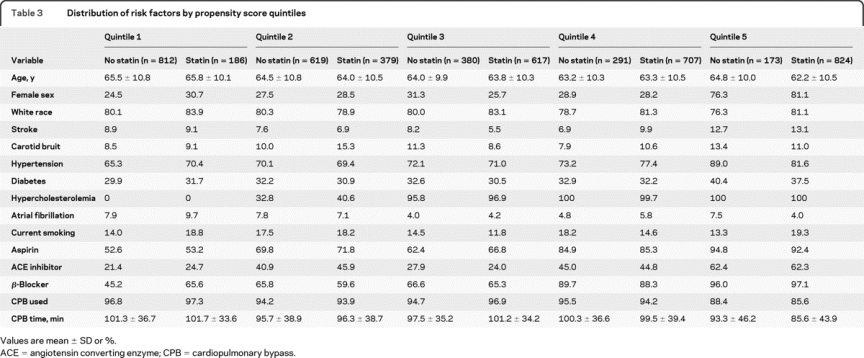
Variables used to construct the propensity score model are described in Methods. Addition of unbalanced stroke risk factors into the propensity score model resulted in good balance between most risk factors within each quintile (table 3). Because some residual imbalances were noted in the extreme quintiles, the outcome ORs were determined both as averages of all 5 quintiles and limited to quintiles 2–4 (n = 2,993) in order to limit potential bias introduced by outliers.
Table 3 Distribution of risk factors by propensity score quintiles
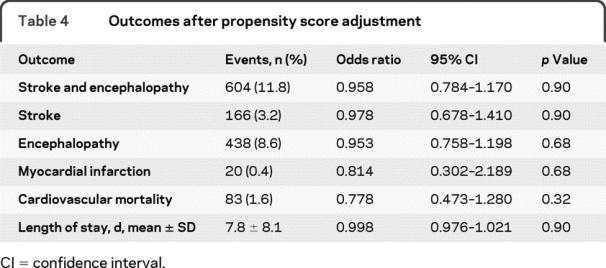
The propensity score-adjusted OR of combined stroke and encephalopathy in statin users was 0.958 (95% CI 0.784-1.170, p = 0.674) averaged across all 5 quintiles. When the analysis was limited to quintiles 2–4, the OR was similar (1.189, 95% CI 0.922–1.534, p = 0.182). The ORs of the individual secondary outcomes also did not show significant treatment effects of statin use (table 4).
Table 4 Outcomes after propensity score adjustment
DISCUSSION
Several previous reports have identified preoperative statin use as beneficial in lowering the incidence of post-CABG complications. We hypothesized that this would be the case for neurologic morbidity in the postoperative period. However, after adjustment for imbalances in stroke risk factors among statin users and nonusers, we found that preoperative statin use does not decrease the odds of stroke and encephalopathy after CABG. The ORs of other postoperative outcomes including MI, cardiovascular death, and length of hospital stay were similarly unaffected by statin use.
These findings contrast with several studies of statin use prior to arterial bypass surgery,11,12 carotid endarterectomy,13,14 and other noncardiac vascular surgical procedures.25–27 Using a similar study design, statin use prior to carotid endarterectomy was associated with a threefold reduction in the incidence of postoperative stroke.14 The benefit of statins for stroke risk in these populations has been attributed to direct effects on serum cholesterol concentration and plaque stability as well as a number of “pleiotropic” effects on endothelial function, oxidative stress, and tissue reperfusion.15 Because surgical manipulation of the aorta or carotid artery may cause stroke from embolization of atherosclerotic plaque, statins could potentially reduce stroke by reducing atherosclerotic burden and stabilizing plaque.28
The mechanisms of stroke after CABG likely differ from noncardiac vascular surgical procedures. Cardiopulmonary bypass may result in stroke by systemic hypotension and diffuse embolization of fibrin or gaseous material as demonstrated by transcranial Doppler ultrasonography.29,30 The majority of strokes are recognized within 48 hours of surgery.1,2,30 Although statin use is unlikely to protect against hypotension or acute embolization from cardiopulmonary bypass, patients undergoing CABG are at risk for other common mechanisms of ischemic stroke related to intracranial and extracranial atherosclerosis.30 While some reports have indicated that statins are associated with a reduced incidence of atrial fibrillation after CABG,31 it has not been clearly shown that atrial fibrillation is a significant cause of stroke postoperatively, especially when most strokes are identified early.
Prior observational studies have yielded conflicting results on the impact of pre-CABG statin use on the incidence of postoperative stroke, mortality, MI, and atrial fibrillation.22,24,32–34 One study20 reported a 30% reduction in morbidity and 50% reduction in mortality among patients taking statins prior to a variety of cardiac surgical procedures. In a study of patients undergoing isolated CABG with cardiopulmonary bypass, the investigators21 reported a 50% reduction in 30-day mortality among patients using statins preoperatively but no reduction in the rates of stroke or MI. Using a similar design to the present study, another group investigated the influence of preoperative statin use on the incidence of death, atrial fibrillation, stroke, and other outcome measures using a database of 2,497 patients.23 Patients receiving statins had a similar incidence of adverse outcomes compared to those not on statins. The authors reported a low incidence of postoperative stroke (1.1%, n = 28) which was also similar between the 2 groups. These results support the findings of earlier studies.35,36
The true incidence of stroke after CABG is likely underestimated for several reasons. Because post-CABG stroke is more likely to be caused by embolization and relative hypotension during cardiopulmonary bypass,6,8,10 most patients develop multiple strokes in arterial watershed territories.6 Multiple small infarcts are more likely to present with global encephalopathy rather than a focal neurologic deficit.6 For this reason, global deficits may be attributed to postoperative delirium and neither neurologic consultation nor imaging are undertaken. In addition, many patients do not undergo diffusion-weighted MRI after CABG because the use of temporary pacer wires, implantable pacer/defibrillators, or aortic balloon counterpulsation devices precludes MRI.6 Because post-CABG strokes are often small, multiple, and distributed in watershed territories, they may be indistinguishable from chronic ischemic white matter changes on noncontrast head CT studies.
The combined endpoint of stroke and encephalopathy was chosen in the present study to maximize the chances of capturing all patients with ischemic neurologic deficits. Although postoperative encephalopathy is often multifactorial, risk factors for post-CABG stroke and encephalopathy significantly overlap.1 Similar to stroke, post-CABG encephalopathy increases the length of hospital stay, morbidity, mortality, and discharge to a nursing facility.1,7 In addition, studies of routine diffusion-weighted MRI after CABG demonstrate a high incidence of clinically unsuspected stroke among patients with postoperative encephalopathy.3,10 Together, these factors suggest that stroke and encephalopathy lie on a spectrum of ischemic injury after CABG.
This study has some important limitations. As this was not a randomized, controlled trial, it is possible that patients who were taking statins prior to CABG had significant differences in access to health care, medical comorbidities, and other factors compared to those not taking statins. We identified risk factors for statin use and used a propensity score model to separate patients into quintiles of propensity for statin use. We achieved balance among most stroke risk factors within each quintile, although some factors remained unbalanced in the extreme quintiles. Outcomes were not significantly different when quintiles 1 and 5 were removed from the analysis. Despite this cautious design, it is possible that unrecorded factors that influence statin use were not included in the database or the statistical model.
Another important limitation is that the duration of statin use before surgery was not known. The optimal duration of statin use for plaque stabilization is unknown.28 If a preponderance of patients were on statins short term before surgery, it could potentially weaken the statistical power to detect differences between the groups. Although the agent and dosage of statin medications were recorded, we did not analyze the effect of statin potency or dosage on outcomes because of relatively small numbers in each subgroup. Other than factors associated with cardiopulmonary bypass, potentially important intraoperative variables such as blood pressure or surgical technique were not collected. Although these variables could influence stroke outcomes, there is no obvious reason why systematic differences would exist between statin users and nonusers.
In an ideal study, all patients would undergo detailed preoperative and postoperative neurologic assessments to minimize ascertainment bias. Given the large number of patients included, the cost of undertaking this design would be prohibitive for an observational cohort study. This study was designed to maximize the probability of capturing all patients with ischemic neurologic injury after CABG. To be as inclusive as possible, we included all patients with stroke and encephalopathy in the primary endpoint. A trained researcher screened every patient for encephalopathy and stroke on a daily basis. All patients with stroke and many patients with encephalopathy were also evaluated by a board-certified neurologist with subspecialty training in vascular neurology. Although the study was designed to minimize ascertainment bias, some residual bias is possible because subtle cases of encephalopathy—which could represent stroke—may have been overlooked.
Another limitation of the database is that proximate causes of encephalopathy were not prospectively identified. In a previous publication, however, the incidence of acute renal failure, infection, and oversedation were considered to be low and most cases of encephalopathy did not have an obvious precipitant.1 A retrospective attempt to determine underlying causes of encephalopathy by chart review would likely introduce errors resulting in underestimation of ischemic neurologic injury. Although the data undoubtedly include patients with nonischemic etiologies of encephalopathy, there is no reason to suspect that such patients would be systematically overrepresented according to prior statin use.
Because ischemic complications of CABG are usually symptomatic in the early postoperative period, this study focused on the acute incidence of stroke and encephalopathy during the hospitalization. Although statins were not found to be protective against these short-term outcomes, this study was not designed to test whether long-term functional, cognitive, or quality-of-life measures were affected by statin use. The absence of long-term follow-up data from these patients is another important limitation of this study.
A randomized, placebo-controlled trial would be required to definitively determine whether preoperative statin use reduces stroke after CABG. Such a trial is unlikely to occur for several reasons. Given the compelling evidence for secondary prevention of cardiovascular morbidity and mortality, most patients undergoing CABG have definite indications for statins.37 Randomizing such patients to placebo would, therefore, be ethically challenging. A trial would also potentially involve delaying CABG for the initiation of statins. Given the negative results of this study and others, it does not seem justifiable to delay a potentially life-saving procedure to initiate statins.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. S.L. Zeger and L.D. Pham.
DISCLOSURE
Dr. Koenig and M.A. Grega report no disclosures. M.M. Bailey has received research support from the NIH/NINDS [35610 (Senior Research Program Coordinator)] and the Dana Foundation. L.D. Pham reports no disclosures. Dr. Zeger served on a scientific advisory board for Merck Serono; serves as coeditor of Biostatistics and on the editorial board of Springer Statistics and the Annual Review of Public Health; receives royalties from publishing Analysis of Longitudinal Data (Oxford University Press, 2002); receives research support from the NIH/NINDS [35610 (Coinvestigator)], the Dana Foundation, and the Johns Hopkins Medical Institution; and served as expert for Merck Serono in a suit against British insurance companies about Vioxx in British court. Dr. Baumgartner receives royalties from publishing the Johns Hopkins Manual of Cardiothoracic Surgery (McGraw Hill, 2006); and receives research support from NIH/NHLBI [31238-10 (Principal Investigator)]. Dr. McKhann serves on a safety monitoring board for Merck Serono; serves as Scientific Advisor to the Dana Foundation; and receives research support from the NIH/NINDS [RO1-35610 (Principal Investigator)].
Address correspondence and reprint requests to Dr. Matthew Koenig, The Johns Hopkins Hospital, 600 N. Wolfe St., Meyer 8-140, Baltimore, MD 21287 mkoenin1@jhmi.edu
Editorial, page 2058
e-Pub ahead of print on November 11, 2009, at www.neurology.org.
Supported by grant 35610 from the National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD, and by the Dana Foundation, New York, NY.
Disclosure: Author disclosures are provided at the end of the article.
Received February 3, 2009. Accepted in final form September 4, 2009.
REFERENCES
- 1.McKhann GM, Grega MA, Borowicz LM, et al. Encephalopathy and stroke after coronary artery bypass grafting: incidence, consequences, and prediction. Arch Neurol 2002;59:1422–1428. [DOI] [PubMed] [Google Scholar]
- 2.McKhann GM, Grega MA, Borowicz LM, Baumgartner WA, Selnes OA. Stroke and encephalopathy after cardiac surgery. An update Stroke 2006;37:562–571. [DOI] [PubMed] [Google Scholar]
- 3.Wityk RJ, Goldsborough MA, Hillis A, et al. Diffusion- and perfusion-weighted brain magnetic resonance imaging in patients with neurologic complications after cardiac surgery. Arch Neurol 2001;58:571–576. [DOI] [PubMed] [Google Scholar]
- 4.Restrepo L, Wityk RJ, Grega MA, et al. Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke 2002;33:2909–2915. [DOI] [PubMed] [Google Scholar]
- 5.McKhann GM, Grega MA, Borowicz LM, Selnes OA, Baumgartner WA, Royall RM. Encephalopathy and stroke after coronary artery bypass grafting. Curr Treat Options Cardiovasc Med 2004;6:171–178. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman RF, Sherman PM, Grega MA, et al. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke 2006;37:2306–2311. [DOI] [PubMed] [Google Scholar]
- 7.Wolman R, Nussmeier NA, Aggarwal A, et al. Cerebral injury after cardiac surgery: identification of a group at extraordinary risk: multicenter study of perioperative ischemia research group (McSPI) and the ischemia research education foundation (IREF) investigators Stroke. 1999;30:514–522. [DOI] [PubMed] [Google Scholar]
- 8.Rankin JM, Silbert PL, Yadava OP, Hankey GJ, Stewart-Wynne EG. Mechanism of stroke complicating cardiopulmonary bypass surgery. Aust NZ J Med 1994;24:154–160. [DOI] [PubMed] [Google Scholar]
- 9.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998;55:1475–1482. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman RF, Hillis AE, Grega MA, et al. Early postoperative cognitive dysfunction and blood pressure during coronary artery bypass graft operation. Arch Neurol 2007;64:1111–1114. [DOI] [PubMed] [Google Scholar]
- 11.Ward RP, Leeper NJ, Kirkpatrick JN, Lang RM, Sorrentino MJ, Williams KA. The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery. Int J Cardiol 2005;104:264–268. [DOI] [PubMed] [Google Scholar]
- 12.Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg 2008;47:774–781. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005;36:2072–2076. [DOI] [PubMed] [Google Scholar]
- 14.McGirt MJ, Perler BA, Brooke BS, et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J Vasc Surg 2005;42:829–836. [DOI] [PubMed] [Google Scholar]
- 15.Williams TM, Harken AH. Statins for surgical patients. Ann Surg 2008;247:30–37. [DOI] [PubMed] [Google Scholar]
- 16.Hindler K, Shaw AD, Samuels J, Fulton S, Collard CD, Riedel B. Improved postoperative outcomes associated with preoperative statin therapy. Anesthesiology 2006;105:1260–1272. [DOI] [PubMed] [Google Scholar]
- 17.Heart Protection Study Collaborative Group. Effects of cholesterol lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363:757–767. [DOI] [PubMed] [Google Scholar]
- 18.Shah SJ, Waters DD, Barter P, et al. Intensive lipid lowering with atorvastatin for secondary prevention in patients after coronary artery bypass surgery. J Am Coll Cardiol 2008;51:1938–1943. [DOI] [PubMed] [Google Scholar]
- 19.Liakopoulos OJ, Choi YH, Haldenwang PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. Eur Heart J 2008;29:1548–1559. [DOI] [PubMed] [Google Scholar]
- 20.Clark LL, Ikonomidis JS, Crawford FA, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8-year retrospective cohort study. J Thorac Cardiovasc Surg 2006;679–685. [DOI] [PubMed] [Google Scholar]
- 21.Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation 2004;110(suppl II):45–49. [DOI] [PubMed] [Google Scholar]
- 22.Dotani MI, Elnicki DM, Jain AC, Gibson CM. Effect of preoperative statin therapy and cardiac outcomes after coronary artery bypass grafting. Am J Cardiol 2000;86:1128–1130. [DOI] [PubMed] [Google Scholar]
- 23.Subramaniam K, Koch CG, Bashour A, et al. Preoperative statin intake and morbid events after coronary artery bypass grafting. J Clin Anesth 2008;20:4–11. [DOI] [PubMed] [Google Scholar]
- 24.Aboyans V, Labrousse L, Lacroix P, et al. Predictive factors of stroke in patients undergoing coronary bypass grafting: statins are protective. Eur J Cardiothorac Surg 2006;30:300–304. [DOI] [PubMed] [Google Scholar]
- 25.Poldermans D, Bax JJ, Kertai MD. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation 2003;107:1848–1851. [DOI] [PubMed] [Google Scholar]
- 26.Durazzo AE, Machado FS, Ideoka DT. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004;39:967–976. [DOI] [PubMed] [Google Scholar]
- 27.O'Neil-Callahan K, Katsimaglis G, Tepper MR, et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the statins for risk reduction in surgery (StaRRS) study. J Am Coll Cardiol 2005;45:336–342. [DOI] [PubMed] [Google Scholar]
- 28.Perler BA. The effect of statin medications on perioperative and long-term outcomes following carotid endarterectomy or stenting. Semin Vasc Surg 2007;20:252–258. [DOI] [PubMed] [Google Scholar]
- 29.Borger MA, Ivanov J, Weisel RD, Rao V, Peniston CM. Stroke during coronary bypass surgery: principal role of cerebral macroemboli. Eur J Cardiothorac Surg 2001;19:627–632. [DOI] [PubMed] [Google Scholar]
- 30.Hogue CW, Gottesman RF, Stearns J. Mechanisms of cerebral injury from cardiac surgery. Crit Care Clin 2008;24:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard PA, Barnes BJ. Potential uses of statins to prevent atrial fibrillation after coronary artery bypass surgery. Ann Pharmacother 2008;42:253–258. [DOI] [PubMed] [Google Scholar]
- 32.Mariscalco G, Lorusso R, Klersy C, et al. Observational study on the beneficial effect of preoperative statins in reducing atrial fibrillation after coronary surgery. Ann Thorac Surg 2007;84:1158–1164. [DOI] [PubMed] [Google Scholar]
- 33.Hindler K, Eltzschig HK, Fox AA, Body SC, Shernan SK, Collard CD. Influence of statins on perioperative outcomes. J Cardiothorac Vasc Anesth 2006;20:251–258. [DOI] [PubMed] [Google Scholar]
- 34.Filion KB, Pilote L, Rahme E, Eisenberg MJ. Perioperative use of cardiac medical therapy among patients undergoing coronary artery bypass graft surgery: a systematic review. Am Heart J 2007;154:407–414. [DOI] [PubMed] [Google Scholar]
- 35.Ali IS, Buth KJ. Preoperative statin use and in-hospital outcomes following heart surgery in patients with unstable angina. Eur J Cardiothorac Surg 2005;27:1051–1056. [DOI] [PubMed] [Google Scholar]
- 36.Ali IS, Buth KJ. Preoperative statin use and outcomes following cardiac surgery. Int J Cardiol 2005;103:12–18. [DOI] [PubMed] [Google Scholar]
- 37.Turley AJ, Roberts AP, Morley R, Thornley AR, Owens WA, de Belder MA. Secondary prevention following coronary artery bypass grafting has improved but remains sub-optimal: the need for targeted follow-up. Interact Cardiovasc Thorac Surg 2008;7:231–234. [DOI] [PubMed] [Google Scholar]