Abstract
Transcranial magnetic stimulation (TMS) allows the testing of various inhibitory processes in human motor cortex. Here we aimed at gaining more insight into the underlying physiology by studying the interactions between short-interval intracortical inhibition (SICI) and short-latency afferent inhibition (SAI). SICI and SAI were examined in a slightly contracting hand muscle of healthy subjects by measuring inhibition of a test motor-evoked potential conditioned by a sub-threshold motor cortical magnetic pulse (S1) or an electrical pulse (P) applied to the ulnar nerve at the wrist, respectively. SICI alone and SAI alone had similar magnitude when S1 intensity was set to 90% active motor threshold and P intensity to three times the perceptual sensory threshold. SICI was reduced or even disinhibited when P was co-applied, and SAI was reduced or disinhibited when S1 was co-applied. These interactions did not depend on the exact timing of arrival of P and S1 in motor cortex. A control experiment with a S1 intensity lowered to 70% active motor threshold excluded a contribution by short-interval intracortical facilitation. Finally, SICI with co-applied P correlated linearly with SICI alone with a slope of the regression line close to 1 whereas SAI did not correlate with SAI when S1 was co-applied with a slope of the regression line close to zero. Data indicate that S1 largely eliminates the effects of P when applied together, suggesting dominance of S1 over P. Findings strongly support the idea that SICI and SAI are mediated through two distinct and reciprocally connected subtypes of GABAergic inhibitory interneurons with convergent projections onto the corticospinal neurons. Furthermore, dominance of S1 over P is compatible with the notion that the SICI interneurons target the corticospinal neurons closer to their axon initial segment than the SAI interneurons.
Introduction
TMS allows distinction of various inhibitory and excitatory processes in the human motor cortex. Several forms of inhibition can be segregated on the basis of their electrophysiological and pharmacological profiles, and their mutual interactions (Chen, 2004). For instance, SICI occurs at interstimulus intervals between S1 and S2 of 1–5 ms and increases by pharmacological enhancement of neurotransmission through the GABAA receptor (Kujirai et al. 1993; Ziemann et al. 1996a; Di Lazzaro et al. 2000a, 2006; Ilic et al. 2002), whereas long-interval intracortical inhibition occurs at intervals of 50–200 ms and increases by enhancement of neurotransmission through the GABAB receptor (Valls-Sole et al. 1992; McDonnell et al. 2006; Müller-Dahlhaus et al. 2008). SICI is reduced in the presence of long-interval intracortical inhibition, which is consistent with GABAB receptor-mediated presynaptic auto-inhibition of GABAAergic interneurons (Sanger et al. 2001; Müller-Dahlhaus et al. 2008). Another form of motor cortical inhibition is SAI, which refers to the inhibition of a test MEP by a conditioning electrical stimulus given to a mixed nerve of the contralateral hand about 20 ms earlier (Tokimura et al. 2000). Pharmacological profiling distinguishes SAI from SICI because only SAI but not SICI decreases with blockade of cholinergic M1 receptors (Di Lazzaro et al. 2000b). In addition, the classical benzodiazepine lorazepam increases SICI but decreases SAI (Di Lazzaro et al. 2005a,b;), and zolpidem, a positive modulator at the GABAA receptor with high affinity to the α1 subtype of the GABAA receptor, does not affect SICI but decreases SAI (Di Lazzaro et al. 2007). These data strongly suggest that SICI and SAI are mediated by at least partially distinct inhibitory neuronal circuits in the central nervous system. It is still, however, an unresolved question if and how SICI and SAI interact. This is an important question, given the potential relevance of SICI and SAI as neurophysiological surrogate markers for neurological and psychiatric disorders (Chen et al. 2008), and given recent work that has substantially advanced our insight into the physiology of TMS measures of motor cortical inhibition through analysis of their interactions (Sanger et al. 2001; Daskalakis et al. 2002, 2004; Sailer et al. 2002; Kukaswadia et al. 2005; Lee et al. 2007; Müller-Dahlhaus et al. 2008). Here we have designed three experiments in order to examine carefully and extensively the interactions between SICI and SAI in healthy subjects.
Methods
Subjects
The study consisted of three experiments. Ten healthy subjects (mean age, 30.0 ± 4.4 years; 9 male) participated in Experiment 1, seven (mean age, 30.6 ± 4.7 years; all male) participated in Experiment 2, and eight (mean age, 30.0 ± 8.2 years; 7 male) participated in Experiment 3. Most subjects took part in more than one experiment. All subjects were right-handed according to the Edinburgh Inventory (Oldfield, 1971). Written informed consent was obtained from all subjects. The study was performed according to the latest revision of the Declaration of Helsinki and was approved by the ethics committee of the hospital of the Goethe-University of Frankfurt, Germany.
Recording and stimulation procedures
Subjects were seated comfortably in a reclining chair. Surface EMG was recorded from the abductor digiti minimi (ADM) muscle of the right hand, using Ag–AgCl electrodes in a belly-tendon montage, with the active electrode placed over the motor point and the reference electrode on the proximal interphalangeal joint of the little finger. The EMG signal was amplified and filtered (10 Hz–2 kHz) (Counterpoint Electromyograph, Dantec Electronics, Skovlunde, Denmark), digitized (sampling rate 4 kHz) (CED micro 1401 laboratory interface, Cambridge Electronic Design, Cambridge, UK) and then fed into a personal computer. In-house programmed data collection and conditional averaging software (Spike 2 for Windows, Version 3.05, Cambridge Electronic Design) was used for off-line analysis.
Focal transcranial magnetic stimulation (TMS) was applied over the hand area of the left motor cortex through a figure-of-eight coil (outer diameter of each loop, 9 cm; peak magnetic field ∼1.5 T) using two Magstim 200 magnetic stimulators (Magstim, Whitland, UK) which were connected to a BiStim module (Magstim) throughout all measurements. The stimulating coil was placed flat on the skull with the handle pointing backwards and rotated 45 deg away from the midline. This way, the monophasic current induced in the brain flowed from lateral–posterior to medial–anterior, approximately perpendicular towards the assumed line of the central sulcus, and the corticospinal neurons are activated predominantly transsynaptically (Di Lazzaro et al. 2008). The optimal coil position was determined as the site where stimulation at slightly supra-threshold stimulus intensity produced consistently the largest MEP in the contralateral ADM. This site was marked on the scalp with a pen in order to ensure a constant coil placement throughout the experiment. Active motor threshold (AMT) was obtained during slight isometric contraction of the right ADM (5–10% of maximum voluntary contraction). AMT was determined to the nearest 1% of maximum stimulator output and was defined as the lowest stimulus intensity that elicited a MEP > 100 μV in the curve average of five trials (Ziemann et al. 1998). All experiments were conducted during slight isometric contraction of the ADM (5–10% of maximum strength). The level of contraction was continuously monitored by audiovisual feedback of the EMG signal recorded at high gain (50 μV per division). The rationale for conducting all experiments during slight muscle contraction was to ascertain that identical experiments could be conducted in neurological patients who are incapable of maintaining full relaxation, such as patients with movement disorders. Previous reports showed that it is feasible to investigate SICI and SAI during muscle contraction (Tokimura et al. 2000; Ilic et al. 2002; Zoghi et al. 2003; Ortu et al. 2008). We are aware that the same experiments but with the target muscle at rest may have led to different findings, but this is considered unlikely given similar preliminary results from one previous study conducted at rest (Stefan et al. 2002) (see Discussion).
Experiment 1. Interactions between SICI and SAI
SICI was tested according to an established paired-pulse TMS protocol (Kujirai et al. 1993; Ziemann et al. 1996b), using a sub-threshold conditioning pulse (S1) of 90% AMT followed by a supra-threshold test pulse (S2). S2 was adjusted to elicit a test MEP of on average 1.5 mV in peak-to-peak amplitude in the active ADM when given alone. SICI was tested at five different ISIs (1.0, 1.5, 2.1, 2.7 and 3.0 ms) in five separate blocks of trials. These ISIs were selected because it was shown that SICI consists of at least two physiologically distinct phases of inhibition, one at an ISI of about 1.0 ms, and another one at ISIs of approximately 2.5–4 ms (Fisher et al. 2002; Hanajima et al. 2003; Roshan et al. 2003; Vucic et al. 2006). SICI was expressed as the ratio of the mean conditioned MEP over the mean unconditioned MEP in the same block of trials (S1S2/S2).
SAI was tested in the same blocks of trials according to an established protocol (Tokimura et al. 2000). The conditioning electrical pulse (P) was applied to the ulnar nerve at the wrist of the right arm through a bipolar electrode (cathode proximal) using a constant current square-wave pulse (duration, 1 ms) of three times perceptual sensory threshold intensity. P was followed by the magnetic test pulse to the contralateral left motor cortex (S2) at a fixed ISI of N20+ 2 ms. N20 refers to the peak latency of the individually determined earliest cortical component of the standard ulnar nerve somatosensory-evoked potential recordings (active electrode over C3′, reference electrode over Fz according to the 10–20 International EEG system, bandpass filter 3 Hz–2 kHz, rate of stimulation 3.3 Hz, average of 300 trials). N20 was 20.3 ± 0.4 ms in Experiment 1, 20.7 ± 0.7 ms in Experiment 2, and 20.2 ± 0.4 ms in Experiment 3. This interval of N20+ 2 ms was chosen because it resulted in consistent inhibition of the test MEP in previous experiments (Tokimura et al. 2000). Most previous studies used median nerve stimulation and MEP recording from the first dorsal interosseus muscle for SAI measurements (Tokimura et al. 2000; Di Lazzaro et al. 2002). However, SAI was qualitatively very similar, no matter if the median nerve was stimulated and MEPs were recorded from the first dorsal interosseus or abductor pollicis brevis muscle (Tokimura et al. 2000), or if MEPs were recorded from the first dorsal interosseus muscle and the median or ulnar nerve was stimulated (Di Lazzaro et al. 2005b). Magnitude of SAI in the present study (see Results section) was similar to SAI in those previous studies. This indicates that SAI can be reliably tested by recording from different intrinsic hand muscles and by afferent stimulation of different forearm nerves. Still, it remains to be tested to what extent the interactions between SICI and SAI demonstrated in this study for the ADM hold true for any of the other intrinsic hand muscles with different functional roles.
SAI was expressed as the ratio of the mean conditioned MEP over the mean unconditioned MEP in the same block of trials (PS2/S2).
Each of the five blocks of trials (each block assigned to one of the 5 ISIs of SICI) consisted of three conditions (A–C in Table 1): S2 alone, S1S2 and PS2. Ten repeats were delivered per condition in randomized order. The mean intertrial interval was 6 s with a random variation of ± 25% to limit anticipation of the next trial.
Table 1.
Stimulus conditions for all experiments
| Condition | P | S1 | S2 | S2* |
|---|---|---|---|---|
| A | MEP1.5mV | |||
| B | 0.9 × AMT | MEP1.5mV | ||
| C | 3 × PT | MEP1.5mV | ||
| D | cMEP1.5mV | |||
| E | 0.9 × AMT | cMEP1.5mV | ||
| F | 3 × PT | cMEP1.5mV | ||
| G | 3 × PT | 0.9 × AMT | cMEP1.5mV |
P, electrical pulse to the right ulnar nerve; 3 × PT, three times perceptual threshold; S1, conditioning magnetic stimulus to left motor cortex; AMT, active motor threshold; S2, test magnetic stimulus to left motor cortex; MEP1.5mV, unconditioned test motor-evoked potential of 1.5 mV in peak-to-peak amplitude; S2*, test stimulus to left motor cortex of adjusted intensity; cMEP1.5mV, conditioned test motor-evoked potential (by P or S1) of 1.5 mV in peak-to-peak amplitude. In Experiment 1, the interstimulus interval between P and S2 (or S2*) was fixed at N20+ 2 ms, while the interval between S1 and S2 (or S2*) varied (1.0, 1.5, 2.1, 2.7 and 3.0 ms). In Experiment 2, the interstimulus interval between P and S2 (or S2*) varied (N20+ 2 ms, N20+ 4.1 ms), while the interval between S1 and S2 (or S2*) was fixed at 2.1 ms. In Experiment 3, the interstimulus interval between S1 and S2 (or S2*) was 2.1 ms, and that between P and S2 (or S2*) was N20+ 2 ms.
Another five blocks of trials were run to test the interactions between the inhibitory effects of S1 and P. In these blocks, everything remained the same as for conditions A–C in Table 1, except that the intensity of S2 was increased (S2*) so that the MEP amplitude of PS2* matched the MEP amplitude of S2 (∼1.5 mV) in the block of trials containing conditions A–C. Furthermore, one condition was added in which all three pulses were given in the same trial (PS1S2*). Therefore, these blocks of trials consisted of four conditions (D–G in Table 1): S2* alone, S1S2*, PS2* and PS1S2*. SICI and SAI were expressed as above (S1S2*/S2* and PS2*/S2*, respectively).
The interactions between the inhibitory effects of S1 and P on S2* were analysed within each pair of blocks of trials that tested the same ISI between S1 and S2. Two main analyses were performed: (1) PS1S2*/PS2* tests the effect of S1 on S2* with co-applied P. This can be directly compared to the two measurements of SICI (conditions B/A and E/D in Table 1): the effects of S1 on S2 (S1S2/S2) where PS2* and S2 are matched for amplitude (∼1.5 mV), and the effects of S1 on S2* (S1S2*/S2*) where PS2* and S2* are matched for stimulus intensity. Four outcomes would be possible: PS1S2*/PS2* < SICI indicates enhancement of the inhibitory effect of S1 on S2* with co-applied P; PS1S2*/PS2*= SICI indicates no effect of P on the inhibitory effect of S1 on S2*; PS1S2*/PS2* > SICI indicates reduction of the inhibitory effect of S1 on S2* by P; PS1S2*/PS2* > 1 indicates disinhibition; (2) PS1S2*/S1S2* tests the effects of P on S2* with co-applied S1. This can be directly compared to the two measurements of SAI (conditions C/A and F/D in Table 1): the effects of P on S2 (PS2/S2) where S1S2* and S2 were matched for amplitude (see Results), and the effects of P on S2* (PS2*/S2*) where S1S2* and S2* are matched for stimulus intensity. The interpretation of the four possible outcomes is analogous to PS1S2*/PS2* (see above).
The interaction of the inhibitory effects between P and S1 on S2* was explored further by correlation analyses between SICI (independent variables, conditions B/A and E/D in Table 1) and PS1S2*/PS2* (dependent variable, conditions G/F in Table 1). This way it is possible to see with greater detail to which extent different levels of SICI are changed with co-applied P. Analogous correlation analyses were performed between SAI (independent variables, conditions C/A and F/D in Table 1) and PS1S2*/S1S2* (dependent variable, conditions G/E in Table 1).
To assess the effects of the temporal order of the arrival of inputs from S1 and P in motor cortex on their interactions with S2*, the above correlation analyses were stratified according to the ISI between S1 and S2 (≤ 1.5 ms vs.≥ 2.1 ms). The rationale for this stratification came from epidural cervical spinal cord recordings of the descending corticospinal volley elicited by S2. These suggested that S1 and P inhibit the same or similar neuronal elements in motor cortex, which are those responsible for the generation of the so-called late indirect waves (Di Lazzaro et al. 1998; Tokimura et al. 2000). Furthermore, the onset of SAI was between N20 and N20+ 1 ms (cf. Fig. 5 in Tokimura et al. 2000). Since the interval between P and S2* was always N20+ 2 ms in Experiment 1, it is likely that the onset of the effects of S1 on S2* preceded those of P when the interval between S1 and S2* exceeded 2 ms while the reverse is true for the intervals of 1.0 and 1.5 ms. Two principal outcomes are possible from this stratified correlation analysis: the regression lines do or do not differ, suggesting that the exact temporal order of onsets of the S1 and P effects does or does not play a role, respectively.
Experiment 2. Interactions between SICI and SAI – effects of the interval between P and S2
In order to analyse the effects of temporal order further, this experiment varied the interval between P and S2 (or S2*) while now the ISI between S1 and S2 (or S2*) was kept fixed at 2.1 ms. Two intervals were investigated: N20+ 2 ms (as in Experiment 1) and N20+ 4.1 ms. According to the line of arguments in Experiment 1 (see above), it is very likely that the onset of effects of S1 precede those of P with N20+ 2 ms while the reverse is true with N20+ 4.1 ms. Otherwise, design and data analysis of Experiment 2 were the same as in Experiment 1.
Experiment 3. Interactions between SICI and SAI using a reduced intensity of S1 of 70% AMT
Reduction of SICI during slight target muscle contraction (10% of maximum voluntary contraction) compared to rest is partially explained by activation of short-interval intracortical facilitation (SICF) when using an ISI of 2–3 ms and a S1 intensity of 90% AMT (Ortu et al. 2008). Therefore, the interactions between SICI and SAI in Experiments 1 and 2 (see below, Results section) could have been caused by an interaction between SICI and SAI, or SICF and SAI, or both. Experiment 3 addressed this issue by using a S1 intensity of 70% AMT where SICI can be studied in isolation without potential contamination by SICF (Ortu et al. 2008). Experiment 3 was restricted to an ISI = 2.1 ms between S1 and S2 as there was no indication for an effect of ISI in Experiment 1. Otherwise, Experiment 3 was identical to Experiment 1.
Statistical procedures
Repeated measures analyses of variance (rmANOVAs) were applied to test the within-subject effects of measure (SICI, SAI or MEP amplitude) and isi (for details, see Results). Whenever appropriate, post hoc comparisons were performed, using two-tailed paired t tests. The relations between SICI without vs. with co-application of P and SAI without vs. with co-application of S1 were tested by linear regression analyses. Statistical significance was assumed if P < 0.05. All data are given as means ±s.e.m. if not indicated otherwise.
Results
Experiment 1. Interactions between SICI and SAI
Representative data from one subject
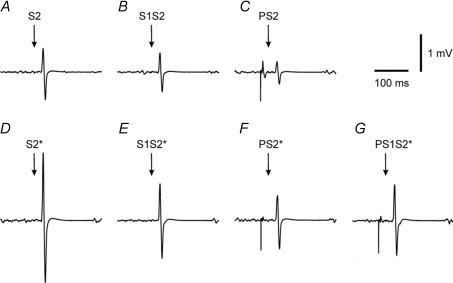
EMG tracings from one representative subject illustrate the findings of Experiment 1 (Fig. 1): PS1S2*/PS2*= 1.32 > S1S2/S2 = 0.76 (comparison matched for amplitude of the denominator i.e. PS2* (Fig. 1F) ≈ S2 (Fig. 1A)), and PS1S2*/PS2*= 1.32 > S1S2*/S2*= 0.57 (comparison matched for stimulus intensity of the test pulse). Furthermore, PS1S2*/S1S2*= 0.95 > PS2/S2 = 0.43 (comparison roughly matched for amplitude of the denominator; in fact S1S2* (Fig. 1E) was even slightly larger than S2 (Fig. 1A), which probably leads to underestimation of the reduction of SAI with co-application of P), and PS1S2*/S1S2*= 0.95 > PS2*/S2*= 0.41 (comparison matched for stimulus intensity of the test pulse). In summary, data from this subject point to disinhibition of SICI when co-applying P, and reduction of SAI when co-applying S1.
Figure 1. Interactions between SICI and SAI in one subject (Experiment 1).
All traces are averages of 10 trials of EMG recordings from the contracting right ADM of one representative subject. Conditions in A–G correspond to conditions A–G in Table 1. From these data the following MEP ratios were calculated: SICI alone (S1S2/S2 = 0.76; S1S2*/S2*= 0.57), SICI with co-applied P (PS1S2*/PS2*= 1.32), SAI alone (PS2/S2 = 0.43; PS2*/S2*= 0.41), and SAI with co-applied S1 (PS1S2*/S1S2*= 0.95). Note the match of MEP amplitude between S2 and PS2*.
Group data
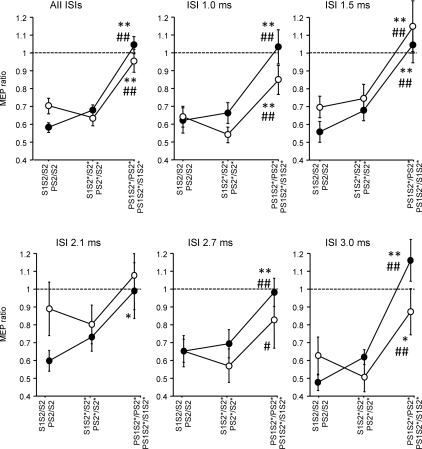
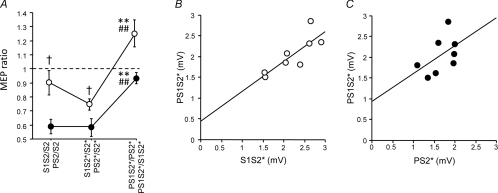
The rmANOVA with the within-subject effects of sici (S1S2/S2, S1S2*/S2* and PS1S2*/PS2*) and isi between S1 and S2 (1.0, 1.5, 2.1, 2.7 and 3.0 ms) revealed a significant effect of sici (F2,18= 24.9; P < 0.0001) while the effect of isi (F4,36= 1.65; P= 0.18) and the interaction between sici and isi (F8,72= 0.63, P= 0.75) were not significant. Post hoc testing showed less inhibition for PS1S2*/PS2* compared to S1S2/S2 (P < 0.0001), and for PS1S2*/PS2* compared to S1S2*/S2* (P < 0.0001), indicating that the inhibitory effect of S1 on S2* was attenuated when co-applying P (Fig. 2, open circles).
Figure 2. Interactions between SICI and SAI – effects of the interval between S1 and S2 (Experiment 1).
SICI alone (S1S2/S2, S1S2*/S2*) vs. SICI with co-applied P (PS1S2*/PS2*, open circles) and SAI alone (PS2/S2, PS2*/S2*) vs. SAI with co-applied S1 (PS1S2*/S1S2*, filled circles) are shown as grand average across all ISIs between S1 and S2, and separately for the single ISIs (1.0, 1.5, 2.1, 2.7 and 3.0 ms). Values less than 1 (dashed horizontal lines) indicate inhibition. S1 inhibited PS2* significantly less than S2 or S2* alone, irrespective of ISI. In some instances, PS1S2*/PS2* even resulted in values larger than 1, indicating disinhibition. Similarly, P inhibited S1S2* significantly less than S2 or S2* alone, irrespective of ISI. Again, in some instances PS1S2*/S1S2* resulted in values larger than 1, indicating disinhibition. Significant differences between PS1S2*/PS2* and S1S2/S2, or between PS1S2*/S1S2* and PS2/S2 are indicated by * (P < 0.05) or ** (P < 0.01). Significant differences between PS1S2*/PS2* and S1S2*/S2*, or between PS1S2*/S1S2* and PS2*/S2* are indicated by # (P < 0.05) or ## (P < 0.01). All data are means ± 1 s.e.m. of 10 subjects.
Similarly, the rmANOVA with the within-subject effects of sai (PS2/S2, PS2*/S2* and PS1S2*/S1S2*) and isi between S1 and S2 revealed a highly significant effect of sai (F2,18= 49.8; P < 0.0001) while the effect of isi (F4,36= 0.04; P= 1.00) and the interaction between sai and isi (F8,72= 0.93, P= 0.50) were not significant. Post hoc testing showed less inhibition for PS1S2*/S1S2* compared to PS2/S2 (P < 0.0001), and for PS1S2*/S1S2* compared to PS2*/S2* (P < 0.0001), indicating that the inhibitory effect of P on S2* was attenuated when co-applying S1 (Fig. 2, filled circles).
The rmANOVA with the within-subject effects of mep amplitude (MEP amplitude elicited by S2, PS2* and S1S2*) and isi revealed no effect of mep amplitude (F2,18= 1.95, P= 0.15; S2: 1.48 ± 0.07 mV, PS2*: 1.52 ± 0.08 mV, S1S2*: 1.35 ± 0.09 mV) or isi (F4,36= 1.90, P= 0.13), or interaction between mep amplitude and isi (F8,72= 1.15, P= 0.34). Therefore, the above comparisons of PS1S2*/PS2* with S1S2/S2, and of PS1S2*/S1S2* with PS2/S2 (Fig. 2) were adequately matched for MEP amplitude.
Furthermore, amplitudes of the direct muscle response (M wave amplitude) elicited by P were not different across conditions (PS2: 0.24 ± 0.05 mV, PS2*: 0.28 ± 0.08 mV, PS1S2*: 0.29 ± 0.1 mV; pair-wise comparisons by t tests, all P > 0.3), indicating that there were no differences in the effectiveness of electrical stimulation of the ulnar nerve which could explain any of the above interactions between S1 and P.
Finally, the level of voluntary isometric contraction of the right ADM, as quantified by the root mean square of the EMG signal in the 100 ms prior to stimulation was not different between conditions (S2: 44 ± 4 μV, S1S2: 43 ± 4 μV, PS2: 43 ± 4 μV, S2*: 51 ± 5 μV, S1S2*: 51 ± 5 μV, PS2*: 50 ± 5 μV, PS1S2*: 50 ± 5 μV; pair-wise comparisons by t tests, all P > 0.4). Therefore, there were no differences in the level of target muscle contraction between conditions, which may have accounted for the interactions between S1 and P.
The data in Fig. 2 suggest that decreasing the intensity of the test pulse from S2* to S2 resulted in a trend towards a decrease of SICI, but a trend towards an increase of SAI. This was further tested by an rmANOVA, which compared the differences S1S2*/S2*– S1S2/S2 and PS2*/S2*– PS2/S2. This revealed a highly significant effect of difference (F1,9= 12.3, P= 0.0001) while the effect of isi and the interaction between isi and difference were not significant, indicating that SICI decreased with decreasing intensity of the test pulse while at the same time SAI increased. The SICI data are in good agreement with previous observations (Kujirai et al. 1993; Ziemann et al. 1996b; Sanger et al. 2001; Daskalakis et al. 2002; Sailer et al. 2002) while effects of test pulse intensity on SAI have not been reported yet.
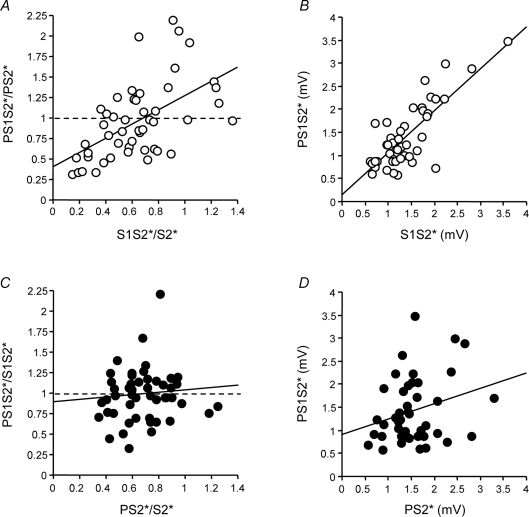
The correlation analysis between S1S2*/S2* (SICI) and PS1S2*/PS2* (SICI with co-application of P) (Fig. 3A) demonstrated a significant linear correlation between the two measures (R2= 0.31, P < 0.0001), indicating that the inhibitory effect of S1 on S2* directly relates to the inhibitory effect of S1 on S2* when co-applying P. The slope of the regression line was close to 1 (0.87). The y-intercept was significantly above zero (0.41, P= 0.003), indicating, in accord with the data in Fig. 2, that the presence of P resulted in a reduction of the inhibitory effect of S1 on S2*. The linear correlation with a slope close to 1 could be interpreted as maintenance of the inhibitory effects of S1 no matter whether or not P was co-applied. In contrast, the correlation analysis between PS2*/S2* (SAI) and PS1S2*/S1S2* (SAI with co-application of S1) (Fig. 3C) revealed a very different result: here, no correlation was found (R2= 0.008, P= 0.55), indicating that the inhibitory effect of P on S2* did not relate to the effects of P on S2* with co-application of S1. The slope of the regression line was close to zero (0.15) and the y-intercept was close to 1 (0.94), extending the data in Fig. 2 by showing that co-application of S1 leads to abolition of the inhibitory effects of P on S2*, irrespective of the magnitude of the inhibitory effect by P.
Figure 3. Correlations between SICI without vs. with co-applied P (A), between SAI without vs. with co-applied S1 (C), and between MEP amplitude of S1S2* (B) or PS2* (D) with MEP amplitude of PS1S2* (Experiment 1).
A, SICI alone (S1S2*/S2*) is plotted against SICI with co-applied P (PS1S2*/PS2*). Each data point is from one single subject (n= 10) and one ISI between S1 and S2 (5 different ISIs). Values less than 1 (dashed line) indicate inhibition. The thick continuous line is the regression line. B, MEP amplitude (in mV) of S1S2* is plotted against that of PS1S2*. C, SAI alone (PS2*/S2*) is plotted against SAI with co-applied S1 (PS1S2*/S1S2*). D, MEP amplitude (in mV) of PS2* is plotted against that of PS1S2*. Arrangement and conventions in B–D are otherwise the same as in A.
In conjunction, these data provide evidence for the proposition that S1 largely abolished the effects of P, or in other words, that the inhibitory effects of S1 on S2* are dominant over those of P on S2*. If this is correct, then MEP amplitude of S1S2* (condition E in Table 1) should strongly correlate with that of PS1S2* (condition G) with slope close to 1 and y-intercept close to 0, whereas MEP amplitude of PS2* (condition F) and PS1S2* (condition G) should show no correlation. This was confirmed by the respective correlation analyses: MEP amplitude of S1S2* correlated significantly with that of PS1S2* (R2= 0.61, P < 0.0001) with a slope of 0.91 and a y-intercept of 0.15 (Fig. 3B), whereas MEP amplitude of PS2* did not correlate with that of PS1S2* (R2= 0.07, P > 0.05, slope 0.33, y-intercept 0.90, Fig. 3D).
One critical issue in validating this interpretation is the analysis of the magnitude of the inhibitory effects of P and S1 when given in the absence of the other input because dominance of S1 over P would be easily explained if the inhibitory effects of S1 in the absence of P were already stronger than those of P in the absence of S1. This was, however, not the case. The rmANOVA with the within-subject effects of inhibition (S1S2*/S2*vs. PS2*/S2*) and isi (1.0, 1.5, 2.1, 2.7 and 3.0 ms) showed no significant effects of inhibition, isi or the interaction between inhibitionand ISI (all P > 0.15, Fig. 2).
Another critical issue to be tested in this regard is the temporal order of effects by P vs. S1 onto S2 (and S2*). The significant interactions between the inhibitory effects of P and S1 on S2 (and S2*) (Fig. 2) strongly suggest that they converge, at least partially, onto the same motor cortical neuronal circuit. Theoretically, the proposed dominance of S1 over P could then be explained by occlusion when S1 always arrived in advance of P. However, linear regression analyses stratified into ISIs ≤ 1.5 ms vs. ISIs > 1.5 ms revealed very similar correlations and regression line characteristics as those across all ISIs shown in Fig. 3. In particular, the regression analysis of MEP amplitude of S1S2*vs. that of PS1S2* showed a correlation of R2= 0.54 (P= 0.0008) with a slope of 1.06 and a y-intercept of −0.04 for ISIs ≤ 1.5 ms, and a correlation of R2= 0.66 (P < 0.0001) with a slope of 0.83 and a y-intercept of 0.23 for ISIs > 1.5 ms. This strongly suggests that the exact temporal order of arrival of inhibitory inputs elicited by S1 and P into the motor cortical circuit is not critical for the proposed dominance of S1 over P. In addition, occlusion cannot explain disinhibition (i.e. PS1S2*/S1S2* > 1.0) which in many instances occurred (see Figs 2 and 3).
Experiment 2. Interactions between SICI and SAI – effects of the interval between P and S2
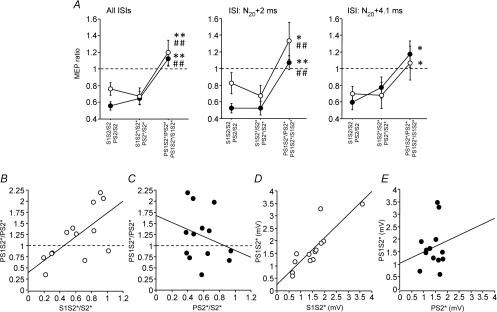
The rmANOVA with the within-subject effects sici (S1S2/S2, S1S2*/S2* and PS1S2*/PS2*) and isi (N20+ 2 ms and N20+ 4.1 ms) showed a significant effect of sici (F2,12= 11.7, P= 0.0003) while the effect of isi (F1,6= 0.49, P= 0.50) and the interaction between sici and ISI (F2,12= 0.70, P= 0.51) were not significant (Fig. 4A). Post hoc testing showed less inhibition for PS1S2*/PS2* compared to S1S2/S2 and S1S2*/S2* (Fig. 4A). Similarly, the rmANOVA with the within-subject effects of sai (PS2/S2, PS2*/S2* and PS1S2*/S1S2*) and isi (N20+ 2 ms and N20+ 4.1 ms) showed a significant effect of sai (F2,12= 14.9, P < 0.0001) while the effect of isi (F1,6= 3.64, P= 0.08) and the interaction between sai and ISI (F2,12= 0.36, P= 0.70) were not significant (Fig. 4A). Post hoc testing showed less inhibition for PS1S2*/S1S2* compared to PS2/S2 and PS2*/S2* (Fig. 4A).
Figure 4. Interactions between SICI and SAI – effects of the interval between P and S2 (Experiment 2).
A, SICI alone (S1S2/S2, S1S2*/S2*) vs. SICI with co-applied P (PS1S2*/PS2*, open circles) and SAI alone (PS2/S2, PS2*/S2*) vs. SAI with co-applied S1 (PS1S2*/S1S2*, filled circles) are shown as grand average across the two tested ISIs between P and S2, and separately for the single ISIs (N20+ 2 ms, N20+ 4.1 ms). Values less than 1 (dashed lines) indicate inhibition. S1 inhibited PS2* significantly less than S2 or S2* alone, irrespective of ISI. In some instances, PS1S2*/PS2* even resulted in values larger than 1, indicating disinhibition. Similarly, P inhibited S1S2* significantly less than S2 or S2* alone, irrespective of ISI. Again, in some instances PS1S2*/S1S2* resulted in values larger than 1, indicating disinhibition. Significant differences between PS1S2*/PS2* and S1S2/S2, or between PS1S2*/S1S2* and PS2/S2 are indicated by * (P < 0.05) or ** (P < 0.01). Significant differences between PS1S2*/PS2* and S1S2*/S2*, or between PS1S2*/S1S2* and PS2*/S2* are indicated by # (P < 0.05) or ## (P < 0.01). All data are means ± 1 s.e.m. of 7 subjects. B, SICI alone (S1S2*/S2*) is plotted against SICI with co-applied P (PS1S2*/PS2*). Each data point is from one single subject (n= 7) and one ISI between P and S2 (2 different ISIs). Values less than 1 (dashed line) indicate inhibition. The thick continuous line is the regression line. C, SAI alone (PS2*/S2*) is plotted against SAI with co-applied S1 (PS1S2*/S1S2*). D, MEP amplitude (in mV) of S1S2* is plotted against that of PS1S2*. E, MEP amplitude (in mV) of PS2* is plotted against that of PS1S2*. Arrangement and conventions in C–E are otherwise the same as in B.
The rmANOVA with the main effects of mep amplitude (MEP amplitude elicited by S2, PS2* and S1S2*) and isi revealed no effect of mep amplitude (F2,12= 0.38, P= 0.69; S2: 1.38 ± 0.08 mV, PS2*: 1.44 ± 0.09 mV, S1S2*: 1.54 ± 0.21 mV) or ISI (F1,6= 1.90, P= 0.13), or interaction between mep amplitude and ISI (F2,12= 1.15, P= 0.34). Therefore, the comparisons of PS1S2*/PS2* with S1S2/S2 (Fig. 4A), and of PS1S2*/S1S2* with PS2/S2 (Fig. 4A) were adequately matched for MEP amplitude.
Furthermore, M wave amplitudes elicited by P were not different across conditions (PS2: 0.22 ± 0.04 mV, PS2*: 0.21 ± 0.03 mV, PS1S2*: 0.20 ± 0.03 mV; pair-wise comparisons by paired t tests, all P > 0.3), indicating that there were no differences in the effectiveness of electrical stimulation of the ulnar nerve between conditions that can be attributed to explain the interactions between S1 and P shown above.
Finally, the level of voluntary isometric contraction of the right ADM, as quantified by the root mean square of the EMG signal in the 100 ms prior to stimulation was not different across conditions (S2: 43 ± 5 μV, S1S2: 41 ± 4 μV, PS2: 38 ± 4 μV, S2*: 38 ± 3 μV, S1S2*: 36 ± 3 μV, PS2*: 42 ± 4 μV, PS1S2*: 40 ± 4 μV; pair-wise comparisons by t tests, all P > 0.05). This excludes the possibility that differences between conditions in the level of target muscle contraction can be attributed to the interactions between S1 and P.
The magnitude of the inhibitory effect of S1 on S2 was slightly less than the inhibitory effect of P on S2 (F1,6= 6.31, P= 0.027) (Fig. 4A) while the effect of ISI and the interaction between inhibition and isi were not significant. The inhibitory effects of S1 vs. P on S2* were not different (F1,6= 0.15, P= 0.70) (Fig. 4A).
The correlation analyses (Fig. 4B–C) confirmed the results from Experiment 1: the magnitude of the inhibitory effect of S1 on S2* when co-applying P correlated linearly with the magnitude of the inhibitory effect of S1 on S2* alone (R2= 0.45, P= 0.01, slope 1.33, y-intercept 0.39, Fig. 4B). In contrast, the inhibitory effect of P on S2* when co-applying S1 did not correlate with the inhibitory effect of P on S2* alone (R2= 0.07, P= 0.39, Fig. 4C).
In conjunction, these data provide additional evidence for the proposition that S1 essentially abolished the effects of P, or in other words, that the inhibitory effects of S1 on S2* are dominant over those of P on S2*. In analogy to the line of arguments in Experiment 1, this was further confirmed by the following correlation analyses: MEP amplitude of S1S2* correlated significantly with that of PS1S2* (R2= 0.72, P= 0.0002) with a slope of 0.93 and a y-intercept of 0.26 (Fig. 4D), whereas MEP amplitude of PS2* did not correlate with that of PS1S2* (R2= 0.03, P > 0.05, slope 0.45, y-intercept 1.03, Fig. 4E). Finally, regression lines for the correlation of MEP amplitude of S1S2* with that of PS1S2* were very similar when subdividing the data into the two ISIs between P and S2 of N20+ 2 ms (R2= 0.96, P= 0.0001, slope 0.89, y-intercept 0.22) vs. N20+ 4.1 ms (R2= 0.53, P= 0.05, slope 1.34, y-intercept −0.17). This suggests that the exact timing of the arrival of P and S1 in the inhibitory motor cortical circuit was not critical for this correlation.
In summary, Experiments 1 and 2 strongly support the view that the inhibitory effects of S1 on a test MEP elicited by S2* dominate over those of P, and that this effect cannot be explained by differences in the magnitude of the inhibitory effect of S1 vs. P on S2* alone, and not by the exact temporal order of arrival of S1 relative to P in the activated motor cortical circuit. However, one recent study demonstrated that SICF is recruited in addition to SICI if a S1 intensity of 90% AMT is used in the voluntarily contracting target muscle (Ortu et al. 2008). In order to exclude a significant contribution of SICF to the observed interactions between S1 and P, we conducted Experiment 3, using a S1 intensity of 70% AMT. This recruits SICI but not SICF (Ortu et al. 2008) and, therefore, allows study of the interactions of SAI with SICI in isolation.
Experiment 3. Interactions between SICI and SAI using a reduced intensity of S1 of 70% AMT
RmANOVA showed a significant effect of SICI (F2,14= 12.0, P= 0.0009). Post hoc testing showed less inhibition for PS1S2*/PS2* compared to S1S2/S2 (P= 0.005), and for PS1S2*/PS2* compared to S1S2*/S2* (P= 0.0003), indicating that the inhibitory effect of S1 on S2* was attenuated when co-applying P (Fig. 5A, open circles). Furthermore, rmANOVA revealed a significant effect of sai (F2,14= 28.0, P < 0.0001). Post hoc testing showed less inhibition for PS1S2*/S1S2* compared to PS2/S2 (P < 0.0001), and for PS1S2*/S1S2* compared to PS2*/S2* (P < 0.0001), indicating that the inhibitory effect of P on S2* was attenuated when co-applying S1 (Fig. 5A, filled circles).
Figure 5. SICI without vs. with co-applied P, and SAI without vs. with co-applied S1 at a reduced S1 intensity of 70% AMT (Experiment 3).
A, SICI alone (S1S2/S2, S1S2*/S2*) vs. SICI with co-applied P (PS1S2*/PS2*, open circles) and SAI alone (PS2/S2, PS2*/S2*) vs. SAI with co-applied S1 (PS1S2*/S1S2*, filled circles) are shown. Values less than 1 (dashed horizontal line) indicate inhibition. S1 inhibited PS2* significantly less than S2 or S2* alone. Similarly, P inhibited S1S2* significantly less than S2 or S2* alone. Significant differences between PS1S2*/PS2* and S1S2/S2, and between PS1S2*/S1S2* and PS2/S2 are indicated by ** (P < 0.01). Significant differences between PS1S2*/PS2* and S1S2*/S2*, and between PS1S2*/S1S2* and PS2*/S2* are indicated by ## (P < 0.01). Significant differences between S1S2/S2 vs. PS2/S2, and between S1S2*/S2*vs. PS2*/S2* are indicated by † (P > 0.05). All data are means ± 1 s.e.m. of 8 subjects. B, MEP amplitude (in mV) of S1S2* is plotted against that of PS1S2* with the regression line indicated. C, MEP amplitude (in mV) of PS2* is plotted against that of PS1S2* with the regression line indicated.
mep amplitude (MEP amplitude elicited by S2, PS2* or S1S2*) was significantly different across stimulus conditions (F2,14= 9.7, P= 0.002; S2: 1.60 ± 0.08 mV, PS2*: 1.67 ± 0.12 mV, S1S2*: 2.22 ± 0.18 mV). Post hoc testing showed that this effect was explained by larger MEP amplitudes elicited by S1S2* when compared to those elicited by S2 (P= 0.013) and PS2* (P= 0.003), while MEP amplitudes elicited by S2 and PS2* were not different (P= 0.67). Therefore, the above comparison of PS1S2*/PS2* with S1S2/S2 was adequately matched for amplitude, while this was not the case for the comparison of PS1S2*/S1S2* with PS2/S2 (Fig. 5A). The larger S1S2* amplitude is explained by the lower intensity and consequently less effective inhibitory effect of S1 when compared to Experiment 1. However, this does not pose a problem for data interpretation, as attenuation of the inhibitory effects of P on S2* by co-application of S1 was, if anything, underestimated.
M wave amplitudes elicited by P were not different across conditions (PS2: 0.53 ± 0.22 mV, PS2*: 0.73 ± 0.50 mV, PS1S2*: 0.70 ± 0.45 mV; pair-wise comparisons by paired t tests, all P > 0.6). Therefore, differences in effectiveness of the electrical stimulation of the ulnar nerve were not detected and cannot explain any of the above interactions between S1 and P.
Finally, the level of voluntary isometric contraction of the right ADM, as quantified by the root mean square of the EMG signal in the 100 ms prior to stimulation was not different across conditions (S2: 37 ± 5 μV, S1S2: 39 ± 6 μV, PS2: 39 ± 6 μV, S2*: 49 ± 10 μV, S1S2*: 46 ± 9 μV, PS2*: 47 ± 10 μV, PS1S2*: 46 ± 8 μV; pair-wise comparisons by t tests, all P > 0.1). This excludes the possibility that differences in the level of target muscle contraction between conditions explained the interactions between S1 and P.
Correlation analyses were performed for MEP amplitudes only because the very small variance of PS2*/S2*, and in particular S1S2*/S2* (see Fig. 5A) and the small number of data points (n= 8) rendered correlation analyses of inhibitions (SICI alone vs. SICI with co-application of P; SAI alone vs. SAI with co-application of S1) not useful. The MEP amplitude of S1S2* correlated significantly with PS1S2* (R2= 0.67, P= 0.012) with a slope of the regression line of 0.72 and a y-intercept of 0.45 (Fig. 5B). In contrast, PS2* did not correlate significantly but showed a trend towards a positive correlation with PS1S2* (R2= 0.26, P= 0.20), with a slope of 0.67 and a y-intercept of 0.93 (Fig. 5C).
Discussion
The following main findings emerged from this study: (1) the inhibitory effects of P and S1 on the test MEP amplitude elicited by S2 mutually reduce each other; (2) this interaction does not depend critically on the timing of arrival of P and S1 in motor cortex on a timescale of a few milliseconds; and (3) the interaction is dominated by the effects of S1 over P.
Physiological interpretation of the interactions between SICI and SAI
Several previous TMS studies demonstrated the feasibility to assess interactions between two inputs into human motor cortex by comparing the single effects with those of one input when co-applying the other one (Sanger et al. 2001; Daskalakis et al. 2002, 2004; Sailer et al. 2002; Kukaswadia et al. 2005; Lee et al. 2007; Müller-Dahlhaus et al. 2008). Only one study has addressed briefly the interaction between SICI and SAI (Stefan et al. 2002). Similar to the present study, it was found that SICI is reduced in the presence of SAI, but the nature of this interaction has not been explored any further (Stefan et al. 2002). In particular, changes in SAI by co-application of S1 and the relative weight of the interaction between S1 and P on the MEP elicited by S2* were not examined. In addition, the inhibitory effects of S1 and P when given alone were not carefully matched. Therefore, these data have to be regarded as preliminary when compared to those of the present experiments. However, in contrast to the present study, the previous experiments were conducted in the voluntarily relaxed target muscle (Stefan et al. 2002) so that it is likely that similar interactions between S1 and P exist in the resting and active muscle.
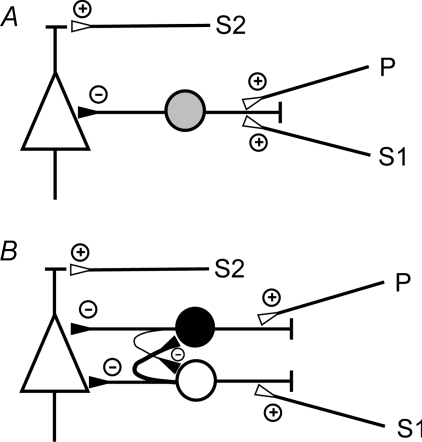
It is a widely accepted view that SICI and SAI exert their inhibitory effects onto corticospinal neurons through cortical inhibitory interneurons (Kujirai et al. 1993; Ziemann et al. 1996a; Di Lazzaro et al. 1998, 2000a, 2007; Tokimura et al. 2000; Ilic et al. 2002). Our finding that SICI is reduced in the presence of SAI, and vice versa– SAI is reduced in the presence of SICI, is in agreement with two simple connectivity models shown in Fig. 6. The model in Fig. 6A assumes convergence of the SICI and SAI inputs onto a common inhibitory interneuron. Typically, the combined EPSP at a common target neuron is less than the arithmetic sum of the single EPSPs. This is because the increased conductance of each active synapse lowers the input resistance, shunting current that otherwise would go to changing the charge of the membrane capacitance (Shepherd & Koch, 1990). In addition, each active synapse moves the membrane potential closer to the synaptic reversal potential, thereby reducing the driving force associated with the conductance change (Shepherd & Koch, 1990). This reduction of the combined EPSP would result in a reduced excitation of the common inhibitory interneuron by one input in the presence of the other input, resulting, in turn, in a reduced inhibition of the corticospinal neuron than would occur if both the EPSPs from the SICI and SAI input would summate at the common inhibitory interneuron in a linear manner. In the other simple connectivity model, SICI and SAI are mediated by separate inhibitory interneurons with convergent inputs onto a common corticospinal neuron (Fig. 6B). The observed mutual reduction of the inhibitory effects of SICI and SAI can also be explained by current shunting, now directly at the proposed common corticospinal neuron, because this interaction of synaptic conductance does equally apply to IPSPs (Shepherd & Koch, 1990).
Figure 6. Simple connectivity models to explain the interactions between SICI and SAI.
A, the excitatory input pathways of SICI and SAI converge onto the same inhibitory interneuron (grey circle), which in turn synapses onto a corticospinal neuron (triangle). B, the excitatory input pathways of SICI and SAI synapse onto distinct subtypes of inhibitory interneurons (black and white circle), which in turn synapse onto a common corticospinal neuron (triangle) with the SICI input located closer to the axon initial segment than the SAI input. The interneurons mutually inhibit each other, with a stronger inhibition from the SICI interneuron onto the SAI interneuron (symbolized by a thicker axon) than the other way round. Small circles with a plus indicate excitatory synapses, while small circles with a minus indicate inhibitory synapses. In addition, both models contain an excitatory input to the corticospinal neuron, which when activated by S2 results in elicitation of a test MEP.
Both models can explain the observation that the exact timing of the SICI and SAI input is not critical for their interaction because EPSPs from SICI and SAI input would integrate at the inhibitory interneuron in the model in Fig. 6A, and IPSPs would integrate at the corticospinal neuron in Fig. 6B over the durations of these EPSPs and IPSPs, which are in the order of ∼10–20 ms (Thomson et al. 2002).
For both models it is true that the maximum possible interaction effect would be complete shunting of one synaptic conductance in the presence of the other one. Therefore, current shunting cannot explain disinhibition. However, in many instances, we observed that PS1S2*/PS2* > 1, i.e. the inhibitory effect of S1 on S2*, turned to facilitation when co-applying P, or PS1S2*/S1S2* > 1, i.e. the inhibitory effect of P on S2*, turned to facilitation when co-applying S1 (Figs 2–5). This can be best explained by assuming that the pathways of SICI and SAI mutually inhibit each other (Fig. 6B), a proposition that fits to data from other studies, which showed that different subtypes of cortical inhibitory interneurons exert reciprocal synaptic inhibition onto each other (Tamas et al. 1998; Gibson et al. 1999; Thomson et al. 2002).
Another explanation for the observed mutual reduction of inhibition between SICI and SAI might be saturation of inhibition, i.e. the inhibition produced by a single input was already at or close to the maximum possible inhibition. This is indeed a possibility because the stimulus intensity used in Experiments 1 and 2 for S1 (90% AMT) is typically optimal for producing maximum inhibition (Ziemann et al. 1996b; Peurala et al. 2008). However, saturation of inhibition would also not explain disinhibition. To address this issue further, Experiment 3 employed less effective SICI. This limited current shunting and eliminated the potential problem of saturation of inhibition. Still, the interaction between SICI and SAI was a highly significant mutual reduction of inhibition (Fig. 5A). This provides additional support for the view that, in the absence of saturation of inhibition and powerful current shunting, this interaction must come from reciprocal synaptic inhibition of inhibitory interneurons (Fig. 6B). In addition, Experiment 3 used a S1 intensity of 70% AMT which is below the threshold for activation of SICF in the voluntarily contracting target muscle (Ortu et al. 2008). As the interactions between S1 and P were very similar to those observed in Experiments 1 and 2, it can be concluded that SICI rather than SICF was essential for causing these interactions.
Additional evidence from this and previous studies supports the assumption made in the model of Fig. 6B that distinct inhibitory interneurons mediate SICI and SAI. First, in line with previous reports (Sanger et al. 2001; Daskalakis et al. 2002; Sailer et al. 2002), SICI tended to decrease with decreasing intensity of the test pulse from S2* to S2, whereas SAI tended to increase (Figs 2, 4A and 5A). One possible explanation for this dissociation is that the interneurons mediating SICI inhibit preferentially high-threshold corticospinal neurons while those mediating SAI inhibit preferentially low-threshold ones. However, it is very likely that a significant overlap (convergence of SICI and SAI interneurons onto the same corticospinal neurons) exists in order to explain the dominance of S1 over P (see below). Another important argument in support of different subtypes of inhibitory interneurons for SICI and SAI comes from their dissociable pharmacological profiles: only SAI but not SICI is reduced by scopolamine, an antagonist at cholinergic M1 receptors, and is enhanced by acetylcholinesterase inhibitors (Di Lazzaro et al. 2000b; Fujiki et al. 2006). This fits with the notion that the main subtypes of cortical GABAergic inhibitory interneurons can be distinguished by their response to cholinergic stimulation (Kawaguchi, 1997; Xiang et al. 1998). Furthermore, the benzodiazepine lorazepam enhances SICI but reduces SAI (Di Lazzaro et al. 2005a,b;), while zolpidem, a high-affinity positive modulator at the α1 subtype of the GABAA receptor, leaves SICI unaltered and decreases SAI (Di Lazzaro et al. 2007).
Dominance of SICI over SAI
Although the inhibitory effects of S1 were reduced when co-applying P, and vice versa– the inhibitory effects of P were reduced when co-applying S1, these interactions were not the same. Even if the inhibitory effects of S1 and P on the MEP amplitude elicited by S2* were carefully matched when given alone (Experiments 1 and 2), P resulted in an offset of the inhibitory action of S1 on S2* (positive y-intercepts in Figs 3A and 4B), but did not otherwise affect this inhibition. This is indicated by the significant linear correlations between S1S2*/S2* and PS1S2*/PS2* (Figs 3A and 4B) with slopes of the regression line close to 1. In contrast, S1 resulted in a disappearance of the inhibitory effects of P on S2* (y-intercepts close to 1 in Figs 3C and 4C), independent of the magnitude of the inhibitory effect of P (no correlation between PS2*/S2* and PS1S2*/S1S2*, slopes of regression lines close to 0; cf. Figs 3C and 4C). This fundamentally different behaviour strongly suggests that S1 essentially abolished the action of P when the two pulses were given together (i.e. dominance of S1 over P). This was further substantiated by additional linear regression analyses between the MEP amplitude of S1S2*vs. that of PS1S2*. If S1 abolished the effect of co-applied P the regression analysis should result in a highly linear correlation with slope close to 1 and y-intercept close to 0. This is exactly what we found (Figs 3B and 4D). In addition, as also expected from the proposed dominance of S1 over P, the regression analyses of the MEP amplitude of PS2* with that of PS1S2* did not show significant correlation (Figs 3D and 4E). Even if the inhibitory effect of S1 was weaker than that of P when given alone (Experiment 3, Fig. 5A), the dominance of S1 over P was still demonstrable by a significant linear correlation of the MEP amplitude of S1S2* with that of PS1S2* (Fig. 5B). However, with the weaker S1 a trend towards a significant linear correlation between the MEP amplitude of PS2* with that of PS1S2* (Fig. 5C) developed. This suggests that the dominance of S1 over P can shift, if the inhibitory effects of S1 relative to those of P become sufficiently small.
This dominance of S1 over P cannot be explained by a model that proposes convergence of SICI and SAI inputs onto the same inhibitory interneuron (Fig. 6A). It can be explained, however, by a connectivity model that proposes distinct subtypes of inhibitory interneurons (Fig. 6B), if the additional assumption is made that e.g. the interneurons mediating SICI synapse onto corticospinal neurons closer to the axon initial segment than those interneurons mediating SAI (Fig. 6B). This would put SICI into an extremely powerful position to control action potential generation while SAI rather offsets excitability of corticospinal neurons by inhibition distant from the site of action potential generation. Another, mutually not exclusive explanation for the observed dominance of S1 over P could be that the inhibition of SAI interneurons by SICI interneurons is more powerful than vice versa (Fig. 6B). The dominance of S1 over P is in accord with other evidence in the literature that SICI constitutes strategically important and powerful motor cortical inhibition as it has the lowest threshold of all TMS excitability measures (Kujirai et al. 1993; Ziemann et al. 1996b; Ilic et al. 2002).
In summary, this study alludes to the possibility that TMS is capable of exploring neuronal circuitry in human motor cortex even down to the level of different subtypes of interneurons by testing the interactions between physiologically and pharmacologically distinct TMS measures of inhibition.
Acknowledgments
H. Alle: Institute of Neurophysiology, Neuroscience Research Center, Charité-Universtitätsmedizin Berlin, Germany.
Glossary
Abbreviations
- ADM
abductor digiti minimi
- AMT
active motor threshold
- M1
muscarinic type 1
- MEP
motor-evoked potential
- P
conditioning electrical stimulus applied to the ulnar nerve for testing short-latency afferent inhibition
- S1
sub-threshold conditioning magnetic pulse for testing short-interval intracortical inhibition
- S2
supra-threshold test pulse for eliciting a test motor-evoked potential
- SAI
short-latency afferent inhibition
- SICI
short-interval intracortical inhibition
- TMS
transcranial magnetic stimulation
Author contributions
All authors (H.A., T.H., L.K., U.Z.) contributed to (1) conception and design, analysis and interpretation of data; (2) drafting the article; and (3) final approval of the version to be published. In addition, H.A. and T.H. acquired the experimental data reported in this paper.
References
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rösler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000a;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in human motor cortex. Exp Brain Res. 2000b;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali PA. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005a;564:661–668. doi: 10.1113/jphysiol.2004.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, Saturno E, Pilato F, Masullo C, Rothwell JC. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: A TMS study. Clin Neurophysiol. 2007;118:2207–2214. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short latency afferent inhibition. J Physiol. 2005b;569:315–323. doi: 10.1113/jphysiol.2005.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimulat. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Hikawa T, Abe T, Ishii K, Kobayashi H. Reduced short latency afferent inhibition in diffuse axonal injury patients with memory impairment. Neurosci Lett. 2006;405:226–230. doi: 10.1016/j.neulet.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Furubayashi T, Iwata NK, Shiio Y, Okabe S, Kanazawa I, Ugawa Y. Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res. 2003;151:427–434. doi: 10.1007/s00221-003-1455-z. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78:1743–1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R. Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol. 2005;563:915–924. doi: 10.1113/jphysiol.2004.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Gunraj C, Chen R. The effects of inhibitory and facilitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol. 2007;580:1021–1032. doi: 10.1113/jphysiol.2006.126011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus JF, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex – a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol. 2008;586:5147–5159. doi: 10.1113/jphysiol.2008.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peurala SH, Müller-Dahlhaus JFM, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Cunic DI, Chen R. Effects of peripheral sensory input on cortical inhibition in humans. J Physiol. 2002;544:617–629. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Koch C. Dentric electrotonus and synaptic integration. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York: Oxford University Press; 1990. pp. 439–473. [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Vucic S, Howells J, Trevillion L, Kiernan MC. Assessment of cortical excitability using threshold tracking techniques. Muscle Nerve. 2006;33:477–486. doi: 10.1002/mus.20481. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996b;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi M, Pearce SL, Nordstrom MA. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol. 2003;550:933–946. doi: 10.1113/jphysiol.2003.042606. [DOI] [PMC free article] [PubMed] [Google Scholar]