Abstract
Pregnancy is characterized by peripheral insulin resistance, which is developed in parallel with a plasma increase of maternal hormones; these include prolactin, placental lactogens, progesterone and oestradiol among others. Maternal insulin resistance is counteracted by the adaptation of the islets of Langerhans to the higher insulin demand. If this adjustment is not produced, gestational diabetes may be developed. The adaptation process of islets is characterized by an increase of insulin biosynthesis, an enhanced glucose-stimulated insulin secretion (GSIS) and an increase of β–cell mass. It is not completely understood why, in some individuals, β–cell mass and function fail to adapt to the metabolic demands of pregnancy, yet a disruption of the β–cell response to maternal hormones may play a key part. The role of the maternal hormone 17β-oestradiol (E2) in this adaptation process has been largely unknown. However, in recent years, it has been demonstrated that E2 acts directly on β–cells to increase insulin biosynthesis and to enhance GSIS through different molecular mechanisms. E2 does not increase β–cell proliferation but it is involved in β–cell survival. Classical oestrogen receptors ERα and ERβ, as well as the G protein-coupled oestrogen receptor (GPER) seem to be involved in these adaptation changes. In addition, as the main production of E2 in post-menopausal women comes from the adipose tissue, E2 may act as a messenger between adipocytes and islets in obesity.
‘Plasticity is defined as the ability of the genotype to produce different phenotypes in response to different environments’ (Crews & McLachlan, 2006). The endocrine pancreas is a very plastic tissue with the capability to change in response to variations in the metabolic state of the organism, such as those produced during pregnancy and obesity. During these two different states peripheral insulin resistance is manifested, generating an environment that requires a higher production of insulin to counteract the lower insulin sensitivity. Therefore, β–cells adapt to peripheral insulin resistance by increasing their secretory response, as well as their cell mass. If β–cells fail to adapt, blood glucose concentration will rise to pathological levels. As a consequence, either gestational diabetes, in the case of pregnancy, or type II diabetes, in the case of obesity, will develop. Additionally, in the case of type II diabetes, genetic predisposition, glucolipotoxicity, cytokines and other factors affect β–cell mass and function, defects that are both required for the onset of type II diabetes (Kahn et al. 2009). In the case of gestational diabetes, it is still unclear why β–cell function does not adapt to the metabolic demands of pregnancy (Kuhl, 1998; Kim et al. 2002). Although genetic predisposition has been suggested to play a role (Reece et al. 2009), alterations in β–cell viability and function in response to the hormonal milieu of pregnancy may be involved as well (Branisteanu & Mathieu, 2003). The onset of gestational diabetes in humans occurs during the second trimester of pregnancy, when progesterone and oestrogen levels increase (Fig. 1) (Guyton & Hall, 2001). Both progesterone and oestrogen receptors are expressed in islets of Langerhans and regulate β–cell viability and function.
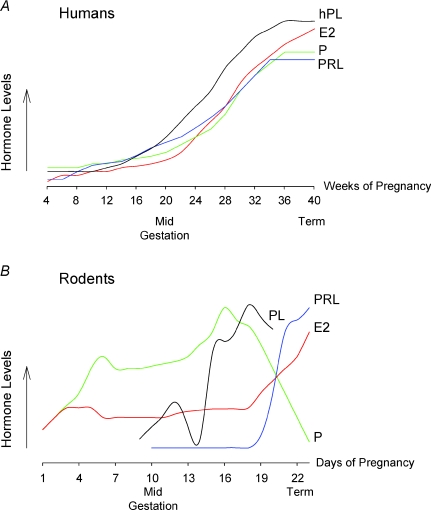
Figure 1. Scheme of the changes in hormone levels during pregnancy in humans and rodents.
A, in humans, there is an increase in serum placental lactogen (hPL), prolactin (PRL), progesterone (P) and 17β-oestradiol (E2) during pregnancy. Data are from Guyton & Hall (2001) and Freemark (2001). B, in rodents, during early pregnancy there is an increase in E2 and P levels as well as two early surges of PRL (not represented); PLs begin to increase at about day 10. During late pregnancy there is a strong increase in E2, as well as in PRL and PLs; however, P decreases. This scheme is based on data from Parsons et al. (1992); Cesen-Cummings et al. (2000); and Soares (2004).
In this report we will discuss the possible role that oestrogens and oestrogen receptors may have in the adaptation of the endocrine pancreas to the insulin resistance generated during pregnancy. Additionally, we will speculate on whether this role may be extended to obesity as well.
Changes in β-cells during pregnancy
During late pregnancy, mothers develop severe insulin resistance reducing their glucose disposal by up to 50% (Catalano, 1999; Freemark, 2006). This maternal insulin resistance is necessary to ensure an appropriate supply of nutrients to the fetus. The rise of maternal hormones in humans coincides with the development of maternal insulin resistance. In humans, prolactin, progesterone and oestrogens increase during pregnancy. At the beginning of gestation, progesterone and 17β-oestradiol are secreted by the corpus luteum in moderate amounts. The placenta takes over the progesterone production and oestrogen (oestradiol, oestrone and oestriol) synthesis during the remaining pregnancy period. The increase in progesterone secretion is enormous, rising up to 10-fold in a normal pregnancy (Guyton & Hall, 2001) (Fig. 1). Oestrogen concentrations rise throughout pregnancy up to 30-fold at term (Fig. 1) (Guyton & Hall, 2001). In fact, the levels of E2 rise from approximately 7 ng ml−1 near the end of the first trimester, to 28–30 ng ml−1 at term. The rate of production of this hormone is so markedly increased that it has been estimated that a woman produces more oestrogen during pregnancy than a normal ovulatory woman could produce in 150 years (Becker et al. 2001). Prolactin and placental lactogens also rise from week 12 of gestation (Fig. 1) (Freemark, 2001).
In rodents, the second half of pregnancy is characterized by the increase in placental lactogen (PL) and prolactin (PRL) levels, as well as other hormones such as progesterone (P) and, particularly, 17β-oestradiol (E2) (Fig. 1) (Barkley et al. 1979; Parsons et al. 1992; Soares, 2004). PL begins to rise at about day 10 of pregnancy, yet placental steroids, oestrogens and progesterone also increase their concentration in plasma with advancing pregnancy. Therefore, E2 and progesterone increase in parallel to the rise of PL in humans (Beck et al. 1965) as well as in rodents during late pregnancy (Barkley et al. 1977; Parsons et al. 1992; Cesen-Cummings et al. 2000; Soares, 2004) (Fig. 1).
In both humans and animal models, maternal hormones are key factors in the development of maternal insulin resistance (Freemark, 2006). Despite this condition, the required extra insulin production is maintained throughout late gestation, which allows the blood glucose concentration to remain within the physiological range. This is possible because the islets of Langerhans adapt to the environment generated throughout the course of pregnancy. Studies performed in rodents indicate that three main mechanisms are triggered in islets to enhance insulin secretion at normal glucose levels, these are: augmented insulin biosynthesis, enhanced sensitivity of GSIS and increased β–cell mass. In rodents, changes in β–cell physiology are produced during the second half of pregnancy (Sorenson & Brelje, 2009), which coincides with the increase in peripheral insulin resistance (Gonzalez et al. 2003; Alonso et al. 2009).
In the next part of this review we will go through the role that maternal hormones play in the adaptation of β–cell mass and function to counteract maternal insulin resistance, with special attention to the important function that oestrogens and oestrogen receptors may play in this process. The majority of results reviewed in the next sections have been described in rodents, unless otherwise stated.
The role of placental lactogens, prolactin and prolactin receptors
Studies performed in animal models have established clear roles for PL and/or PRL during pregnancy. These hormones reproduce in vitro and in vivo changes in islets similar to those that occur during pregnancy: expansion of islet cell mass, an increase in insulin biosynthesis and enhanced GSIS (Brelje & Sorenson, 1991; Sorenson & Brelje, 2009). Lactogens act through binding to PRL receptors (PRLrs) and subsequent activation of downstream signalling pathways, including JAK2/STAT5, PI3K/Akt, ERK1/2, adenylate cyclase/cAMP and intracellular calcium (Brelje et al. 2002; Amaral et al. 2003, 2004). Transgenic mice with a targeted expression of PL (RIP-mPL1 mice) in the β–cell show an enhanced β–cell proliferation, as well as islet mass (Vasavada et al. 2000). These mice are resistant to the diabetogenic and cytotoxic effects of the β–cell toxin, streptozotocin, which indicates a protective role for PL in the β–cell (Vasavada et al. 2000; Fujinaka et al. 2004). In addition, it has been demonstrated, both in insulinoma cells and in primary culture of β–cells, that PL and PRL induce β–cell replication and inhibit β–cell apoptosis through JAK2/STAT5 activation. The involvement of menin in the β–cell replication (Karnik et al. 2007) and Bcl-XL in the protective effect of PRL has been described (Fujinaka et al. 2007; Hügl & Merger, 2007). In addition, PRL induces a decrease in the expression of Fork-head box O1 (FoxO1), peroxisome proliferator activator receptor α (PPARα) and carnitine palmitoyltransferase 1 (CPT–1), which would promote β–cell division, and an increase in glucose transporter 2 mRNA that may enhance GSIS (Arumugam et al. 2008). The phenotype of the PRLr knock-out mice indicates an important role for lactogenic signalling in normal islet development and function. These mice present a reduced β–cell mass of 25–40%, decreased insulin content and abnormal GSIS. Additionally, PRLr KO mice are glucose intolerant (Freemark et al. 2002). The importance of PRLr signalling during pregnancy has been recently demonstrated. Since homozygous PRLr null mice are sterile, Huang et al. (2009) studied islets growth and function during pregnancy in heterozygous PRLr(+/–) females. These mice are glucose intolerant and have a diminished β–cell mass increment during pregnancy, probably through a decrease of cell proliferation rather than a change in the apoptotic rate (Huang et al. 2009). Progesterone may have a counteractive effect since progesterone receptors have a role in decreasing β–cell mass (Picard et al. 2002).
In addition to the PL action on β–cell mass, they also induce important changes related to insulin production and secretion. It has been known since 1976 that islets increase insulin biosynthesis during pregnancy as part of their adaptation (Bone & Taylor, 1976). PRL plays a role in the regulation of insulin biosynthesis (Brelje et al. 1993) and it promotes insulin gene transcription (Fleenor & Freemark, 2001). Although PL and PRL do not elicit an acute action on GSIS, they enhance GSIS in several manners. In vitro, PRL enhances glucokinase activity, raises 2–fold the glucose transporter 2 levels as well as increases glucose utilization and oxidation (Fig. 2). These changes are similar to those produced during pregnancy in rats between days 15 and 20 (Weinhaus et al. 1996).
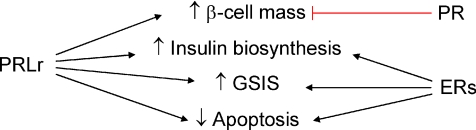
Figure 2. End-points regulated by prolactin receptor (PRLr), progesterone receptor (PR) and oestrogen receptors (ERs) in rodent islets of Langerhans.
PRLr is involved in the increment of β–cell mass, insulin biosynthesis, glucose-stimulated insulin secretion (GSIS) and decrease of apoptosis. PR has been demonstrated to participate in reducing β–cell mass. ERs are involved in incrementing insulin biosynthesis and GSIS, and decreasing apoptosis.
Role of oestrogens and oestrogen receptors
The level of E2 changes during the second part of pregnancy in rodents and it has been suggested to be involved in the development of maternal insulin resistance (Gonzalez et al. 2002, 2003; Barros et al. 2009). E2 exerts profound effects on insulin biosynthesis and GSIS, which resemble those induced during pregnancy. E2 and the oestrogen receptor ERα play a role in protecting β–cells from injury-stimulated apoptosis (Contreras et al. 2002; Le May et al. 2006). However, they do not influence either β–cell division or β–cell mass in a non-pathological situation (Alonso-Magdalena et al. 2008) (Fig. 2).
E2 acutely enhances GSIS when applied at physiological concentrations, both in vitro and in vivo (Nadal et al. 1998; Alonso-Magdalena et al. 2006). It has been demonstrated that E2 triggers the synthesis of cGMP, which in turn activates protein kinase G (PKG). ATP-dependent potassium channels (KATP) then close in a PKG-dependent manner, causing the plasma membrane to depolarize and enhancing glucose-induced [Ca2+]i signals (Ropero et al. 1999). Very likely this process is responsible for the E2-induced insulin secretion mentioned above (Nadal et al. 1998). Additional to its effect on insulin release, the increase of [Ca2+]i by E2 is involved in the rapid activation of cAMP-response element binding protein (CREB) (Quesada et al. 2002), a key transcription factor involved in β–cell division and survival (Jhala et al. 2003; Hussain et al. 2006; Jansson et al. 2008). The insensitivity of these rapid responses to the anti-oestrogen ICI182,780 as well as the different pharmacological profile of a membrane binding site identified in β–cells led us to suggest that a non-classical membrane oestrogen receptor (ncmER) was responsible for these actions (Nadal et al. 2000; Ropero et al. 2002). However, new results indicate that extranuclear ERβ is involved in the rapid regulation of KATP channel activity by E2 and subsequent insulin release (authors’ unpublished observations). The fact that ERβ triggers rapid actions in β–cells does not exclude the presence of other ncmERs. Indeed, two membrane molecules have been described as behaving like ncmERs in β–cells and therefore they may be the ncmER previously reported (Nadal et al. 2000): the sulphonylurea receptor (SUR1) expressed in β–cells and the G protein-coupled oestrogen receptor GPER (formerly named GPR30). It is of note, however, that both molecules trigger their actions at pharmacological rather than physiological E2 concentrations. Binding to SUR1 and regulation of apoptosis was demonstrated for E2 concentrations as high as 100 μm (Ackermann et al. 2009). Therefore, a physiological role for SUR1 as ncmER is still undemonstrated. Recently, GPR30/GPER was proposed as a novel oestrogen receptor (Revankar et al. 2005; Thomas et al. 2005). It is present in β–cells and it mediates rapid E2-induced insulin release, although only at supraphysiological concentrations of E2 (5 μm) (Martensson et al. 2009). A new role for GPER in the protection of β–cells from apoptosis has been recently described at 10 nm E2 (Liu et al. 2009). In any case, whether GPER works in β–cells as a proper oestrogen receptor or it is recruited by membrane ERs (Levin, 2009) is still a matter of debate.
In addition to the acute effect of E2, it has been known for a long time that it exerts a long-term regulation of insulin biosynthesis (Sutter-Dub, 2002). Using genetically modified mice, it has been demonstrated that direct activation of the oestrogen receptor ERαin vivo and in vitro regulates pancreatic insulin levels at physiological concentrations (Alonso-Magdalena et al. 2008). Together with the increase of insulin biosynthesis, islets incubated in the presence of E2 presented an enhanced GSIS (Adachi et al. 2005; Alonso-Magdalena et al. 2006, 2008).
In summary, E2, a hormone that increases its levels during late pregnancy potentiates insulin biosynthesis and GSIS, two factors that traditionally have been uniquely attributed to PL and PRL. Therefore, E2, together with PL and PRL, may be another important hormone that participates in the adaptation of insulin production to comply with the increased metabolic demand of pregnancy.
Is E2 an adipose-derived hormone?
Leptin, adiponectin and resistin, among others, are hormones released by adipocytes with important roles in blood glucose homeostasis. Leptin can modulate blood glucose levels through its direct effect on α- and β-cells, which produce the inhibition of glucagon and insulin secretion, respectively (Tuduri et al. 2009). In the case of adiponectin, low concentrations of this hormone are correlated with insulin resistance, type II diabetes and the metabolic syndrome (Kadowaki et al. 2006). It has also been reported that adiponectin can exert direct actions on β–cells to increase insulin secretion (Okamoto et al. 2008). It has been proposed that resistin may have a role in insulin resistance and type II diabetes in obesity. However, there is still ongoing debate about the physiological actions of this hormone in rodents and humans (Kusminski et al. 2005). Stromal cells from the adipose tissue have been shown to produce oestrogens (Simpson et al. 1981). It has been well established that in obesity there is a decrease in steroid hormone-binding globulins as the body mass index (BMI) increases in both pre- and post-menopausal women. Moreover, there is a direct association between oestrogen levels and BMI in post-menopausal women (Lukanova et al. 2004; Cleary & Grossmann, 2009). Oestrogen signalling through the oestrogen receptor ERα is important in the development of obesity and insulin resistance. ERα(–/–) mice are obese and insulin resistant and humans with ERα mutations suffer insulin resistance as well (Heine et al. 2000; Ropero et al. 2008; Nadal et al. 2009). Oestrogen signalling through the novel GPER may be important in the aetiology of obesity and type II diabetes, although it is still a matter of controversy. While a group reported that male and female GPER-deficient mice are obese (Haas et al. 2009), others have described an opposite effect in females of different GPER(–/–) mice, developed using a cre/lox approach (Martensson et al. 2009). Other investigators have not observed any changes in weight and glucose tolerance or insulin resistance in GPER(–/–) female mice (Liu et al. 2009). In post-menopausal women, oestrogens are produced mainly in the adipose tissue as a conversion of androgens or other oestrogens, principally oestrone, and its production is not regulated by feedback mechanisms (Siiteri, 1987). Therefore, in addition to other signalling molecules released by the adipose tissue, E2 may participate as an adipose-derived hormone in the ERα-mediated enhancement of insulin biosynthesis (Alonso-Magdalena et al. 2008) and in the potentiation of GSIS through ERs other than ERα to help β–cells adapt to the higher demand of insulin during obesity.
Concluding remarks
Oestradiol potentiates the insulin secretory response after the activation of cGMP-dependent protein kinase, and promotes insulin biosynthesis and resistance to apoptosis. These E2-triggered actions are some of those used by the islets of Langerhans to adapt to insulin resistance states, such as pregnancy and obesity. Therefore, together with other hormones and extracellular signalling molecules, E2 may be an important signal involved in β–cell plasticity. Nevertheless, it should be noted that, if these oestrogenic actions occur at an inappropriate time, or at doses not within the physiological range, they may cause adverse effects such as insulin resistance (Nadal et al. 2009).
Acknowledgments
The authors’ laboratories are supported by the Spanish Ministry of Education and Science, grants BFU2007-67607 and BFU2008-01492 and Generalitat Valenciana. CIBERDEM is an initiative of Instituto de Salud Carlos III.
References
- Ackermann S, Hiller S, Osswald H, Losle M, Grenz A, Hambrock A. 17β–Estradiol modulates apoptosis in pancreatic β–cells by specific involvement of the sulfonylurea receptor (SUR) isoform SUR1. J Biol Chem. 2009;284:4905–4913. doi: 10.1074/jbc.M807638200. [DOI] [PubMed] [Google Scholar]
- Adachi T, Yasuda K, Mori C, Yoshinaga M, Aoki N, Tsujimoto G, Tsuda K. Promoting insulin secretion in pancreatic islets by means of bisphenol A and nonylphenol via intracellular estrogen receptors. Food Chem Toxicol. 2005;43:713–719. doi: 10.1016/j.fct.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Alonso A, Ordóñez P, Fernández R, Moreno M, Llaneza P, Patterson AM, González C. 17β–Estradiol treatment is unable to reproduce p85α redistribution associated with gestational insulin resistance in rats. J Steroid Biochem Mol Biol. 2009;116:160–170. doi: 10.1016/j.jsbmb.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic β–cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ERα. PLoS One. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral ME, Cunha DA, Anhe GF, Ueno M, Carneiro EM, Velloso LA, Bordin S, Boschero AC. Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J Endocrinol. 2004;183:469–476. doi: 10.1677/joe.1.05547. [DOI] [PubMed] [Google Scholar]
- Amaral ME, Ueno M, Carvalheira JB, Carneiro EM, Velloso LA, Saad MJ, Boschero AC. Prolactin-signal transduction in neonatal rat pancreatic islets and interaction with the insulin-signalling pathway. Horm Metab Res. 2003;35:282–289. doi: 10.1055/s-2003-41303. [DOI] [PubMed] [Google Scholar]
- Arumugam R, Horowitz E, Lu D, Collier JJ, Ronnebaum S, Fleenor D, Freemark M. The interplay of prolactin and the glucocorticoids in the regulation of β–cell gene expression, fatty acid oxidation, and glucose-stimulated insulin secretion: implications for carbohydrate metabolism in pregnancy. Endocrinology. 2008;149:5401–5414. doi: 10.1210/en.2008-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley MS, Geschwind II, Bradford GE. The gestational pattern of estradiol, testosterone and progesterone secretion in selected strains of mice. Biol Reprod. 1979;20:733–738. doi: 10.1095/biolreprod20.4.733. [DOI] [PubMed] [Google Scholar]
- Barkley MS, Michael SD, Geschwind II, Bradford GE. Plasma testosterone during pregnancy in the mouse. Endocrinology. 1977;100:1472–1475. doi: 10.1210/endo-100-5-1472. [DOI] [PubMed] [Google Scholar]
- Barros R, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERα and ERβ in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab. 2009;297:E124–E133. doi: 10.1152/ajpendo.00189.2009. [DOI] [PubMed] [Google Scholar]
- Beck P, Parker ML, Daughaday WH. Radioimmunologic measurement of human placental lactogen in plasma by a double antibody method during normal and diabetic pregnancies. J Clin Endocrinol Metab. 1965;25:1457–1462. doi: 10.1210/jcem-25-11-1457. [DOI] [PubMed] [Google Scholar]
- Becker KL, Bilezikian JP, Bremner WJ, Hung W, Kahn CR. Principles and Practice of Endocrinology and Metabolism. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Bone AJ, Taylor KW. Mitabolic adaptation to pregnancy shown by increased biosynthesis of insulin in islets of Langerhans isolated from pregnant rat. Nature. 1976;262:501–502. doi: 10.1038/262501a0. [DOI] [PubMed] [Google Scholar]
- Branisteanu DD, Mathieu C. Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab. 2003;14:54–56. doi: 10.1016/s1043-2760(03)00003-1. [DOI] [PubMed] [Google Scholar]
- Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132:879–887. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- Brelje TC, Sorenson RL. Role of prolactin versus growth hormone on islet B-cell proliferation in vitro: implications for pregnancy. Endocrinology. 1991;128:45–57. doi: 10.1210/endo-128-1-45. [DOI] [PubMed] [Google Scholar]
- Brelje TC, Svensson AM, Stout LE, Bhagroo NV, Sorenson RL. An immunohistochemical approach to monitor the prolactin-induced activation of the JAK2/STAT5 pathway in pancreatic islets of Langerhans. J Histochem Cytochem. 2002;50:365–383. doi: 10.1177/002215540205000308. [DOI] [PubMed] [Google Scholar]
- Catalano PM. Pregnancy and lactation in relation to range of acceptable carbohydrate and fat intake. Eur J Clin Nutr. 1999;53(Suppl. 1):S124–S131. doi: 10.1038/sj.ejcn.1600753. [DOI] [PubMed] [Google Scholar]
- Cesen-Cummings K, Copland JA, Barrett JC, Walker CL, Davis BJ. Pregnancy, parturition, and prostaglandins: defining uterine leiomyomas. Environ Health Perspect. 2000;108(Suppl. 5):817–820. doi: 10.1289/ehp.00108s5817. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. 17β–Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002;74:1252–1259. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147:S4–10. doi: 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- Fleenor DE, Freemark M. Prolactin induction of insulin gene transcription: roles of glucose and signal transducer and activator of transcription 5. Endocrinology. 2001;142:2805–2810. doi: 10.1210/endo.142.7.8267. [DOI] [PubMed] [Google Scholar]
- Freemark M. Ontogenesis of prolactin receptors in the human fetus: roles in fetal development. Biochem Soc Trans. 2001;29:38–41. doi: 10.1042/0300-5127:0290038. [DOI] [PubMed] [Google Scholar]
- Freemark M. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm Res. 2006;65(Suppl. 3):41–49. doi: 10.1159/000091505. [DOI] [PubMed] [Google Scholar]
- Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N, Breant B, Kelly PA. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- Fujinaka Y, Sipula D, Garcia-Ocana A, Vasavada RC. Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic β–cell survival. Diabetes. 2004;53:3120–3130. doi: 10.2337/diabetes.53.12.3120. [DOI] [PubMed] [Google Scholar]
- Fujinaka Y, Takane K, Yamashita H, Vasavada RC. Lactogens promote β cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem. 2007;282:30707–30717. doi: 10.1074/jbc.M702607200. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Alonso A, Fernandez R, Patterson AM. Regulation of insulin receptor substrate–1 in the liver, skeletal muscle and adipose tissue of rats throughout pregnancy. Gynecol Endocrinol. 2003;17:187–197. doi: 10.1080/gye.17.3.187.197. [DOI] [PubMed] [Google Scholar]
- Gonzalez CG, Alonso A, Balbin M, Diaz F, Fernandez S, Patterson AM. Effects of pregnancy on insulin receptor in liver, skeletal muscle and adipose tissue of rats. Gynecol Endocrinol. 2002;16:193–205. [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Tratado de Fisiología Médica. 10th edn. Mexico: McGraw-Hill Interamericana; 2001. [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of β–cell mass during pregnancy. Endocrinology. 2009;150:1618–1626. doi: 10.1210/en.2008-1003. [DOI] [PubMed] [Google Scholar]
- Hügl SR, Merger M. Prolactin stimulates proliferation of the glucose-dependent β–cell line INS–1 via different IRS-proteins. JOP. 2007;8:739–752. [PubMed] [Google Scholar]
- Hussain MA, Porras DL, Rowe MH, West JR, Song WJ, Schreiber WE, Wondisford FE. Increased pancreatic β–cell proliferation mediated by CREB binding protein gene activation. Mol Cell Biol. 2006;26:7747–7759. doi: 10.1128/MCB.02353-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson D, Ng AC, Fu A, Depatie C, Al Azzabi M, Screaton RA. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc Natl Acad Sci U S A. 2008;105:10161–10166. doi: 10.1073/pnas.0800796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic β–cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Zraika S, Utzschneider KM, Hull RL. The β cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia. 2009;52:1003–1012. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreatic β–cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- Kuhl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care. 1998;21(Suppl. 2):B19–B26. [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond) 2005;109:243–256. doi: 10.1042/CS20050078. [DOI] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic β–cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology. 2009;150:1563–1565. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. Importance of extranuclear estrogen receptor α and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009 doi: 10.2337/db09-0257. DOI 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R. Body mass index, circulating levels of sex-steroid hormones, IGF–I and IGF-binding protein–3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic β–cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304:63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci U S A. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Rovira JM, Laribi O, Leon-quinto T, Andreu E, Ripoll C, Soria B. Rapid insulinotropic effect of 17β-estradiol via a plasma membrane receptor. FASEB J. 1998;12:1341–1348. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Ohara-Imaizumi M, Kubota N, Hashimoto S, Eto K, Kanno T, Kubota T, Wakui M, Nagai R, Noda M, Nagamatsu S, Kadowaki T. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51:827–835. doi: 10.1007/s00125-008-0944-9. [DOI] [PubMed] [Google Scholar]
- Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- Picard F, Wanatabe M, Schoonjans K, Lydon J, O’Malley BW, Auwerx J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to β–cell proliferation. Proc Natl Acad Sci U S A. 2002;99:15644–15648. doi: 10.1073/pnas.202612199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada I, Fuentes E, Viso-Leon MC, Soria B, Ripoll C, Nadal A. Low doses of the endocrine disruptor bisphenol–A and the native hormone 17β-estradiol rapidly activate transcription factor CREB. FASEB J. 2002;16:1671–1673. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373:1789–1797. doi: 10.1016/S0140-6736(09)60515-8. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signalling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Ropero AB, Alonso-Magdalena P, Quesada I, Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008;73:874–879. doi: 10.1016/j.steroids.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Ropero AB, Fuentes E, Rovira JM, Ripoll C, Soria B, Nadal A. Non-genomic actions of 17β–oestradiol in mouse pancreatic β–cells are mediated by a cGMP-dependent protein kinase. J Physiol. 1999;521:397–407. doi: 10.1111/j.1469-7793.1999.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropero AB, Soria B, Nadal A. A nonclassical estrogen membrane receptor triggers rapid differential actions in the endocrine pancreas. Mol Endocrinol. 2002;16:497–505. doi: 10.1210/mend.16.3.0794. [DOI] [PubMed] [Google Scholar]
- Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45:277–282. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Ackerman GE, Smith ME, Mendelson CR. Estrogen formation in stromal cells of adipose tissue of women: induction by glucocorticosteroids. Proc Natl Acad Sci U S A. 1981;78:5690–5694. doi: 10.1073/pnas.78.9.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC. Prolactin receptors are critical to the adaptation of islets to pregnancy. Endocrinology. 2009;150:1566–1569. doi: 10.1210/en.2008-1710. [DOI] [PubMed] [Google Scholar]
- Sutter-Dub MT. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic B cell, the adipocyte and other cell types. Steroids. 2002;67:77–93. doi: 10.1016/s0039-128x(01)00142-8. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Tuduri E, Marroqui L, Soriano S, Ropero AB, Batista TM, Piquer S, Lopez-Boado MA, Carneiro EM, Gomis R, Nadal A, Quesada I. Inhibitory effects of leptin on the pancreatic α-cell function. Diabetes. 2009;58:1616–1624. doi: 10.2337/db08-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada RC, Garcia-Ocana A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF. Targeted expression of placental lactogen in the β cells of transgenic mice results in β cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- Weinhaus AJ, Stout LE, Sorenson RL. Glucokinase, hexokinase, glucose transporter 2, and glucose metabolism in islets during pregnancy and prolactin-treated islets in vitro: mechanisms for long term up-regulation of islets. Endocrinology. 1996;137:1640–1649. doi: 10.1210/endo.137.5.8612496. [DOI] [PubMed] [Google Scholar]