Abstract
Activation of neurons in the dorsomedial hypothalamus (DMH) evokes increases in mean arterial pressure (MAP), sympathetic activity, heart rate (HR) and respiratory activity. Results of previous studies suggest that the DMH-evoked increases in MAP and HR are mediated by neurons within the periaqueductal grey (PAG), but a recent study has proposed that the converse is also true, i.e. that increases in MAP and HR evoked from the PAG depend upon neuronal activity in the DMH. In this study in anaesthetized rats, we examined the functional relationship between the DMH and PAG in regulating renal sympathetic nerve activity (RSNA) and respiratory activity (determined by measuring phrenic nerve activity (PNA)). Bilateral microinjections of the neuronal inhibitor muscimol into the DMH virtually abolished the increases in MAP, RSNA and PNA burst rate and amplitude evoked from the dorsolateral (dl) PAG. In contrast, multiple bilateral injections of much larger (10 times) doses of muscimol or of the local anaesthetic lignocaine into sites in the dlPAG at three different rostrocaudal levels did not reduce the magnitude or duration of the sympathetic vasomotor and respiratory responses evoked by disinhibition of neurons in the DMH. Thus, sympathetic vasomotor and respiratory responses generated from the dlPAG are dependent upon neuronal activity in the DMH, but not the converse. The results of this study together with those of previous studies indicate that the PAG regulates cardiovascular and respiratory function via both ascending projections to the DMH and descending projections to the ventral medulla, that originate from different PAG subregions.
Introduction
The dorsomedial hypothalamus (DMH) and midbrain periaqueductal grey (PAG) are both brain regions that are known to have a critical role in regulating the cardiovascular changes associated with defensive behaviour (Hilton, 1982; Carrive, 1993; Lovick, 1993; Bandler et al. 2000; Keay & Bandler, 2001; DiMicco et al. 2002). Inhibition of neurons in the DMH has been shown to greatly reduce the increases in mean arterial pressure (MAP), heart rate (HR) and adrenocorticotropic hormone (ACTH) release evoked by air jet stress in conscious rats (Stotz-Potter et al. 2006a,b;). In anaesthetized rats, activation of neurons in the DMH evokes a range of responses, including increases in MAP, HR, sympathetic vasomotor activity, sympathetically mediated thermogenesis and respiratory activity (DiMicco et al. 2002; Cao et al. 2004; McDowall et al. 2007), similar to the responses that occur during naturally evoked defensive behaviour (Grossman, 1983; DiMicco et al. 2002).
Activation of neurons in the lateral or dorsolateral (l/dl) columns of the PAG also evokes increases in MAP and HR (Carrive, 1993; Bandler et al. 2000; Keay & Bandler, 2001). Furthermore, da Silva et al. (2003) reported that after inhibition of neurons within the l/dlPAG (by microinjection of the neuronal inhibitor muscimol), increases in MAP and HR evoked by disinhibition of the DMH were greatly reduced, leading to the proposal that neurons in the l/dlPAG were part of the descending pathways mediating these responses evoked from the DMH. Very recently, however, de Menezes et al. (2009) have reported that, conversely, the increases in MAP and HR evoked by activation of neurons in the l/dlPAG were greatly reduced by muscimol microinjection into the DMH. This finding indicates that these responses are dependent upon neuronal activity in the DMH, leading to the suggestion that a direct or indirect ascending pathway from the l/dlPAG to the DMH mediates, at least in part, the increases in MAP and HR evoked from the former region (de Menezes et al. 2009). These observations therefore challenge the generally accepted view that PAG neurons regulate autonomic activity entirely via descending projections to nuclei in the lower brainstem (Carrive, 1993; Lovick, 1993).
Apart from effects on MAP and HR, activation of neurons in the l/dlPAG can evoke marked increases in the sympathetic vasomotor outflow to different vascular beds, including the renal, gut and skeletal muscle vascular beds (Carrive, 1993), as well as respiratory activity (Huang et al. 2000; Hayward et al. 2004), but it is not known whether these effects also are dependent on neuronal activity in the DMH. In this study, we have examined this question by testing the effect of bilateral inhibition of neurons in the DMH on the increases in renal sympathetic nerve activity (RSNA) and respiratory activity evoked from the PAG. Conversely, we have also examined whether the increases in these variables evoked from the DMH depend upon activation of neurons in the PAG.
Methods
General procedures
Experiments were performed on a total of 28 male Sprague–Dawley rats (380–580 g) supplied by University of Sydney Laboratory Animal Services. All experimental procedures were approved by the Animal Ethics Committee of the University of Sydney and were carried out in accordance with the Guidelines for Animal Experimentation of the National Health and Medical Research Council of Australia. Anaesthesia was initially induced by inhalation of isoflurane (2.0–2.5% in oxygen-enriched air). The trachea was then intubated and body temperature was maintained in the range of 37–38°C with a heating pad. Catheters were placed in a femoral artery and a femoral vein for the recording of pulsatile arterial pressure and drug injection, respectively. After the surgery, the isoflurane anaesthesia was gradually withdrawn while being replaced by urethane (1.3 g kg−1i.v. with supplementary doses of 0.1 g kg−1i.v., if required). In some experiments, the steroid anaesthetic alfaxalone was administered instead of urethane. In these experiments, alfaxalone (10 mg ml−1) was continuously infused i.v. throughout the experiment via an infusion pump at a rate of 5–10 mg kg−1 h−1. The adequacy of anaesthesia was verified by the absence of the corneal reflex and a withdrawal response to nociceptive stimulation of a hind paw. A tracheotomy was performed to maintain an unobstructed airway and all animals were allowed to breathe freely.
The rat was then mounted in a stereotaxic apparatus with the incisor bar fixed 3.5 mm below the interaural line, and the renal and phrenic nerves exposed and prepared for recording as described previously (McDowall et al. 2007). Small portions of the dorsal surfaces of the cortex and colliculi were exposed to allow insertion of micropipettes into the DMH and PAG, respectively. The MAP and HR signals were derived from the pulsatile arterial pressure signal via a low-pass filter and a rate meter, respectively. All signals were recorded on a computer using a PowerLab system (AD Instruments). Chart software was used to rectify and integrate the RSNA and phrenic nerve activity (PNA) signals, and to compute the rate and amplitude of the bursts of PNA.
Microinjections of various compounds were made into sites in the DMH or PAG using a glass micropipette held vertically in a micromanipulator. The vehicle solution was artificial cerebrospinal fluid adjusted to pH 7.4. In all cases the injectate also contained green or red fluorescent latex microspheres (0.5%, Lumafluor), to facilitate the later histological verification of microinjection sites. Microinjections were made by pressure, using a previously described method (Fontes et al. 2001). At the end of each experiment, the rat was killed with an overdose of pentobarbital sodium, the brain was removed, and after fixation in 4% paraformaldehyde solution, coronal sections (50 μm) were cut on a freezing microtome and mounted onto glass slides. The labelled microinjection sites were identified by examining the sections using a fluorescence microscope and were then mapped onto standard sections of the atlas by Paxinos & Watson (2004).
Experimental procedures
In the first series of experiments performed in 12 rats, microinjections of d,l-homocysteic acid (DLH, 5 nmol in 50 nl) were made into the PAG before and after bilateral microinjections of the GABA receptor agonist muscimol (0.1 nmol in 50 nl) into the DMH. In preliminary experiments, it was found that large increases in RSNA were most commonly obtained when the micropipette tip was centred at the co-ordinates 7.6 mm caudal to bregma, 0.3–0.4 mm lateral to the midline and 4.0 mm ventral to the dura surface. If a large increase in RSNA (>80% baseline) was not obtained from this site, further microinjections were made into nearby sites until a large response was evoked. In most cases, only one microinjection was required, but in some one or two additional microinjections were required. Following this control microinjection, there was a waiting period of approximately 10 min to allow the cardiorespiratory variables (MAP, HR, RSNA, and PNA burst rate and amplitude) to return to their pre-injection baseline levels. In six experiments, a microinjection of muscimol was made into the DMH on one side (3 ipsilateral and 3 contralateral to the DLH microinjection site in the PAG), after which a second DLH microinjection was made into the same PAG site as for the control microinjection. After a further waiting period to allow the cardiorespiratory variables to stabilize, a microinjection of muscimol was made into the DMH on the opposite side to the first muscimol micoinjection, and a third DLH microinjection was made into the PAG. In two other experiments, the procedure was the same as in the first group except that only two microinjections were made into the PAG, before and after bilateral microinjections of muscimol into the DMH. Finally, in four control experiments, the procedure was the same as in the first group except that the vehicle solution instead of muscimol was injected into the DMH.
For the muscimol microinjections into the DMH, the tip of the micropipette was positioned stereotaxically 3.1 mm caudal to bregma, 0.5 mm lateral to the midline, and 8.6 ventral to the dura surface, as determined using a standard rat brain atlas (Paxinos & Watson, 2004). This site lies within the region in the DMH where large increases in MAP, HR, RSNA and PNA burst rate are consistently evoked by microinjections of the GABA receptor antagonist bicuculline (Fontes et al. 2001; Horiuchi et al. 2004, McDowall et al. 2007).
In the second series of experiments performed in 16 rats, a microinjection of bicuculline (10 pmol in 20 nl) was made into the DMH before and after multiple microinjections of muscimol or the local anaesthetic lignocaine into the dlPAG. For the microinjection into the DMH, the tip of the micropipette was positioned stereotaxically at 3.1 mm caudal to bregma, 0.5 mm lateral to the midline, and 8.6 mm ventral to the dura surface. For microinjections into the PAG, the tip of the micropipette was first positioned stereotaxically 7.6 mm caudal to bregma, 0.5 mm lateral to the midline and 4.4 mm ventral to the dura surface. A microinjection of a large dose (1 nmol in 100 nl) of muscimol was made into this site and then into the contralateral site at the same level, followed by bilateral microinjections into sites with the same mediolateral and dorsoventral co-ordinates but at levels 0.8 mm rostral (6.8 mm caudal to bregma) and 0.7 mm caudal (8.3 mm caudal to bregma). Thus, a total dose of 6 nmol of muscimol in 600 nl were made into these six sites centred in the dlPAG. In eight other experiments, the procedure was the same except that (1) lignocaine (100 nl of 2% solution) was injected into the same six sites instead of muscimol (n= 4), or (2) the rats were anaesthetized with the steroid anaesthetic alfaxalone instead of urethane (n= 4).
Statistical analysis
The changes in MAP, HR, RSNA, PNA burst amplitude and burst rate evoked by microinjections of DLH into the PAG under control conditions and after unilateral or bilateral injections of muscimol or vehicle solution into the DMH were compared using one-way ANOVA. The same procedure was used to compare the changes in these variables evoked by microinjections of bicuculline into the DMH under control conditions and after bilateral injections of muscimol or lignocaine into the PAG. Pair-wise comparisons were made using the t test with application of the Helm step-down procedure for multiple comparisons where appropriate (Shaffer, 1986). A P value of < 0.05 was regarded as statistically significant. All values are presented as mean ±s.e.m.
Results
Effect of muscimol microinjections into the DMH on cardiorespiratory responses evoked from the PAG
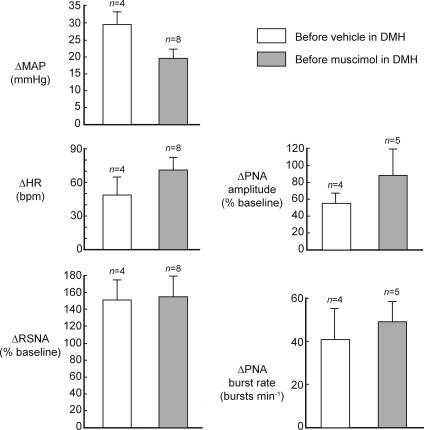
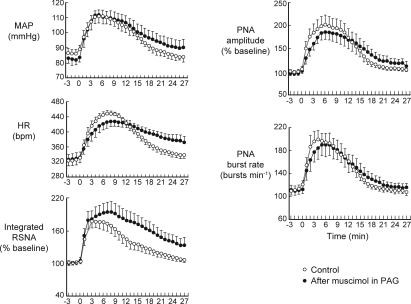
Microinjections of DLH (5 nmol) were made into sites in the PAG in 12 experiments. The centres of these injections were mainly located within the dlPAG at levels extending from approximately 6.8 to 7.8 mm caudal to bregma (Fig. 1). The baseline levels of MAP, HR and PNA burst rate before injections of muscimol into the DMH (n= 8) were 99 ± 5 mmHg, 342 ± 10 beats min−1 and 103 ± 6 bursts min−1, respectively. After bilateral microinjections of muscimol into the DMH, the baseline levels of these variables were 96 ± 3 mmHg, 324 ± 13 beats min−1 and 93 ± 3 bursts min−1, respectively, and were not significantly different from the pre-muscimol values (P > 0.05 in all cases). Similarly, in the four control experiments the baseline levels of all these variables were not significantly different after bilateral injections of the vehicle solution into the DMH. Microinjections of DLH into these PAG sites consistently evoked increases in MAP, HR and RSNA but had more variable effects on PNA amplitude and burst rate (Figs 2 and 3). In most experiments large increases were evoked (e.g. Fig. 3), but in three experiments DLH microinjection evoked little change in PNA burst amplitude and burst rate (<10% of the baseline level). The data from these three experiments were therefore not used for the calculations of mean PNA burst amplitude and burst rate before and after muscimol injections into the DMH (Figs 2 and 4).
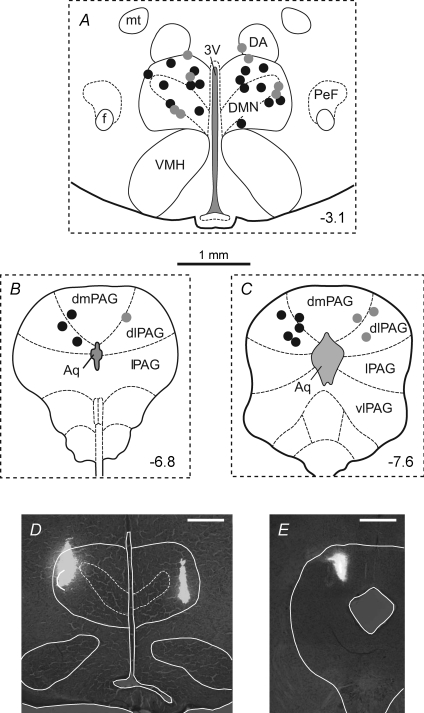
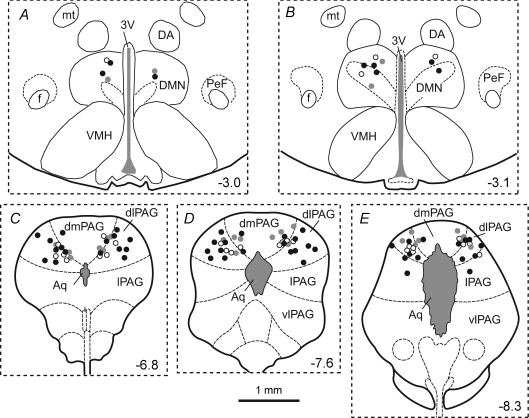
Figure 1. Centres of sites of injection of DLH in the PAG and of muscimol or vehicle solution in the DMH.
Coronal sections of the rat brain from the atlas of Paxinos & Watson (2004), illustrating centres of injection sites of either muscimol (0.1 nmol) or the vehicle solution into the DMH (A), and of DLH (5 nmol) into the PAG (B and C). The number in each panel indicates the distance of that section in millimetres from bregma. Injection sites within the DMH and PAG from the same series of experiments are indicated by matching symbols. Black circles indicate experiments in which DLH was injected into the PAG before and after injections of muscimol into the DMH (n= 8), and the grey circles indicate experiments in which DLH was injected into the PAG before and after injections of vehicle solution into the DMH (n= 4). Examples of injection sites in the DMH and PAG are shown in D and E, respectively. The scale bar in these photomicrographs represents 0.5 mm. Abbreviations: 3V, third ventricle; Aq, aqueduct; DA, dorsal hypothalamic area; DMN, dorsomedial hypothalamic nucleus; dmPAG, dorsomedial PAG; f, fornix; mt, mamillothalamic tract; PeF, perifornical area; vlPAG, ventrolateral PAG. Note that the DMH is defined as the DMN together with the DA.
Figure 2. Effects of microinjection of DLH into the dlPAG.
Responses shown are the mean maximal changes in mean arterial pressure (MAP), heart rate (HR), renal sympathetic nerve activity (RSNA), and phrenic nerve activity (PNA) burst amplitude and rate evoked by microinjection of DLH (5 nmol) into the dlPAG before injection of either muscimol or vehicle solution into the DMH.
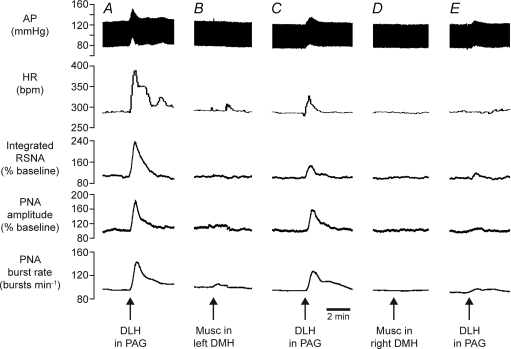
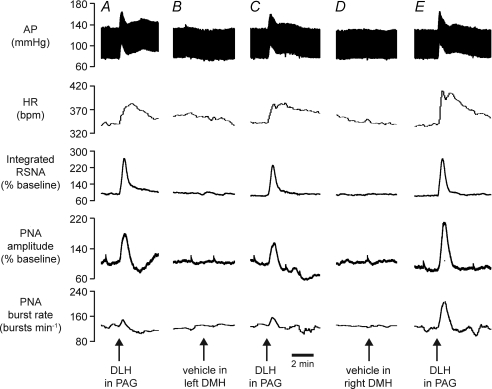
Figure 3. Example of effects of inhibition of DMH on responses evoked from the dlPAG.
Chart recordings from one experiment showing changes in arterial pressure (AP), HR, RSNA, PNA burst amplitude and rate evoked by microinjection of DLH (5 nmol) in the dlPAG (A), followed by microinjection of muscimol (Musc, 0.1 nmol) in the left DMH (B), then microinjection of DLH into the same PAG site again (C), followed by microinjection of muscimol into the right DMH (D), and then finally a third microinjection of DLH into the same PAG site (E).
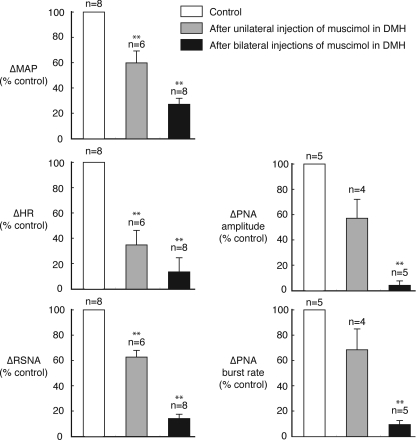
Figure 4. Effects of inhibition of DMH on responses evoked from the dlPAG.
Grouped data showing effects of unilateral and bilateral microinjections of muscimol (0.1 nmol) into the DMH on the cardiorespiratory responses evoked by microinjection of DLH (5 nmol) in the PAG. **P < 0.01 (compared to control).
Microinjections of muscimol into the DMH on the side ipilateral (n= 3) or contralateral (n= 3) to the PAG injection site resulted in reductions in the magnitudes of the changes in MAP, HR, RSNA, and PNA burst amplitude and rate evoked by DLH microinjections into the PAG (e.g. Fig. 3). There was no significant difference (P > 0.5 in all cases) when the effects of muscimol injections on the ipsilateral side were compared to those on the contralateral side, and so these data were pooled (Fig. 4). After a subsequent microinjection of muscimol into the DMH on the other side, there were further large reductions in the magnitudes of the evoked responses (Figs 3 and 4). In particular, after bilateral muscimol injections into the DMH, the increases in RSNA, PNA burst amplitude and PNA burst rate were reduced to 14%, 4% and 8% of their respective control pre-injection values (Fig. 4). In confirmation of a previous study by de Menezes et al. (2009), the increases in MAP and HR were also greatly reduced, to 27% and 13% of their respective control values.
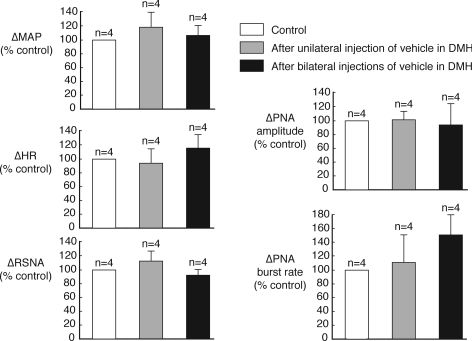
In contrast to the effects of muscimol, neither unilateral nor bilateral microinjections of vehicle solution into the DMH had any significant effect on any of the cardiorespiratory variables measured (Figs 5 and 6).
Figure 5. Example of effects of vehicle microinjections in DMH on responses evoked from the dlPAG.
Chart recordings from one control experiment showing changes in cardiorespiratory variables evoked by microinjection of DLH (5 nmol) in the dlPAG (A), followed by microinjection of vehicle in the left DMH (B), then microinjection of DLH into the same PAG site again (C), followed by microinjection of vehicle into the right DMH (D), and then finally a third microinjection of DLH into the same PAG site (E).
Figure 6. Effects of vehicle microinjections in DMH on responses evoked from the dlPAG.
Grouped data showing lack of significant effects of unilateral and bilateral microinjections of vehicle solution into the DMH on the cardiorespiratory responses evoked by microinjection of DLH (5 nmol) in the PAG.
Effect of muscimol microinjections into the PAG on cardiorespiratory responses evoked from the DMH
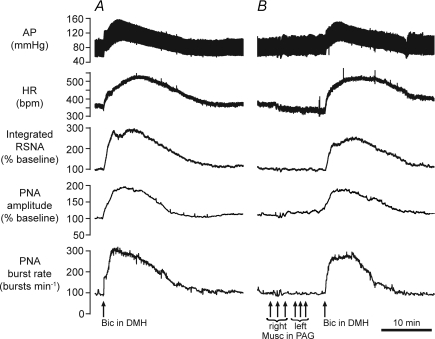
In eight experiments, the cardiorespiratory responses evoked by a microinjection of bicuculline into the DMH were determined before and after bilateral injections of muscimol (each of 1 nmol in 100 nl) into the PAG at three different levels. Most of these injections were centred in the dlPAG, in the region from which DLH microinjections evoked large sympathoexcitatory and responses (Fig. 7). An example of the results from one experiment is shown in Fig. 8, and the grouped results are shown in Figs 9 and 10. After multiple muscimol injections into the PAG, there was no significant change in the magnitudes of the maximum evoked increases in MAP, HR, RSNA, and PNA burst amplitude and rate (Fig. 10), although the time courses of the evoked changes in MAP, HR and RSNA were more prolonged in comparison to the control experiments (Fig. 9). For example, in the experiments in which muscimol was injected into the PAG the MAP, HR and RSNA were all still significantly elevated with respect to their baseline pre-injection levels (P < 0.05 in all cases) at 27 min after bicuculline injection into the DMH, whereas in the control experiments all of these variables were not significantly different from their respective baseline pre-injection levels (P > 0.05 in all cases) at the same time point (Fig. 9).
Figure 7. Centres of sites of injection of bicuculline in the DMH and of muscimol or lignocaine in the PAG.
Coronal sections of the rat brain from the atlas of Paxinos & Watson (2004), illustrating centres of injection sites of bicuculline (10 pmol) in the DMH (A and B), and of muscimol or lignocaine in the PAG (C, D and E). The number in each panel indicates the distance of that section in millimetres from bregma. Injection sites within the DMH and PAG from the same series of experiments are indicated by matching symbols. In all experiments bicuculline was injected into the DMH before and after bilateral injections of either muscimol or lignocaine at three rostrocaudal levels of the PAG (i.e. 6 injections in each experiment). Black circles indicate experiments in which muscimol was injected into the PAG (n= 8), grey circles experiments in which lignocaine was injected into the PAG (n= 4), and the open circles experiments in which muscimol was injected into the PAG but the rats were anaesthetized with alfaxalone instead of urethane (n= 4).
Figure 8. Example of effects of muscimol injections in the PAG on responses evoked from the DMH.
Chart recordings from one experiment showing changes in cardiorespiratory variables evoked by microinjection of bicuculline (Bic, 10 pmol) in the DMH before (A) and after (B) multiple bilateral injections of muscimol (Musc, 1 nmol each injection) into the dlPAG.
Figure 9. Time course of responses evoked from DMH before and after muscimol injections in the PAG.
Grouped results (n= 8) showing average time course of changes in cardiorespiratory variables evoked by microinjection of bicuculline in the DMH before (Control) and after multiple bilateral injections of muscimol into the dlPAG. The numbers on the x-axis represent the times in minutes before and after microinjection of bicuculline into the DMH.
Figure 10. Effects of inhibition of dlPAG on responses evoked from the DMH.
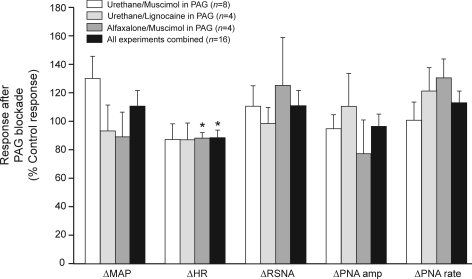
Grouped data showing maximum changes in cardiorespiratory variables evoked by a bicuculline microinjection into the DMH after multiple bilateral injections of muscimol or lignocaine into the dlPAG, where the responses are calculated as a percentage of the bicuculline-evoked response before injections in the PAG (control responses). Three groups of experiments were performed, that differed according to whether muscimol or lignocaine was injected into the PAG, or whether the rats were anaesthetized with urethane or lignocaine. There was no significant difference in the relative responses (measured as a percentage of the control response) between the 3 different experimental conditions for all of the cardiorespiratory variables (P= 0.17–0.99), and so the data were pooled. None of the responses were significantly different from their respective control response, except for the HR response which was slightly reduced. *P < 0.05 (compared to 100%).
In four other experiments, lignocaine was injected into the PAG (Fig. 7) instead of muscimol. After multiple lignocaine injections into the PAG, there was also no significant change in the magnitudes of the maximum increases in MAP, HR, RSNA, and PNA burst amplitude and rate evoked by bicuculline microinjection into the DMH (Fig. 10). In a final group of four experiments, the effects of muscimol in the PAG (Fig. 7) was tested in rats anaesthetized with the steroid anaesthetic alfaxalone. In these experiments there was also no significant change in the magnitudes of the maximum evoked increases before and after muscimol in the PAG for any of the cardiorespiratory variables, with the exception of the increase in HR, which was slightly reduced (by 11 ± 3%, P < 0.05).
There was no difference between the three experimental conditions (muscimol or lignocaine injected into the PAG under urethane anaesthesia, or muscimol injected into the PAG under alfaxalone anaesthesia) in regard to the relative magnitudes of the evoked increases in MAP, HR, RSNA, PNA burst amplitude or rate (measured with respect to the pre-injection control levels) after injections into the PAG (P= 0.17–0.99). The data for all three groups of experiments were therefore pooled (Fig. 10). There was no significant change in the relative magnitudes of the responses for the pooled data before and after injections in the PAG for any of the cardiorespiratory variables, with the exception of HR, which was slightly reduced (by 12%, P < 0.05, Fig. 10).
Discussion
In this study the major findings are that increases in RSNA and respiratory activity evoked from the dlPAG are virtually abolished by inhibition of neurons in the DMH. In contrast, however, inhibition of neurons in the dlPAG did not significantly affect the increases in RSNA and respiratory activity evoked by activation of neurons in the DMH. These results therefore indicate that in the anaesthetized rat, sympathetic vasomotor and respiratory responses evoked by activation of neurons in the dlPAG depend upon neuronal activity in the DMH, but not the converse.
Although the centres of the muscimol injections were all confirmed to be within the DMH, it could be argued that the inhibition of the PAG-evoked responses was due to diffusion of muscimol to the paraventricular nucleus (PVN), which also regulates RSNA and respiratory activity (Schlenker et al. 2001). This is very unlikely, however, because de Menezes et al. (2009) demonstrated that a microinjection into the PVN of the same dose (0.1 nmol) of muscimol as used in our study did not reduce the increases in MAP and HR evoked from the l/dlPAG, although when this dose was injected into the DMH the responses were virtually abolished. Consistent with this, we have previously shown that microinjection of a larger dose (0.4 nmol) into the PVN did not reduce the magnitude of increases in MAP, HR or RSNA evoked by bicuculline microinjection into the ispilateral DMH (Fontes et al. 2001). We therefore conclude that the reduction of the sympathetic vasomotor and respiratory responses evoked from the l/dlPAG is due to inhibition of neurons in the DMH, not the PVN.
Role of DMH in mediating increases in RSNA evoked from PAG
The anatomical connections of the dlPAG are distinctly different from those of other PAG subregions. In marked contrast to all other PAG subregions (lateral, ventrolateral, ventromedial and dorsomedial subregions), all of which have direct projections to the ventral medulla (Carrive et al. 1989; Carrive, 1993; Farkas et al. 1998; van Bockstaele et al. 1991), there are no direct projections from neurons in the dlPAG to the medulla (Carrive, 1993; Bandler & Shipley, 1994). There are, however, major direct projections from the dlPAG to the cuneiform nucleus in the midbrain and to the superior lateral parabrachial nucleus in the pons (Carrive, 1993; Bandler & Shipley, 1994; Krout et al. 1998). Both of these nuclei in turn project to the hypothalamus, including the DMH (Fulwiler & Saper, 1984; Bester et al. 1997; Lam et al. 1997). There is also a minor direct projection from the dlPAG to the DMH (Thompson & Swanson, 1998). It is therefore possible that these ascending direct and indirect connections from the dlPAG to the DMH mediate the increases in RSNA arising from activation of PAG neurons.
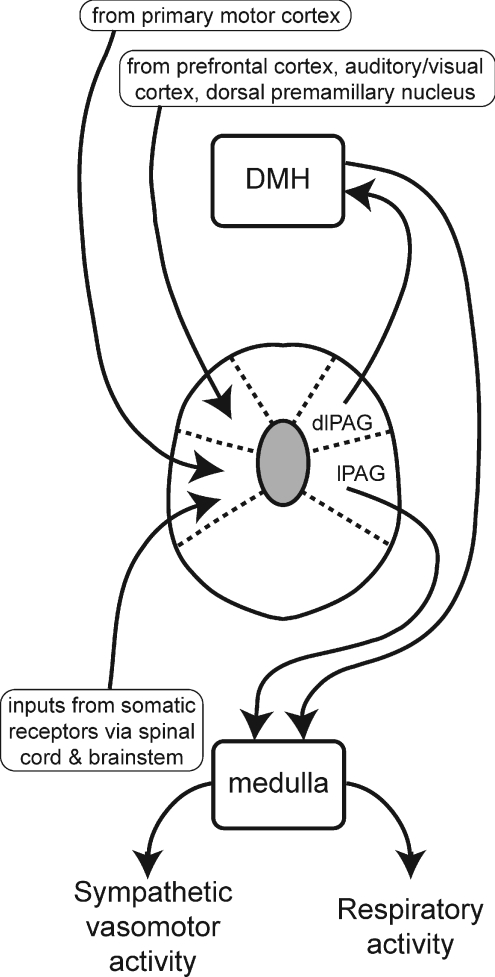
Although the centres of the DLH injection sites were mostly within the dlPAG, it is likely that neurons in the adjacent PAG subregions (lPAG and dorsomedial PAG) would also have been activated by DLH injections, given the close proximity of these PAG subregions. As mentioned above, both of these subregions project directly to the ventral medulla, including the rostral ventromedial and rostral ventrolateral regions (van Bockstaele et al. 1991), which are major origins of premotor neurons regulating the renal sympathetic outflow (Sly et al. 1999). Our results indicate, however, that if this descending pathway was activated by DLH microinjections into the PAG, it was not sufficient to evoke an increase in RSNA in the absence of inputs to sympathetic premotor neurons originating from the DMH. As proposed by de Menezes et al. (2009) in regard to PAG-evoked increases in MAP and HR, the PAG-evoked increase in RSNA may depend upon a facilitatory summation, at the level of renal sympathetic premotor neurons in the ventral medulla, of direct descending inputs from the PAG and inputs mediated via the DMH (as shown schematically in Fig. 11).
Figure 11. Schematic diagram showing proposed major connections between DMH, PAG and the medulla subserving changes in sympathetic vasomotor and respiratory activity.
The lines with arrows indicate proposed connections, that could be either direct (monosynaptic) or indirect (polysynaptic). The inputs to the different PAG subregions that are shown represent some, but not all, of the major inputs as described in previous studies. On the basis of results obtained in this study combined with those obtained from previous studies we propose the hypothesis that the dlPAG regulates sympathetic vasomotor activity and respiratory activity via ascending connections with neurons in the DMH, whereas the lPAG regulates these variables via descending connections with neurons in the medulla.
Role of DMH in mediating increases in respiratory activity evoked from PAG
As previously reported (Huang et al. 2000; Hayward et al. 2004), stimulation of neurons in the dlPAG also increased respiratory rate and depth. Our finding that the large increases in PNA burst amplitude and rate evoked from the dlPAG were almost completely abolished after bilateral injections of muscimol into the DMH shows that this respiratory response, like the cardiovascular responses, is dependent on neuronal activity in the DMH. It is interesting to note that Hayward et al. (2004) found that the increase in respiratory frequency evoked from the dlPAG was reduced by 90% after bilateral muscimol injections into the lateral parabrachial nucleus. This nucleus includes the superior lateral subnucleus which, as mentioned above, receives a major direct input from the dlPAG and projects to the hypothalamus (Fulwiler & Saper, 1984; Bester et al. 1997; Krout et al. 1998). Thus, the respiratory responses evoked from the dlPAG may also be mediated via an ascending pathway that includes neurons in the DMH as well as the parabrachial nucleus.
Role of PAG in mediating cardiovascular and respiratory responses evoked from DMH
da Silva et al. (2003) found that the increases in MAP and HR evoked by disinhibition of neurons in the DMH were greatly reduced after a muscimol injection into the l/dlPAG. To reconcile this finding with the observation that a muscimol injection into the DMH greatly reduces the increases in MAP and HR evoked from the l/dlPAG, de Menezes et al. (2009) proposed the hypothesis that neurons in both the DMH and PAG communicate bidirectionally as a functionally unified network, such that inhibition of activity in either the DMH or PAG would be sufficient to prevent cardiovascular responses evoked from the other region. To test this hypothesis with respect to sympathetic vasomotor and respiratory responses, we examined the effect of inhibition of neurons in the dlPAG on the increases in RSNA and PNA burst rate and amplitude evoked from the DMH. Our results showed that these responses were not significantly reduced by muscimol injections into the dlPAG. In fact, the duration of the evoked renal sympathoexcitatory response was significantly increased. The fact that muscimol injections failed to reduce these responses is unlikely to be due to an inadequate dose of the drug, because bilateral injections, each of 1 nmol in 100 nl, were made at three different rostrocaudal levels of the PAG to ensure maximum coverage of the dlPAG. The dose of muscimol in each injection was much greater than the dose of muscimol (0.1 nmol in 50 nl) in each of the two bilateral injections into the DMH that were sufficient to virtually abolish responses evoked from the PAG. In addition, we found that the sympathetic vasomotor and respiratory responses evoked from the DMH were not reduced when the local anaesthetic lignocaine was injected instead of muscimol, or when the rats were anaesthetized with the steroid alfaxalone instead of urethane. Our results therefore do not support the hypothesis that neurons in the dlPAG and DMH are connected reciprocally, such that responses evoked from either of these regions is dependent upon the neuronal activity in the other region.
In contrast to the findings of da Silva et al. (2003), we found that multiple injections of muscimol or lignocaine into the dlPAG also did not reduce the increase in MAP evoked from the DMH, and resulted in only a slight reduction in the evoked increase in HR. The reasons for this apparent discrepancy are not clear. Although in the study by da Silva et al. (2003) the injection sites were in the lPAG as well as the dlPAG, the locations of the injection sites in their study appear to correspond quite closely with the locations of the sites in our study. Furthermore, in their study a single unilateral injection was made into the PAG at the level 7.8 mm caudal to bregma whereas in our study bilateral injections of muscimol or lignocaine were made into the PAG at a very similar level (7.6 caudal to bregma) and also into the levels 0.5 mm rostral and caudal, to ensure maximum coverage of the dlPAG. Given that we made multiple injections of a relatively large dose (1 nmol) of muscimol, it is very unlikely that in our experiments we failed to inhibit a critical group of neurons in the dlPAG that were consistently blocked by a single injection in the study by da Silva et al. (2003).
An important difference between our study and that of da Silva et al. (2003) is that in their studies the rats were conscious, whereas in our study the rats were anaesthetized. It has been previously suggested that in conscious rats the PAG is a source of tonic excitatory input to the DMH, which is essential for allowing activation of DMH neurons after blockade of their tonic inhibitory GABAergic inputs (Samuels et al. 2004). If that were the case, then the fact that in our experiments DMH-evoked responses were not reduced after inhibition of the PAG implies that in anaesthetized rats there is another source of tonic excitation of DMH neurons, independent of the PAG, that is not active in the conscious state. While this possibility cannot be ruled out, it seems to be unlikely, especially in view of the fact that the lack of effect of muscimol injections in the PAG on DMH-evoked responses was also observed in rats anaesthetized with alfaxalone, which has a different mechanism of anaesthesia to that of urethane (Davis et al. 1984; Hara & Harris, 2002).
Injections of bicuculline into the DMH of conscious rats evokes a marked increase in locomotor activity (DiMicco et al. 2002; da Silva et al. 2003). After injection of muscimol in the l/dlPAG, however, de Menezes et al. (2006) found that the increase in locomotor activity evoked from the DMH was significantly reduced. It is therefore possible that the increase in MAP and HR evoked from the DMH in conscious rats may be in part secondary to locomotor activity. If so, this could explain the marked reduction in the HR response to disinhibition of the DMH after inhibition of the PAG in conscious rats.
Finally, it is interesting to note that de Menezes et al. (2006) also found that, in conscious rats, injection of a relatively small dose (100 pmol) of muscimol into the l/dlPAG significantly reduced (by approximately 50%) both the thermogenic response (increase in core body temperature) and locomotor response evoked by disinhibition of the DMH, but had no significant effect on the evoked increase in MAP and only a modest effect (22% reduction) on the evoked increase in HR. This finding, which is consistent with the results of our study, suggests that the descending pathway from the DMH to the l/dlPAG may have a more important role in mediating thermogenic and behavioural responses evoked from the DMH, rather than increases in MAP and HR. In any case, the findings of our study indicate that this descending pathway does not have an essential role in mediating the increases in RSNA and respiratory activity evoked by disinhibition of the DMH.
Concluding remarks
The results of our study, taken together with those of de Menezes et al. (2009) indicate that a wide range of physiological responses, including increases in MAP, HR, RSNA, and respiratory activity and thermogenic responses that are generated by activation of neurons in the dlPAG, all depend upon the activity of neurons in the DMH. As illustrated in Fig. 11, we propose that all these effects are mediated by ascending projections from the PAG to the DMH. It has also been found that the stress-evoked increase in ACTH is also partly mediated by neurons in the l/dlPAG (de Menezes et al. 2008) and it has been suggested (de Menezes et al. 2009) that these neurons may also regulate ACTH secretion via an ascending pathway to DMH neurons that in turn project to the PVN.
As discussed above, our findings do not support the hypothesis proposed by de Menezes et al. (2009) that stress-evoked cardiovascular and respiratory responses depend upon reciprocal connections between the DMH and PAG. Instead, we propose that these responses are mediated both by ascending projections from the dlPAG to the hypothalamus and descending projections from the lPAG to the medulla (Fig. 11). This hypothesis is compatible with the view that descending pathways from the PAG to the brainstem can mediate stress-evoked cardiorespiratory responses (Carrive, 1993; Lovick, 1993). In fact, it is very interesting that Carrive et al. (1989) found that in the decerebrate cat, pressor and vasoconstrictor responses were only evoked by microinjections of DLH into sites in the lPAG that contained neurons that projected directly to the rostral ventrolateral medulla. In contrast, DLH microinjections into the dlPAG evoked no significant cardiovascular response. Since the hypothalamus is removed in the decerebrate preparation, this observation in the cat is entirely consistent with our finding in the rat that sympathetic vasomotor responses evoked from the dlPAG are dependent upon neuronal activity in the DMH.
If inputs to the dlPAG that drive cardiorespiratory responses do not arise primarily from the DMH, what is their origin? Just as the lPAG and dlPAG have quite different output connections, they also have very different afferent connections. In particular, as illustrated in Fig. 11, the dlPAG receives major inputs from a number of cortical regions, including the medial frontal cortex and the primary auditory and secondary visual cortices (Carrive, 1993; Bandler & Shipley, 1994; Vianna & Brandao, 2003), and a particularly strong input from the dorsal premamillary nucleus in the hypothalamus (Canteras & Swanson, 1992). The dorsal premamillary nucleus has a pivotal role in processing inputs that normally trigger defensive behaviour, such as exposure to a predator, and lesions of this nucleus greatly reduce such defensive responses (Cezario et al. 2008). Similarly, inputs from the cortical regions may also signal external threats that trigger defensive responses. In contrast, the lPAG does not receive major inputs from the above regions, but does receive major inputs from several regions that do not project to the dlPAG, such as the sensory nuclei in the spinal cord and brainstem (Bandler & Shipley, 1994; Vianna & Brandao, 2003) (Fig. 11).
In conclusion, the results of this study together with the findings of previous functional and anatomical studies indicate that ascending projections from the dlPAG to the DMH are a critical component of the central pathways subserving the cardiovascular and respiratory responses associated with defensive behaviour.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia and the Australian Research Council.
Glossary
Abbreviations
- 3V
third ventricle
- ACTH
adrenocorticotropic hormone
- Aq
aqueduct
- DA
dorsal hypothalamic area
- dl
dorsolateral
- DLH
d,l-homocysteic acid
- dm
dorsomedial
- DMH
dorsomedial hypothalamus
- DMN
dorsomedial hypothalamic nucleus
- f
fornix
- HR
heart rate
- l
lateral
- MAP
mean arterial pressure
- mt
mamillothalamic tract
- Musc
muscimol
- PAG
periaqueductal grey
- Pef
perifornical area
- PNA
phrenic nerve activity
- PVN
paraventricular nucleus
- RSNA
renal sympathetic nerve activity
- vl
ventrolateral
- VMH
ventromedial hypothalamic nucleus
Author contributions
J.H. had a major role in the conception and design of the study, in the analysis and interpretation of data, in the drafting of the article and in revising it critically for its important intellectual content. L.M.McD. contributed to the conception and design of the study, to the interpretation of the data, and to revising the article critically for its important intellectual content. R.A.L.D. had a major role in the conception and design of the study, in the analysis and interpretation of data, in the drafting of the article and in revising it critically for its important intellectual content. All authors gave final approval of the version to be published.
References
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1997;383:245–281. doi: 10.1002/(sici)1096-9861(19970707)383:3<245::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. The dorsal premammillary nucleus: an unusual component of the mammillary body. Proc Natl Acad Sci U S A. 1992;89:10089–10093. doi: 10.1073/pnas.89.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behaviour: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R, Dampney RAL. Viscerotopic control of regional vascular beds by discrete groups of neurons within the midbrain periaqueductal gray. Brain Res. 1989;493:385–390. doi: 10.1016/0006-8993(89)91176-1. [DOI] [PubMed] [Google Scholar]
- Cezario AF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS. Hypothalamic sites responding to predator threats – the role of the dorsal premammillary nucleus in unconditioned and conditioned antipredatory defensive behaviour. Eur J Neurosci. 2008;28:1003–1015. doi: 10.1111/j.1460-9568.2008.06392.x. [DOI] [PubMed] [Google Scholar]
- da Silva LG, de Menezes RC, dos Santos RA, Campagnole-Santos MJ, Fontes MA. Role of periaqueductal gray on the cardiovascular response evoked by disinhibition of the dorsomedial hypothalamus. Brain Res. 2003;984:206–214. doi: 10.1016/s0006-8993(03)03157-3. [DOI] [PubMed] [Google Scholar]
- Davis DW, Hawkins RA, Mans AM, Hibbard LS, Biebuyck JF. Regional cerebral glucose utilization during Althesin anaesthesia. Anesthesiology. 1984;61:362–368. doi: 10.1097/00000542-198410000-00002. [DOI] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Fontes MAP, DiMicco JA. Microinjection of muscimol into caudal periaqueductal gray lowers body temperature and attenuates increases in temperature and activity evoked from the dorsomedial hypothalamus. Brain Res. 2006;1092:129–137. doi: 10.1016/j.brainres.2006.03.080. [DOI] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Fontes MAP, DiMicco JA. Cardiovascular and thermal responses evoked from the periaqueductal grey require neuronal activity in the hypothalamus. J Physiol. 2009;587:1201–1215. doi: 10.1113/jphysiol.2008.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Sarkar S, Fontes MAP, DiMicco JA. Microinjection of muscimol into the periaqueductal gray suppresses cardiovascular and neuroendocrine response to air jet stress in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R881–R890. doi: 10.1152/ajpregu.00181.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Farkas E, Jansen ASP, Loewy AD. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Res. 1998;792:179–192. doi: 10.1016/s0006-8993(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Fontes MAP, Tagawa T, Polson JW, Cavanagh SJ, Dampney RAL. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol. 2001;280:H2891–H2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Grossman P. Respiration, stress, and cardiovascular function. Psychophysiology. 1983;20:284–300. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anaesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Hayward LF, Castellanos M, Davenport PW. Parabrachial neurons mediate dorsal periaqueductal gray evoked respiratory responses in the rat. J Appl Physiol. 2004;96:1146–1154. doi: 10.1152/japplphysiol.00903.2003. [DOI] [PubMed] [Google Scholar]
- Hilton SM. The defence-arousal system and its relevance for circulatory and respiratory control. J Exp Biol. 1982;100:159–174. doi: 10.1242/jeb.100.1.159. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MAP, Dampney RAL. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol. 2004;287:R824–R832. doi: 10.1152/ajpregu.00221.2004. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Subramanian SH, Balnave RJ, Turman AB, Moi Chow C. Roles of periaqueductal gray and nucleus tractus solitarius in cardiorespiratory function in the rat brainstem. Respir Physiol. 2000;120:185–195. doi: 10.1016/s0034-5687(00)00107-9. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Krout KE, Jansen AS, Loewy AD. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol. 1998;401:437–454. [PubMed] [Google Scholar]
- Lam W, Gundlach AL, Verberne AJ. Neuronal activation in the forebrain following electrical stimulation of the cuneiform nucleus in the rat: hypothalamic expression of c-fos and NGFI-A messenger RNA. Neuroscience. 1997;78:1069–1085. doi: 10.1016/s0306-4522(96)00527-1. [DOI] [PubMed] [Google Scholar]
- Lovick TA. The periaqueductal gray-rostral medulla connection in the defence reaction: efferent pathways and descending control mechanisms. Behav Brain Res. 1993;58:19–25. doi: 10.1016/0166-4328(93)90087-7. [DOI] [PubMed] [Google Scholar]
- McDowall LM, Horiuchi J, Dampney RAL. Effects of disinhibition of neurons in the dorsomedial hypothalamus on central respiratory drive. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1728–R1735. doi: 10.1152/ajpregu.00503.2007. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 2004. [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Schlenker E, Barnes L, Hansen S, Martin D. Cardiorespiratory and metabolic responses to injection of bicuculline into the hypothalamic paraventricular nucleus (PVN) of conscious rats. Brain Res. 2001;895:33–40. doi: 10.1016/s0006-8993(01)02011-x. [DOI] [PubMed] [Google Scholar]
- Shaffer JP. Modified sequentially rejective multiple test procedures. J Am Stat Assoc. 1986;91:826–831. [Google Scholar]
- Sly DJ, Colvill L, McKinley MJ, Oldfield BJ. Identification of neural projections from the forebrain to the kidney, using the virus pseudorabies. J Auton Nerv Syst. 1999;77:73–82. [PubMed] [Google Scholar]
- Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996a;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996b;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol. 1991;309:305–327. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- Vianna DM, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Med Biol Res. 2003;36:557–566. doi: 10.1590/s0100-879x2003000500002. [DOI] [PubMed] [Google Scholar]