Abstract
Background:
Methylenedioxymethamphetamine (MDMA, “ecstasy”) is a popular recreational drug of abuse and a selective brain serotonin neurotoxin. Functional consequences of MDMA neurotoxicity have defied ready characterization. Obstructive sleep apnea (OSA) is a common form of sleep-disordered breathing in which brain serotonin dysfunction may play a role. The present study sought to determine whether abstinent recreational MDMA users have an increased prevalence of OSA.
Methods:
We studied 71 medically healthy recreational MDMA users and 62 control subjects using all-night sleep polysomnography in a controlled inpatient research setting. Rates of apneas, hypopneas, and apnea hypopnea indices were compared in the 2 groups, controlling for body mass index, age, race, and gender.
Results:
Recreational MDMA users who had been drug free for at least 2 weeks had significantly increased rates of obstructive sleep apnea and hypopnea compared with controls. The odds ratio (95% confidence interval) for sleep apnea (mild, moderate, and severe combined) in MDMA users during non-REM sleep was 8.5 (2.4–30.4), which was greater than that associated with obesity [6.9 (1.7–28.2)]. Severity of OSA was significantly related to lifetime MDMA exposure.
Conclusions:
These findings suggest that prior recreational methylenedioxymethamphetamine use increases the risk for obstructive sleep apnea and lend support to the notion that brain serotonin neuronal dysfunction plays a role in the pathophysiology of sleep apnea.
GLOSSARY
- AHI
= apnea hypopnea index;
- BMI
= body mass index;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- MDMA
= methylenedioxymethamphetamine;
- NREM
= non-REM;
- OR
= odds ratio;
- OSA
= obstructive sleep apnea;
- PSG
= polysomnography;
- SCID-I
= Scheduled Diagnostic Interview for DSM-IV;
- SDB
= sleep-disordered breathing.
Obstructive sleep apnea (OSA) is a highly prevalent sleep disorder that is estimated to afflict approximately 15 million adult Americans1 and is characterized by repeated episodes of apnea or hypopnea during sleep. The pathophysiology of sleep apnea is incompletely understood. However, the consequences of OSA can be serious and include daytime sleepiness, cognitive dysfunction, metabolic syndrome, and cardiovascular disease.2 Recently, OSA has also been identified as an independent risk factor for death related to cardiovascular causes.3,4
The neurobiologic underpinnings of OSA are not fully understood. However, there has been increased interest in the role of central serotonin systems in its pathogenesis. Brain serotonin neurons modulate sleep and breathing patterns through a variety of different mechanisms. For example, serotonin neurons influence circadian changes in sleep propensity,5 mediate cortical arousal,6 act as carbon dioxide chemoreceptors that modulate respiratory drive,7 and innervate muscles that maintain pharyngeal patency.8–11 In addition, there is serotonin neuronal input to the respiratory rhythm generator in the brainstem pre-Bötzinger complex, as well as to spinal respiratory motor neurons.12,13 Therefore, although the pathophysiology of sleep apnea is complex and may involve a variety of mechanisms,14 there is reason to believe that altered brain serotonin neuronal function may be involved.
Methylenedioxymethamphetamine (MDMA, “ecstasy”) is a popular recreational drug of abuse and a potent selective brain serotonin neurotoxin.15,16 Studies in animals, including nonhuman primates, have demonstrated that MDMA leads to lasting dose-related and protracted reductions in a variety of brain serotonin axonal markers, and neuroanatomical studies with immunochemical markers suggest that losses of axonal markers are secondary to a distal axotomy of serotonin neurons.17,18 PET imaging studies using radioligands that bind to serotonin transporters provide evidence that MDMA is also neurotoxic in humans.19–21 Although functional consequences of MDMA-induced brain serotonin neurotoxicity are not well understood, available data suggest that abstinent MDMA users have alterations in sleep architecture22 and cognitive function.23–26
The present study was conducted in abstinent, otherwise healthy MDMA users whose use characteristics have previously been shown to lead to persistent losses of brain serotonin transporters suggestive of serotonin neurotoxicity. In particular, MDMA users with similar use patterns in studies with identical inclusion criteria have previously been found to have a reduction in brain serotonin transporters as measured by PET.19 The purpose of this study was to determine whether abstinent MDMA users showed evidence of sleep-disordered breathing (SDB).
METHODS
Subjects.
All participants (n = 71 MDMA users; n = 62 controls) were medically healthy individuals who responded to recruitment materials posted in newspapers and fliers looking for “club drug users.” The same recruitment materials were used for both MDMA users and controls, and potential subjects were not aware that we were specifically interested in the effects of MDMA. Inclusion in the MDMA subject group required a self-report of 25 or more separate lifetime uses of MDMA because such subjects have previously been shown to have lasting reductions in brain serotonin axonal markers.19,27 Inclusion in the control group required that subjects had never used MDMA. Inclusion criteria for all subjects included willingness to abstain from illicit substance use for 2 weeks before study participation, normal results on all medical screening measures (see below), and negative drug screens (with the exception of marijuana, which can remain positive for at least 3 weeks). Exclusion criteria included regular use of prescribed psychotropic medications, previous diagnosis of a sleep disorder, major medical illness, drug or alcohol dependence, history of significant head injury, and presence of an Axis I psychiatric diagnosis in which serotonin has been implicated (major depression, bipolar affective disorder, obsessive compulsive disorder, panic disorder, psychosis).
To determine whether interested individuals would be included in the study, potential subjects first underwent an initial institutional review board–approved scripted phone screen. Questions in the script were designed to determine whether subjects met inclusion and exclusion criteria. All subjects who met study criteria in the phone screen (for either the MDMA or the control group) were then invited for face-to-face screening. At the time of the face-to-face screen, subjects provided informed written consent before undergoing further evaluation. In addition to drug testing, additional screening measures included routine blood chemistries, HIV screening, complete blood counts, and urine testing for drugs of abuse, including cannabinoids, opiates, cocaine metabolites, alcohol, and amphetamines. If, after laboratory and medical examination, individuals continued to meet full inclusion criteria, they were immediately enrolled and admitted. Notably, before coming for face-to-face screening, subjects had been informed that they needed to abstain from all illicit drug use for 2 weeks, from any alcohol consumption for at least 3 days, that they would be screened for illicit drug use at the time of the screening, and that would be excluded from participation if drug or alcohol screens were positive. All subjects were also interviewed using the Scheduled Diagnostic Interview for DSM-IV (SCID-I).28 MDMA (and other drug) use was assessed during the initial phone screen and by a standardized drug questionnaire, nursing assessment upon admission to the inpatient Clinical Research Unit, the SCID-I, and an MDMA-specific questionnaire. If subjects provided inconsistent information, they were excluded from study participation.
Standard protocol approvals, registrations, and patient consents.
The research described in this article was approved by an institutional investigational review board. All subjects provided informed, written consent to participate in the research.
Polysomnography.
The research took place in a federally funded inpatient clinical research unit. While on the clinical research unit, the sleep schedule was regulated, with a “lights-out” time of 11:00 pm and a “lights-on” time of 7:00 am. Participants were not permitted to sleep before lights-out time or sleep past lights-on time. The overnight polysomnography (PSG) consisted of continuous recordings of right and left electrooculographic leads, 4 electroencephalographic leads (C3–A3, C4–A1, O1–A2, O2–A1), submental electromyogram, and bilateral anterior tibialis. The electrooculograms and EEGs were recorded at a minimum of 100 Hz and up to 500 Hz, and EMGs were recorded at 200 Hz. Respiration of the participant was monitored by a nasal pressure cannula at the nostrils, a thermistor/thermocouple at the nose and mouth, and thoracic and abdominal strain gauges. Continuous recording of the participant's oxyhemoglobin saturation was obtained by an oximeter. Scoring of the overnight PSG was visually scored by certified polysomnographers from the Johns Hopkins Sleep Disorders Center, following American Academy of Sleep Medicine Guidelines.29 Apnea and hypopnea measures were characterized as obstructive, central, or mixed. Polysomnographers who scored sleep records were blind to subjects’ group designation (i.e., MDMA vs control). The apnea hypopnea index (AHI) was defined as the mean number of apneas plus hypopneas per hour of sleep, the apnea index was defined as the mean number of apneas per hour, and the hypopnea index was defined as the mean number of hypopneas per hour. Severity of OSA was defined using conventional definitions (AHI <5 = normal; AHI 5–14 = mild OSA; AHI 15–29 = moderate OSA; AHI ≥30 = severe OSA).
Statistical analysis.
Between-group comparisons were performed using a multiple linear regression model comparing disordered breathing rates in MDMA users and control subjects. Stepwise variable selection was used to identify potential significant covariates, including known risk factors for SDB: body mass index (BMI), sex, race, and age. In addition, given the larger number of males in the MDMA group and known gender differences in the prevalence of SDB, we also conducted an analysis comparing SDB measures in males only (see supplemental data on the Neurology® Web site at www.neurology.org). The significance level used for determining the entry and removal of the covariates from the model was a p value ≤0.15. The variable remained in the model only if it retained a p value ≥0.10. A multiple logistic regression model was also used to determine the odds ratio (OR) for mild, moderate, and severe obstructive sleep apnea (combined) in MDMA users. Binary variables were created using the AHI during total sleep, during non-REM (NREM) sleep, and during REM sleep. The latent variable was defined as 1 if the AHI was greater than 5 events per hour and 0 otherwise. For the purpose of the logistic regression, age was studied as a continuous variable, whereas BMI was categorized into underweight (BMI <18.5), normal (18.5 ≤ BMI < 25), overweight, (25 ≤ BMI < 30), and obese (30 ≤ BMI < 40).
To assess the potential association between the total number of lifetime MDMA exposures and disordered breathing measures in the MDMA population, Pearson partial correlation coefficients were determined. In these analyses, an individual MDMA exposure was defined as MDMA use in a single setting (e.g., a dance party) and sometimes involved multiple doses over several hours. The same stepwise selection process as described above was used to identify covariates. A similar procedure was conducted to explore the potential relationship between prior cocaine use and SDB, because cocaine has the potential to produce microvascular brain injury that could potentially influence our findings. Statistics were conducted using MATLAB (The Mathworks, Inc., Natick, MA) and SAS (SAS Institute Inc., Cary, NC).
RESULTS
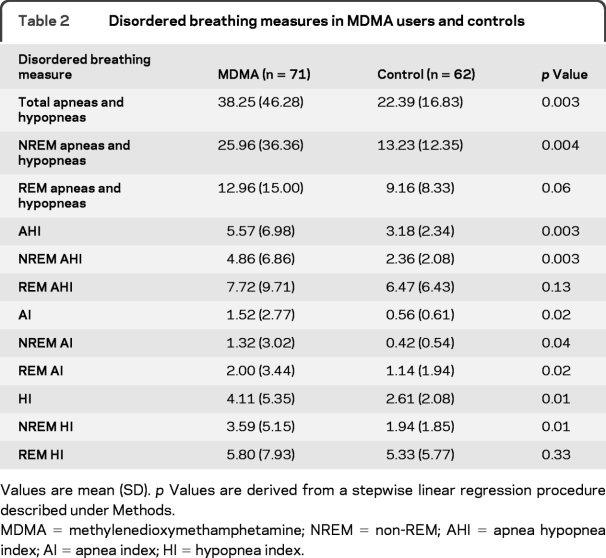
Demographics and recreational drug use characteristics of subjects are provided in table 1. The 2 groups did not significantly differ with regard to age or BMI. The proportion of male subjects was higher in the MDMA group, and the proportion of African Americans was higher in the control group. MDMA users, as a group, used more recreational drugs in the past than control subjects.
Table 1 Demographics of MDMA and control subject groups
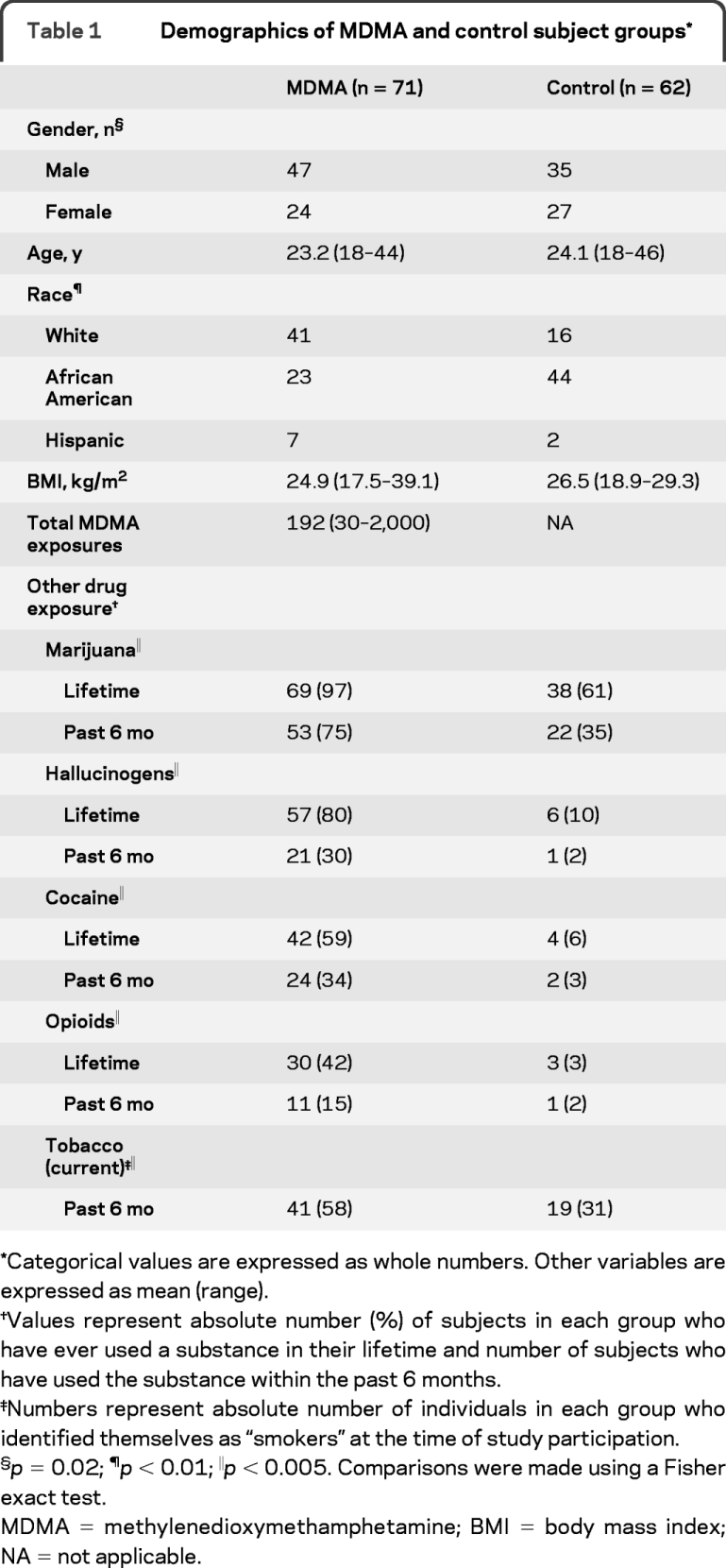
MDMA users had greater numbers and rates of apneas and hypopneas when compared with controls (table 2 and figure 1), with 80% of the apneas in MDMA users identified as being of the obstructive type, 14% being of the mixed type, and the remaining 6% being of the central type. When data were analyzed using conventional definitions of OSA severity, rates of mild OSA (AHI 5–14) were similar in the 2 groups, but moderate (AHI 15–29) and severe (AHI ≥30) OSA were only observed in the MDMA group (figure 1). Of the 24 MDMA subjects who met criteria for OSA, 22 were aged 31 years or younger. A Fisher exact test (1-tailed) revealed that differences in the rate of moderate and severe sleep apnea observed in the 2 groups were not secondary to chance (p = 0.001; table e-1).
Table 2 Disordered breathing measures in MDMA users and controls
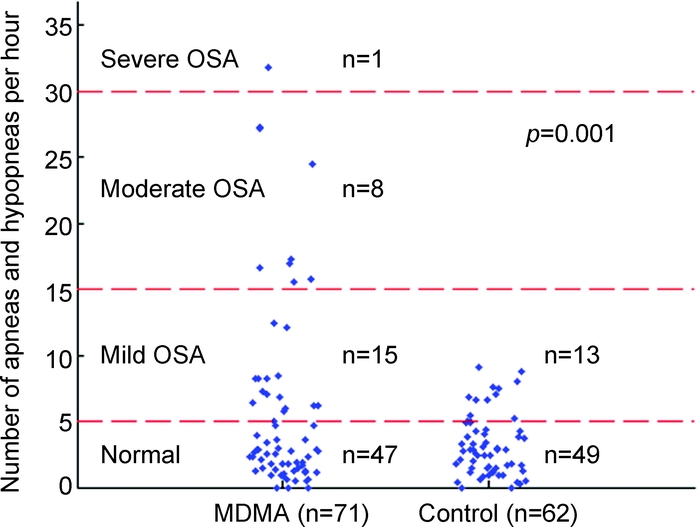
Figure 1 Apnea hypopnea indices in abstinent MDMA users and controls
Blue diamonds represent apnea hypopnea index values for subjects in methylenedioxymethamphetamine (MDMA) and control groups. OSA = obstructive sleep apnea.
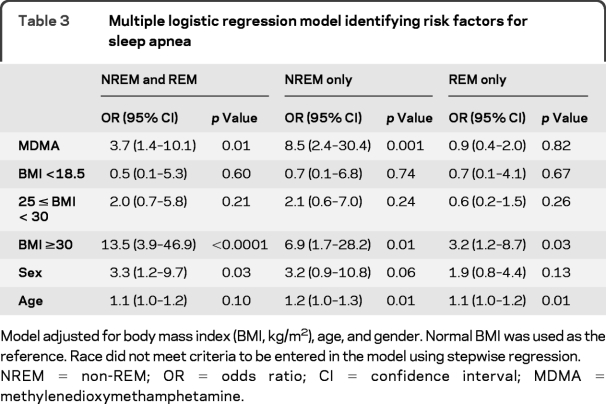
Logistic regression during REM, NREM, and total sleep revealed that MDMA users were at increased risk for meeting criteria for sleep apnea (mild, moderate, and severe combined), particularly during NREM sleep (table 3). During NREM sleep, MDMA users had more than 8 times the odds of apnea and hypopnea episodes than controls. When only male subjects were considered, the OR for SDB during NREM sleep increased to 11 (table e-2). Prior MDMA use increased the odds for sleep apnea during NREM sleep to a greater degree than BMI in this study population.
Table 3 Multiple logistic regression model identifying risk factors for sleep apnea
Greater lifetime MDMA use was associated with increased rates of sleep apnea (r = 0.44, p = 0.001; figure 2). Partial correlations between lifetime MDMA use and sleep apnea and hypopnea indices (controlling for age, gender, BMI, and race) demonstrated a significant relationship between these measures and prior MDMA exposure (table e-3). Prior use of cocaine was not found to be associated with SDB.
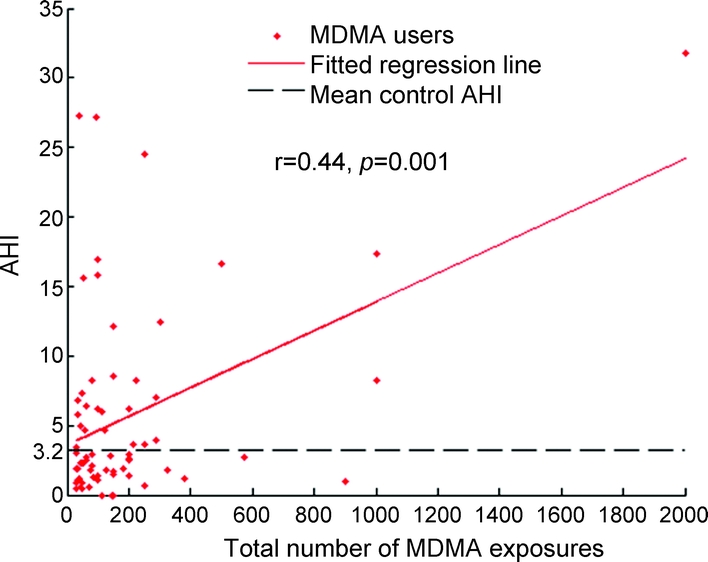
Figure 2 Apnea hypopnea index vs total lifetime MDMA use
AHI = apnea hypopnea index; MDMA = methylenedioxymethamphetamine.
DISCUSSION
The present study indicates that prior use of the recreational drug and brain serotonin neurotoxin, MDMA, is a risk factor for sleep apnea in otherwise healthy young adults, independent of age, male gender, race, and obesity. Further, the relationship between MDMA use and sleep apnea seems to be directly related to extent of prior MDMA exposure, with greater lifetime MDMA use associated with increased rates of sleep apnea. These findings lend support to the view that brain serotonin systems play a role in the pathophysiology of OSA8,10–13 and suggest that sleep apnea is a potential functional consequence of recreational MDMA use.
Although the present findings further implicate brain serotonin dysfunction in sleep apnea and hypopnea, the precise mechanisms involved are not clear. Increased rates of apnea and hypopnea in abstinent recreational MDMA consumers could be related to loss of airway patency due to altered serotonergic input to the hypoglossal motor nucleus.8–11 Alternatively, sleep apnea in these young individuals could result from faulty chemoreception by serotonergic neurons,7 disruption of the respiratory rhythm generator,12 or defective serotonergic modulation of respiratory motoneurons.13 Preclinical models of MDMA-induced serotonin neurotoxicity may also be useful in clarifying these important issues.
Functional consequences of MDMA-induced neurotoxicity in human recreational users have been difficult to identify. The most consistent behavioral differences that have been reported in abstinent MDMA users have been subtle cognitive deficits,23–26 behavioral impulsivity,30,31 altered sleep architecture,30 and altered neuroendocrine function.32,33 The present study provides evidence that MDMA exposure increases the risk for obstructive sleep apnea, with rates of sleep apnea and hypopnea being directly related to prior MDMA exposure. As discussed below, there may be a relationship among OSA and cognitive deficits and impulsivity in abstinent MDMA users, but it remains to be clarified.
Apneas and hypopneas, by disrupting sleep, may contribute to cognitive deficits found in MDMA users.22–26 This possibility is raised by the fact that chronic sleep disruption is known to have a deleterious effect on daytime cognitive functioning.34 Thus, arousals during sleep secondary to nocturnal hypopneas and apneas could play a role in subtle changes in cognitive function that have been reported in abstinent MDMA users. However, none of the previous polysomnographic studies in MDMA users30 have found significant decreases in sleep latency in MDMA users, suggesting that excessive daytime sleepiness is not the way in which SDB leads to cognitive deficits in abstinent MDMA users. Notably, another aspect of cognitive function that has been found to be altered in MDMA users is impulse control.30,31 There is a substantial body of literature indicating that sleep loss in adolescents increases impulsive and risk-taking behavior.35,36 Although intriguing, it remains to be determined whether sleep loss is responsible for behavioral findings in MDMA users and, further, whether serotonin neurotoxicity underlies sleep abnormalities and impulsive tendencies in the same individuals.
Limitations of the present study should be acknowledged and include the fact that MDMA users, as a rule, experiment with multiple substances, most commonly marijuana. In theory, one or several of these other substances could play a role in the increased incidence of sleep apneas and hypopneas found in the present group of MDMA users. However, although many drug classes have the potential to influence breathing and respiratory patterns acutely, there are no data suggesting that any of the recreational drugs used by these subjects has long-lasting effects on respiratory function or serotonin neurons. Although some subjects did test positive for marijuana, marijuana use has not been identified as a risk factor for sleep apnea, and exclusion of these subjects did not influence the study outcome. Indeed, cannabinoids have been proposed as a potential treatment for obstructive sleep apnea.37 Cocaine, which has the potential to produce microvascular brain injury, was not associated with SDB in the present study. Another limitation of this study is that drug use reports were retrospective, and self-reported prior drug use is subject to error. However, we have previously found reductions of brain serotonin transporters, as measured by PET, to be significantly associated with self-reported lifetime MDMA exposure.19 Finally, concerns regarding the role of other drugs of abuse in the present findings are mitigated by the relationship between extent of prior MDMA exposure and sleep apnea and hypopnea in the current study, the known dose-related neurotoxic effects of MDMA toward brain serotonin neurons,15–18 and the fact that serotonin has been previously implicated in OSA.8
Additional research will be necessary to determine whether sleep abnormalities in MDMA users play a role in subtle cognitive deficits and impulsivity that have previously been identified in this population. Studies that evaluate the relationship between brain serotonin markers and measures of SDB will strengthen conclusions regarding the role of serotonin neurotoxicity in sleep apnea in MDMA users. Preclinical and clinical studies coupling respiratory physiology methodology with functional neuroimaging approaches may be required to determine the neurobiological basis for our findings.
AUTHOR CONTRIBUTIONS
Statistical analyses were performed by Francis P. Sgambati, BSE, and reviewed by Nae-Yuh Wang, PhD (Interdisciplinary Center for Translational Research Biostatistician).
ACKNOWLEDGMENT
The authors thank Dr. Nae-Yuh Wang for his biostatistical review of this manuscript; Emily Dotter, Kristen Kelley, Michael Wilson, and Michael Palermo for research assistance; and the CRU/ICTR nursing staff for their participation in the execution of these studies.
DISCLOSURE
Dr. McCann has served on a scientific advisory board and speakers’ bureau for Jazz Pharmaceuticals; has received honoraria from the Public Health Service and the Veterans Administration (grant reviews); and receives research support from the NIH [NIDA R01 DA010217 (PI), NIDA R01 DA16563 (PI), NIDA 1R01 DA017231-01 A1 (Coinvestigator), NIDA 2 R01 DA05707 (Coinvestigator), NIDA 2 R01 DA05938 (Coinvestigator), and NICHHD 1 R01 HD050202-01 (Coinvestigator)]. Mr. Sgambati reports no disclosures. Dr. Schwartz may accrue revenue on US Patent 6457472 (Issued: October 1, 2002): Transtracheal insufflation for treatment of obstructive sleep apnea; serves as a scientific advisor to Apnex, Scientific Advisor, and Cardiac Concepts; and receives research support from the NIH [HL50381 (PI), SCCOR program (Coinvestigator), HL37379 (Coinvestigator), and NCRR (Sleep Core Director, ICTR and Clinical Research Unit)]. Dr. Ricaurte receives research support from the NIH [NIDA K05 DA01796401 (PI), NIDA 5 R01 DA05938 (PI), and NICHHD 1 R01 HD050202-01 (PI)].
Supplementary Material
Address correspondence and reprint requests to Dr. Una D. McCann, Department of Psychiatry, The Johns Hopkins School of Medicine, 5501 Hopkins Bayview Circle, Room 5B71c, Baltimore, MD 21224 umccann@jhmi.edu
Editorial, page 1947
Supplemental data at www.neurology.org
e-Pub ahead of print on December 2, 2009, at www.neurology.org.
Supported by Public Health Service grants DA16563 (McCann), DA05938 and DA01796401 (Ricaurte), and NCRR grant M01RR002719 (Ford).
Disclosure: Author disclosures are provided at the end of the article.
Received March 12, 2009. Accepted in final form August 12, 2009.
REFERENCES
- 1.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008;118:1080–1111. [DOI] [PubMed] [Google Scholar]
- 2.Pack AI. Advances in sleep-disordered breathing. Am J Respir Crit Care Med 2006;173:7–15. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 5.Challet E. Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 2007;148:5648–5655. [DOI] [PubMed] [Google Scholar]
- 6.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005;437:1257–1263. [DOI] [PubMed] [Google Scholar]
- 7.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 2004;5:449–461. [DOI] [PubMed] [Google Scholar]
- 8.Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea: therapeutic potential. Am J Respir Med 2003;2:21–29. [DOI] [PubMed] [Google Scholar]
- 9.Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2 A and 2C receptors in brainstem motoneurons. Eur J Neurosci 2003;17:1179–1188. [DOI] [PubMed] [Google Scholar]
- 10.Horner RL. Control of genioglossus muscle by sleep state-dependent neuromodulators. Adv Exp Med Biol 2008;605:262–267. [DOI] [PubMed] [Google Scholar]
- 11.Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol 2008;164:179–196. [DOI] [PubMed] [Google Scholar]
- 12.Tryba AK, Peña F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 2006;26:2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahamed S, Mitchell GS. Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol 2008;586:2171–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis 2009;51:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green AR, Mechan AO, Elliott JM, O'shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 2003;55:463–508. [DOI] [PubMed] [Google Scholar]
- 16.Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav 2008;90:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci 1988;8:2788–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatzidimitriou G, McCann UD, Ricaurte GA. Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/−)3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery. J Neurosci 1999;19:5096–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCann UD, Szabo Z, Seckin E, et al. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology 2005;30:1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (“ecstasy”) users. Br J Psychiatry 1999;176:63–69. [DOI] [PubMed] [Google Scholar]
- 21.Thomasius R, Zapletalova P, Petersen K, et al. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol 2006;20:211–225. [DOI] [PubMed] [Google Scholar]
- 22.McCann UD, Ricaurte GA. Effects of (+/−) 3,4-methylenedioxymethamphetamine (MDMA) on sleep and circadian rhythms. Sci World J 2007;7:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrott AC. Human research on MDMA (3,4-methylene-dioxymethamphetamine) neurotoxicity: cognitive and behavioural indices of change. Neuropsychobiology 2000;42:17–24. [DOI] [PubMed] [Google Scholar]
- 24.Zakzanis KK, Campbell Z, Jovanovski D. The neuropsychology of ecstasy (MDMA) use: a quantitative review. Hum Psychopharmacol 2007;22:427–435. [DOI] [PubMed] [Google Scholar]
- 25.Kalechstein AD, De La Garza R II, Mahoney JJ III, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology 2007;189:531–537. [DOI] [PubMed] [Google Scholar]
- 26.de Win MM, Jager G, Booij J, et al. Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain 2008;131(pt 11):2936–2945. [DOI] [PubMed] [Google Scholar]
- 27.McCann UD, Ridenour A, Shaham Y, Ricaurte GA. Serotonin neurotoxicity after (+/−) 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”): a controlled study in humans. Neuropsychopharmacology 1994;10:129–138. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), clinician version. Arlington, VA: American Psychiatric Publishing Inc; 1997. [Google Scholar]
- 29.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 30.Morgan MJ, Impallomeni LC, Pirona A, Rogers RD. Elevated impulsivity and impaired decision-making in abstinent ecstasy (MDMA) users compared to polydrug and drug-naive controls. Neuropsychopharmacology 2006;31:1562–1573. [DOI] [PubMed] [Google Scholar]
- 31.Quednow BB, Kühn KU, Hoppe C, Westheide J, Maier W, Daum I, Wagner M. Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (“ecstasy”). Psychopharmacology 2007;189:517–530. [DOI] [PubMed] [Google Scholar]
- 32.McCann UD, Eligulashvili V, Mertl M, Murphy DL, Ricaurte GA. Altered neuroendocrine and behavioral responses to m-chlorophenylpiperazine in 3,4-methylenedioxymethamphetamine (MDMA) users. Psychopharmacology 1999;147:56–65. [DOI] [PubMed] [Google Scholar]
- 33.Gerra G, Zaimovic A, Ferri M, et al. Long-lasting effects of (+/−)3,4-methylenedioxymethamphetamine (ecstasy) on serotonin system function in humans. Biol Psychiatry 2000;47:127–136. [DOI] [PubMed] [Google Scholar]
- 34.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 35.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann NY Acad Sci 2004;1021:276–291. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med 2005;3:113–133. [DOI] [PubMed] [Google Scholar]
- 37.Carley DW, Paviovic S, Janelidze M, Radulovacki M. Functional role for cannabinoids in respiratory stability during sleep. Sleep 2002;25:391–398. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.