Abstract
Cholinergic innervation of the prefrontal cortex is critically involved in arousal, learning and memory. Dysfunction of muscarinic acetylcholine receptors and their downstream signalling pathways has been identified in mental retardation. To assess the role played by the muscarinic receptors at the hippocampal–frontal cortex synapses, an important relay in information storage, we used a newly developed frontal slice preparation in which hippocampal afferent fibres are preserved. Transient activation of muscarinic receptors by carbachol results in a long-lasting depression of synaptic efficacy at the hippocampal but not cortical pathways or local circuitry. On the basis of a combination of electrophysiological, pharmacological and anatomical results, this input-specific muscarinic modulation can be partially attributed to the M2 subtype of muscarinic receptors, possibly through a combination of pre- and postsynaptic mechanisms.
Introduction
The prefrontal cortex (PFC) receives and integrates information from multiple brain regions and its function is the target of many neuromodulators including acetylcholine. For example, cholinergic input to the PFC regulates attention (Robbins et al. 1989; Muir et al. 1992; Hasselmo, 1995), memory (Beninger et al. 1992; DeSousa et al. 1994), and anxiety induction processes (Berntson et al. 1998). Dysfunction of muscarinic acetylcholine receptors (mAChRs) and mAChR-mediated signalling pathways has been linked to Fragile X mental retardation (Volk et al. 2007), Alzheimer's disease (Fisher, 2008), and normal ageing processes (Joseph et al. 1993).
Cholinergic afferents to the prelimbic and infralimbic cortex, the major components of the medial prefrontal cortex (mPFC), rise primarily from the basal forebrain nucleus basalis (Gaykema et al. 1991). Among five mAChR subtypes, M1 and M2 receptors are the predominant form expressed in the mPFC. M1 receptors are mainly localized in postsynaptic compartments of glutamatergic synapses, whereas M2 receptors are found both pre- and postsynaptically (Volpicelli & Levey, 2004). Activation of mAChRs results in an array of effects involving different cellular mechanisms in various brain regions. These effects can be excitatory or inhibitory, mediated by M1, M2 and M4 receptors. For instance, M1 mAChR activation increases temporal summation of synaptic events in the prefrontal cortex by down-regulating Kir2 channels (Carr & Surmeier, 2007). However, in hippocampus (Scheiderer et al. 2006; Volk et al. 2007) and cortex (Kirkwood et al. 1999; Massey et al. 2001; McCoy & McMahon, 2007), the non-selective muscarinic agonist carbachol (CCh) induces long-term depression of synaptic transmission (CCh-LTD). This form of plasticity is mediated by M1 receptors and dependent on extracellular signal-regulated kinase (ERK) signalling pathways and protein synthesis mechanisms (McCoy & McMahon, 2007; Volk et al. 2007; McCoy et al. 2008; Scheiderer et al. 2008).
Among a number of glutamatergic afferents projecting to the mPFC, the input from the hippocampus is especially important. Previous work has shown that the CA1 and subiculum of ventral hippocampus send ipsilateral, unidirectional projections that terminate on neurons in the mPFC (Jay & Witter, 1991; Jay et al. 1992). The functional integrity of the hippocampal–mPFC network, and the flow of information between these two brain regions, are critical to the proper functioning of the mPFC in memory formation (O’Donnell & Grace, 1995; Seamans et al. 1995; O’Donnell et al. 2002; Goto & O’Donnell, 2003).
Employing a newly developed frontal slice preparation on which hippocampal afferent fibres are preserved and can be selectively activated (Parent et al. 2009), we are equipped to test whether the synaptic properties of this pathway allow for input-specific regulation by neuronal activities and modulators. The goal of this study is to investigate the effects and temporal profile of the activation of mAChR on hippocampal–mPFC synapses, with an emphasis on agonist-induced synaptic long-term depression.
Methods
Preparation of frontal slices containing prelimbic cortex
The University of Minnesota Institutional Animal Care and Use Committee approved the use of animals for the studies described below. Experiments included in these studies comply with all polices and regulations, as detailed by Drummond (2009).
Coronal slices containing medial prefrontal cortex were prepared from 8- to 15-week-old mice following standard procedures (Parent et al. 2009). Briefly, animals were anaesthetized by a lethal dose mixture of ketamine and xylazine and perfused through the heart with ice-cold cutting solution containing (in mm): 240 sucrose, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, and 7 MgCl2. Prior to use, the cutting solution was saturated with 95% O2–5% CO2 and frozen. Both hemispheres were quickly removed and coronally sliced at 300–350 μm thickness using a HM 650V microtome (Microm International GmbH). After incubation in a holding chamber containing normal aCSF for at least 30 min at room temperature, slices were transferred into the recording chamber. The bath solution (aCSF) contained (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2.0 CaCl2, 1.0 MgCl2, and 15 dextrose. All recordings were conducted at 31–33°C with a perfusion speed of 1.5–2 ml min−1.
A Zeiss Axioskop 2 FS, fitted with ×40 water-immersion objective and differential interference contrast (DIC), was used to view slices. Light in the near infrared range (740 nm), in conjunction with a contrast-enhancing camera, was used to visualize individual neurons.
Electrophysiological recordings and synaptic stimulations
A Dagan 700A and an Axopatch 200B amplifier were used for whole-cell current- and voltage-clamp recordings, respectively. All recordings were made from neurons located in the prelimbic region of PFC, with recording pipettes (4–8 MΩ) containing (in mm): 120 potassium gluconate, 20 KCl, 10 Hepes, 0.2 EGTA, 2 Mg2Cl, 4 Na2ATP, 0.3 Tris-GTP, and 14 phosphocreatine (pH 7.25 with KOH). The hippocampal axonal bundle was stimulated electrically with a glass microelectrode (1–3 MΩ) filled with aCSF and controlled by a fine micromanipulator at a resolution of 1 μm. The distance between the recording and stimulus electrode was between 550 and 700 μm (Parent et al. 2009). Pulse generation and data acquisition were controlled with custom software written in the IGOR Pro environment. Test stimuli were delivered every 30 s unless otherwise stated. A hyperpolarizing current pulse was injected into the cell after the test stimulus to monitor the input resistance and series resistance throughout recording. Slope measurements of EPSPs were made from a line fitted to the rising phase of the EPSP. To measure paired pulse facilitation (PPF), two successive synaptic stimuli were delivered and EPSCs recorded. The PPF ratio was calculated as EPSC2/EPSC1 in amplitude.
Drugs
BAPTA tetrapotassium (Molecular Probes) was dissolved directly into the pipette solution. All other drugs were obtained from Tocris Bioscience. Carbachol, pirenzepine (M1 blocker), and CGP52432 were dissolved in distilled water, AF-DX 116 (M2 blocker), picrotoxin, nifedipine in DMSO, and dl-aminophosphonovalerate (APV) in NaOH. These drugs were diluted freshly from frozen stock aliquots. Because of the light-sensitivity of nifedipine, care was taken to minimize light exposure during these experiments.
Statistics
Data were expressed as mean ±s.e. Student's t test or ANOVA test was applied.
Results
Activation of mAChR induced long-term depression of synaptic efficacy in a pathway-specific manner
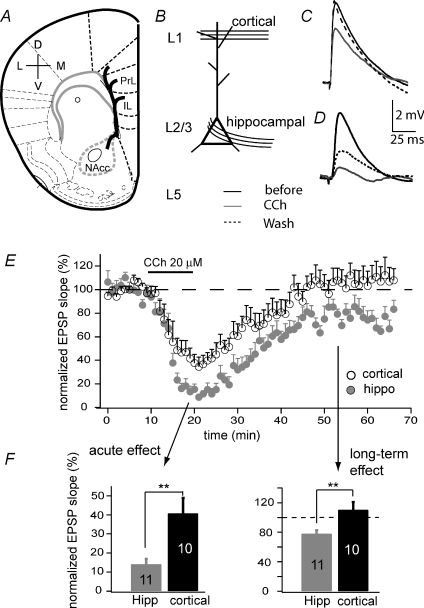
In a modified coronal slice preparation developed recently (Parent et al. 2009), we were able to identify hippocampal afferent fibres before they project into the prelimbic region of the mPFC (Fig. 1A and B). In response to electrical stimulation of these visually identified fibres, pyramidal neurons in layers 2/3 exhibited consistent, monosynaptic excitatory postsynaptic potentials (EPSPs) in whole-cell current clamp mode (Fig. 1D), when the resting membrane potential was constantly held at −70 mV. Bath application of the subtype-non-selective muscarinic agonist carbachol (CCh, 20 μm) for 10 min suppressed the EPSP slope to 17.2 ± 3.3% of control (n= 11). Following washout of the agonist, EPSPs recovered partially; 40–45 min after removing CCh, late phase synaptic responses were 76.6 ± 5.7% of the original (Fig. 1D and E). Since layer 5 pyramidal neurons also receive hippocampal inputs, we tested how the hippocampal–layer 5 pathway responded to CCh. Similar to layer 2/3 terminating fibres, hippocampal fibres terminating on pyramidal neurons in layer 5 exhibited acute reduction (to 10.1 ± 3.4%) in response to CCh and long-lasting depression (to 75.9 ± 5.7%, n= 4) when CCh was washed out. We focused on layer 2/3 neurons for the rest of the studies.
Figure 1. Acute and long-term suppression of EPSPs in the mPFC.
A, schematic diagram showing a coronal slice preparation on which hippocampal projections to mPFC were preserved. The bold curvy line indicates the projection trajectory. PrL: prelimbic; IL: infralimbic region. B, recording configuration highlighting layer 2/3 (L2/3) pyramidal neurons that receive both cortical inputs at layer 1 (L1) and hippocampal afferents. Selective stimulation of these pathways resulted in input-specific EPSPs (C and D). C, representative cortical EPSPs before, during and 30 min after application of 20 μm carbachol (CCh). D, representative hippocampal EPSPs before, during and 30 min after application of 20 μm CCh. E, comparison of the time course of CCh-induced EPSP suppression at the cortical and hippocampal pathways. F, EPSP suppression at different phases (**P < 0.01).
This ∼25% long-term depression of the hippocampal synapses in mPFC, induced by a brief CCh exposure, showed a similarity in kinetics and amplitude to CCh-LTD as observed in other cortical regions (McCoy & McMahon, 2007). In addition to synaptic changes, CCh application also depolarized membrane potential by 5–10 mV and increased firing frequency in response to a current injection of 700 ms (data not shown), consistent with previous reports of the muscarinic modulation of membrane excitability (Carr & Surmeier, 2007). Unlike the long-term suppression of synaptic responses, effects on intrinsic membrane properties were reversible.
In addition to pyramidal neurons, muscarinic receptors are expressed in interneurons (Hajos et al. 1998). Although the waveform of synaptic responses recorded in the absence of GABA receptor blockers did not show obvious contributions from inhibitory postsynaptic responses (IPSPs), we cannot rule out the possibility that the long-lasting suppression of the synaptic events is due to the indirect effects of CCh on GABAergic transmission. To test this possibility, we next performed a similar experiment in the presence of GABAA and GABAB receptor blockers picrotoxin (20–50 μm) and CGP52432 (3 μm). Under this condition, transient exposure to CCh still suppressed EPSPs to 69.0 ± 6.0% of baseline responses (n= 5, P < 0.05). The kinetics and degree of CCh-LTD showed no significant difference from previous observations when no blockers were included (P= 0.4). These results suggest that inhibitory activity does not contribute directly to the formation of CCh-LTD at hippocampal–mPFC glutamatergic synapses.
In this slice preparation, we were also able to selectively activate fibres from other cortical regions by positioning the stimulus electrode in mPFC layer 1 (Fig. 1B). Laminar stimulation in other layers within the mPFC may activate a mix of local network and extrinsic inputs. We first examined separately, and then combined, the muscarinic modulation data for these two conditions because results were similar. Specifically, CCh application elicited a quick reduction of EPSPs (to 37.4 ± 7.0%, n= 10; Fig. 1C and E). However, unlike the long-lasting depression induced at the hippocampal pathway, EPSPs evoked by laminar stimulation recovered completely within 30 min of CCh washout (Fig. 1E and F).
Taken together, the hippocampal–mPFC synapses exhibit input-specific LTD that is induced by activation of muscarinic receptors but not dependent on inhibitory activity.
Pathway-specific depression was accompanied by presynaptic changes
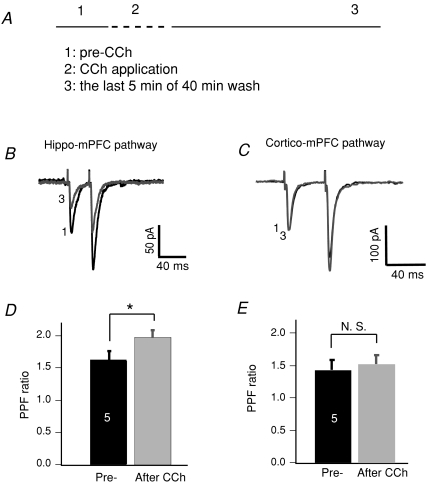
Hippocampal inputs projecting to either layer 2/3 or layer 5 of the mPFC exhibited CCh-induced LTD. One common feature shared by these two pathways is their presynaptic origin. To address whether any presynaptic mechanisms were involved in the synaptic suppression, we recorded synaptic currents, in voltage clamp mode, in response to two consecutive stimulations with an interval of 40 ms (Fig. 2B and C). During the brief 10 min exposure to CCh, EPSCs were heavily suppressed, making it unreliable to measure the paired pulse facilitation (PPF) ratio. We thus evaluated the PPF ratio prior to CCh application and 40 min after CCh removal (Fig. 2A).
Figure 2. Changes in PPF ratio associated with the hippocampal pathway.
A, experimental procedure showing three phases: pre-CCh, CCh application and CCh washout. B, averaged EPSC traces in response to two consecutive stimulations of the hippocampal–mPFC pathway, taken from phases 1 and 3, were superimposed. Note that the first EPSC remained suppressed after CCh was washed out. C, traces of EPSCs in response to stimulation of cortical pathways. Note that the first EPSC was completely recovered from CCh-induced acute depression. D, comparison of the PPF ratio of hippocampal synapses before CCh application and after CCh washout (**P < 0.01). The PPF ratio was calculated as EPSC2/EPSC1. E, comparison of the PPF ratio of cortical/local circuitry synapses before CCh application and after CCh washout (N. S.: non-significant).
EPSCs from the hippocampal pathway generally exhibited a PPF ratio of 1.6 ± 0.1 (n= 5). EPSCs recovered but not completely 30–45 min after CCh was washed out, consistent with what we have observed in EPSP recordings. Furthermore, the decreased EPSCs were accompanied by an increased PPF ratio (2.0 ± 0.1, P < 0.05; Fig. 2D). EPSCs recorded from activation of non-hippocampal pathways, i.e. laminar stimulation, did not show changes in the PPF ratio (n= 6; Fig. 2E). An increased PPF associated with the sustained phase at the hippocampal–mPFC pathway suggests that presynaptic mechanisms may contribute to the CCh-LTD.
The role of M2 AChR in synaptic depression of hippocampal–mPFC pathway
Previous work has demonstrated that M1 AChR mediates CCh-induced LTD in the hippocampus (Scheiderer et al. 2006) and cortex (Kirkwood et al. 1999; Massey et al. 2001), possibly through postsynaptic mechanisms. With the data presented above suggesting the involvement of presynaptic mechanisms, we wondered which subtype(s) of muscarinic receptors contribute to CCh-induced LTD in the hippocampal–mPFC pathway.
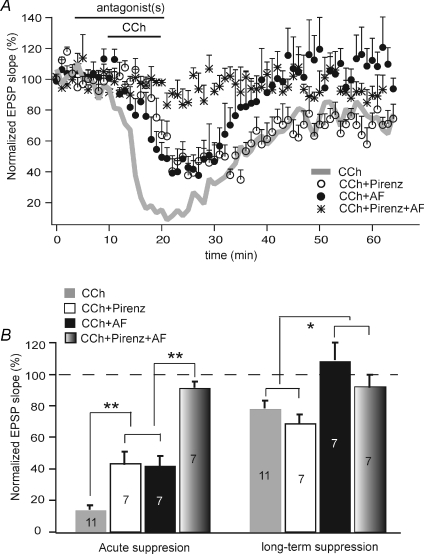
To this end, we combined CCh with receptor subtype-selective antagonists to dissect mechanisms underlying acute vs. long-lasting depression induced by CCh (Fig. 3). In the presence of M1-selective blocker pirenzepine (2 μm), the CCh-induced acute reduction in EPSP slope was attenuated from the CCh-only condition but still significant (to 48.4 ± 6.8% of baseline transmission, P < 0.05, n= 7). However, pirenzepine did not appear to block or attenuate CCh-LTD. Under this condition, synaptic responses in the late phase remained suppressed to 69.0 ± 5.4% of baseline (n= 7, P < 0.05) and the degree of suppression was comparable to that in the absence of pirenzepine (P= 0.36). On the contrary, the M2-selective blocker AF-DX 116 (2 μm) completely blocked CCh-induced LTD. Specifically, in the presence of AF-DX 116, CCh acutely reduced the EPSP slope to 44.7 ± 5.6% of baseline response but EPSPs were completely recovered to 110.6 ± 11.7% after CCh was removed (n= 7). M1 and M2 blockers together completely eliminated both the acute and the long-term effects of CCh on EPSPs (Fig. 3).
Figure 3. Involvement of different subtypes of mAChR in various phases of CCh-induced synaptic suppression of the hippocampal pathway.
A, time course of changes in EPSP slope of the hippocampal synapses under various conditions: CCh alone, CCh + M1 antagonist pirenzepine (2 μm), CCh + M2 antagonist AF-DX 116 (2 μm), and CCh + M1 + M2 antagonists. B, summarized EPSP reduction in both acute and sustained phases (*P < 0.05; **P < 0.01).
It has been suggested that pirenzepine at 2 μm may block M4 receptors in addition to M1 (Dorje et al. 1991; Marino et al. 1998). We thus tested whether a lower concentration would show different effects on the acute depression induced by CCh. Co-application of CCh and 75 nm pirenzepine reduced the EPSP slope to 24.5 ± 3.8% of baseline within 15 min (n= 3). This degree of acute synaptic suppression was indistinguishable from that induced by CCh alone (P= 0.37), but significantly larger than that caused by the combination of CCh and 2 μm pirenzepine (P < 0.05). The difference in attenuating acute suppression between the two concentrations may indicate the possible involvement of M4.
Taken together, these data suggest that multiple muscarinic receptors may contribute to the acute suppression of synaptic transmission; however, M2 receptors appear solely responsible for CCh-induced long-term depression.
Synaptic activity is required for CCh-LTD during mAChR activation
Is activation of M2 the only mechanism that is required to establish CCh-induced LTD? One intriguing phenomenon attracted our attention while pursuing the mechanisms of CCh-mediated LTD. We noticed that certain levels of synaptic activity during CCh application at the hippocampal–mPFC pathway were required to induce LTD.
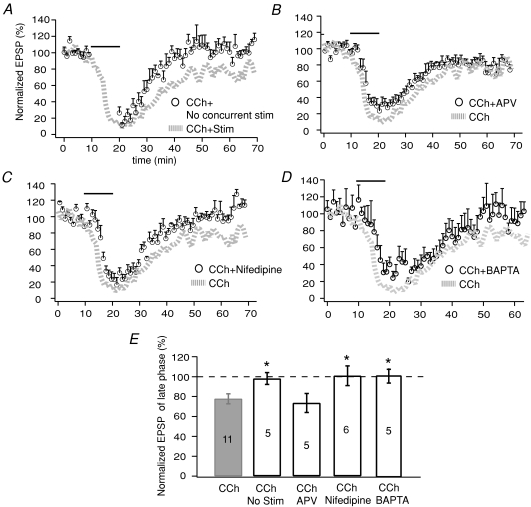
As stated in Methods, we normally delivered electrical stimulation to the hippocampal afferent fibres every 30 s (i.e. 0.033 Hz) to obtain EPSPs throughout the course of an experiment. To assess the role of activity in CCh-LTD, we next halted electrical stimulation only during the 10 min of CCh application. We recorded EPSPs immediately before and after application; the degree of acute reduction in EPSP slope, in the absence of concurrent electrical stimulation, was similar to that of the normal condition. Contrarily, LTD was completely prevented by the lack of concurrent stimulation. The EPSP slope after CCh washout was not different from the pre-drug level (98.6 ± 5.5, n= 5, P > 0.05; Fig. 4A).
Figure 4. Involvement of synaptic activation in CCh-LTD.
A, comparison of CCh effects between two conditions: with and without concurrent synaptic stimulation during 10 min CCh application. B, time course of EPSP suppression when APV (50 μm) was co-applied with CCh. C, time course of EPSP suppression when nifedipine (10 μm) was co-applied with CCh. D, time course of EPSP suppression by CCh when BAPTA (5 μm) was included in recording pipettes throughout the experiment. E, summarized data showing the effects of concurrent stimulation, APV, nifedipine, or intracellular BAPTA on CCh-induced LTD. Statistical comparisons were made between each experimental condition and CCh alone (*P < 0.05).
How does concurrent stimulation contribute to CCh-LTD? Electrical stimulation during CCh application evokes glutamate release that then activates NMDA and AMPA receptors. Furthermore, AMPARs mediate EPSPs in dendrites that activate L-type Ca2+ channels, the low-threshold VGCCs (Magee & Johnston, 1995). Thus, we questioned whether blockade of NMDA receptors (NMDARs) or L-type Ca2+ channels would prevent CCh-induced LTD despite the presence of concurrent stimulation.
In the presence of the NMDAR blocker APV (50 μm), EPSP was still suppressed by CCh to 33.6 ± 5.5% and remained at 73.4 ± 9.6% (n= 5) after CCh was washed out, indicating little involvement of NMDAR (Fig. 4B). The L-type Ca2+ channel blocker nifedipine at 10 μm did not alter the degree of acute synaptic depression by CCh (38.6 ± 5.9%). However, the long-term suppression after CCh washout was completely blocked, i.e. EPSP was recovered to 101.0 ± 9.8% of control level (n= 6) in the presence of nifedipine (Fig. 4C). These results suggest that Ca2+ influx through L-type Ca2+ channels, but not NMDARs, is required to establish the long-term suppressive effects of CCh.
Since the neuronal responses suppressed by CCh were mainly subthreshold individual EPSPs, with no involvement of action potentials throughout the recording, we hypothesized that the amount of Ca2+ influx through L-type channels may be moderate, thus a moderate concentration of BAPTA may be able to chelate intracellular Ca2+ changes. We included 5 mm BAPTA in the pipette solution and then induced CCh-LTD. While the acute suppression of hippocampal transmission persisted, 101.0 ± 7.4% recovery of EPSP was observed in the late phase (n= 5), indicating that chelating intracellular Ca2+ with BAPTA blocked the CCh-LTD.
Discussion
The present results reveal input-specific modulation of hippocampal synapses in response to muscarinic receptor activation in the mPFC region. Specifically, these synapses exhibit acute and long-term depression in the presence of, and after the removal of, mAChR agonists, respectively. While multiple muscarinic receptors may be involved in the acute phase, only M2 receptors appear to mediate the long-term suppressive effect. On the contrary, synapses that originate from cortical projections or the local circuitry are modulated by muscarinic activation only in an acute way.
What are the mechanisms of long-term muscarinic suppression (CCh-LTD) at the hippocampal–mPFC synapses? We present several lines of evidence suggesting that CCh-LTD is due to a reduction of presynaptic transmitter release via the M2 receptor subtype. First, CCh-LTD was not associated with any changes to input resistance, despite a reversible membrane depolarization, indicating that the suppression has synaptic relevance. Second, although the nearly complete elimination of EPSCs during CCh application prevented the measurement of PPF ratios in the acute phase, PPF measured during the late phase was increased. This observation supports the hypothesis that reduction of hippocampal synaptic responses is probably mediated, at least in part, by reduced presynaptic transmitter release. Finally, complete blockade of the acute suppression of hippocampal EPSP by the combination of subtype-specific antagonists demonstrates the involvement of multiple receptors including M1, M2 and M4 in immediate effects of CCh. However, only application of the M2 antagonist completely abolished the long-term muscarinic suppression, pointing to a central role for M2 receptors.
CCh-LTD has been observed at various synapses in the cortex, including cortex–cortex connections (Massey et al. 2001) and layer 4–layer 2/3 synapses (Kirkwood et al. 1999; McCoy & McMahon, 2007). However, in the mPFC region, we failed to induce CCh-LTD at synapses originating from cortical projections or the local circuitry. We speculate that a possible lack of presynaptic M2 at these synapses prevents CCh-LTD.
How does a presynaptic M2 receptor regulate neurotransmitter release? M2 receptors have been reported to localize at both cholinergic and glutamatergic presynaptic terminals (Rouse et al. 2000; Volpicelli & Levey, 2004). Activation of M2 autoreceptors, which preferentially couple with Gi type of G-proteins, increases the frequency of presynaptic K+ channel openings (Caulfield et al. 1993), inhibits Ca2+ channels (Segal, 1989; Bernheim et al. 1992; Caulfield et al. 1993), and interferes with the presynaptic release machinery by direct protein–protein interactions (Linial et al. 1997). All these actions lead to decreased presynaptic release of acetylcholine. The role of M2 receptors localized on non-cholinergic terminals is not well understood due to the difficulty of accessing a specific afferent. Regardless, immunostaining reveals that M2 receptors are present on both excitatory and inhibitory terminals in the hippocampus (Rouse et al. 2000). Stimulation of these receptors inhibits glutamate release from hippocampal synaptosomes (Marchi & Raiteri, 1989). An in vivo study in which field EPSPs were recorded in the entorhinal cortex in response to stimulation of the piriform cortex (Hamam et al. 2007), also supports the notion that neurotransmission can be suppressed by presynaptic cholinergic mechanisms. Although both cholinergic and non-cholinergic synapses can be inhibited presynaptically, it remains speculative whether these effects share the same mechanisms at both types of terminals. Furthermore, we do not understand which signalling pathways, located presynaptically or downstream of M2 receptors, are required to cause long-lasting depression that goes beyond the initial activation of M2.
While presynaptic cholinergic mechanisms are clearly involved, to some extent, in the induction of CCh-LTD, we do not know what other players or signalling pathways are involved in the expression and maintenance of long-lasting depression at the hippocampal–mPFC pathway. Previous work reveals the activity dependence of CCh-LTD in other brain regions (Kirkwood et al. 1999; McCoy & McMahon, 2007); we suspect this is also the case for hippocampal–mPFC synapses. In our experiments, stimulation was normally delivered to the hippocampal afferents at 0.033 Hz. One phenomenon worthy of noting is that when no concurrent stimulation of the afferent fibres was present during the CCh application, the acute suppressive effect of CCh persisted but not the long-lasting one. What mechanism does this observation reveal? It appears that activation of other synaptic components (presynaptic, postsynaptic, or both) is required to achieve CCh-LTD. Our data further suggest that Ca2+ influx through L-type Ca2+ channels, but not NMDA receptors, represent a postsynaptic component that mediates the observed effects of CCh. In both the visual cortex (McCoy & McMahon, 2007) and the hippocampus (Volk et al. 2007; Scheiderer et al. 2008), postsynaptic M1 receptor-dependent LTD appears to require ERK1/2 activation and protein synthesis, possibly leading to enhanced endocytosis of cell surface glutamate receptors (Volk et al. 2007). It would be of interest to further identify downstream signalling pathways that would eventually lead to and maintain the long-lasting suppression of hippocampal synaptic transmission in mPFC.
The requirement of two simultaneously active mechanisms, i.e. presynaptic acetylcholine binding to M2 receptors and activation of postsynaptic L-type Ca2+ channels, suggests the dependence of CCh-LTD on coincidental events. In other words, release of ACh and subsequent binding to presynaptic M2 receptors is not sufficient to achieve sustained reduction of synaptic strength, unless the synapse is also active during the ACh exposure. The significance of M2-mediated selective long-term suppression of hippocampal input to the mPFC and the behavioural consequences of these findings remain to be further investigated.
Acknowledgments
We thank Marc Parent for technical assistance, and Mu Sun, Paulo Kofuji, Esam El-Fakahany, and Amber Lockridge for comments on the manuscript. This work was supported by the National Institutes of Health (NS049129).
Glossary
Abbreviations
- CCh
carbachol
- APV
dl-aminophosphonovalerate
- ERK
extracellular signal-regulated kinase
- LTD
long-term depression
- mAChR
muscarinic acetylcholine receptor
- mPFC
medial prefrontal cortex
- PFC
prefrontal cortex
- PPF
paired pulse facilitation
Author contributions
L.W. conceived, designed and performed experiments, analysed and interpreted data, and approved the final version of the manuscript. L.-L.Y. conceived and designed experiments, analysed and interpreted data, drafted the manuscript, and approved the final version of the manuscript. All experiments were performed at the University of Minnesota, USA.
References
- Beninger RJ, Ingles JL, Mackenzie PJ, Jhamandas K, Boegman RJ. Muscimol injections into the nucleus basalis magnocellularis of rats: selective impairment of working memory in the double Y-maze. Brain Res. 1992;597:66–73. doi: 10.1016/0006-8993(92)91506-a. [DOI] [PubMed] [Google Scholar]
- Bernheim L, Mathie A, Hille B. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1992;89:9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav Brain Res. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol. 2007;97:3432–3438. doi: 10.1152/jn.00828.2006. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Robbins J, Higashida H, Brown DA. Postsynaptic actions of acetylcholine: the coupling of muscarinic receptor subtypes to neuronal ion channels. Prog Brain Res. 1993;98:293–301. doi: 10.1016/s0079-6123(08)62411-5. [DOI] [PubMed] [Google Scholar]
- DeSousa NJ, Beninger RJ, Jhamandas K, Boegman RJ. Stimulation of GABAB receptors in the basal forebrain selectively impairs working memory of rats in the double Y-maze. Brain Res. 1994;641:29–38. doi: 10.1016/0006-8993(94)91811-2. [DOI] [PubMed] [Google Scholar]
- Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer's disease – the pivotal role of brain M1 receptors. Neurodegener Dis. 2008;5:237–240. doi: 10.1159/000113712. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Gaál G, Traber J, Hersh LB, Luiten PG. The basal forebrain cholinergic system: efferent and afferent connectivity and long-term effects of lesions. Acta Psychiatr Scand Suppl. 1991;366:14–26. doi: 10.1111/j.1600-0447.1991.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Altered prefrontal cortexnucleus accumbens information processing in a developmental animal model of schizophrenia. Ann N Y Acad Sci. 2003;1003:398–401. doi: 10.1196/annals.1300.035. [DOI] [PubMed] [Google Scholar]
- Hajos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Hamam BN, Sinai M, Poirier G, Chapman CA. Cholinergic suppression of excitatory synaptic responses in layer II of the medial entorhinal cortex. Hippocampus. 2007;17:103–113. doi: 10.1002/hipo.20249. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modelling the physiological basis of behaviour. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. Contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci. 1992;4:1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Cutler R, Roth GS. Changes in G protein-mediated signal transduction in aging and Alzheimer's disease. Ann N Y Acad Sci. 1993;695:42–45. doi: 10.1111/j.1749-6632.1993.tb23024.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M, Ilouz N, Parnas H. Voltage-dependent interaction between the muscarinic ACh receptor and proteins of the exocytic machinery. J Physiol. 1997;504:251–258. doi: 10.1111/j.1469-7793.1997.251be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy P, Norton TT, McMahon LL. Layer 2/3 synapses in monocular and binocular regions of tree shrew visual cortex express mAChR-dependent long-term depression and long-term potentiation. J Neurophysiol. 2008;100:336–345. doi: 10.1152/jn.01134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy PA, McMahon LL. Muscarinic receptor dependent long-term depression in rat visual cortex is PKC independent but requires ERK1/2 activation and protein synthesis. J Neurophysiol. 2007;98:1862–1870. doi: 10.1152/jn.00510.2007. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol. 1995;487:67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M, Raiteri M. Interaction acetylcholineglutamate in rat hippocampus: involvement of two subtypes of M-2 muscarinic receptors. J Pharmacol Exp Ther. 1989;248:1255–1260. [PubMed] [Google Scholar]
- Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-d-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1998;95:11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Bhabra G, Cho K, Brown MW, Bashir ZI. Activation of muscarinic receptors induces protein synthesis-dependent long-lasting depression in the perirhinal cortex. Eur J Neurosci. 2001;14:145–152. doi: 10.1046/j.0953-816x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- Muir JL, Dunnett SB, Robbins TW, Everitt BJ. Attentional functions of the forebrain cholinergic systems: effects of intraventricular hemicholinium, physostigmine, basal forebrain lesions and intracortical grafts on a multiple-choice serial reaction time task. Exp Brain Res. 1992;89:611–622. doi: 10.1007/BF00229886. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp108. DOI 10.1093/cercor/bhp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, Marston HM, Wilkinson J, Jones GH, Page KJ. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behav Brain Res. 1989;35:221–240. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Hamilton SE, Potter LT, Nathanson NM, Conn PJ. Muscarinic-induced modulation of potassium conductances is unchanged in mouse hippocampal pyramidal cells that lack functional M1 receptors. Neurosci Lett. 2000;278:61–64. doi: 10.1016/s0304-3940(99)00914-3. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, McCutchen E, Thacker EE, Kolasa K, Ward MK, Parsons D, Harrell LE, Dobrunz LE, McMahon LL. Sympathetic sprouting drives hippocampal cholinergic reinnervation that prevents loss of a muscarinic receptor-dependent long-term depression at CA3-CA1 synapses. J Neurosci. 2006;26:3745–3756. doi: 10.1523/JNEUROSCI.5507-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiderer CL, Smith CC, McCutchen E, McCoy PA, Thacker EE, Kolasa K, Dobrunz LE, McMahon LL. Coactivation of M1 muscarinic and α1 adrenergic receptors stimulates extracellular signal-regulated protein kinase and induces long-term depression at CA3-CA1 synapses in rat hippocampus. J Neurosci. 2008;28:5350–5358. doi: 10.1523/JNEUROSCI.5058-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Segal M. Presynaptic cholinergic inhibition in hippocampal cultures. Synapse. 1989;4:305–312. doi: 10.1002/syn.890040406. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in Fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]