Abstract
The intestine is an oestrogen responsive organ and circulatory oestrogens suppress Cl− secretion across the epithelium of the colon to promote fluid retention at the luteal stage of the menstrual cycle. Ion transporters in the colon which are involved in Cl− secretion show differential expression between males and females as do the signalling protein kinase intermediates involved in acutely regulating these transporters. Work from our laboratory has identified the KCNQ1/KCNE3 channel as one of the principal targets for oestrogen-induced signalling cascades in the distal colon. Through inhibition of the KCNQ1 channel, basolateral K+ recycling is decreased so reducing the favourable electrochemical gradient for Cl− extrusion at the apical membrane. The actions of oestrogen on non-reproductive tissues such as the colon, kidney, lung and sweat gland will affect whole body electrolyte and fluid homeostasis and also have consequences for reproductive potential.
Introduction
Oestrogen is the primary female reproductive hormone and exerts biological effects through the modulation of transcriptional and cell signalling processes in target tissues (Simpson, 2003). Oestrogen regulates cell growth and differentiation in primary and secondary reproductive organs but also acts on what are generally regarded as non-reproductive tissues expressing oestrogen receptors (ERs). The role of oestrogen in modulating the physiology of non-reproductive tissues is an emerging area of investigation. ERs are expressed in the respiratory tract, kidney, sweat gland and colon as well as at sites in the brain (Kuiper et al. 1997). The prominence of secretory and absorptive epithelia among the list of oestrogen target tissues suggests an important role for oestrogen in the regulation of whole body electrolyte and fluid balance through the modulation of ion transport processes (Crocker, 1971; Harvey et al. 2001). Over the course of the oestrous cycle large fluctuations in circulatory oestrogen concentrations are experienced by the female body and these changes modulate the structure and function of reproductive tissues, particularly the uterus to facilitate embryo implantation. Through oestrogen synthesis and release by the ovaries, the ion transport activities of epithelial tissues such as the distal colon are also synchronized with the female reproductive cycle. The importance of oestrogen action on epithelial ion transport to the physiological processes of conception, implantation and pregnancy are still unknown.
The two isoforms of the oestrogen receptor, ERα and ERβ, show marked differential tissue expression and biological roles. ERα is predominantly expressed in breast, uterus, vagina and other tissue types (Gustafsson, 1999; Hall et al. 2001). ERβ is predominantly found in ovary, testis, spleen, lung, hypothalamus and thymus with some expression noted in breast tissue (Hall et al. 2001). The receptors act as ligand-dependent transcription factors to positively or negatively regulate the expression of a broad repertoire of oestrogen responsive genes. While bound to oestrogen, these receptors interact with specific DNA sequences to facilitate the assembly of the transcription pre-initiation complexes at gene promoter sites. It is also now clear that ERs interact with adjacent transcription factors at cis elements in promoter sequences to regulate transcription in a far more subtle manner than was previously imagined. Reports over the last two decades have shown that oestrogen and other steroid hormones also stimulate the rapid activation of protein kinase signalling cascades in target tissues that potentiate and modulate the direct transcriptional effects of the nuclear ERs. These rapid ‘non-genomic’ signalling events are initiated at membrane localized oestrogen receptors (Pappas et al. 1995; Pietras et al. 2001; Pedram et al. 2002), which may be splice variants of the nuclear receptor (Razandi et al. 1999, 2003) or may be truncated forms of the ERα associated with the cell membrane through acylation (Marino et al. 2006). Even though the rapid activation of protein kinases by oestrogen has been established, the biological role of these responses is only now being elucidated. We have identified a role for oestrogen-induced protein kinase signalling cascades in the suppression of fluid secretion in the distal colon which represents a novel and important facet in the hormone's range of physiological effects (O’Mahony & Harvey, 2008).
Oestrogen and colonic physiology
The distal colon is the principal site for water conservation in the body. The transport of ions, particularly Na+ absorption and Cl− secretion across the epithelium, determines the rate of water loss and recovery from the lumen of the colon. Disruption of this equilibrium results in conditions such as secretory diarrhoea. The absorption of water from the faeces is coupled to Na+ absorption through the Na+/H+ exchanger or through the epithelial Na+ channel (ENaC). Cl− driven secretion serves to hydrate the mucosa and also lubricates the passage of the faecal matter through the colon. Cl− is transported into the epithelial cells of the colon at the basolateral surface through the Na+–K+–2Cl− (NKCC1) co-transporter and exits the cell across the apical membrane into the lumen through the cystic fibrosis transmembrane conductance regulator (CFTR). The Na+,K+-ATPase at the basolateral membrane provides the driving force for apical Cl− secretion and ENaC-dependent Na+ absorption, while the electrochemical gradient is maintained by K+ recycling through basolateral K+ channels such as KCNQ1 (Liao et al. 2005). KCNQ1 plays a crucial role in regulating the rate of salt and water transport across a number of epithelia including those of the small intestine, nephron and colon (Jespersen et al. 2005). The transporters engaged in electrolyte transport processes across the colon and other epithelia are subject to precise transcriptional control while acutely acting secretagogues such as forskolin or carbachol modulate transporter activity through the stimulation of signalling cascades coupled to intracellular cAMP or Ca2+, respectively. We have found that oestrogen suppresses the secretagogue-induced Cl− secretion in the distal colon suggesting that the hormone may also have an antisecretory role in normal colon physiology (Fig. 1; Condliffe et al. 2001). The observation that antagonism of basolateral K+ recycling also attenuates Cl− secretion and so mimics the effects of oestrogen in the colon gave an insight into a potential mechanism by which oestrogen could produce its antisecretory effect (McNamara et al. 1999). Furthermore, in cardiac tissue oestrogen suppresses the KCNQ1-mediated K+ current suggesting this channel subunit could be a potential molecular target for oestrogen antisecretory action (Moller & Netzer, 2006).
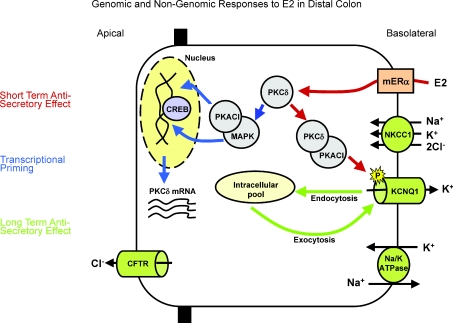
Figure 1. Mechanism of rapid oestrogen inhibition of cAMP activated chloride ion secretion and subsequent induction of fluid retention in the isolated distal colon from female rats.
Cl− ions enter the cell via the basolateral NKCC1 protein and exit via apical CFTR. Na+ and K+ recycling occur via the Na+,K+-ATPase pump and the KCNQ1 channel at the basolateral membrane. Oestrogen rapidly activates PKCδ (2 min), which in turn activates PKA. The kinase complex translocates to the plasma membrane and associates with the cytoplasmic region of the KCNQ1 channel. PKA induces a rapid serine phosphorylation of the channel. Phosphorylation of the KCNQ1 channel results in an inhibition of K+ recycling and in turn inhibition of Cl− secretion. In addition to inducing the rapid and antisecretory effect, oestrogen induces a transcriptional response via CREB resulting in the upregulation of expression of PKCδ and downregulation of the expression of ion transporters. In this way the rapid ‘non-genomic’ effects of oestrogen promote transcriptional priming of signalling intermediates of the antisecretory response and also feed forward to sustain the antisecretion through inhibition of ion transporter expression and activation of inhibitory protein kinases.
Oestrogen and the colonic epithelium
The epithelium of the colon expresses both ERα and ERβ (Thomas et al. 1993). However, in the crypts of the proximal colon ERα is expressed more highly at the base of the crypt while ERβ expression is predominant in the mid-section of the crypt and in the lumen surface cells (Cho et al. 2007). This segregation of ER isoform expression suggests antagonistic roles for the two receptors and differential effects of circulatory oestrogen on the physiological function of the cells located at different sites along the crypt. Progenitor cell proliferation at the base of the crypt gives way to enterocyte differentiation in the midsection and shedding of senescent or apoptotic cells at the lumen surface. ERβ has been proposed as a tumour suppressor in colorectal cancer (CRC). ERβ expression is selectively lost during tumour progression through methylation-dependent gene silencing (Foley et al. 2000) and hormone replacement therapy (HRT) reduces the incidence of CRC in postmenopausal women (Nelson et al. 2002; Chlebowski et al. 2004). Recent data suggests that HRT may also be protective in suppressing episodes of inflammatory bowel disease in post-menopausal women through the antiinflammatory effects of oestrogen (Kane & Reddy, 2008). The role of oestrogen in regulating normal colon physiology and ion transport is less well characterized. However, studies with ERβ−/− mice demonstrated a role for the receptor in cell differentiation and the organization of colon tissue architecture through regulation of cell to cell interactions such as tight junction and desmosome assembly (Wada-Hiraike et al. 2006). In our studies, we have used isolated rat colonic crypts as an experimental model to study the role of oestrogen in suppressing agonist-induced Cl− secretion and K+ recycling. KCNQ1 is a voltage-gated, low conductance K+ channel activated during cAMP-stimulated Cl− secretion in the colon (Kunzelmann et al. 2001). We found that the activity of this channel was suppressed following oestrogen treatment at physiological levels of the hormone. The oestrogen effect was non-additive to the effects of Chromanol 293B, a specific blocker of KCNQ1, so confirming this channel as an important effector of the oestrogen antisecretory response. The antisecretory action of oestrogen is superimposed upon an ion transporter protein expression profile that differs between males and females. NKCC1, CFTR and Na+/K+-ATPase α-subunit are all expressed at lower abundance in colonic crypts isolated from female rats compared to males (O’Mahony & Harvey, 2008). The KCNQ1 channel subunit is not differentially expressed in the male and female colonic epithelium; however the expression of the KCNE3 regulatory subunit is less abundant in females compared to males (O’Mahony & Harvey, 2008).
Oestrogen-induced signalling in the colon
Oestrogen suppresses forskolin-induced secretion in the distal colon within 10 min of hormone treatment suggesting that the effect is at least in part modulated by a signalling cascade rather than by a direct transcriptional response (Condliffe et al. 2001). The involvement of a protein kinase signalling cascade was confirmed by the sensitivity of the oestrogen effect to PKC antagonism (Doolan & Harvey, 1996; McNamara et al. 1999; Condliffe et al. 2001). The phosphorylation of membrane transporters is an important regulatory mechanism contributing either to the direct activation of the transporter or to its localization at the cell membrane. KCNQ1 exists at the cell membrane as part of a multi-molecular complex with the A-kinase anchoring protein Yotiao and a regulatory β subunit. KCNQ1 is phosphorylated at residue Ser27 by membrane associated PKA to modulate its activity in the heart (Kurokawa et al. 2004). In the distal colon KCNQ1 is in complex with the KCNE3 regulatory subunit. In common with the antisecretory effect of oestrogen on the colon, the suppression of KCNQ1 activity by oestrogen in the colon was female sex specific (O’Mahony et al. 2007). We also found that oestrogen stimulated the rapid activation of PKA in isolated female colonic crypts but not in crypts isolated from male rats (O’Mahony et al. 2007). The level of PKA phosphorylation induced by oestrogen correlates with the degree of stimulation or suppression of KCNQ1 activity. The activation of different PKA isoforms, which localize to different cellular sites, has different physiological consequences. PKA activation in oestrogen treated colonic crypts is localized to the cytosol rather than to the cell membrane (Doolan et al. 2000). PKA isoform II is anchored to the cell membrane while PKA I is distributed to pools in the cytosol (Tasken & Aandahl, 2004). Oestrogen treatment stimulates the physical association of the cytosolic PKA-CI isoform with KCNQ1 whereas the membrane associated PKA is unaffected. The physical interaction of PKA-CI–KCNQ1 correlates with the oestrogen-induced serine phosphorylation of the KCNQ1 channel protein (O’Mahony et al. 2007). The oestrogen-induced inhibition of Cl− secretion and KCNQ1 activity and the stimulation of PKA-dependent phosphorylation of KCNQ1 were antagonized by prior inhibition of PKCδ (O’Mahony et al. 2007). Recent data points to the differential expression of key protein kinase signalling intermediates in tissues from males as compared to females. At least some of these differentially regulated molecules contribute to steroid-induced responses or are themselves transcriptionally regulated by oestrogen (Maizels et al. 1996). Sex differences in PKCδ abundance has been found in aortic smooth muscle (Kanashiro & Khalil, 2001) and in the liver (Zangar et al. 1995). The abundance of PKCδ in isolated colonic crypts from rats at the oestrus stage of the reproductive cycle was on average fourfold higher in females compared to males, while females at the pro-oestrus stage of the cycle express PKCδ at low levels equivalent to males (O’Mahony et al. 2007). Through the modulation of protein kinase expression, varying circulating levels of oestrogen determine the sensitivity of particular cell signalling cascades to the rapid actions of the hormone. Oestrogen-induced PKCδ expression effectively creates a positive feedback loop that amplifies PKCδ-dependent oestrogen rapid and genomic responses at the peak of circulatory levels of oestrogen (O’Mahony et al. 2009). More recently this genomic regulation of PKCδ in the female rat distal colon has been demonstrated to be via a novel membrane ERα (memERα) (O’Mahony et al. 2009). The memERα is expressed in rat colonic crypts from the male and female but with a reduced expression in the male tissue compared to female (O’Mahony et al. 2009).
Conclusion
Oestrogen modulates the rate of fluid secretion across the female colonic epithelium over the course of the oestrous cycle. Cl− and fluid secretion are attenuated at the oestrus phase of the cycle by the suppression of basolateral K+ recycling through the KCNQ1 channel. Oestrogen acutely suppresses KCNQ1 activity through phosphorylation of the channel α subunit, which is dependent on the activation of PKCδ and cytosolic PKA. The expression of PKCδ by the colonic epithelium is modulated throughout the oestrous cycle to amplify the kinase-dependent regulation of KCNQ1 during the oestrus phase. Precise regulation of Cl− secretion by the colon at key stages of the reproductive cycle facilitates fluid conservation at a time that is coincident with uterine restructuring and represents a novel facet of the role of oestrogen in the physiology of reproduction.
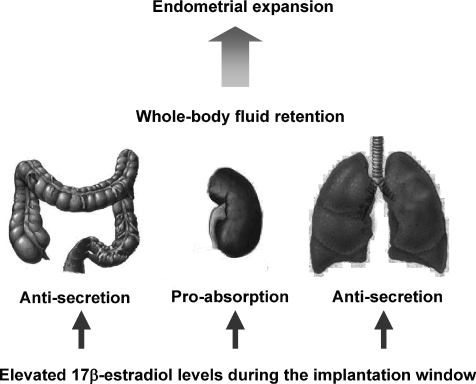
The intestinal tract has previously not been thought of as a sex-steroid targeted organ. Interorgan interactions have previously been reported between the reproductive, urinary and intestinal systems (Nelson et al. 2002). The identification of functional ER in the intestine indicate a physiological role of oestrogen in the intestine (Thomas et al. 1993). This review highlights the important role for oestrogen in the distal colon in contributing to the regulation of whole body fluid and electrolyte regulation in the female. In preparation for the implantation of the blastocyst, the endometrial lining must expand its vasculature and this is associated with a significant increase in tissue wet weight. High levels of oestrogen result in fluid retention as a result of conservation of large volumes of water from organs such as the kidney, lung and large intestine (Fig. 2). This process is a rapid and reversible event as whole body fluid retention of water is cyclical and occurs over short periods of time. In this manner the intestine responds to fluctuating levels of oestrogen to rapidly and dynamically reduce water loss via inhibition of the Cl− secretory pathway.
Figure 2.
High circulating levels of oestrogen occurring during the implantation window of the oestrous cycle (and with hormone replacement therapy and the contraceptive pill) produce an inhibition of chloride ion secretion in the intestine and lung and increase the absorption of sodium in the kidney, resulting in increased fluid retention which is necessary for endometrial expansion and implantation of the blastocyst
References
- Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, Rosenberg CA, Taylor VM, Harris R, Chen C, Adams-Campbell LL, White E. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- Cho NL, Javid SH, Carothers AM, Redston M, Bertagnolli MM. Estrogen receptors α and β are inhibitory modifiers of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res. 2007;67:2366–2372. doi: 10.1158/0008-5472.CAN-06-3026. [DOI] [PubMed] [Google Scholar]
- Condliffe SB, Doolan CM, Harvey BJ. 17β-oestradiol acutely regulates Cl− secretion in rat distal colonic epithelium. J Physiol. 2001;530:47–54. doi: 10.1111/j.1469-7793.2001.0047m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker AD. Variations in mucosal water and sodium transfer associated with the rat oestrous cycle. J Physiol. 1971;214:257–264. doi: 10.1113/jphysiol.1971.sp009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan CM, Condliffe SB, Harvey BJ. Rapid non-genomic activation of cytosolic cyclic AMP-dependent protein kinase activity and [Ca2+]i by 17β-oestradiol in female rat distal colon. Br J Pharmacol. 2000;129:1375–1386. doi: 10.1038/sj.bjp.0703193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan CM, Harvey BJ. Modulation of cytosolic protein kinase C and calcium ion activity by steroid hormones in rat distal colon. J Biol Chem. 1996;271:8763–8767. doi: 10.1074/jbc.271.15.8763. [DOI] [PubMed] [Google Scholar]
- Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor β in malignant human colon. Cancer Res. 2000;60:245–248. [PubMed] [Google Scholar]
- Gustafsson JA. Estrogen receptor β: a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signalling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Harvey BJ, Condliffe S, Doolan CM. Sex and salt hormones: rapid effects in epithelia. News Physiol Sci. 2001;16:174–177. doi: 10.1152/physiologyonline.2001.16.4.174. [DOI] [PubMed] [Google Scholar]
- Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: from gene to physiological function. Physiology (Bethesda) 2005;20:408–416. doi: 10.1152/physiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280:C34–45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- Kane SV, Reddy D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1193–1196. doi: 10.1111/j.1572-0241.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Hubner M, Schreiber R, Levy-Holzman R, Garty H, Bleich M, Warth R, Slavik M, von Hahn T, Greger R. Cloning and function of the rat colonic epithelial K+ channel KVLQT1. J Membr Biol. 2001;179:155–164. doi: 10.1007/s002320010045. [DOI] [PubMed] [Google Scholar]
- Kurokawa J, Motoike HK, Rao J, Kass RS. Regulatory actions of the A-kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc Natl Acad Sci U S A. 2004;101:16374–16378. doi: 10.1073/pnas.0405583101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T, Wang L, Halm ST, Lu L, Fyffe RE, Halm DR. K+ channel KVLQT1 located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl− secretion. Am J Physiol Cell Physiol. 2005;289:C564–575. doi: 10.1152/ajpcell.00561.2004. [DOI] [PubMed] [Google Scholar]
- Maizels ET, Shanmugam M, Lamm ML, Hunzicker-Dunn M. Hormonal regulation of PKC-δ protein and mRNA levels in the rabbit corpus luteum. Mol Cell Endocrinol. 1996;122:213–221. doi: 10.1016/0303-7207(96)03885-3. [DOI] [PubMed] [Google Scholar]
- Marino M, Ascenzi P, Acconcia F. S-palmitoylation modulates estrogen receptor α localization and functions. Steroids. 2006;71:298–303. doi: 10.1016/j.steroids.2005.09.011. [DOI] [PubMed] [Google Scholar]
- McNamara B, Winter DC, Cuffe JE, O'Sullivan GC, Harvey BJ. Basolateral K+ channel involvement in forskolin-activated chloride secretion in human colon. J Physiol. 1999;519:251–260. doi: 10.1111/j.1469-7793.1999.0251o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Netzer R. Effects of estradiol on cardiac ion channel currents. Eur J Pharmacol. 2006;532:44–49. doi: 10.1016/j.ejphar.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- O’Mahony F, Alzamora R, Betts V, LaPaix F, Carter D, Irnaten M, Harvey BJ. Female gender-specific inhibition of KCNQ1 channels and chloride secretion by 17β-estradiol in rat distal colonic crypts. J Biol Chem. 2007;282:24563–24573. doi: 10.1074/jbc.M611682200. [DOI] [PubMed] [Google Scholar]
- O’Mahony F, Alzamora R, Chung KL, Thomas W, Harvey BJ. Genomic priming of the anti-secretory response to estrogen in rat distal colon throughout the estrous cycle. Mol Endocrinol. 2009 doi: 10.1210/me.2008-0248. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony F, Harvey BJ. Sex and estrous cycle-dependent rapid protein kinase signalling actions of estrogen in distal colonic cells. Steroids. 2008;73:889–894. doi: 10.1016/j.steroids.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labelling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signalling by steroid to transcription and cell biology. J Biol Chem. 2002;277:50768–50775. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Nemere I, Szego CM. Steroid hormone receptors in target cell membranes. Endocrine. 2001;14:417–427. doi: 10.1385/ENDO:14:3:417. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signalling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Xu X, Norfleet AM, Watson CS. The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology. 1993;132:426–430. doi: 10.1210/endo.132.1.8419141. [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor β in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103:2959–2964. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangar RC, Reiners JJ, Jr, Novak RF. Gender-specific and developmental differences in protein kinase C isozyme expression in rat liver. Carcinogenesis. 1995;16:2593–2597. doi: 10.1093/carcin/16.10.2593. [DOI] [PubMed] [Google Scholar]