Abstract
Objective:
To assess the effectiveness of the selegiline transdermal system (STS) in reversing HIV-induced metabolic brain injury (as measured by proton magnetic resonance spectroscopy [MRS]) and in decreasing oxidative stress, measured by CSF protein carbonyl concentration.
Methods:
Sixty-two subjects with HIV-associated cognitive impairment were coenrolled in a 24-week placebo-controlled study (AIDS Clinical Trial Group protocol A5090) and were randomly assigned to receive STS 3 mg/24 h, STS 6 mg/24 h, or matching placebo. Cognitive performance was evaluated using the neuropsychological z score (NPZ)-8 and NPZ-6, as well as cognitive domain scores. Subjects underwent proton MRS at study entry and weeks 12 and 24. CSF protein carbonyl was measured at baseline and week 24.
Results:
A slight increase in N-acetyl aspartate/creatine from baseline to week 24 was found in the basal ganglia (p = 0.023) and centrum semiovale (p = 0.072) of the placebo group compared with the STS groups; however, there were no significant changes when the absolute metabolite concentrations were analyzed. The levels of choline/creatine in the midfrontal cortex were also significantly higher during the week 12 visit in the combined STS groups. This persisted to the week 24 visit (p = 0.002). Evaluation of the change in NPZ-8, NPZ-6, and cognitive domain scores from baseline to weeks 12 and 24 revealed no significant differences between treatment arms. Protein carbonyl analysis revealed no significant changes among the groups.
Conclusion:
In this 24-week study, the selegiline transdermal system (STS) had no effect on either magnetic resonance spectroscopy (MRS) metabolites or oxidative stress, as measured by CSF protein carbonyl concentration. The lack of effect on these biomarkers is also reflected in the lack of cognitive improvement in the STS groups compared to placebo.
Level of evidence:
This study provides Class II evidence that STS had no effect on either MRS metabolites or oxidative stress, as measured by CSF protein carbonyl concentration over a period of 24 weeks.
GLOSSARY
- ADC
= dementia complex;
- Cho
= choline;
- Cr
= creatine;
- DNPH
= 2,4-dinitrophenylhydrazine;
- FOV
= field of view;
- Glx
= glutamate/glutamine;
- HAART
= highly active antiretroviral therapy;
- MI
= myoinositol;
- MRS
= magnetic resonance spectroscopy;
- NAA
= N-acetylaspartate;
- NEX
= number of excitations;
- NPZ
= neuropsychological z score;
- PBS
= phosphate-buffered saline;
- SNR
= signal-to-noise ratio;
- STS
= selegiline transdermal system;
- TE
= echo time;
- TR
= repetition time.
Proinflammatory products and reactive oxygen species secreted by infected or activated microglia and macrophages have been implicated in the pathogenesis of HIV-associated neurologic disorders.1 Additionally, elevated markers of oxidative stress have been reported in the CSF of subjects with HIV-associated cognitive impairment.2–5 Despite highly active antiretroviral therapy (HAART), levels of oxidative stress can remain elevated in HIV-infected subjects with cognitive impairment,3 and HAART alone does not fully prevent the occurrence of cognitive impairment.6 Antioxidant intervention may provide a useful adjunctive therapy to HAART in the treatment of HIV-associated cognitive impairment. The AIDS Clinical Trial Group has conducted a phase 2, placebo-controlled, double-blind study of the selegiline transdermal system (STS) in the treatment of HIV-associated cognitive impairment (study A5090).7 Selegiline is a monoamine oxidase B inhibitor capable of decreasing oxygen-free radicals, increasing the formation of the antioxidant enzymes superoxide dismutase and catalase, and providing additional neuroprotection by enhancing the synthesis of neurotrophic factors.8-13 Results of 2 prior placebo-controlled pilot studies of oral selegiline and STS in subjects with HIV-associated cognitive impairment suggested improvement in several tests of psychomotor speed.14,15
A5090 results demonstrated no significant benefit in either cognitive or functional outcome in subjects treated with selegiline compared with those who received placebo.7 However, it is possible that the exposure to STS in A5090 was too short to translate in a measurable cognitive or functional effect. The open-label extension of A509016 did not fully address this issue. We have conducted a substudy of A5090 using magnetic resonance spectroscopy (MRS) and CSF protein carbonyl concentration as biomarkers of CNS injury, which we hypothesized would be able to measure response to selegiline before observable clinical or functional changes.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was reviewed and approved by the institutional review board at each participating institution. All subjects signed a written informed consent before enrollment. This study was registered with clinicaltrials.gov under identifier NCT00027040.
Subjects.
One hundred twenty-eight HIV-infected subjects with cognitive impairment were enrolled in A5090 and were randomly assigned to receive STS 3 mg/24 h, STS 6 mg/24 h, or matching placebo for 24 weeks.7 Randomization was stratified by plasma HIV viral load (undetectable, <200 copies/mL, vs detectable, ≥200 copies/mL) and clinical stage of the AIDS dementia complex (ADC; 0.5 vs 1.0 or higher). Sixty-two subjects were coenrolled in this substudy at sites with MRS expertise. Cognitive impairment was defined as performance at least 1 SD below the mean on 2 or more independent neuropsychological tests, or at least 2 SDs below the mean on 1 neuropsychological test using a standard neuropsychological battery. Cognitive performance was evaluated using the NPZ-8 (average z score of 8 neuropsychological tests) and NPZ-6 (average z-score of 6 neuropsychological tests), as well as cognitive domain scores (average z score of neuropsychological tests corresponding to cognitive domain).
Imaging.
MRI was performed at study entry and weeks 12 and 24 on commercial GE Signa (Harvey, IL) 1.5-T scanners. Sagittal T1-weighted images were collected with the following parameters: 5-mm slice thickness, echo time (TE)/repetition time (TR) = 20/600, field of view (FOV) = 24 cm, and matrix size = 256 × 256, and number of excitations (NEX) = 1. Axial fast spin echo images were collected according to the following parameters: 5-mm slice thickness, TE1/TE2/TR = 30/80/2,500, FOV = 24 cm, matrix size = 245 × 192, echo train length = 8, bandwidth = 32 KHz, and NEX = 1.
Single-voxel proton spectra were acquired from 3 regions of interest using the commercially available GE PROBE-P PRESS sequence. Voxels 20 × 20 mm2 (slice thickness = 15 mm) were prescribed graphically from axial MR images in the midline of the frontal lobes (gray matter), right (or left) midfrontal centrum semiovale (white matter), and right (or left) basal ganglia (deep gray matter). Shimming was performed on each voxel using the automated FID-based algorithm from GE. Water suppressed spectra were collected according to the following parameters: TE/TR = 35/3,000, bandwidth = 2,500 Hz, 128 averages, and NEX = 8. Additionally, single-scan, fully relaxed water spectra were collected from each voxel with 8 different echo times. LCModel spectral analysis software17 was used to calculate the metabolite ratios of N-acetylaspartate (NAA) to creatine (Cr), choline (Cho) to Cr, myoinositol (MI) to Cr, and the combined peak of glutamate/glutamine (Glx) to Cr. Absolute quantitation of these metabolites was performed using the technique described by Kreis et al.18 and yielded metabolite concentrations corrected for the percentage of CSF in each voxel.
CSF protein carbonyl.
CSF was obtained at baseline and week 24. The level of protein oxidation was determined by an Oxidized Protein Detection Kit (Oxyblot, Chemicon, Temecula, CA). Equal amounts of protein (1 μg) from each CSF sample were loaded on the blot, and the intensity of the bands was quantitated by densitometry. Samples were incubated for 20 minutes with 6% (wt/vol) sodium dodecyl sulfate and 2,4-dinitrophenylhydrazine (DNPH) in 10% (vol/vol) trifluoroacetic acid, vortexing every 5 minutes, and then neutralized in Oxyblot neutralization solution. Samples were transferred to nitrocellulose membrane by slot-blotting technique. After the transfer, membranes were blocked with 5% (wt/vol) skim milk (in phosphate-buffered saline [PBS] with 0.1% (vol/vol) Tween-20) overnight at 2° to 8°C. The nitrocellulose membrane was exposed to a primary rabbit anti-DNPH protein antibody from Chemicon Oxyblot (1:150 working dilution) for 1 hour, and then to a secondary antibody (anti-rabbit immunoglobulin G coupled to horseradish peroxidase, Sigma, St. Louis, MO]) diluted in the blocking solution 1:5,000 for 60 minutes at room temperature. Membranes were washed after every step in washing buffer (PBS with 0.1% Tween-20). The nitrocellulose membrane was then developed by an EHP plus kit (GE Healthcare) and exposed to film, until the bands of oxidized proteins appeared. Blots were analyzed using BioRad (Hercules, CA) Quantity One software.
Statistical analysis.
Power calculations were based on our previous cross-sectional work19 and longitudinal work.20 Assumptions were based on the correlation of successive MRS evaluations obtained on the same individuals. Under most scenarios, we determined that with a minimum of 48 subjects, there would be adequate power (i.e., >80%) to detect similar differences between baseline and week 24 as we had seen cross-sectionally in the study by Chang et al.19 between neurologically impaired and nonimpaired HIV-infected patient groups.
Comparisons of continuous measures between groups were performed using the Kruskal-Wallis test, and comparisons of frequencies between groups were performed using the Fisher exact test.
To evaluate changes in MRS metabolite ratios over time, analysis of covariance models were applied to the 2 postbaseline MRS evaluations adjusted for baseline metabolite ratio levels using Box-Cox transformed measures for the longitudinal models of the metabolite levels. An autoregressive order 1, variance/covariance matrix was used to model the covariance structure of the model. In all resulting comparisons, the 2 selegiline arms have been pooled because there was never a significant difference observed between the 3-mg/24 h and 6-mg/24 h doses. Similar models were applied to assess absolute MRS metabolite changes over time.
Comparisons of the median protein carbonyl concentrations between groups were performed using the Kruskal-Wallis test.
RESULTS
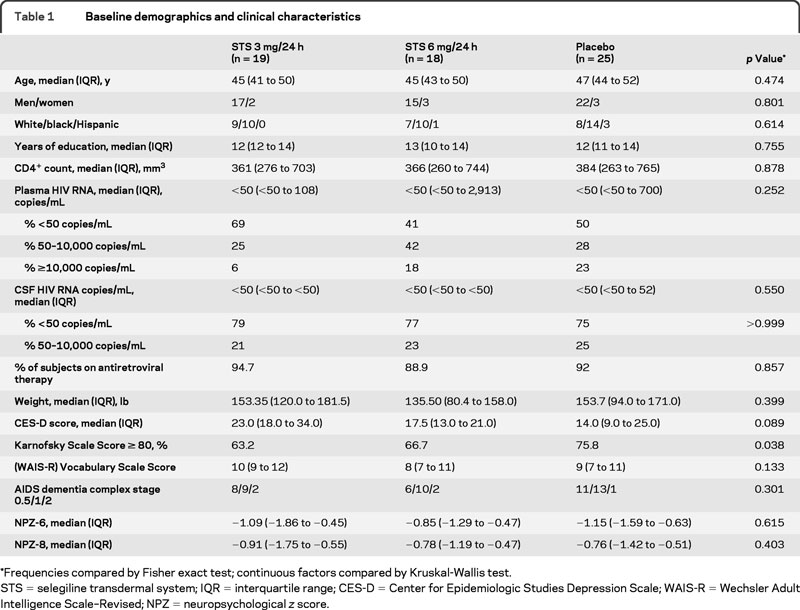
The 62 subjects enrolled in this substudy were predominantly male (87%) and African American (55%) (table 1). The median age at baseline was 46 years, with a median education level of 12.5 years. The 3 treatment groups did not differ significantly at baseline with respect to these demographic characteristics, ADC stage, HIV viral load, CD4+ count, NPZ scores (table 1), or cognitive domain scores (data not shown). No significant differences in proton MRS metabolite ratios were detected between treatment arms at baseline, with the exception of Glx/Cr in the centrum semiovale (table e-1 on the Neurology® Web site at www.neurology.org).
Table 1 Baseline demographics and clinical characteristics
As observed in the parent study, evaluation of the change in NPZ-8 and NPZ-6 scores (figure 1), as well as cognitive domain scores (data not shown) from baseline to weeks 12 and 24 revealed no significant differences between treatment arms.
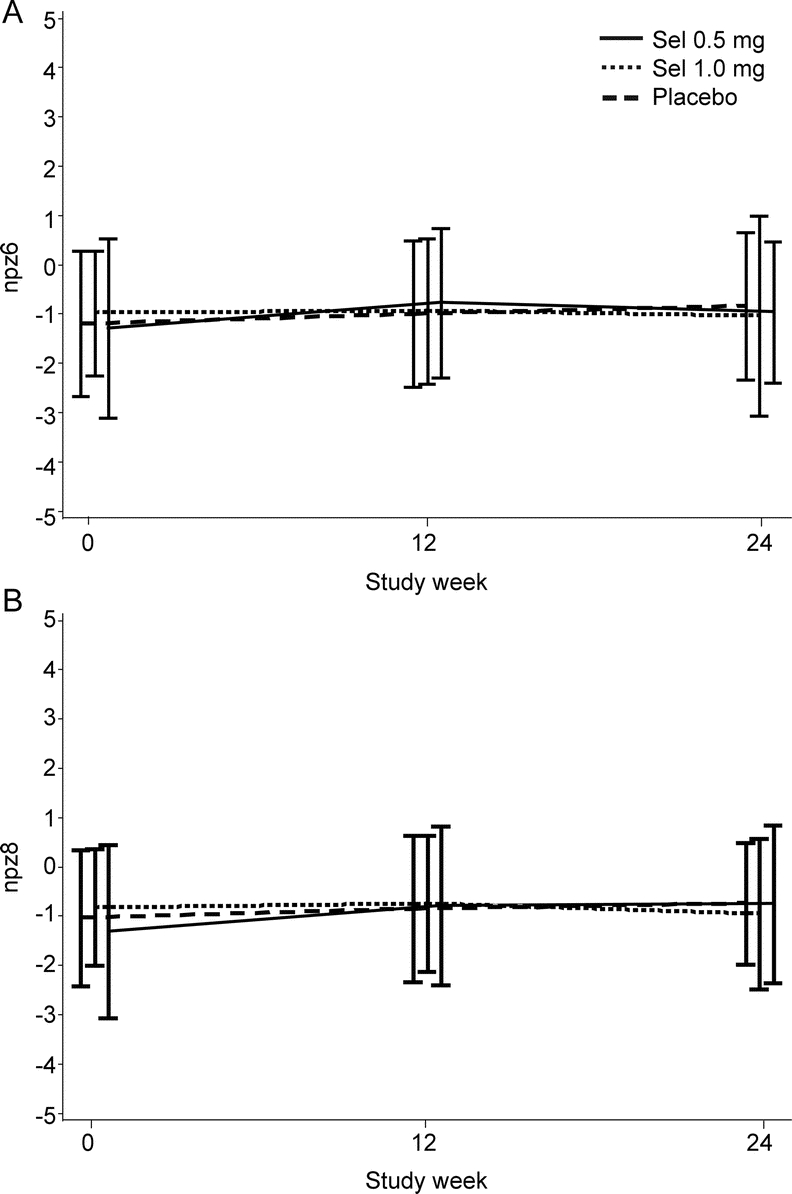
Figure 1 Changes in NPZ scores by treatment arm.
(A) Neuropsychological z score (NPZ)-6. (B) NPZ-8.
Changes in MRS metabolite ratios from baseline to weeks 12 and 24 are reported in table e-2. There was a slight increase in NAA/Cr in the basal ganglia (figure 2A) and in the centrum semiovale (figure 2B) of the placebo group compared with the STS groups (basal ganglia, p = 0.023; centrum semiovale, p = 0.072), although the change in the absolute concentration on NAA was not significant. The combined STS groups also demonstrated higher levels of Cho/Cr (figure 2C) in the midfrontal cortex (p = 0.002) when compared with the placebo group after adjusting for baseline levels of the metabolite. This difference in Cho was still present when the concentration of Cho was analyzed, although the significance was reduced (p = 0.046). As shown in table e-2, no other comparison results were significantly different among the groups. Analyses using absolute metabolite concentrations (data not shown) were similar to using their ratio to Cr, with the exception of those reported above.
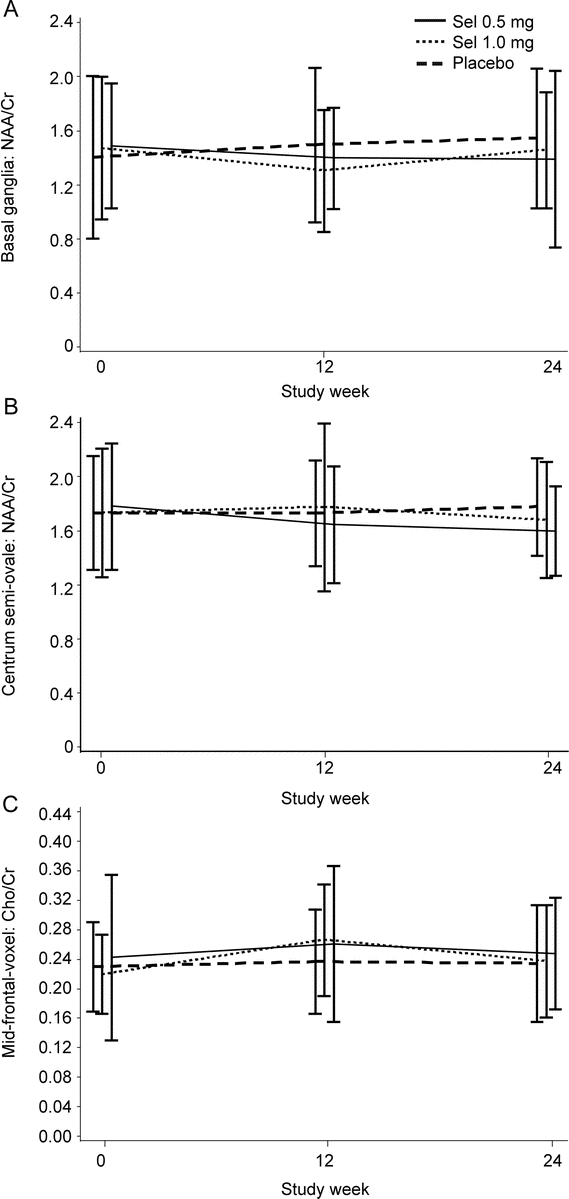
Figure 2 Means ± 2 SDs of metabolite ratios with between-group differences in change from baseline to week 24.
(A and B) Slight increases in N-acetylaspartate (NAA)/creatine (Cr) in the basal ganglia and in the centrum semiovale of the placebo group compared with the selegiline transdermal system (STS) groups (p = 0.023 and p = 0.072, respectively). Change in the absolute concentration of NAA was not significant. (C) Combined STS groups had higher levels of choline (Cho)/Cr in the midfrontal cortex (p = 0.002) compared with the placebo group after adjusting for baseline levels of the metabolite. A smaller difference in Cho (p = 0.046) was present when the absolute concentration of Cho was analyzed.
Protein carbonyl concentration in CSF (available in 47 subjects) decreased from baseline to week 24 in both placebo and STS groups; however, there was no significant difference among the groups (table 2). Only a small subset of subjects had both protein carbonyl CSF concentrations and MRS data available at baseline (8 were on placebo, and 11 were on STS). There were no significant correlations between protein carbonyl concentrations and MRS measures. Lower baseline protein carbonyl concentrations were associated with a greater increase in NAA/Cr in the centrum semiovale at weeks 12 and 24 (p = 0.005). A trend was also observed between higher concentrations of protein carbonyl at baseline and a greater increase of MI/Cr in the basal ganglia at weeks 12 and 24 (p = 0.063).
Table 2 Protein carbonyl changes by treatment arm
DISCUSSION
The use of the selegiline transdermal system (STS) in this cohort of HIV-infected individuals with cognitive impairment was not associated with either improvement in brain metabolites (as measured by MRS) or decreases in oxidative stress levels (as measured by CSF protein carbonyl concentrations) when compared with placebo. These results are complementary to the lack of cognitive improvement in the STS groups compared with placebo observed in this substudy and the larger parent study.7 The trend toward a decrease in NAA/Cr in the basal ganglia and centrum semiovale and increase in Cho/Cr in the frontal cortex in the STS groups compared with the placebo group raises additional questions regarding the neuroprotective role of STS in HIV-infected individuals. Although most in vitro and animal investigations have suggested that selegiline is neuroprotective,21 including some models of HIV-associated neurotoxicity,2,22 there have been reports in the simian immunodeficiency model that selegiline may worsen CNS pathology, possibly mediated by the resulting increase in dopaminergic activity.23,24
To our knowledge, the combination of MRS and protein carbonyl as the outcome of a clinical trial has not been reported before. MRS is a noninvasive technique that allows in vivo assessment of brain metabolites, including NAA (a marker of neuronal integrity), Cho (a marker of membrane damage), and MI (an osmolyte and astrocyte marker elevated in inflammatory and neoplastic disorders).25 Several studies indicate that MRS provides a sensitive and robust in vivo method to monitor the pattern and course of CNS injury in HIV infection.26-30 Particularly relevant to the use of MRS as a biomarker is the evidence that increases in NAA/Cr concentration and decreases in MI/Cr concentration occur in response to antiretroviral therapy and are associated with improved cognitive performance.26,31 Furthermore, MRS sensitivity in monitoring CNS injury is underscored by its ability to detect changes even before clinical manifestations of diseases.28,32,33 The usefulness of MRS in measuring CNS injury has been demonstrated in several other neurodegenerative diseases, including Parkinson disease,34 multiple system atrophy,35 multiple sclerosis,36 Huntington disease,32 and cortical dementias,33 as well as in toxic or neoplastic CNS injury.25
The MRS protocol used in this study reflected the imaging capability of the sites involved at the time of the study. Having 3-T data might have been beneficial, but there were not enough 3-T systems available when the study was designed. Furthermore, although higher field strength probably would have afforded some increases in the signal-to-noise ratio (SNR) compared with 1.5 T, gains in SNR do not necessarily translate into improvements in metabolite ratio reliability. For example, whole-brain NAA levels were not more reproducible at 3.0 T compared with 1.5 T.37 Finally, we used a single-voxel approach to maximize the detection of changes in brain regions known to be affected by HIV neuropathology.
The second biomarker, protein carbonyl, is a marker of protein oxidation and has been used to assess the impact of oxidative stress in aging and neurodegeneration.38-41 Furthermore, recent animal studies suggest that protein carbonyl concentrations may be responsive to antioxidant treatment.41 We have previously shown that CSF concentration of protein carbonyl is increased in HIV-infected individuals with cognitive impairment compared with HIV-infected subjects without cognitive impairment and compared with HIV-negative controls.2 It should be emphasized that CSF represents a limited window for assessing oxidative stress in the brain; however, it constitutes the closest compartment to the brain that we can easily and safely access.
We hypothesized that protein carbonyl concentration would have an inverse correlation with NAA/Cr and a positive correlation with Cho/Cr and MI/Cr, but we did not find significant correlations at baseline. However, lower CSF protein carbonyl concentration at baseline was associated with subsequent significant increase of NAA/Cr in the frontal white matter. One possible interpretation of this finding is that changes in levels of oxidative stress predate changes measured via MRS. Unfortunately, only a small number of subjects had both MRS and CSF protein carbonyl measured, because the MRS and lumbar puncture were optional in the parent study, thereby limiting the power of assessing the relationship between CSF protein carbonyl and MRS-measured metabolites.
Overall, the concordance between the lack of clinical response and lack of improvement in MRS-measured brain metabolites in this study and the concordance between trends in cognitive improvement and significant increases in NAA/Cr in 2 previous clinical trials in HIV-associated cognitive impairment20,42 reinforce the usefulness of MRS as a biomarker in HIV-associated cognitive impairment. The responsiveness of CSF protein carbonyl concentrations in predicting clinical outcomes merits further evaluation.
We are cognizant that a larger sample size would have provided greater power in assessing the effect of selegiline on brain metabolites and protein carbonyl. However, based on the results of this study that found no improvement in brain metabolites or in markers of oxidative stress and combined with the lack of clinical improvement observed in the larger parent study,7 we conclude that there are no strong bases for future development of STS as a neuroprotective compound for the treatment of HIV-associated cognitive impairment.
AUTHOR CONTRIBUTIONS
Statistical analyses were conducted by Constantin T. Yiannoutsos, PhD.
ACKNOWLEDGMENT
The authors thank Michelle Gaugh, MA, as a contributor in manuscript preparation, including technical assistance and editing for nonintellectual content.
STUDY FUNDING
A5114, NCT00027040 was supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases grants AI38858, AI68636, AI68634, AI069465, AI-069511-02, AI 069434, AI 69432, and AI27660; the Neurologic AIDS Research Consortium funded by the National Institute of Neurologic Diseases and Stroke, NS32228; the General Clinical Research Center Units funded by the National Center for Research Resources grants RR025005, 5-MO1 RR00044, and UO1-AI 032783-14; CFAR grants 5-P30-AI-045008-09, P30 MH075673, and 2-P01 MH064570-05; and the National Institute of Mental Health, MH64409.
DISCLOSURE
Dr. Schifitto receives research support from the NIH [MH64409 (PI)]. Dr. Yiannoutsos has received honoraria from the National Cancer Institute, the Susan G. Komen Breast Cancer Foundation, and the Annals of Surgery; and received research support from the NIH [NIAID 5U01AI069911 (PI), NCI 5U01AI069911 supplement (PI), NICHD 5U01AI069911 supplement (PI), NIMH 5 U01 MH083545-02 (PI), and NINDS 5R01NS036524 (Coinvestigator)]. Dr. Ernst receives research support from NRI Inc., the NIH [NIDA 5K02DA016991-03 (PI), NIMH 2R01MH061427-04-A1 (Coinvestigator), NINDS 2R01NS036524 (Coinvestigator), NIDA 1R01DA021016-03 (Coinvestigator), NCRR 1P20RR011091-14 (Coinvestigator), NCRR G12RR003061-23 (Coinvestigator), NINDS 1U54NS056883-01 (Coinvestigator), NIDA 1R01DA021146 (PI)], and Queen's Medical Center. Dr. Navia receives research support from the NIH [NINDS NS36524 (PI)]. Dr. Nath serves as Associate Editor of the Journal of Neurovirology; may accrue revenue from the following pending patents: Tat as an Immunogen [Filed: 4/2002]; Diosgenin for Treatment of Neurodegenerative Diseases [JHU Ref 4382 (Filed: 2004)], Role of Kv Channels in Neuroregeneration and Protection [JHU Ref 4986 (Filed: 2006)], Role of Lominoid Compounds as Neuroprotective Agents [JHU Ref 5038 (Filed: 2006)], and Tat ELISA [JHU Ref 5087 (Filed: 2006)]; served as a consultant to and received stock options from Nerveda Inc.; and receives research support from the NIH [NINDS R01NS039253 (PI), NINDS R01AI058842-01A2 (PI), P30 MH075673-01 (PI), R01NS056884 (PI), R25MH080661 (PI), NIDA R01DA024593 (PI), and NINDS R01NS055628 (PI)]. Dr. Sacktor receives research support from the NIH [NIAID AI-35042 (Coinvestigator), NIMH N01MH22005 (Coinvestigator), NIMH MH71150 (PI), NINDS U01-NS32228 (PI), NIMH P30-MH075673 (Core PI), NIMH MH058076 (Coinvestigator), and NIMH MH083465 (PI)]. Ms. Anderson reports no disclosures. Dr. Marra receives royalties from publishing an article in UpToDate (current to date) and from the textbook Infections of the Central Nervous System, 3rd ed. (Lippincott Williams & Wilkins, 2004); and receives research support from the NIH [1R01 NS/AI 34235 (PI), N01 MH22005 (Coinvestigator), and U01 NS 32228 (Site PI)]. Dr. Clifford has served on scientific advisory boards or as a consultant for Biogen Idec, Elan Corporation, Roche, Forest Laboratories, Inc., Genentech, Inc., GlaxoSmithKline ($2500) Millennium Pharmaceuticals, Inc., Schering-Plough Corp., regarding HIV 2007 ($300) Bristol-Myers Squibb, and Genzyme Corporation; received a speaker honorarium from GlaxoSmithKline; receives research support from Pfizer Inc., Schering-Plough Corp., Bavarian Nordic, NeurogesX, GlaxoSmithKline, Tibotec Therapeutics, Boehringer Ingelheim, Gilead Sciences, Inc., and Biogen Idec; and receives research support from the NIH [UO1 NS32228 (PI), UO1 AI69495 (PI), NIMH 22005 CHARTER Project (Site PI), NIDA RO3 DA022137(Coinvestigator), NIMH MH058076 (Site PI), and R21 3857-53187 (PI)].
Supplementary Material
APPENDIX
A5114 site acknowledgements: Katherine Carter, PA-C, Ilene Wiggins, RN (Johns Hopkins Adult AIDS CRS); Jianhui Zhong, PhD, Mary Adams, RN (University of Rochester CTU); Shelia Dunaway, MD, Margot Perrin, RN, MPH (University of Washington ACTU); Ronald J. Ellis, MD, PhD, Kathleen Nuffer, NP, Susan Cahill, RN (University of California, San Diego CTU); Beau Ances, MSc, MD, PhD, Joyce Okawa, RN, MSEd (University of Pennsylvania CTU); Elyse Singer, MD, Suzette Chafey, NP (UCLA Care Center CTU); Linda Chang, MD (University of Hawaii).
Address correspondence and reprint requests to Dr. Giovanni Schifitto, 1351 Mount Hope Ave., Suite 223, Rochester, NY 14620 giovanni.schifitto@ctcc.rochester.edu
Editorial, page 1942
See also page 1982
Supplemental data at www.neurology.org
e-Pub ahead of print on November 4, 2009, at www.neurology.org.
Study funding: Funding information is provided at the end of the article.
Disclosure: Author disclosures are provided at the end of the article.
Received February 17, 2009. Accepted in final form August 3, 2009.
REFERENCES
- 1.Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis 2006;21:1–17. [DOI] [PubMed] [Google Scholar]
- 2.Turchan J, Pocernich CB, Gairola C, et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology 2003;60:307–314. [DOI] [PubMed] [Google Scholar]
- 3.Sacktor N, Haughey N, Cutler R, et al. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol 2004;157:176–184. [DOI] [PubMed] [Google Scholar]
- 4.Haughey NJ, Cutler RG, Tamara A, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol 2004;55:257–267. [DOI] [PubMed] [Google Scholar]
- 5.Bandaru VV, McArthur JC, Sacktor N, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology 2007;68:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 2002;8:136–142. [DOI] [PubMed] [Google Scholar]
- 7.Schifitto G, Zhang J, Evans SR, et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 2007;69:1314–1321. [DOI] [PubMed] [Google Scholar]
- 8.Carrillo MC, Kitani K, Kanai S, Sato Y, Miyasaka K, Ivy GO. (−)-Deprenyl increases activities of superoxide dismutase and catalase in certain brain regions in old male mice. Life Sci 1994;54:975–981. [DOI] [PubMed] [Google Scholar]
- 9.Salo PT, Tatton WG. Deprenyl reduces the death of motoneurons caused by axotomy. J Neurosci Res 1992;31:394–400. [DOI] [PubMed] [Google Scholar]
- 10.Tatton WG, Greenwood CE. Rescue of dying neurons: a new action for deprenyl in MPTP parkinsonism. J Neurosci Res 1991;30:666–672. [DOI] [PubMed] [Google Scholar]
- 11.Matsui Y, Kumagae Y. Monoamine oxidase inhibitors prevent striatal neuronal necrosis induced by transient forebrain ischemia. Neurosci Lett 1991;126:175–178. [DOI] [PubMed] [Google Scholar]
- 12.Ansari KS, Yu PH, Kruck TP, Tatton WG. Rescue of axotomized immature rat facial motoneurons by R(−)-deprenyl: stereospecificity and independence from monoamine oxidase inhibition. J Neurosci 1993;13:4042–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatton WG, Chalmers-Redman RME. Modulation of gene expression rather than monoamine oxidase inhibition: (−)-deprenyl-related compounds in controlling neurodegeneration. Neurology 1996;47:S171–S183. [DOI] [PubMed] [Google Scholar]
- 14.The Dana Consortium on the Therapy of HIV Dementia and Related Cognitive Disorders. Safety and tolerability of thioctic acid and deprenyl in HIV dementia. Neurology 1998;50:645–651. [DOI] [PubMed] [Google Scholar]
- 15.Sacktor N, Schifitto G, McDermott MP, Marder K, McArthur JC, Kieburtz K. Transdermal selegiline in HIV-associated cognitive impairment: pilot placebo-controlled study. Neurology 2000;54:233–235. [DOI] [PubMed] [Google Scholar]
- 16.Evans SR, Yeh T-M, Sacktor N, et al. Selegiline transdermal system (STS) for HIV-associated cognitive impairment: open-label report of ACTG 5090. HIV Clin Trials 2007;8:437–446. [DOI] [PubMed] [Google Scholar]
- 17.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 18.Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain, II: metabolite concentrations. J Magn Res B 1993;102:9–19. [Google Scholar]
- 19.Chang L, Lee PL, Yiannoutsos CT, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage 2004;23:1336–1347. [DOI] [PubMed] [Google Scholar]
- 20.Schifitto G, Navia BA, Yiannoutsos CT, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS 2007;21:1877–1886. [DOI] [PubMed] [Google Scholar]
- 21.Ebadi M, Brown-Borg H, Ren J, et al. Therapeutic efficacy of selegiline in neurodegenerative disorders and neurological diseases. Curr Drug Targets 2006;7:1513–1529. [DOI] [PubMed] [Google Scholar]
- 22.Turchan-Cholewo J, Liu Y, Gartner S, et al. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis 2006;23:109–119. [DOI] [PubMed] [Google Scholar]
- 23.Czub S, Koutsilieri E, Sopper S, et al. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol 2001;101:85–91. [DOI] [PubMed] [Google Scholar]
- 24.Czub S, Czub M, Koutsilieri E, et al. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol 2004;107:216–226. [DOI] [PubMed] [Google Scholar]
- 25.Lin A, Ross BD, Harris K, Wong W. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx 2005;2:197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang L, Ernst T, Leonido-Yee M, et al. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology 1999;53:782–789. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology 1999;52:100–108. [DOI] [PubMed] [Google Scholar]
- 28.Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology 1999;52:995–1003. [DOI] [PubMed] [Google Scholar]
- 29.Salvan AM, Vion-Dury J, Confort-Gouny S, Nicoli F, Lamoureux S, Cozzone PJ. Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res Hum Retroviruses 1997;13:1055–1066. [DOI] [PubMed] [Google Scholar]
- 30.Yiannoutsos CT, Ernst T, Chang L, et al. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage 2004;23:928–935. [DOI] [PubMed] [Google Scholar]
- 31.Stankoff B, Tourbah A, Suarez S, et al. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology 2001;56:112–115. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds J, Prost RW, Mark LP. Heterogeneity in 1H-MRS profiles of presymptomatic and early manifest Huntington's disease. Brain Res 2005;1031:82–89. [DOI] [PubMed] [Google Scholar]
- 33.Godbolt AK, Waldman AD, MacManus DG, et al. MRS shows abnormalities before symptoms in familial Alzheimer disease. Neurology 2006;66:718–722. [DOI] [PubMed] [Google Scholar]
- 34.Oz G, Terpstra M, Tkac I, et al. Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: detection of high GABA concentrations. Magn Reson Med 2006;55:296–301. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe H, Fukatsu H, Katsuno M, et al. Multiple regional 1H-MR spectroscopy in multiple system atrophy: NAA/Cr reduction in pontine base as a valuable diagnostic marker. J Neurol Neurosurg Psychiatry 2004;75:103–109. [PMC free article] [PubMed] [Google Scholar]
- 36.Rovaris M, Gambini A, Gallo A, et al. Axonal injury in early multiple sclerosis is irreversible and independent of the short-term disease evolution. Neurology 2005;65:1626–1630. [DOI] [PubMed] [Google Scholar]
- 37.Benedetti B, Rigotti DJ, Liu S, Filippi M, Grossman RI, Gonen O. Reproducibility of the whole-brain N-acetylaspartate level across institutions, MR scanners, and field strengths. AJNR Am J Neuroradiol 2007;28:72–75. [PMC free article] [PubMed] [Google Scholar]
- 38.Smith CD, Carney JM, Starke-Reed PE, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA 1991;88:10540–10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience 2001;103:373–383. [DOI] [PubMed] [Google Scholar]
- 40.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid [beta]-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med 2007;43:658–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Opii WO, Joshi G, Head E, et al. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer's disease. Neurobiol Aging 2008;29:51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schifitto G, Peterson DR, Zhong J, et al. Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report. Neurology 2006;66:919–921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.