Abstract
Background:
Traditionally, benign essential blepharospasm (BEB) is considered a disorder caused by basal ganglia dysfunction. Electrophysiologic and brain imaging studies suggest pathologic changes in excitability in the primary motor cortex (MC), anterior cingulate (AC), and secondary motor areas, such as premotor (PMC) and supplementary motor cortices (SMA).
Methods:
In this pilot study of 7 patients with BEB, we experimentally reduced cortical excitability of 4 areas: MC (first dorsal interosseus area), PMC, SMA, and AC, each with 3 noninvasive techniques: low-frequency repetitive transcranial magnetic stimulation (lfrTMS), continuous theta burst stimulation (cTBS), and cathodal transcranial direct current stimulation (tDCS). Primary outcome was the clinical effects on blepharospasm (blink rate observation by an investigator blinded to the intervention and subjective rating by the patient); secondary outcome was the blink reflex recovery curve (BRR).
Results:
lfrTMS resulted in a significant improvement over all 4 brain areas for physician rating, patient rating, and BRR, whereas cTBS and tDCS showed only trends for improvement in physician rating, but no improvements for patient rating and BRR. lfrTMS had a significantly higher effect over AC than MC for physician rating, but no differences were seen for other pairwise comparisons of stimulated brain areas.
Conclusions:
Electrophysiologic and clinical improvements by functional inhibition of the medial frontal areas using low-frequency repetitive transcranial magnetic stimulation suggests that hypersensitivity of the anterior cingulate is directly or indirectly involved in the pathophysiology of benign essential blepharospasm. Inhibition of these areas using low-frequency repetitive transcranial magnetic stimulation could provide a therapeutic tool and is worthy of a larger study.
GLOSSARY
- AC
= anterior cingulate;
- aMT
= active motor threshold;
- ANOVA
= analysis of variance;
- BEB
= benign essential blepharospasm;
- BG
= basal ganglia;
- BRR
= blink reflex recovery;
- cTBS
= continuous theta burst stimulation;
- GPi
= globus pallidus internus;
- lfrTMS
= low-frequency repetitive transcranial magnetic stimulation;
- MC
= motor cortex;
- OO
= orbicularis oculi;
- PMC
= premotor cortex;
- rMT
= resting motor threshold;
- SMA
= supplementary motor cortex;
- SNr
= substantia nigra pars reticulata;
- tDCS
= transcranial direct current stimulation.
Benign essential blepharospasm (BEB) is a form of focal dystonia and is characterized by excessive involuntary closure of the eyelids due to spasm of the orbicularis oculi muscles (OO).1 The antagonistic activity between the OO and the levator palpebrae muscles is disturbed in some patients, and a minority of patients have involuntary levator palpebrae inhibition.2 BEB substantially impacts the health status of the afflicted individual and can lead to serious depression.3 Despite normal visual acuity, in severe cases, patients are functionally blind.
Dystonia is thought to arise from aberrant brain plasticity and a lack of surround inhibition in the motor system.4 In patients with BEB, electrophysiologic studies have shown shortened silent periods of the facial muscles.5 This suggests hyperexcitability of cortical neurons, likely representing a lack of inhibition of the neurons that supply the facial muscles. PET studies have shown increased glucose uptake in the right posterior and left AC, frontal nonprimary motor areas, and the PMC.6,7 Neuroanatomic findings in the monkey indicated that efferents from SMA and AC project bilaterally to upper facial muscles.8 In a recent study, lfrTMS over primary and secondary motor areas in an inhibitory stimulation mode was used to modulate writer’s cramp.9 Stimulation of the PMC but not of the primary motor cortex (MC) improved tracking errors, pen pressure, and prolonged TMS-induced silent periods.
Studies on the interactions between primary and nonprimary motor areas, especially mesial frontal areas in BEB, may provide important clues leading to better understanding of the pathophysiology of BEB. Earlier studies showed that lfrTMS, TBS in a continuous mode, and cathodal tDCS applied over cortical areas reduces cortical excitability.9,10,11 We conducted this pilot study to examine the effects of 3 different stimulation techniques applied to 4 different motor centers of the brain to affect symptoms of BEB. This may have therapeutic implications in the future.
METHODS
In this prospective, randomized, observer-blinded pilot study, 7 right-handed patients (2 male and 5 female, age 62.6 ± 7.8 years) with BEB participated and met the following inclusion criteria: clinical diagnosis of idiopathic BEB or Meige syndrome; age between 18 and 85 years; normal findings in the medical history, physical and neurologic examination (except for BEB); no prior use of neuroleptics; and no treatment with antidepressants, antiepileptic medication, anticholinergic drugs, or muscle relaxants within the past 4 weeks (for patient characteristics, see table 1). All patients except patient 5 had a history of botulinum toxin injection; however, the last injection was >3 months ago. Patients received general information about stimulation techniques and sites but no concrete information before each particular experiment.
Table 1 Characteristics of included patients
Standard protocol approvals, registrations, and patient consents.
Before inclusion in the study, written informed consent was obtained from all patients. A signed patient consent-to-disclose form was obtained for videos of any recognizable patient. This study was approved by the NIH Institutional Review Board.
Every patient came for 10 visits, each separated by at least 2 days. In random order, we performed the following stimulation paradigms: lfrTMS over MC, PMC, SMA, AC; cTBS over MC, PMC, SMA, AC; tDCS over MC/PMC and SMA/AC.
Stimulation techniques.
We chose 3 stimulation techniques that have been well documented to reduce cortical excitability noninvasively and are well tolerated by patients. All 3 techniques have been proposed as potential therapeutic tools,10–12 but have not been used in BEB or compared to each other.
lfrTMS.
Recent studies have reported suppressive effects on cortical excitability at frequencies as low as 0.2 and 0.3 Hz.9,12 We applied lfrTMS at a frequency of 0.2 Hz. A total of 180 stimuli (15 minutes per session) were delivered to each of the 4 areas (MC, PMC, SMA, and AC). Stimulation intensity was set at a stimulator output of 90% resting motor threshold (rMT) of the FDI over MC for both the MC and PMC, and 90% rMT of the OO for AC. The SMA was stimulated at 100% active motor threshold (aMT) of the leg representation area of the MC.9
cTBS.
We applied cTBS (3 TMS pulses at 50 Hz, repeated every 200 msec) in a continuous paradigm for 40 seconds (600 pulses uninterrupted) at an intensity of 80% aMT of the FDI over MC for both the MC and PMC, 80% aMT of the OO for AC, and 80% of the aMT of the leg area of the MC for SMA. Previous work showed suppressive effects of cTBS on cortical excitability at this intensity.11
tDCS.
Bipolar tDCS (1 mA for 20 minutes) was delivered by a battery-driven stimulator via 2 conductive-rubber electrodes, placed in saline-soaked sponges (5 × 7 cm), positioned over the left MC/PMC or SMA/AC (cathode) and Oz (anode, international 10–20 system).10
Stimulation areas.
MC.
A figure-of-eight stimulation coil was connected to a Magstim 200 stimulator (outside diameter of one half-coil 8.7 cm). The coil was placed over an area 2 cm anterior to a point that is 3.5 cm lateral to Cz (international 10–20 system), with the handle pointing backwards (45 degree angle) and parallel to the midline. We identified the “hot spot” for the MEP of the right FDI and noted the resting motor threshold (rMT, 5 MEPs out of 10 stimuli) at that location.
PMC.
As defined in earlier studies,9,13 the stimulation site was determined to be 2 cm anterior and 1 cm medial to the hot spot.
SMA.
The SMA stimulation site was determined to be 2 cm anterior to the leg representation (tibialis anterior muscle) of the MC.14
AC.
As used earlier,15 to determine the stimulation site for AC, a standard 9-cm circular coil was placed over Fz and then moved over the midline of the brain in 0.5-cm steps in the anterior direction, until the point of maximum MEP in the OO (with a latency of 6–8 msec) was reached (about 3.5 cm medial and 5.5 cm anterior to MC).
Outcome measures.
The primary outcome measure was the clinical effects on BEB symptoms. Five-minute videos of eye blinks before and after stimulation were assessed by a blinded rater. The secondary outcome was subjective rating by the patients. The primary and secondary outcome measures tested clinical changes in BEB. The BRR was used as a third measure to evaluate neurophysiologic correlates to the clinical endpoint measures.
Physician rating.
An investigator who was not present during the experiments and blinded to the intervention rated the videos. Clinical evaluation was expressed in percent change before and after stimulation, including eye blink rate, number of sustained blinks, and time of eye closure. An eye blink was defined as any visible, bilateral, and synchronous contraction of the OO muscle, causing eyelid drop. Blink rate was expressed as blinks per minute. Sustained spasms of the OO muscle were not considered blinks and were counted separately. The rater counted the time (seconds) of eye closure with a stopwatch whenever blinks caused a prolonged eye closure (eyes shut >2 seconds).
Patient rating.
Patients rated their symptoms before and after stimulation on a 7-point nominal scale: 1) excellent, 2) very good, 3) good, 4) average, 5) slightly worse than usual, 6) bad, 7) very bad.
Blink reflex recovery.
Subjects opened their eyes gently during stimulation and stayed in a relaxed position. Paired electrical stimuli (conditioning and test) were delivered at an interstimulus interval of 0.2 seconds. Stimulus intensity was 3 times the threshold of the R2s response (lowest intensity with 5 out of 10 R2s responses). Responses with artifacts due to involuntary movements were rejected. To avoid habituation, a rest period of 25–35 seconds was maintained between trials. The low pass filter was set at 3 kHz and the high pass filter at 1 Hz. All responses were stored digitally. In an offline analysis procedure, performed fully automatically, reflex responses were digitally bandpass filtered within a range of more than 100 Hz to minimize DC offsets and slow eye drifts and below 900 Hz to reduce the high-frequency noise. Then, the responses were full wave rectified and we computed the average of 6 trials. Peak amplitude of R2 was calculated within a window from 30 to 60 msec to avoid stimulation artifacts. We obtained R2 recovery values by dividing the size of R2test [R2t] by the size of conditioning response [R2c].
Statistics.
Data were analyzed using SPSS version 15.0 for Windows. All statistical tests were 2-tailed, and Alpha was set at 0.05. Since the physician rating was measured in percentages, an arc sine transformation was computed to use parametric tests. To analyze the effects of stimulation treatment, we conducted a 2 (time points) × 4 (stimulation areas) repeated measures analysis of variance (ANOVA) for each stimulation technique. Main effect comparisons were corrected using the Bonferroni procedure. If there was a significant violation of homogeneity of variance, as assessed by the Mauchly Sphericity Test, the Greenhouse-Geisser Epsilon was used to calculate a more conservative p value for each F ratio. As treatment was confounded with time, we used descriptive analysis to investigate the data of each of the 3 outcome variables over time. No relevant changes of data could be explained through sequence or treatment day. Pearson correlations among the 3 outcome measures were computed to examine whether a multiple endpoint adjustment was necessary. Since none of the correlations reached a level of r = 0.5 (r physician rating–BRR = 0.138, r physician rating–patient rating = 0.388, and r patient rating–BRR = 0.267), there was no need for the adjustment.
RESULTS
Patients tolerated all 3 stimulation techniques over the 4 brain areas without any problems and with no adverse events. Stimulation intensity (mean/SD) for lfrTMS over MC was 54 ± 13.7% stimulator output, over PMC 52.5 ± 8.4%, over SMA 62.3 ± 16.5%, and over AC 60.6 ± 5.6%; for TBS stimulation intensity over MC was 45.6 ± 8.2%, over PMC 46.6 ± 9.8%, over SMA 49 ± 5.6%, and over AC 47.7 ± 8.8%.
lfrTMS resulted in a significant improvement over all 4 brain areas for physician rating, patient rating, and BRR, whereas cTBS and tDCS showed only trends for improvement in physician rating and no significant improvements for the other outcome measures (table 2 and figure 1).
Table 2 Two (time points) × 4 (stimulation areas) repeated measures analysis of variance for each stimulation technique
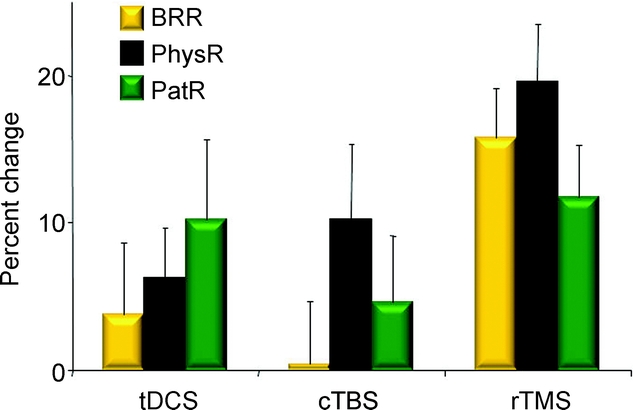
Figure 1 Comparison of the 3 stimulation techniques
X-axis: stimulation techniques, tDCS = transcranial direct current stimulation; cTBS = continuous theta burst stimulation; lfrTMS = repetitive low-frequency transcranial magnetic stimulation; Y-axis: percent change before and after stimulation; BRR = blink reflex recovery; PhysR = physician rating; PatR = patient rating. Error bars represent standard errors for the estimated least-squares means.
Due to the broad sponges used in tDCS, stimulation could not be targeted over each of the 4 brain areas separately. Therefore, tDCS was performed together over the frontal areas (SMA/AC) and the dorsal areas (MC/PMC) (figure 2). To study the 4 brain areas separately, we employed the only effective stimulation technique (lfrTMS) and compared stimulation effects over the 4 brain areas in a post hoc analysis (figure 3). A repeated measures ANOVA with the difference between the 2 time points for each brain area as fixed factors and subject as the random factor showed a significant difference between brain areas for physician rating, a trend for subjective rating, but no significant difference for BRR. Pairwise comparisons revealed a significant higher effect over AC than MC for physician rating (p = 0.007). No differences were seen for all other pairwise comparisons (MC-PMC, MC-SMA, PMC-SMA, PMC-AC, and SMA-AC; p > 0.05).

Figure 2 Stimulation effects over the frontal (supplementary motor cortex/anterior cingulate) and dorsal (motor cortex/premotor cortex) brain areas for the 3 outcome measures
X-axis: outcome measures; BRR = blink reflex recovery; PhysR = physician rating; PatR = patient rating; Y-axis: percent change before and after stimulation. Error bars represent standard errors for the estimated least-squares means.

Figure 3 Comparison of the 4 brain areas (motor cortex/premotor cortex/supplementary motor cortex/anterior cingulate): Stimulation with lfrTMS
X-axis: outcome measures; BRR = blink reflex recovery; PhysR = physician rating; PatR = patient rating; Y-axis: percent change before and after stimulation. Error bars represent standard errors for the estimated least-squares means.
DISCUSSION
We used 3 different noninvasive stimulation techniques to identify the most effective technique over the most sensitive brain area to reduce symptoms of BEB. We found significant improvements with lfrTMS for all 3 outcome measures, whereas cTBS and tDCS did not demonstrate significant improvements. When comparing stimulation effects with lfrTMS over the 4 brain areas, lfrTMS over AC had the highest mean stimulation value. However, AC stimulation was statistically higher only compared to MC. Pairwise comparisons between the other brain areas did not show significant differences, which might be due to the small sample size of this pilot study. Since we did not find a reliable facial M1 area in an earlier study15 and it is controversial whether upper facial responses can be produced by stimulating the MC,15–17 we chose the FDI as the target area for M1. Although the hand representation area, especially the thumb and index finger, is located next to the upper face and eye area,18 identifying and utilizing the facial M1 area for lfrTMS might have resulted in more effective stimulation over M1. As the depth of targeted brain areas varied between the different brain areas, stimulation intensities were based on motor evoked potentials. However, reduced cortical excitability as a result of low frequency repetitive TMS is most likely caused by changes in excitability of inhibitory cortical interneurons, and matching MEP amplitudes may not match an effect on inhibitory interneurons.
While the study design was not sham-controlled, the results obtained here cannot be explained by any placebo effects. First, cTBS and tDCS produced no significant changes in any of the outcome measures, whereas lfrTMS improved not only the subjective patient rating but also physician rating and the electrophysiologic BRR. Second, patients were not informed about the specific brain area that was stimulated or about the stimulation technique although they knew and felt that different techniques were applied. The only subjective stimulation effect that may have influenced patient rating was the way in which patients assessed the comfort of each technique. Most patients assessed lfrTMS and tDCS as “slightly comfortable” or “indifferent,” whereas cTBS was assessed “indifferent” or “slightly uncomfortable.” This may have influenced patient rating and may account for the fact that patient rating was slightly higher for tDCS than for cTBS. Between study days, we did not see carryover effects and statistically, there were no interactions between different stimulation sessions. Stimulation effects of all 3 techniques used in this study have been reported to last less than 1 hour. Therefore, 2 days washout period between stimulation sessions was adequate to avoid carryover effects.
Several electrophysiologic techniques are known to reduce cortical excitability noninvasively. All 3 techniques used in this study, low-frequency subthreshold lfrTMS, TBS in a continuous mode, and cathodal tDCS, have been well documented to reduce cortical excitability.10,11,16 To our knowledge, there is no trial that compared clinical and electrophysiologic effects among these techniques in dystonia. lfrTMS had the strongest clinical and electrophysiologic effect. It is conceivable that the other techniques, cTBS and tDCS, may have resulted in significant changes with a larger sample size, since they showed some small but no significant improvements in our study.
In contrast to the classic view that the primary motor cortex controls facial movements, there is increasing evidence that cortical centers for the upper facial movements, including blinking, predominantly involve the mesial frontal region. Although we did not stimulate a facial M1 area to which we could compare mesial frontal regions, essential stimulation effects over the SMA and AC, as seen in our study, correlate with neuroanatomic findings in primates. A systematic description of corticobulbar projections to musculotopically defined subsections of the facial nucleus has been done in the monkey8: projections arise from MC (M1), SMA (M2), AC (M3) and caudate cingulate (CC), (M4) and ventral lateral PMC (vlPMC) motor cortices. Non-primary motor areas such as the PMC and the SMA receive 2 to 8 times as many thalamocortical projections as the MC. Corticofacial afferents from M1, M4, and vlPMC project primarily to contralateral lower facial muscles. M2 and M3 project bilaterally to upper facial muscles through the medial part of the facial nucleus for M2 afferents and the dorsal and intermediate subnuclei for the M3 afferents.
In current pathophysiologic models of dystonia, disinhibited thalamo-frontal projections due to increased GABA mediated striato-pallidal inhibition and reduced pallido-thalamic inhibition results in increased cortical excitability and disorganized cortical representation.7 Arguments for a cortical role in BEB, notably the cingulate cortex, also come from brain imaging studies. Using PET, decreased glucose metabolism was found in the superior-medial-frontal area and in the striatum19 in sleeping BEB patients. Absence of blinking during sleep indicates that this hypometabolism might reflect a primary abnormality. In awake patients, metabolic activity is increased in cerebellum and pons. The striatal hypometabolism could reflect a primary basal ganglia dysfunction while the hyperactivities could be secondary to the involuntary movements. In a recent fMRI study, eye-closure-related brain activity was found in the rostral anterior cingulate cortex while hand movement-related brain activity was noted in the caudal cingulate cortex. Comparing upper and lower facial movements, selective activity with upper facial movements was found in rostral anterior cingulate and bilateral M1.20 Reducing cortical excitability may on the one hand simply change cortical motor output and hence improve symptoms of BEB. It may, however, secondarily induce a plastic change in the corticobasal-thalamofrontal motor loop, as central plastic adaptations in BEB patients after treatment with botulinum toxin have been documented.21
Dysfunction of the basal ganglia (BG), and consequently on the output structure of BG (globus pallidus internus [GPi]/substantia nigra pars reticulata [SNr]), may also lead to a decrease or loss of the inhibitory control exerted by the BG on the blink reflex circuitry. This would increase the excitability of this circuit. An animal model of blepharospasm suggests that a predisposing condition to develop BEB could be a loss of dopamine-containing neurons in the SNc causing a decreased inhibition in the blink circuit. Decreased inhibition weakening the OO triggered spasms of lid closure in the animals.22 The BG output structure, i.e., SNr, has an inhibitory influence on trigeminal blink reflex excitability: SNr has GABAergic inhibitory projections to the superior colliculus, which in turn has excitatory projections to the nucleus raphe magnus. This nucleus has inhibitory serotonergic (5HT) projections to the spinal trigeminal complex.23 Reducing cortical excitability may modulate corticobasal-thalamofrontal loops that integrate the substantia nigra. According to this concept, by modulating the excitability of SNr, the pathologic decreased 5HT projections located on blink reflex interneurons within the spinal trigeminal complex would resume their physiologic activity, which could explain the physiologic changes in BRR after cortical stimulation, as found in our study.
Finally, it appears that BG dysfunction can have repercussions on the excitability of mesial frontal cortical areas and the blink reflex circuit in the brainstem. Electrophysiologic and clinical improvements resulting from functional inhibition of appropriate cortical regions using lfrTMS, as shown in this study, suggests that hypersensitivity of primary and secondary motor areas is either directly or indirectly involved in BEB pathophysiology. Inhibition of these areas using lfrTMS could provide a therapeutic tool to treat BEB and is worthy of a larger study.
ACKNOWLEDGMENT
The authors thank Devera Schoenberg, MSc, for editing.
DISCLOSURE
Dr. Kranz has received research support from the Max Kade Foundation and has received funding for travel from the NIH/NINDS and Ipsen. Dr. Shamim receives research support from the NIH/NINDS (Intramural Grant) and holds equity interest in Amgen and Medtronic, Inc. Dr. Lin has received research support from the NIH/NINDS (Intramural Grant). G.S. Kranz and Dr. Voller report no disclosures. Dr. Hallett serves as Chair of the Medical Advisory Board for and receives funding for travel from the Neurotoxin Institute; serves as Chair of the Medical Advisory Board of the Benign Essential Blepharospasm Foundation; has received honoraria and/or funding for travel for lectures or educational activities not funded by industry; serves on editorial advisory boards for Clinical Neurophysiology, Western Hemisphere, Brain, Acta Neurologica Scandinavica, Journal of Clinical Neurophysiology, Italian Journal of Neurological Sciences, Medical Problems of Performing Artists, Annals of Neurology, Neurology and Clinical Neurophysiology, The Cerebellum, NeuroRx, Current Trends in Neurology, Faculty of 1000 Biology, European Neurology, Faculty of 1000 Medicine, Brain Stimulation, Journal of Movement Disorders (Korea), and World Neurology; may accrue revenue on US Patent 6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders; US Patent 7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same; receives royalties from publishing from Blackwell Publishers, Cambridge University Press, Springer Verlag, Taylor & Francis Group, Oxford University Press, John Wiley & Sons, and Elsevier; receives research support from Ariston Pharmaceuticals, NIH/NINDS (Intramural Program), and the US Department of Defense (Army); has received license fee payments from the NIH (from Brainsway) for licensing the patent for the H-coil; and with his spouse held stock in Agilent Technologies, Amgen, Amylin Pharmaceuticals, Merck & Co., Monsanto Co New Del, Sanofi-Aventis, Coventry Health Care Inc., Sigma Aldrich Corp., Warner Chilcott Ltd., Pfizer Inc., Genentech, Inc., United Health Group, St. Jude Medical, and Eli Lilly and Company.
Address correspondence and reprint requests to Dr. Gottfried Kranz, Department of Neurology, Medical University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria gottfried.kranz@meduniwien.ac.at
Sponsored by the Human Motor Control Section, National Institute of Neurological Disorders and Stroke, and supported by the Intramural Research Program of the NIH/NINDS.
Disclosure: Author disclosures are provided at the end of the article.
Received April 1, 2009. Accepted in final form September 15, 2009.
REFERENCES
- 1.Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF International Workshop. Neurology 2008;71:1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aramideh M, Eekhof LA, Bour LJ, et al. Electromyography and blink reflex recovery in involuntary eyelid closure: a comparative study. J Neurol Neurosurg Psychiatry 1995;58:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller J, Klemmer G, Wissl J, et al. The impact of blepharospasm and cervical dystonia on health-related quality of life and depression. J Neurol 2002;249:842–684. [DOI] [PubMed] [Google Scholar]
- 4.Hallett M. Dystonia: abnormal movements result from loss of inhibition. In: Fahn S, Hallett M, DeLong M, eds. Advances in Neurology, Volume 94. Philadelphia: Lippincott Williams & Wilkins; 2002:1–7. [PubMed] [Google Scholar]
- 5.Curra A, Romaniello A, Berardelli A, et al. Shortened cortical silent period in facial muscles of patients with cranial dystonia. Neurology 2000;54:130–135. [DOI] [PubMed] [Google Scholar]
- 6.Kerrison JB, Lancaster JL, Zamarripa FE, et al. Positron emission tomography scanning in essential blepharospasm. Am J Ophthalmol 2003;136:846–852. [DOI] [PubMed] [Google Scholar]
- 7.Ceballos-Baumann AO, Passingham RE, Marsden CD, Brooks DJ. Motor reorganization in acquired hemidystonia. Ann Neurol 1995;37:746–757. [DOI] [PubMed] [Google Scholar]
- 8.Morecraft RJ, Louie JL, Herrick JL, et al. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain 2001;124:176–208. [DOI] [PubMed] [Google Scholar]
- 9.Murase N, Rothwell JC, Kaji R, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain 2005;128:104–115. [DOI] [PubMed] [Google Scholar]
- 10.Nitsche MA, Nitsche MS, Klein CC, et al. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 2003;114:600–604. [DOI] [PubMed] [Google Scholar]
- 11.Huang YZ, Edwards JM, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron 2005;45:201–206. [DOI] [PubMed] [Google Scholar]
- 12.Cincotta M, Borgheresi A, Gambetti C, et al. Suprathreshold 0.3 Hz repetitive TMS prolongs the cortical silent period: potential implications for therapeutic trials in epilepsy. Clin Neurophysiol 2003;114:1827–1833. [DOI] [PubMed] [Google Scholar]
- 13.Schluter ND, Rushworth MF, Passingham RE, et al. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements: a study using transcranial magnetic stimulation. Brain 1998;121:785–799. [DOI] [PubMed] [Google Scholar]
- 14.Muri RM, Rivaud S, Vermersch AI, et al. Effects of transcranial magnetic stimulation over the region of the supplementary motor area during sequences of memory-guided saccades. Exp Brain Res 1995;104:163–166. [DOI] [PubMed] [Google Scholar]
- 15.Sohn YH, Voller B, Dimyan M, et al. Cortical control of voluntary blinking: a transcranial magnetic stimulation study. Clin Neurophysiol 2003;115:341–347. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Theoret H, Mottaghy FM, et al. Intracortical inhibition and facilitation in human facial motor area: difference between upper and lower facial area. Clin Neurophysiol 2001;112:1604–1611. [DOI] [PubMed] [Google Scholar]
- 17.Paradiso GO, Cunic DI, Gunraj CA, Chen R. Representation of facial muscles in human motor cortex. J Physiol 2005;567:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schott GD. Penfield’s homunculus: a note on cerebral cartography. J Neurol Neurosurg Psychiatry 1993;56:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchinson M, Nakamura T, Moeller JR, et al. The metabolic topography of essential blepharospasm: a focal dystonia with general implications. Neurology 2000;55:673–677. [DOI] [PubMed] [Google Scholar]
- 20.Hanakawa T, Parikh S, Bruno MK, Hallett M. Finger and face representations in the ipsilateral precentral motor areas in humans. J Neurophysiol 2005;93:2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quartarone A, Sant’Angelo A, Battaglia F, et al. Enhanced long-term potentiation-like plasticity of the trigeminal blink reflex circuit in blepharospasm. J Neurosci 2006;26:716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schicatano EJ, Basso M, Evinger C. Animal model explains the origins of the cranial dystonia benign essential blepharospasm. Neurophysiology 1997;77:2842–2846. [DOI] [PubMed] [Google Scholar]
- 23.Basso MA, Evinger C. An explanation for reflex blink hyperexcitability in Parkinson’s disease: II: nucleus raphe magnus. J Neurosci 1996;16:7318–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindeboom R, De Haan R, Aramideh M, Speelman JD. The blepharospasm disability scale: an instrument for the assessment of functional health in blepharospasm. Mov Disord 1995;10:444–449. [DOI] [PubMed] [Google Scholar]




