Abstract
Development of the cortical map is experience dependent, with different critical periods in different cortical layers. Previous work in rodent barrel cortex indicates that sensory deprivation leads to changes in synaptic transmission and plasticity in layer 2/3 and 4. Here, we studied the impact of sensory deprivation on the intrinsic properties of layer 5 pyramidal neurons located in rat barrel cortex using simultaneous somatic and dendritic recording. Sensory deprivation was achieved by clipping all the whiskers on one side of the snout. Loss of sensory input did not change somatic active and resting membrane properties, and did not influence dendritic action potential (AP) backpropagation. In contrast, sensory deprivation led to an increase in the percentage of layer 5 pyramidal neurons showing burst firing. This was associated with a reduction in the threshold for generation of dendritic calcium spikes during high-frequency AP trains. Cell-attached recordings were used to assess changes in the properties and expression of dendritic HCN channels. These experiments indicated that sensory deprivation caused a decrease in HCN channel density in distal regions of the apical dendrite. To assess the contribution of HCN down-regulation on the observed increase in dendritic excitability following sensory deprivation, we investigated the impact of blocking HCN channels. Block of HCN channels removed differences in dendritic calcium electrogenesis between control and deprived neurons. In conclusion, these observations indicate that sensory loss leads to increased dendritic excitability of cortical layer 5 pyramidal neurons. Furthermore, they suggest that increased dendritic calcium electrogenesis following sensory deprivation is mediated in part via down-regulation of dendritic HCN channels.
Introduction
The maturation of neuronal circuits is strongly dependent on experience and activity (Katz & Shatz, 1996). The rodent barrel cortex offers an excellent model system to investigate such experience-dependent changes in cortical development (Fox, 1992), with previous work indicating that somatosensory maps are highly malleable both during brain development (Van der Loos & Woolsey, 1973; Simons & Land, 1987) and in the adult animal (Buonomano & Merzenich, 1998).
The architecture of the rodent barrel cortex is organised into different columns, where each column or barrel represents a cluster of neurons in layer 4. Each barrel and corresponding supra and infragranular layers respond strongly to the stimulation of the related principal whisker and only weakly to inputs from surrounding whiskers (Armstrong-James & Fox, 1987; Armstrong-James et al. 1991; Fox, 1994; Moore & Nelson, 1998; Zhu & Connors, 1999). This cortical map of the whisker pad is represented topographically (Woolsey & Van der Loos, 1970), and is established early during development (Schlaggar & O’Leary, 1994). Disruption of sensory input to barrel cortex via whisker follicle destruction in the first postnatal week causes structural changes in barrel formation (Van der Loos & Woolsey, 1973; Woolsey & Wann, 1976). These changes during the first postnatal week parallel developmental changes in synaptic plasticity at thalamocortical synapses (Crair & Malenka, 1995; Isaac et al. 1997; Feldman et al. 1998). In contrast, sensory deprivation induced by whisker trimming or plucking can lead to changes in receptive field properties throughout development (Fox, 1992; Diamond et al. 1993), and can occur in a layer specific manner (Diamond et al. 1994; Glazewski & Fox, 1996; Stern et al. 2001; Polley et al. 2004).
The plasticity mechanisms involved in the development and refinement of cortical maps are poorly understood. Previous work in barrel cortex indicates that sensory experience can lead to changes in synaptic transmission and plasticity (Glazewski & Fox, 1996; Allen et al. 2003). Alternatively, plasticity may occur through changes in the intrinsic properties of cortical neurons (Zhang & Linden, 2003). Input–output properties of neurons, as well as the expression of voltage-gated channels, can be modified by experience in vivo (Disterhoft et al. 1986; Sanchez-Andres & Alkon, 1991; Saar et al. 1998; Aizenman et al. 2003; Maravall et al. 2004), and also following changes in activity in neuronal networks in vitro (Desai et al. 1999; Nelson et al. 2003). The plasticity of intrinsic neuronal properties in barrel cortex following changes in sensory experience has not been studied in detail. The only previous study to address this issue observed that the development of spiking properties of layer 2/3 pyramidal neurons is delayed by sensory deprivation during the critical period (Maravall et al. 2004). To date, there are no data on the impact of sensory deprivation on the intrinsic properties of cortical layer 5 pyramidal neurons in somatosensory cortex. Moreover, there is no evidence on whether changes in sensory experience can influence dendritic properties of cortical neurons. These two issues are the focus of the current study.
Methods
Slice preparation
All procedures were performed according to methods approved by the Animal Ethics Committee of the Australian National University. Four- to six-week-old Wistar rats (of either sex) were deeply anaesthetised by isoflurane inhalation (3% in oxygen) and decapitated; the brain was quickly removed and coronal brain slices (300 μm thick) containing barrel cortex were prepared. Throughout the slice preparation, the brain was maintained in ice-cold artificial cerebrospinal fluid (ACSF) of the following composition (in mm): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 25 glucose, 0.5 CaCl2, 6 MgCl2, pH 7.4; oxygenated with carbogen (95% O2–5% CO2). After cutting, slices were immersed in ACSF containing (in mm): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 25 glucose, 2 CaCl2, 1 MgCl2; oxygenated with (95% O2–5% CO2) and maintained at 35°C for 30 min, then stored at room temperature. The same ACSF solution was used for electrophysiological recording.
Sensory deprivation
Sensory deprivation was carried out at two time points relative to the barrel cortex maturation. Whisker trimming started either at post-natal day 3 (before the critical period, n= 44) or at post-natal day 20 (after formation of the cortical map, n= 24). As no significant difference was observed between the two groups, the data have been combined. All whiskers on the right side of the snout were trimmed (length <1 mm) using scissors every day until rats were utilised for electrophysiological experiments. Whiskers on the left side of the face were left intact and gently brushed with the scissors. Light anaesthesia using isoflurane inhalation (3% in oxygen) was used to immobilise animals older than 10 days of age during whisker trimming. Recordings were made from layer 5 pyramidal neurons in barrel cortex from the left hemisphere (deprived neurons), and were compared to recordings from layer 5 pyramidal neurons in barrel cortex from the right hemisphere, which constituted the control condition. In some experiments recordings were made from layer 5 pyramidal neurons in barrel cortex from the left hemisphere of control, non-deprived animals (n= 16). These experiments were carried out to investigate possible left–right asymmetry in the properties of layer 5 pyramidal neurons from barrel cortex in the left and right hemispheres. Cell selection was based on cell health rather than the sensory condition, leading to different numbers of neurons in the control and deprived groups. To minimise experimental bias analysis was performed every 2 weeks on large blocks of data, typically 10–15 cells, with the nature of the sensory condition of analysed neurons only established at the end of the analysis session.
Electrophysiological procedures
Brain slices were transferred to a recording chamber continuously perfused with oxygenated ACSF (95% O2–5% CO2). Barrel cortex and cortical layer 5 were visualised under low magnification using an upright microscope (5× magnification; BX50WI, Olympus, Tokyo, Japan). Somatic and dendritic recordings from layer 5 pyramidal neurons were performed at high magnification (60×) using differential interference contrast (DIC) optics combined with infrared illumination (Stuart et al. 1993). Patch pipettes for somatic and dendritic recording were made from borosilicate glass (Harvard Apparatus, Edenbridge, Kent, UK) pulled to obtain a resistance of 3–5 MΩ and 10–12 MΩ, respectively. For whole-cell current-clamp recordings, glass pipettes were filled with a solution consisting of the following (in mm): 20 KCl, 120 potassium gluconate, 10 Hepes, 4 MgATP, 0.3 Na2GTP, 10 Na2-phosphocreatine; pH 7.3 with KOH and osmolarity set to 280 mosmol l−1 with sucrose. For cell-attached voltage-clamp recordings, pipettes contained (in mm): 120 KCl, 20 TEA-Cl, 5 4-AP, 1 MgCl2, 1 BaCl2, 1 NiCl2, 0.5 CdCl2, 10 Hepes, 5 EGTA and 0.001 TTX; pH 7.35 with KOH and osmolarity set to 280 mosmol l−1 with sucrose. All recordings were obtained at a temperature of 34 ± 1°C.
Dual whole-cell somatic and dendritic current-clamp recordings were obtained using two identical BVC-700A current-clamp amplifiers (Dagan, Mineapolis, MN, USA). Voltage was digitised with an ITC-18 computer interface at 50 kHz (InstruTech, Port Washington, NY, USA). Signals were analog filtered on-line at 10 kHz and acquired using the data acquisition software AxoGraph 4.9 (Axograph Scientific, Australia). Pipette capacitance and bridge balance were compensated on-line with the amplifier circuits. Cells were only included in analysis if the somatic access resistance was less than 20 MΩ and the dendritic access resistance less than 50 MΩ. All neurons included in analysis exhibited a stable somatic resting membrane potential more negative than −65 mV. All membrane potentials have been corrected for an experimentally determined liquid junction potential of ∼12 mV. In voltage-clamp mode, cell-attached recording was performed using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA, USA). Current was analog filtered at 10 kHz and digitally sampled at 20 kHz using AxoGraph 4.9 software. The bath level of the recording chamber was kept to a minimum to reduce pipette capacitance and fast capacitance transients were compensated using the amplifier compensation circuit. The instrumental noise was estimated to be ∼500 fA root mean square at 2 kHz bandwidth. Leak current was subtracted on-line using a P/10 protocol of scaled and averaged leak pulses. Dendritic patch potentials were corrected for a liquid junction potential of −3 mV and the holding potential was set to ∼25 mV positive to the resting membrane potential, assuming a somatic resting membrane potential of ∼−75 mV (Williams & Stuart, 2000b; Gulledge & Stuart, 2003) and corrected for a distance-dependent depolarization of the local dendritic resting membrane potential of ∼1 mV per 100 μm (Stuart et al. 1997; Kole et al. 2006).
Data analysis
The distance of dendritic recordings from the soma was estimated in situ using the linear distance between the dendritic recording site and the beginning of the apical trunk. The amplitude and width of somatic and dendritic action potentials (APs) was measured from threshold, defined as a dV/dt of 50 and 25 V s−1, respectively. Action potential duration was measured at 10% of peak amplitude. The amplitude of the somatic after-hyperpolarization (AHP) and after-depolarization (ADP) was defined as the difference in the resting membrane potential and the minimum and maximum potential after the AP, respectively. The rheobase was defined as the minimum somatic current required to elicit APs and AP velocity was determined from the time difference between the peak of somatic and dendritic APs. Input resistance was obtained from a linear fit to the hyperpolarizing phase of the current to voltage relationship. To investigate the critical frequency for dendritic calcium electrogenesis, trains of APs at frequencies from 20 to 200 Hz were generated at the soma via somatic current injection and the evoked response recorded at the dendrite (Larkum et al. 1999a). The integral of the dendritic voltage was plotted against AP frequency, and the critical frequency defined as the AP frequency corresponding to the peak of the derivative of the dendritic voltage integral versus AP frequency plot. Cells were only included in this analysis if the derivative of the dendritic voltage integral versus AP frequency plot had a peak greater than 20 μV s Hz−1. The current amplitude of Ih obtained from cell-attached recordings (steps from −50 to −150 mV) was converted into conductance density (pS μm−2) based on a reversal potential of 0 mV (Kole et al. 2006), assuming a patch membrane of 4.5 μm2 (Engel & Jonas, 2005), and corrected for the 5.1-fold difference in Ih amplitude due to high external K+ (Kole et al. 2006). Steady-state activation curves were obtained from the amplitude of tail currents following steps to different voltages (Williams & Stuart, 2000b). Data were normalised to the maximum tail current amplitude and fitted with a Boltzmann function: y= 1/[1 + e(V1/2-V)/k]; where V1/2 is the voltage of half-maximal activation, V is the imposed membrane potential and k is the slope factor. Numerical values are given in the text as means ±s.e.m. and the level of statistical significance was set to P < 0.05.
Results
In this study, whole-cell and cell-attached recordings were performed to assess the membrane properties of layer 5 pyramidal neurons within rat barrel cortex under control conditions and following sensory deprivation induced by whisker trimming. Recordings were obtained from neurons with somata between 890 and 1470 μm (1117 ± 58 μm) from the pia (n= 332).
Effects of sensory deprivation on the somatic compartment
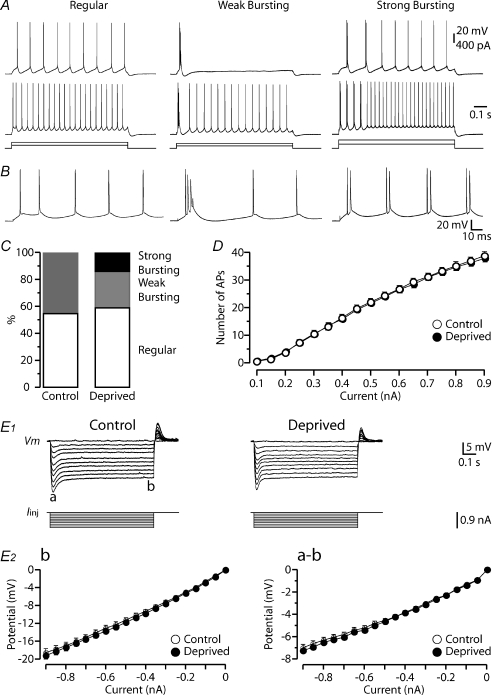
To examine the effects of whisker trimming on somatic membrane properties, we first investigated the voltage response to current injections made into the soma. In response to supra-threshold depolarizing current injections, layer 5 pyramidal neurons exhibited three different firing patterns (Fig. 1A and B) (see Williams & Stuart, 1999). Regular firing was characterised by tonic firing of single APs, whereas cells showing weak burst firing exhibited an initial burst of APs (spike frequency >200 Hz) followed by tonic firing. Strong bursting was characterised by consecutives bursts of APs (2–5 APs, spike frequency >200 Hz, Williams & Stuart, 1999). In control conditions, only regular (28 of 51 neurons) and weak bursting (23 of 51 neurons) firing patterns were observed (55% and 45% respectively; Fig. 1C). After sensory deprivation, the proportion of neurons showing regular firing was similar to that observed in control conditions (59%, 52 of 88 neurons, χ2 test, P= 0.8). In contrast, following sensory deprivation the portion of weak bursting neurons was reduced while the proportion of strong bursting neurons increased (Fig. 1C). Following sensory deprivation weak burst firing was observed in 27% of neurons (24 of 88 neurons), whereas strong burst firing was observed in 14% (12 of 88 neurons) of the neuronal population. The input–output properties of layer 5 pyramidal neurons were unchanged by sensory deprivation (Fig. 1D; deprived neurons: P= 0.96; Wilcoxon's test, for 500 pA current pulse). Similarly, the properties of single somatic APs (threshold, amplitude, rate-of-rise, half-width, AHP, ADP and rheobase) were not changed following sensory deprivation (Table 1).
Figure 1. Impact of sensory deprivation on somatic active and passive properties.
A, traces represent three typical firing patterns recorded from layer 5 pyramidal neurons in barrel cortex, referred to as ‘regular’, ‘weak bursting’ and ‘strong bursting’. B, the first 150 ms of the top traces in A. C, bar graph of the proportion of layer 5 neurons with the different firing patterns for the indicated sensory conditions (control: n= 51; deprived: n= 88; P > 0.05). Note the regular firing pattern remains constant for each experimental condition, with strong bursting encountered solely after sensory deprivation. D, graph showing the input–output properties of recorded neurons after different sensory experiences (number of APs elicited by 900 ms somatic current injections of the indicated amplitudes). The input–output properties were unchanged after sensory deprivation (control: n= 51, open symbols; deprived: n= 88, filled symbols; P > 0.05). E1, voltage response (top) to somatic hyperpolarizing current steps (bottom) for the different conditions. The membrane potential hyperpolarization exhibited a peak (a) follow by a time-dependent ‘sag’ until reaching steady-state (b). E2, graphs of the steady-state (b, left) and peak to steady-state difference (a–b, right) versus current step amplitude for the different conditions (control: n= 51, open symbols; deprived: n= 88, filled symbols; P > 0.05). Sensory deprivation did not change the I–V curves of the recorded neurons. Data are shown as means ±s.e.m.
Table 1.
Loss of sensory deprivation fails to change somatic AP properties
| Threshold (mV) | Amplitude (mV) | dV/dt (V s−1) | Half-Width (ms) | AHP (mV) | ADP (mV) | Rheobase (pA) | n | |
|---|---|---|---|---|---|---|---|---|
| Control | −59.7 ± 0.5 | 90.3 ± 0.8 | 616 ± 17.5 | 0.51 ± 0.02 | 4.7 ± 0.5 | 13.7 ± 1.0 | 266 ± 15 | 51 |
| Deprived | −59.2 ± 0.4 | 91.3 ± 0.6 | 636 ± 13.2 | 0.52 ± 0.01 | 4.4 ± 0.3 | 13.2 ± 0.5 | 270 ± 13 | 88 |
Data are shown as mean ±s.e.m.
In addition, we assessed the voltage response to hyperpolarizing current steps (Fig. 1E). Membrane hyperpolarization showed a peak (Fig. 1E1, a) followed by a time-dependent depolarization called ‘sag’ due to the activation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels that underlie the generation of the Ih current (Fig. 1E1, b). Current–voltage relationships for the peak and steady-state response at the soma were not significantly different following sensory deprivation (Fig. 1E2), indicating no obvious change in input resistance (RN) or HCN expression as seen at the soma (Table 2). Similarly, the resting membrane potential (Vrest) was unchanged by sensory deprivation (Table 2).
Table 2.
Impact of sensory deprivation on resting membrane properties
| Vrest (mV) | Rn (MΩ) | Distance from pia (μm) | n | |
|---|---|---|---|---|
| Control | −76.3 ± 0.5 | 22.8 ± 1.3 | 1095 ± 12 | 51 |
| Deprived | −75.6 ± 0.4 | 22.8 ± 0.9 | 1087 ± 10 | 88 |
Data are shown as means ±s.e.m.
Consequences of sensory deprivation on action potential backpropagation
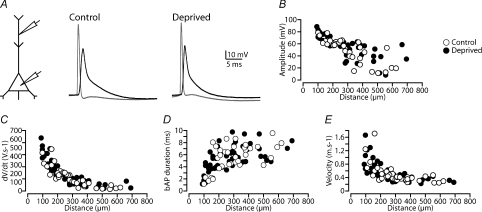
We next used dual somatic and dendritic whole-cell recording to assess the extent and properties of backpropagating APs (bAPs) in control neurons and after sensory deprivation (Fig. 2A). APs were evoked by somatic current injection and recorded simultaneously at the soma and the main apical dendrite (up to 700 μm from the soma) in control neurons (n= 36), and in neurons in barrel cortex that had undergone sensory deprivation (n= 53). The amplitude of bAPs decreased as they propagated along the apical dendrite, and in some cases failed to invade distal dendritic locations (Fig. 2B, see Larkum et al. 2001; Stuart & Hausser, 2001). There was no statistically significant impact of sensory deprivation on the amplitude of bAPs at distal dendritic locations (Fig. 2B; control: 25.5 ± 6.4 mV, 527 ± 28 μm from the soma, n= 7; deprived: 36.9 ± 6.0 mV, 529 ± 31 μm from the soma, n= 9; P= 0.22, one way ANOVA). Similarly, we observed no statistically significant impact of sensory deprivation on the rate-of-rise (Fig. 2C; control: 45.2 ± 9.6 V s−1; deprived: 64.9 ± 11.6 V s−1; P= 0.23), duration (Fig. 2D; control: 7.0 ± 0.6 ms; deprived: 6.8 ± 0.5 ms; P= 0.81), or propagation speed (Fig. 2E; control: 0.35 ± 0.03 m s−1; deprived: 0.33 ± 0.03 m s−1; P= 0.75) of bAPs in neurons from control and deprived cortex (control: 527 ± 28 μm from the soma, n= 7; and deprived: 529 ± 31 μm from the soma, n= 9). These data suggest that sensory deprivation did not influence the properties and/or expression of the dendritic voltage-activated sodium and potassium channels which regulate AP backpropagation into the apical dendrites of cortical layer 5 neurons (Stuart & Sakmann, 1994).
Figure 2. Sensory deprivation doesn't change action potential backpropagation.
A, left, schematic of the experimental configuration. Right, example of somatic (grey) and dendritic bAPs (black) recorded in control and deprived neurons. APs evoked by somatic current injection and bAPs recorded ∼380 μm from the soma. B–E, graphs showing the dependence of backpropagating AP amplitude (B), rate-of-rise (C), duration (D) and velocity (E) on dendritic location for the different experimental conditions (control: open symbols and deprived: filled symbols).
Impact of sensory deprivation on dendritic excitability
Dendritic excitability following sensory deprivation was assessed using trains of APs evoked by somatic current injections at different frequencies. Previous studies in cortical layer 5 pyramidal neurons indicate that trains of bAPs lead to generation of dendritic calcium spikes in a frequency-dependent manner (Larkum et al. 1999a; Williams & Stuart, 2000a). These dendritic calcium spikes are known to be involved in the generation of burst firing in cortical pyramidal neurons (Larkum et al. 1999b; Williams & Stuart, 1999, 2000a). The frequency of bAPs required to evoke a dendritic calcium spike is called the ‘critical frequency’ (Larkum et al. 1999a), and can be used as a measure of dendritic excitability.
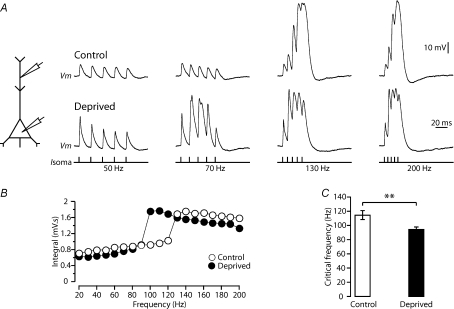
Trains of five APs were elicited by somatic current injection at frequencies ranging from 20 Hz to 200 Hz during dual whole-cell current-clamp recordings from the soma and apical dendrite of layer 5 pyramidal neurons (Fig. 3A). Neurons in control conditions had a critical frequency of 114.1 ± 6.3 Hz (n= 27 of 36), consistent with previous findings (Larkum et al. 1999a; Berger et al. 2003; Kole et al. 2007). Sensory deprivation led to a significant decrease in the critical frequency for generation of dendritic calcium spikes (Fig. 3B–C). On average, the critical frequency was 94.2 ± 3.2 Hz in layer 5 neurons from deprived barrel cortex (Fig. 3C, n= 43 of 53; P= 0.002, Student's t test). This decrease in critical frequency was not due to left–right brain asymmetry, as the critical frequency of layer 5 pyramidal neurons in the left hemisphere under control (non-deprived) conditions (104.0 ± 8.1 Hz; n= 5) was similar to that of layer 5 neurons located in the right (control) hemisphere (P= 0.55, one-way ANOVA).
Figure 3. Sensory deprivation increases dendritic excitability.
A, left, schematic of the experimental configuration. Right, dendritic response (control: 460 μm and deprived: 470 μm from the soma) to trains of five somatic APs at the indicated frequencies (bottom) in recordings from control (top) and deprived (middle) cortex. B, plot of the integral of dendritic membrane potential versus the frequency of AP trains for the experimental conditions. Note the non-linear increase in dendritic integral, indicative of the critical frequency, is lower in deprived neurons (closed symbols) compared to control (open symbols). C, bar graph of the average critical frequency in control (n= 27) and deprived animals (n= 43). Data shown as means ±s.e.m. **P < 0.01.
Impact of sensory deprivation on dendritic HCN channels
What underlies the observed decrease in critical frequency following sensory deprivation? One possibility is that sensory deprivation leads to a decrease in dendritic calcium electrogenesis via modulation of dendritic voltage-gated calcium channels. To investigate this possibility we determined the voltage integral underlying dendritic calcium spikes evoked by supra-critical somatic AP trains in control and deprived neurons. This voltage integral reflects total charge influx and therefore activation of dendritic voltage-gated calcium channels. Inconsistent with the idea that sensory deprivation leads to down-regulation of dendritic calcium channels, the voltage integral underlying dendritic calcium spikes during supra-threshold high-frequency AP trains (180 Hz) was similar in control and deprived neurons (control: 1.34 ± 0.06 mV s, n= 27; deprived: 1.28 ± 0.04 mV s, n= 43; P= 0.40, one way ANOVA).
Previous work indicates a critical role of dendritic HCN channel expression in regulating the critical frequency in cortical layer 5 pyramidal neurons (Berger et al. 2003). HCN channels in layer 5 pyramidal neurons are expressed at high densities at distal dendritic locations (Williams & Stuart, 2000b; Berger et al. 2001; Lorincz et al. 2002; Kole et al. 2006), where they lead to a distance-dependent depolarization of the local dendritic resting membrane potential (Stuart et al. 1997; Kole et al. 2006). Consistent with the idea that the density of dendritic HCN channels is modified following sensory deprivation, the dendritic resting membrane potential of layer 5 neurons from the deprived barrel cortex was on average 4.5 mV more hyperpolarized than that recorded at similar dendritic locations in control non-deprived cortex (deprived: −73.9 ± 0.8 mV, 497 ± 32 μm from the soma, n= 11; compared to control: −69.4 ± 1.0 mV, 448 ± 30 μm from the soma, n= 10; P= 0.002, Student's t test).
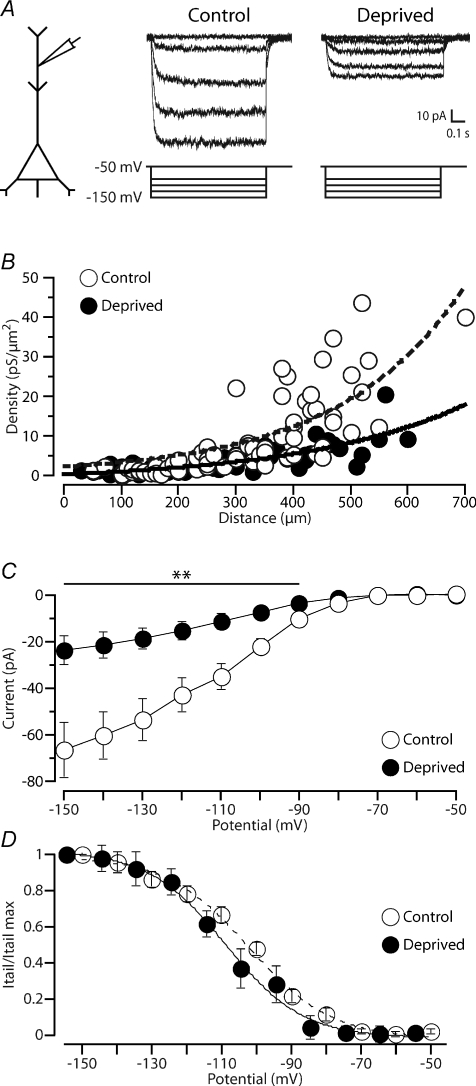
To directly test whether sensory deprivation leads to changes in dendritic HCN channels, we examined HCN currents at different dendritic locations using cell-attached recording (Fig. 4A). The density of HCN current increased exponentially along the main apical dendrite of layer 5 pyramidal neurons in recordings from non-deprived neurons (Fig. 4B, see Kole et al. 2006), with no obvious right–left brain asymmetry in control animals (see online Supplemental Material, Fig. S1; HCN current density right hemisphere: 18.5 ± 2.4 pS μm−2, 470 ± 15 μm from the soma, n= 22; HCN current density left hemisphere: 15.4 ± 1.7 pS μm−2, 515 ± 16 μm from the soma, n= 26; P= 0.46, Tukey's post hoc test). Sensory deprivation (n= 54) led to a distance-dependent decrease in HCN current at distal dendritic locations (Fig. 4A and B). Distal dendritic HCN channel density was significantly decreased in recordings from deprived neurons (HCN current density, left hemisphere: 7.8 ± 1.3 pS μm−2, 487 ± 16 μm from the soma, n= 14) compared to recordings from control (right hemisphere) neurons (P= 0.04, Tukey's post hoc test). On average, the current–voltage relationship for HCN channels at distal dendritic locations (control: 469 ± 23 μm, n= 13; deprived: 482 ± 16 μm, n= 10) was decreased to approximately a third of control levels following sensory deprivation over a broad voltage range (Fig. 4C; −90 to −150 mV; P < 0.01, Tukey's post hoc test). In contrast, the voltage dependence of HCN steady-state activation was unchanged following sensory deprivation (Fig. 4D). The HCN half-maximum voltage for activation (V1/2) was on average −101.5 ± 1.9 mV, with a slope factor (k) of 12.0 ± 1.1 mV, in recordings from neurons under control conditions (n= 13), which was not statistically different (one way ANOVA, P= 0.08 and P= 0.35, respectively) from recordings in neurons from barrel cortex that had undergone sensory deprivation (V1/2: −107.1 ± 2.1 mV; k: 10.1 ± 1.8 mV; n= 10). HCN channel kinetics were also unchanged by sensory deprivation. At distal dendritic locations (400–700 μm from the soma), the time course of Ih activation during voltage steps to −150 mV was best fitted with a double-exponential function. The activation time constants of Ih were 10.8 ± 1.0 ms (τfast) and 41.9 ± 5.8 ms (τslow) in recordings from control neurons (n= 13), with the amplitude of the fast component (τfast) dominating (∼90%). Sensory deprivation via whisker trimming did not significantly influence Ih activation kinetics (τfast= 12.7 ± 1.7 ms, τslow= 66.6 ± 14.9 ms; n= 10; P= 0.33 and P= 0.12, respectively, one way ANOVA), and did not significantly change the relative amplitude of the fast to slow time constants. Together, these data suggest that sensory deprivation leads to down-regulation of HCN channels at distal dendritic locations without significantly changing their properties.
Figure 4. Sensory deprivation leads to a decrease in HCN channel expression at distal dendritic locations.
A, left, schematic diagram of the experimental configuration. Right, examples of HCN current recorded in dendritic cell-attached patches from control (550 μm from the soma) and deprived (530 μm from the soma) neurons. B, plot of dendritic HCN channel density during voltage steps to an estimated membrane potential of −150 mV versus distance of the recording location from the soma in recordings from control (open symbols, n= 68) and deprived (filled symbols, n= 54) neurons. The curves represent exponential fits to the control (dotted curve, distance constant (λ): 219 μm) and deprived (continuous curve, λ: 209 μm) data sets, respectively. C, I–V curve of average peak HCN current at distal dendritic locations (∼470–480 μm from the soma) in recordings from neurons in control conditions (open symbols; n= 13) and following sensory deprivation (filled symbols; n= 10). D, HCN tail current normalized to maximum for control (open symbols, n= 13) and deprived (filled symbols, n= 10) neurons indicating no obvious impact of sensory deprivation on the voltage dependence of steady-state activation of HCN channels. Holding and test potentials were corrected for the measured −4.5 mV shift in resting membrane potential in deprived neurons (see Results). Data shown as means ±s.e.m. **P < 0.01.
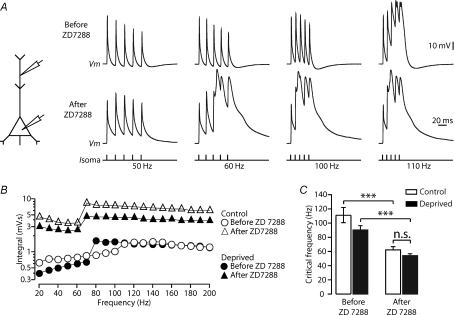
To investigate the potential role of the reduced expression of HCN channels (Fig. 4) on the observed increase in dendritic excitability following sensory deprivation (Fig. 3), we investigated the impact of the HCN channel blocker ZD 7288 on the critical frequency in control and deprived neurons. In these experiments the critical frequency in control neurons was 111.1 ± 10.7 Hz (n= 9) and 90.0 ± 6.1 Hz (n= 10) after sensory deprivation (Fig. 5C, left). Bath application of ZD 7288 (50 μm) caused a hyperpolarisation of the resting membrane potential, which reached steady state within 5–10 min, and led to a significant decrease in the critical frequency (Fig. 5A and B) in both control and deprived animals to ∼60 Hz (Fig. 5C; control: P= 0.0005, deprived: P= 0.00005, Student's t test). No difference in the critical frequency was observed between control and deprived neurons following block of HCN channels (Fig. 5C, right; P= 0.13, Student's t test). This observation suggests that reduction of dendritic HCN channels may explain the observed increase in dendritic calcium electrogenesis following sensory deprivation.
Figure 5. Decreased dendritic Ih contributes to increased dendritic excitability following sensory deprivation.
A, left, schematic diagram of the experimental configuration. Right, dendritic response to trains of five somatic APs at the indicated frequencies (bottom) in a recording from a control neuron before (top) and after (middle) bath application of ZD 7288 (50 μm). B, plot of the dendritic voltage integral versus AP frequency for control (open symbols) and deprived (filled symbols) neurons before (circles) and after (triangles) bath application of ZD 7288 (50 μm). C, block of HCN channels by bath application of ZD 7288 (50 μm) reduced the critical frequency of both control (n= 9) and deprived (n= 10) neurons to a similar extent. Data shown as means ±s.e.m. ***P < 0.001; n.s., non-significant.
Discussion
Using whisker trimming, we provide evidence that sensory deprivation increases the excitability of cortical layer 5 pyramidal neurons. We show that sensory deprivation promotes dendritic calcium electrogenesis and increases burst firing. Furthermore, we find that increased dendritic excitability following sensory deprivation is associated with down-regulation of dendritic HCN channels. This change in excitability is confined to the distal dendrites of layer 5 pyramidal neurons, as we did not observe an impact of sensory deprivation on resting membrane properties at the soma. Furthermore, sensory deprivation did not alter somatic AP properties or AP backpropagation into the apical dendrite.
Cellular mechanisms underlying changes in dendritic excitability
Sensory deprivation did not change the amplitude or duration of single backpropagating APs. These data argue against a specific effect of sensory deprivation on dendritic sodium or potassium channels. In addition, the finding that the integral under dendritic calcium spikes at supra-critical frequencies was similar in control and deprived neurons suggests that dendritic voltage-gated calcium channels were also not affected by sensory deprivation. In contrast, sensory deprivation caused a clear down-regulation of HCN channels (Fig. 4), which are known to play an important role in regulating dendritic membrane potential and excitability (Berger et al. 2003). These data suggest that down-regulation of HCN channels is likely to play a key role in the observed increase in dendritic excitability following sensory deprivation. Consistent with this idea, sensory deprivation was associated with hyperpolarisation of the dendritic membrane potential, and block of HCN channels by ZD 7288 decreased the critical frequency for generation of dendritic calcium spikes in control and deprived neurons to a similar value (see Fig. 5). This action of ZD 7288 was immediate (within 5–10 min after bath application), and therefore is presumably via a direct action on HCN channels rather than via a non-specific action, as described previously by Chevaleyre & Castillo (2002), which develops over the course of 30 min to 1 h.
Sensory deprivation leads to hyperpolarization of the dendritic resting membrane potential. This dendritic hyperpolarization is unlikely to be involved in the observed increase in dendritic excitability, as previous work shows that hyperpolarization of the dendritic membrane potential leads to an increase, rather than a decrease, in critical frequency (Berger et al. 2003). These data indicate that the decrease in critical frequency following sensory deprivation is not due to dendritic hyperpolarisation per se, but rather is due to removal of HCN channels, whose unusual voltage-dependent properties act to dampen dendritic excitability by limiting membrane deviations from the resting membrane potential (Magee, 1999).
While these data are suggestive of an important role of down-regulation of HCN channels in the increase in dendritic excitability observed following sensory deprivation, they do not rule out a contribution of other mechanisms. These could include modulation of other dendritic channels, such as leak channels, calcium-activated K+ or Cl− channels or even ion exchangers such as the Na+/K+-ATPase pump. Future experiments will be required to determine whether other dendritic properties, in addition to HCN channels, are modified by loss of sensory input.
Modulation of dendritic HCN channel expression by activity
In cortical layer 5 pyramidal neurons HCN channels are non-uniformly distributed along the apical dendrite, and are at high densities at distal dendritic locations (Stuart & Spruston, 1998; Williams & Stuart, 2000b; Berger et al. 2001; Lorincz et al. 2002; Kole et al. 2006). They play an important role in setting the resting membrane potential and in regulating excitatory postsynaptic potential summation, and can act to isolate the distal dendritic tuft from the soma (Stuart & Spruston, 1998; Williams & Stuart, 2000b; Berger et al. 2001, 2003). Consistent with this, deletion of genes coding for HCN1 and HCN2 subunits is known to change neuronal excitability and neuronal network behaviour in CA1 pyramidal and thalamocortical neurons (Ludwig et al. 2003; Nolan et al. 2004; Tsay et al. 2007). Ih current in cortical pyramidal neurons is thought to be mediated predominately by the HCN1 and HCN2 subunits (Santoro et al. 2000; Altomare et al. 2003). Sensory deprivation led to a decrease in the density of Ih at distal dendritic locations without an obvious change in activation kinetics or voltage dependence. This suggests the down-regulation of both HCN1 and HCN2 subunits, as occurs in entorhinal cortex and hippocampus following status epilepticus induced by kainate or pilocarpine (Shah et al. 2004; Jung et al. 2007). However, in a genetic absence epilepsy model, down-regulation of Ih in cortical pyramidal neurons is thought to primarily reflect the reduced expression of the HCN1 subunit (Strauss et al. 2004; Kole et al. 2007).
HCN channel density can be modulated in an activity-dependant manner. In epileptic rat models, it has been reported that HCN channels are down-regulated (Shah et al. 2004; Kole et al. 2007), leading to an increase in neuronal excitability. Other studies have shown that depending on the nature of the activity, both up- and down-regulation of HCN channels can be observed (Fan et al. 2005; Brager & Johnston, 2007; Campanac et al. 2008). Given that previous work indicates that long-lasting changes in synaptic activity modulate voltage-gated conductances in a homeostatic manner (Desai et al. 1999; Golowasch et al. 1999; Baines et al. 2001; Aizenman et al. 2003), the observed down-regulation of HCN channels following loss of sensory input may represent a homeostatic change, leading to increased neuronal excitability in the face of reduced synaptic drive. Such a change would act to return the level of network activity in the deprived cortical area to normal. This potential homeostatic change in Ih would be analogous to the observed up-regulation of HCN channels that occurs following an increase in network activity above normal levels (van Welie et al. 2004; Fan et al. 2005).
Sensory deprivation increases the excitability of the mature cortical network
Sensory manipulation affects many aspects of neuronal maturation, such as integrative properties, connectivity and dendritic morphology (see Introduction). In the only other study to observe changes in intrinsic properties of cortical pyramidal neurons following sensory deprivation via whisker trimming, Maravall et al. (2004) described that maturation of spiking behaviour in layer 2/3 pyramidal neurons could be delayed by sensory deprivation, but only if this was performed during the critical period (at 2 weeks of age). This delay in maturation of spiking behaviour in layer 2/3 pyramidal neurons occurred in the absence of changes in somatic active and resting membrane properties. While we also did not observe changes in somatic membrane properties of layer 5 neurons (Fig. 1), following sensory deprivation the percentage of layer 5 pyramidal neurons showing burst firing was increased. This increase in burst firing was associated with an increase in dendritic excitability and was independent of the timing of sensory deprivation (see Methods). This indicates that sensory deprivation can lead to changes in dendritic excitability even in adult rats, consistent with earlier work showing that modified sensory input leads to changes in receptive field properties in the adult animal (Diamond et al. 1993).
Sensory deprivation has functional consequences on somato-dendritic excitability
APs in cortical layer 5 neurons attenuate and broaden as they backpropagate along the main apical dendrite in vitro and in vivo (Stuart et al. 1997; Helmchen et al. 1999). These backpropagating APs are known to activate dendritic calcium channels, triggering dendritic calcium electrogenesis that can feed back to the soma providing a mechanism generating AP burst firing in these neurons (Stuart et al. 1997; Larkum et al. 1999a; Williams & Stuart, 1999). The decrease in dendritic Ih after sensory deprivation reduces the critical frequency for dendritic calcium spikes during AP trains by ∼20 Hz, providing a broader frequency range for eliciting dendritic calcium spikes during axo-somatic AP firing. Indeed, we observed a significant increase in bursting firing in layer 5 pyramidal neurons following sensory deprivation (Fig. 1). Previous work by Kole et al. (2007) in an animal model of absence epilepsy also described an increase in somato-dendritic excitability in layer 5 pyramidal neurons that was associated with a reduction in Ih, and increased burst firing in both layer 5 and layer 2/3 pyramidal neurons in neocortex (Strauss et al. 2004; Kole et al. 2007).
In conclusion, the loss of dendritic HCN channels and associated increase in dendritic excitability and burst firing that occurs following whisker trimming could represent a homeostatic mechanism to increase activity in barrel cortex in response to reduced sensory input. The cellular mechanisms involved in these effects are independent of the timing of sensory deprivation and are likely to involve down-regulation of dendritic HCN channels.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia. We thank Mic Cavazzini and Deborah Heydet for their helpful comments on previous versions of this manuscript.
Glossary
Abbreviations
- ADP,
after depolarization
- AHP,
after hyperpolarization
- AP,
action potential
- bAP,
backpropagated action potential
- DIC,
differential interference contrast
- HCN channel,
hyperpolarization-activated cyclic nucleotide-gated channel
Author contributions
J.D.B. and G.J.S. designed the experiments. J.D.B. carried out and analysed the experiments. J.D.B. and G.J.S. interpreted the data and wrote the manuscript.
Supplemental material
Supplemental Figure S1: Absence of right-left hemisphere asymmetry in HCN channel density in control conditions.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. doi: 10.1016/s0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Altomare C, Terragni B, Brioschi C, Milanesi R, Pagliuca C, Viscomi C, Moroni A, Baruscotti M, DiFrancesco D. Heteromeric HCN1–HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol. 2003;549:347–359. doi: 10.1113/jphysiol.2002.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, Callahan CA, Friedman MA. Thalamo-cortical processing of vibrissal information in the rat. I. Intracortical origins of surround but not centre-receptive fields of layer IV neurones in the rat S1 barrel field cortex. J Comp Neurol. 1991;303:193–210. doi: 10.1002/cne.903030203. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 ‘barrel’ cortex. J Comp Neurol. 1987;263:265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Luscher HR. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–868. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- Berger T, Senn W, Luscher HR. Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J Neurophysiol. 2003;90:2428–2437. doi: 10.1152/jn.00377.2003. [DOI] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Campanac E, Daoudal G, Ankri N, Debanne D. Downregulation of dendritic Ih in CA1 pyramidal neurons after LTP. J Neurosci. 2008;28:8635–8643. doi: 10.1523/JNEUROSCI.1411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Assessing the role of Ih channels in synaptic transmission and mossy fibre LTP. Proc Natl Acad Sci U S A. 2002;99:9538–9543. doi: 10.1073/pnas.142213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci U S A. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, Alkon DL. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci U S A. 1986;83:2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fibre boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, Isaac JT. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron. 1998;21:347–357. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. The cortical component of experience-dependent synaptic plasticity in the rat barrel cortex. J Neurosci. 1994;14:7665–7679. doi: 10.1523/JNEUROSCI.14-12-07665.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Golowasch J, Abbott LF, Marder E. Activity-dependent regulation of potassium currents in an identified neuron of the stomatogastric ganglion of the crab Cancer borealis. J Neurosci. 1999;19:RC33. doi: 10.1523/JNEUROSCI.19-20-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Svoboda K, Denk W, Tank DW. In vivo dendritic calcium dynamics in deep-layer cortical pyramidal neurons. Nat Neurosci. 1999;2:989–996. doi: 10.1038/14788. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D’Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kole MH, Brauer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol. 2007;578:507–525. doi: 10.1113/jphysiol.2006.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Hallermann S, Stuart GJ. Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci. 2006;26:1677–1687. doi: 10.1523/JNEUROSCI.3664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Kaiser KM, Sakmann B. Calcium electrogenesis in distal apical dendrites of layer 5 pyramidal cells at a critical frequency of back-propagating action potentials. Proc Natl Acad Sci U S A. 1999a;96:14600–14604. doi: 10.1073/pnas.96.25.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999b;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons. J Physiol. 2001;533:447–466. doi: 10.1111/j.1469-7793.2001.0447a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/12229. [DOI] [PubMed] [Google Scholar]
- Maravall M, Stern EA, Svoboda K. Development of intrinsic properties and excitability of layer 2/3 pyramidal neurons during a critical period for sensory maps in rat barrel cortex. J Neurophysiol. 2004;92:144–156. doi: 10.1152/jn.00598.2003. [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol. 1998;80:2882–2892. doi: 10.1152/jn.1998.80.6.2882. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40:609–620. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioural role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andres JV, Alkon DL. Voltage-clamp analysis of the effects of classical conditioning on the hippocampus. J Neurophysiol. 1991;65:796–807. doi: 10.1152/jn.1991.65.4.796. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, O’Leary DD. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994;346:80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron. 2004;44:495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987;326:694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol. 1997;505:617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci. 1998;18:3501–3510. doi: 10.1523/JNEUROSCI.18-10-03501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Hausser M. Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci. 2001;4:63–71. doi: 10.1038/82910. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron. 2007;56:1076–1089. doi: 10.1016/j.neuron.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- van Welie I, van Hooft JA, Wadman WJ. Homeostatic scaling of neuronal excitability by synaptic modulation of somatic hyperpolarization-activated Ih channels. Proc Natl Acad Sci U S A. 2004;101:5123–5128. doi: 10.1073/pnas.0307711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J Physiol. 1999;521:467–482. doi: 10.1111/j.1469-7793.1999.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Backpropagation of physiological spike trains in neocortical pyramidal neurons: implications for temporal coding in dendrites. J Neurosci. 2000a;20:8238–8246. doi: 10.1523/JNEUROSCI.20-22-08238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic Ih in neocortical pyramidal neurons. J Neurophysiol. 2000b;83:3177–3182. doi: 10.1152/jn.2000.83.5.3177. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van Der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Wann JR. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. J Comp Neurol. 1976;170:53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Connors BW. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol. 1999;81:1171–1183. doi: 10.1152/jn.1999.81.3.1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.