Abstract
Many physiological processes and pharmacological agents modulate the ryanodine receptor (RyR), the primary sarcoplasmic reticulum (SR) Ca2+ release channel in the heart. However, how such modulations translate into functional effects during cardiac excitation–contraction coupling (ECC) is much less clear. Using a low dose (250 μm) of caffeine we sensitized the RyR and examined SR Ca2+ release using dynamic measurements of cytosolic Ca2+ ([Ca2+]i) and free Ca2+ within the SR ([Ca2+]SR). In field stimulated (1 Hz) rabbit ventricular myocytes, application of 250 μm caffeine caused an initial 33% increase in SR Ca2+ release, which was followed by a decrease in SR Ca2+ load (28%) and steady-state SR Ca2+ release (23%). To investigate the effects of caffeine on local SR Ca2+ release, we measured [Ca2+]SR from individual release junctions during ECC as well as during spontaneous Ca2+ sparks. In intact myocytes during ECC, caffeine increased global fractional SR Ca2+ release by decreasing the [Ca2+]SR level at which local release terminated by 21%. Similarly, in permeabilized myocytes during spontaneous Ca2+ sparks, caffeine decreased the [Ca2+]SR level for release termination by 12%. Finally, we examined if Ca2+ release termination was changed in myocytes from failing hearts, where remodelling processes lead to altered RyR function. In myocytes from failing rabbit hearts, the [Ca2+]SR termination level for Ca2+ sparks was 13% lower than that of non-failing myocytes. Collectively, these data suggest that altering the termination level for local Ca2+ release may represent a novel mechanism to increase SR Ca2+ release and contractility during ECC.
Introduction
In the heart excitation–contraction coupling (ECC) occurs via Ca2+-induced Ca2+ release (CICR), where localized influx of Ca2+ via L-type Ca2+ channels triggers further release of Ca2+ from the SR through ryanodine receptor (RyR) release clusters. The global intracellular Ca2+ increase that activates contraction during ECC is due to the summation of thousands of individual CICR events, which in ventricular myocytes occur at specialized release units where L-type Ca2+ channels in the T-tubule membrane closely approach the RyR release clusters of the junctional SR (for review see Bers, 2001). Elementary Ca2+ release events from these clusters also occur spontaneously, and can be visualized as distinct increases in cytosolic Ca2+, termed Ca2+ sparks (Cheng et al. 1993), or as the reciprocal intra-SR Ca2+ depletion, events called Ca2+ blinks (Brochet et al. 2005). During diastole, relaxation occurs as a result of Ca2+ being sequestered back into the SR via the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), as well as by Ca2+ being removed from the cell, predominantly via the Na+/Ca2+ exchanger (NCX). In the steady-state, the amount of Ca2+ that enters the cell must be extruded, and the amount of Ca2+ that is released during CICR must be pumped back into the SR via SERCA (Eisner et al. 2000). Alterations in this cellular Ca2+ flux balance result in pathological conditions such as arrhythmogenic Ca2+ overload (net cellular Ca2+ gain) and chronic [Ca2+]SR depletion and subsequent decreased contractility as seen in heart failure (net cellular Ca2+ loss).
Heart failure is a complex pathological condition simply defined at the organ level as a decrease in cardiac pump function. At the cellular level the reduction in contractility associates with substantial decreases in [Ca2+]SR and SR Ca2+ release during ECC (Lindner et al. 1998; Pieske et al. 1999; Hobai & O’Rourke, 2001; Pogwizd et al. 2001). Recent evidence suggests that cardiac contraction would be further reduced if it were not for concomitant modulations to RyRs, which sensitize release clusters to release Ca2+. This becomes evident when SR Ca2+ content is experimentally matched, and Ca2+ spark frequency and fractional SR Ca2+ release during ECC are increased in heart failure myocytes compared to non-failing myocytes (Shannon et al. 2003b; Kubalova et al. 2005; Guo et al. 2007). Unfortunately, the modifications which occur in heart failure also create instability during diastole in the form of SR Ca2+ leak (Shannon et al. 2003b; Oda et al. 2005; Wehrens et al. 2005; Belevych et al. 2007) and spontaneous Ca2+ release events such as Ca2+ waves, making these compensatory modifications potentially arrhythmogenic (George, 2008).
Ca2+ release from the SR, and therefore cardiac contraction, is a finely graded process, which allows the heart to adjust its contractility to meet physiological demands. However, for SR Ca2+ release to be graded, robust release termination mechanisms must exist to counteract the inherent positive feedback and all-or-nothing nature of CICR. Based on global [Ca2+]SR measurements during ECC (Shannon et al. 2003a), as well as of spontaneous Ca2+ blinks (Brochet et al. 2005; Terentyev et al. 2008b; Zima et al. 2008b), it is apparent that Ca2+ release does not simply terminate due to exhaustion of releasable Ca2+. Experimental evidence and mathematical models have suggested numerous potential termination mechanisms, including adaptation, inactivation, stochastic attrition and cluster de-activation via a luminal [Ca2+]SR-dependent mechanism (Stern & Cheng, 2004). In previous work we have shown that local Ca2+ release terminates at a set [Ca2+]SR level, irrespective of the initial [Ca2+]SR or Ca2+ release flux (Zima et al. 2008b). These data show that Ca2+ release terminates via a distinct luminal [Ca2+]SR-dependent mechanism, which makes termination mechanisms such as stochastic attrition (which would produce a random [Ca2+]SR termination level) and [Ca2+]i-dependent inactivation (higher [Ca2+]SR termination level with increasing Ca2+ release flux) unlikely. Furthermore, by limiting release flux and maintaining [Ca2+]SR above this [Ca2+]SR termination level, long-lasting Ca2+ release events were visualized, which then likely terminated via [Ca2+]SR-independent mechanisms (Zima et al. 2008a). While these data clearly point to a luminal [Ca2+]SR threshold for release termination, it remains unclear if this threshold can be altered by pharmacological or physiological modulation of the RyR.
The aim of this study was to investigate the mechanisms by which RyR sensitization increases fractional SR Ca2+ release in rabbit ventricular myocytes. For this purpose we pharmacologically sensitized RyRs using a low dose (250 μm) of the classical RyR activator caffeine and monitored [Ca2+]i and [Ca2+]SR during ECC as well as during spontaneous Ca2+ sparks. We then used myocytes isolated from a well-characterized rabbit model of heart failure (Pogwizd, 1995) to investigate if similar effects occur in the failing heart, where endogenous modifications ‘sensitize’ RyRs and increase fractional [Ca2+]SR release. We show that RyR sensitization with caffeine increases fractional SR Ca2+ release via several functional mechanisms. First, release clusters are sensitized to release activation, which increases release junction recruitment and synchronizes release during CICR. Furthermore, RyR sensitization also increases fractional SR Ca2+ release at each individual release junction by decreasing the [Ca2+]SR level where release terminates. In heart failure myocytes strikingly similar effects occurred as part of the remodelling process, suggesting that these mechanisms are also relevant during physiological modulation of the RyR. Taken together, these data suggest that release activation and release termination represent two mechanisms by which SR Ca2+ release is regulated. Modulation of the termination threshold for Ca2+ release may therefore represent an important inotropic mechanism during cardiac ECC.
Preliminary accounts of this work have been published in abstract form (Zima et al. 2007; Domeier et al. 2009).
Methods
Solutions and chemicals
All chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA), unless noted otherwise. Tyrode solution contained (in mm): 135 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, 10 Hepes; pH 7.4 with NaOH. The internal cell solution for permeabilized myocytes contained (in mm): 100 potassium aspartate, 15 KCl, 5 KH2PO4, 5 MgATP, 0.35 EGTA, 0.12 CaCl2, 0.75 MgCl2, 10 phosphocreatine, 5 U ml−1 creatine phosphokinase, 8% dextran (Mr: 40 000), 10 Hepes, 0.04 rhod-2 tripotassium salt or 0.03 fluo-4 pentapotassium salt (Molecular Probes/Invitrogen, Carlsbad, CA, USA); pH 7.2 with KOH. Free [Ca2+] and [Mg2+] of this solution were 150 nm and 1 mm, respectively.
Myocyte isolation
Ventricular myocytes were isolated from New Zealand White rabbits (25 animals, 2.5 kg, Myrtle's Rabbitry, Thompsons Station, TN, USA). Rabbits were anaesthetized with sodium pentobarbital (50 mg kg−1) and hearts were excised and mounted on a Langendorff apparatus. Hearts were retrogradely perfused with nominally Ca2+-free Tyrode solution for 5 min, followed by minimal essential medium Eagle (MEM) solution containing 20 μm Ca2+ and 45 μg ml−1 Liberase Blendzyme 4 (Roche Applied Science, Indianapolis, IN, USA) for 20 min at 37°C. The left ventricular free wall was removed from the heart and digested for an additional 5 min in the enzyme solution at 37°C. Digested tissue was then minced, filtered, and washed in a MEM solution containing 50 μm Ca2+ and 10 mg ml−1 bovine serum albumin. Isolated cells were kept in MEM solution with 50 μm Ca2+ at room temperature (22–24°C) until used for experimentation. In addition, left ventricular myocytes were isolated from rabbits with non-ischaemic heart failure induced by combined aortic insufficiency and stenosis (6 animals) (Pogwizd, 1995). All protocols were approved by the Institutional Animal Care and Use Committee, and comply with US and UK regulations on animal experimentation (Drummond, 2009).
Ca2+ indicator dye loading and general procedures
To load the sarcoplasmic reticulum with the low-affinity Ca2+ indicator fluo-5N, ventricular myocytes were incubated with 5 μm of the membrane permeant fluo-5N/AM (Molecular Probes/Invitrogen) with 0.25% Pluronic F-127 in Tyrode solution for 2.5 h, followed by a 30 min wash, all at 37°C. For cytosolic measurements of Ca2+ in intact myocytes, cells were incubated at room temperature with 10 μm rhod-2/AM (Molecular Probes/Invitrogen) or 10 μm fluo-4/AM (Molecular Probes/Invitrogen) for 10 min, followed by a 20 min wash. Cells were placed on laminin-coated coverslips. Action potentials and ECC were induced by electrical field stimulation using a pair of platinum electrodes, which were connected to a Grass stimulator set at a voltage ∼50% above the threshold. To study individual Ca2+ release events (Ca2+ sparks and blinks) in a precisely controlled intracellular environment, myocytes were permeabilized with 0.005% saponin for 30 s and exposed to the internal cell solution, which contained rhod-2 tripotassium salt or fluo-4 pentapotassium salt (Molecular Probes/Invitrogen). All experiments were performed at room temperature.
Epifluorescence microscopy
Global [Ca2+]SR or [Ca2+]i was monitored using fluo-5N or fluo-4, respectively, with dye excitation at 480 ± 20 nm and emission collected at 535 ± 25 nm. Fluo-4 emission was background subtracted and normalized to baseline fluorescence emission (F0), with changes of [Ca2+]i presented as ΔF/F0 (where ΔF=F−F0). Fluo-5N fluorescence emission was background subtracted and calibrated to [Ca2+]SR using [Ca2+]SR=KD× (F−FMin)/(FMax−F), where KD= 400 μm (Shannon et al. 2003a). FMin is the intra-SR fluorescence in the absence of Ca2+ obtained after complete emptying of [Ca2+]SR with 10 mm caffeine. This value was corrected for the 15% chemical quench of fluo-5N by this concentration of caffeine. FMax was defined as the diastolic fluorescence in the presence of 1 μm isoproterenol during 1 Hz pacing. In the presence of isoproterenol [Ca2+]SR increases to levels where intra-SR fluo-5N is nearly saturated with Ca2+ and this therefore approximates maximal fluo-5N fluorescence (Shannon et al. 2003a).
Confocal microscopy
Confocal microscopy (Radiance 2000/MP, Bio-Rad, UK) was used to simultaneously image [Ca2+]SR (fluo-5N) and [Ca2+]i (rhod-2), with fluo-5N excitation at 488 nm and emission collected at 515 ± 15 nm, and rhod-2 excitation at 543 nm with emission collected at >600 nm. In some experiments [Ca2+]i was monitored alone using fluo-4 with excitation at 488 nm and emission collected at >500 nm. Images were acquired in linescan mode at 3 ms per scan with a pixel size of 0.12 μm. Measurements during ECC were acquired by positioning the scan line in a central region along the short axis of the cell (transverse scan) to minimize motion artifacts. For measurements in permeabilized myocytes the scan line was positioned in the cytosol along the longitudinal axis of the cell. Rhod-2 fluorescence emission is presented as ΔF/F0 (where ΔF=F−F0), while fluo-5N fluorescence was calibrated to [Ca2+]SR or normalized to baseline fluorescence (F/F0) using (F−FMin/F0−FMin). F0 corresponds to diastolic fluorescence at 0.5 Hz pacing in intact myocytes (Fig. 3) or baseline resting fluorescence in permeabilized myocytes (Figs 4 and 6). To directly compare permeabilized myocytes from normal rabbits and heart failure rabbits, SR Ca2+ load was approximated indirectly using the amplitude of the fluo-4 [Ca2+]i transient in response to 10 mm caffeine. Additionally, resting [Ca2+]SR was calculated directly from the fluo-5N fluorescence signal (see above). In permeabilized cells, FMin was the fluorescence signal obtained after application of 10 mm caffeine in a 0 Ca2+ solution containing 5 mm EGTA, while FMax was obtained in the presence of 10 mm caffeine and 10 mm Ca2+. For FMax calibration, 10 μm blebbistatin and 5 mm 2,3-butanedione monoxime were included to prevent cell contraction. Both approaches yielded a [Ca2+]SR in heart failure myocytes that was ∼11% lower than in non-failing myocytes (see Fig. 5F). This 11% value was subsequently used to adjust fluo-5N fluorescence in heart failure myocytes to fluorescence in non-failing myocytes (see dashed line in Fig. 6).
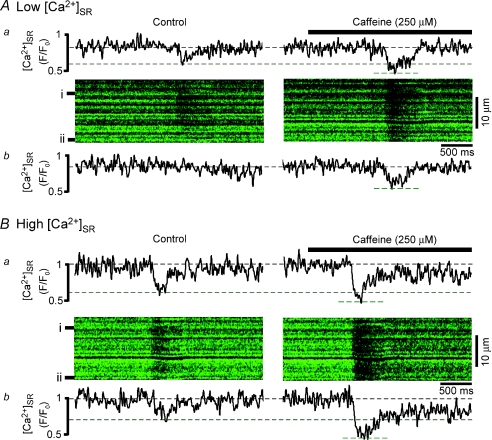
Figure 3. During excitation–contraction coupling caffeine increases release junction recruitment and decreases the [Ca2+]SR at which release terminates.
Confocal line scan images from the same cell of [Ca2+]SR depletions during excitation–contraction coupling at low (A) and high (B) initial [Ca2+]SR under control conditions (left) and after addition of 250 μm caffeine (right). F/F0 profiles of fluorescence from two individual SR release junctions (denoted by black bars to left of images) are shown above (a) and below (b) images. F0 corresponds to fluo-5N fluorescence at high load. Dashed lines denote [Ca2+]SR level prior to field stimulus and at lowest level during release. Note that at low initial SR Ca2+ load, not all release junctions release Ca2+ (e.g. junction ii in Ab). Caffeine lowered the [Ca2+]SR at release termination by 21 ± 2%; n= 15 events from 5 cells, P < 0.05.
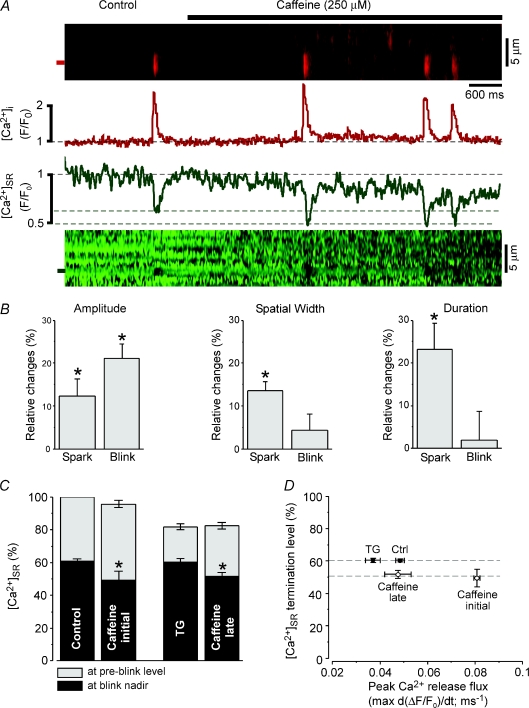
Figure 4. Caffeine decreases the [Ca2+]SR termination level of elementary Ca2+ release events.
A, cytosolic Ca2+ sparks (upper image and F/F0 profile) and their corresponding intra-SR Ca2+ blinks (lower image and F/F0 profile) in the absence and presence of 250 μm caffeine. F/F0 profiles are normalized to baseline fluorescence under control conditions (F0). Dashed lines represent initial [Ca2+]SR, and [Ca2+]SR at blink termination under control conditions and in the presence of caffeine. B, summary data of the relative changes (% increase versus control) in Ca2+ spark and Ca2+ blink amplitude (left), spatial width (FWHM, middle), and duration (FDHM, right) in the presence of caffeine. C, summary data showing effects of caffeine on Ca2+ blink termination level (black bars) at high (control, caffeine initial) and low (TG, caffeine late) [Ca2+]SR. To decrease [Ca2+]SR from control levels and match to SR Ca2+ load observed after sustained caffeine application (caffeine late), [Ca2+]SR was experimentally decreased by adding the SERCA inhibitor thapsigargin (TG, 10 μm). D, summary data showing relationship between peak Ca2+ spark release flux and Ca2+ blink [Ca2+]SR termination level in the absence (TG, Ctrl) and presence (Caff) of 250 μm caffeine. *P < 0.05.
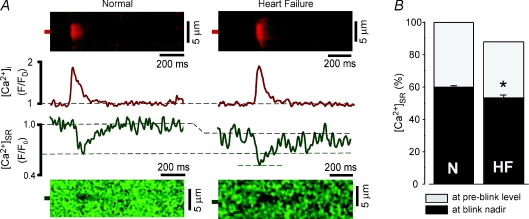
Figure 6. The [Ca2+]SR termination level of Ca2+ sparks is decreased in heart failure myocytes.
A, cytosolic Ca2+ sparks and intra-SR Ca2+ blinks from a normal myocyte (left image and F/F0 profile) and heart failure myocyte (right image and F/F0 profile). Resting fluorescence in heart failure myocytes was normalized to normal myocytes using the value shown in Fig. 5F. B, summary data showing the decreased Ca2+ blink [Ca2+]SR termination level of 53.0 ± 1.9% (n= 11) in heart failure myocytes compared with 60.1 ± 0.9% (n= 82) of control myocytes. *P < 0.05 versus control.
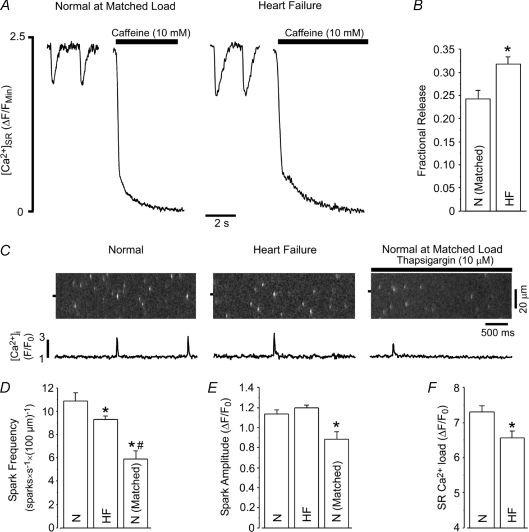
Figure 5. Fractional SR Ca2+ release, Ca2+ spark frequency and Ca2+ spark amplitude are increased in heart failure myocytes.
A, example traces of [Ca2+]SR during 0.5 Hz pacing followed by application of 10 mm caffeine in normal (at matched load) and heart failure myocytes. B, summary data showing the increased fractional SR Ca2+ release during 0.5 Hz pacing in heart failure myocytes when compared to load-matched normal myocytes. Load matching resulted in ΔF/FMin values of 2.60 ± 0.25 (N= 8) and 2.59 ± 0.40 (N= 5) in normal and heart failure myocytes, respectively. C, example images and F/F0 profiles of cytosolic Ca2+ sparks from normal (left), heart failure (middle) and load-matched normal (right) rabbit myocytes. D and E, summary data of Ca2+ spark frequency (D) and Ca2+ spark amplitude (E) from normal (N; n= 37), heart failure (HF; n= 36) and load-matched normal (N matched; n= 17) myocytes. F, SR Ca2+ content was 11% lower in permeabilized heart failure myocytes than in normal myocytes. *P < 0.05 versus normal myocytes, #P < 0.05 versus heart failure myocytes.
Data analysis and statistics
Global SR Ca2+ depletion amplitude (Δ[Ca2+]SR) was calculated as [Ca2+]SR,diastolic–[Ca2+]SR,systolic, while SR fractional Ca2+ release was calculated as Δ[Ca2+]SR/[Ca2+]SR,diastolic. Ca2+ spark frequency (quantified as number of sparks per second and 100 μm of scanned distance; sparks × (100 μm)−1× s−1), amplitude, duration (FDHM), and spatial width (FWHM) were calculated using the SparkMaster algorithm (Picht et al. 2007). Ca2+ spark release flux was calculated from the first derivative of the rhod-2 cytosolic fluorescence during a Ca2+ spark (d(ΔF/F0)/dt; ms−1). For each detected Ca2+ spark, blink amplitude, duration (FDHM), and spatial width (FWHM) were calculated manually. In Fig. 2, SR Ca2+ load was matched between control and caffeine data groups by placing the data in 100 μm bins. For comparisons of normal and heart failure myocytes, cells paced at 0.5 Hz were load-matched by selecting normal myocytes that exhibited SR Ca2+ loads within the range of loads observed in heart failure myocytes. Normal permeabilized myocytes were treated with 10 μm thapsigargin to inhibit SERCA and decrease SR Ca2+ load to the level observed in heart failure myocytes. Statistical comparisons were made using Student's t test for paired or unpaired data, with statistical significance set at P < 0.05. Data are presented as individual observations or as the mean ±s.e.m.; n refers to the number of events (Ca2+ sparks and blinks), N refers to the number of cells.
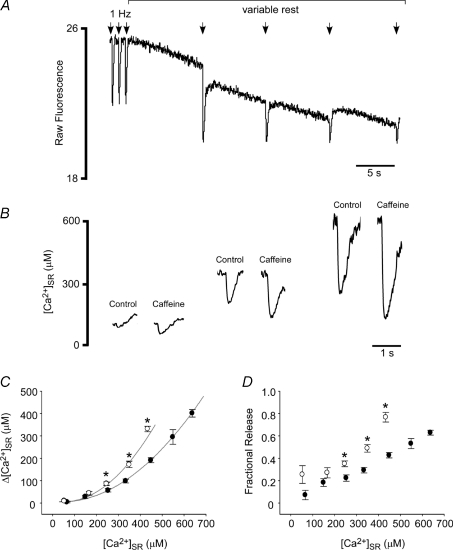
Figure 2. Caffeine increases global SR Ca2+ release over a wide range of [Ca2+]SR.
A, rest–decay protocol used to measure SR Ca2+ release during ECC at various [Ca2+]SR. Electrical stimuli were applied at the arrows. B, example load-matched [Ca2+]SR depletions in the presence (open circles in C,D) and absence (closed circles in C,D) of 250 μm caffeine. C and D, summary data showing effects of caffeine on depletion amplitude (C) and SR fractional release (D). Curves in C were fitted using exponential functions (r2: 0.93 (control), 0.84 (caffeine)). *Significant difference from the SR Ca2+ load-matched control value, P < 0.05.
Results
RyR sensitization with caffeine produces complex effects on SR Ca2+ release in paced myocytes
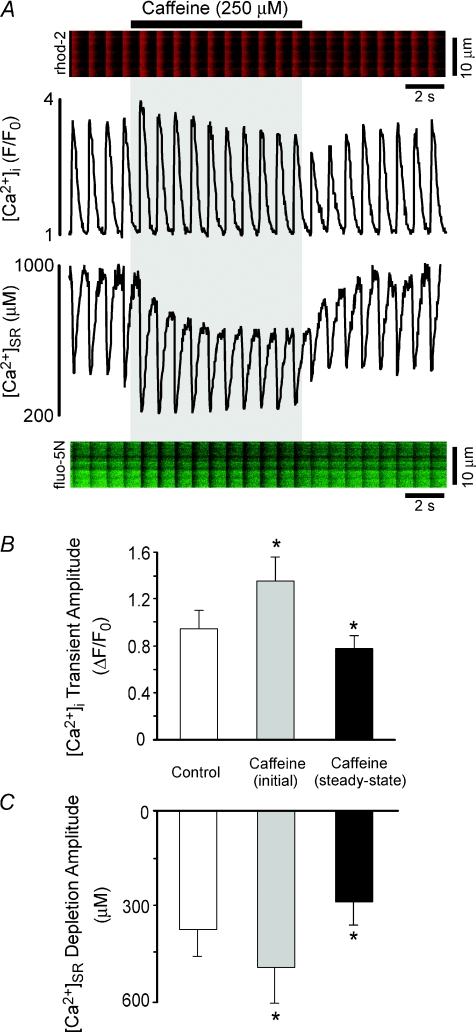
To characterize the mechanisms by which RyR sensitization alters SR Ca2+ release we loaded the SR of rabbit ventricular myocytes with the low affinity Ca2+ indicator fluo-5N. We used a low dose of caffeine (250 μm) to sensitize RyRs and examined its effects on ECC using simultaneous dynamic fluorescence measurements of [Ca2+]i (using the high-affinity Ca2+ indicator rhod-2) and [Ca2+]SR. As illustrated by the example traces of Fig. 1A, application of 250 μm caffeine to myocytes paced at 1 Hz produced an immediate increase in SR Ca2+ release, as shown by the increase in amplitude of both the [Ca2+]i transient and the corresponding [Ca2+]SR depletion. This was followed by a gradual decline in diastolic [Ca2+]SR (Fig. 1A, lower trace) and a subsequent decrease in the amplitude of steady-state Ca2+ release. Upon wash out of caffeine, Ca2+ release was markedly depressed at the lower [Ca2+]SR but then gradually recovered to the control level as the SR refilled to the original [Ca2+]SR. Measurements of [Ca2+]i using rhod-2 in intact myocytes (Fig. 1A) are hampered by accumulation of dye within mitochondria. Therefore, for quantitative measurements of [Ca2+]i we performed separate measurements using fluo-4 (N= 8). Figure 1B,C presents summary data of [Ca2+]i transients and [Ca2+]SR depletions under control conditions, immediately following caffeine addition (caffeine, initial), and in the presence of caffeine after the new steady-state was achieved (caffeine, steady-state). In these experiments, intra-SR Ca2+ depletion amplitude (Fig. 1C) initially increased by 33%, but then subsequently decreased by 23% (versus control) as SR Ca2+ load decreased from 680 ± 110 μm to 492 ± 81 μm in the new steady-state with caffeine (N= 5, P < 0.05). These data indicate that sensitization of the RyR produces only transient effects on SR Ca2+ release due to a subsequent decline in SR Ca2+ load.
Figure 1. RyR sensitization with caffeine produces only transient positive inotropic effects due to unloading of [Ca2+]SR.
A, simultaneous measurements of [Ca2+]i (rhod-2, red image and upper trace) and [Ca2+]SR (fluo-5N, green image and lower trace) before, during (grey shading), and after sensitization of RyRs with 250 μm caffeine. B and C, summary data showing initial and steady-state effects of 250 μm caffeine on [Ca2+]i (B) and [Ca2+]SR (C). Summary data in (B and C) are separate measurements using fluo-4 ([Ca2+]i) and fluo-5N ([Ca2+]SR). *Significant difference from control, P < 0.05.
Caffeine increases fractional SR Ca2+ release by altering release activation and termination
A single stimulus protocol was used to examine the effects of 250 μm caffeine over a wide range of initial [Ca2+]SR. Rabbit ventricular myocytes exhibit the post-rest decay phenomenon. That is, SR Ca2+ content gradually declines after rest from steady-state pacing (Fig. 2A). We applied a single field stimulus after variable periods of rest to assess the amount of Ca2+ released at a given SR Ca2+ content (Fig. 2A). Consistent with previous reports (Guo et al. 2007), the amplitude of SR Ca2+ release displayed a non-linear dependence on diastolic [Ca2+]SR (Fig. 2C, filled symbols). Sensitization of the RyR with caffeine resulted in more SR Ca2+ release at any given [Ca2+]SR (Fig. 2C, open symbols), or leftward shifts in the SR Ca2+ release amplitude versus[Ca2+]SR (Fig. 2C) and fractional SR Ca2+ release versus[Ca2+]SR (Fig. 2D) relationships.
The increase in global fractional release (Fig. 2D) observed in the presence of caffeine could be explained by lowering the activation threshold for SR Ca2+ release (i.e. more release junctions fire in response to CICR), by increasing the synchronization of release junctions, or by increasing the amount of Ca2+ released at each individual release junction. To experimentally differentiate between these possibilities we monitored [Ca2+]SR at individual release junctions during ECC in response to action potential stimulation. As shown in line-scan images of Fig. 3, individual release junctions were observed as regions of high fluo-5N fluorescence spaced ∼1.2 to 1.4 μm apart in the transverse direction of the cell. Fluorescence profiles during Ca2+ release from two such individual release junctions (a and b) are shown in Fig. 3 at low (A) and high (B) [Ca2+]SR in the absence and presence of 250 μm caffeine. Under control conditions at low [Ca2+]SR, release was inhomogeneous and [Ca2+]SR depletion was not observed in all release junctions (Fig. 3A). At the same initial [Ca2+]SR in the presence of 250 μm caffeine, release became homogeneous and well-synchronized (Fig. 3A, caffeine), and intra-SR depletions occurred at all release junctions. Qualitatively similar results of caffeine increasing release junction recruitment during CICR were observed in four additional cells. Strikingly, at the release junctions that fired under control conditions, the [Ca2+]SR at which release terminated was lower in the presence of caffeine. At high [Ca2+]SR, release was homogeneous under both control conditions and in the presence of caffeine (Fig. 3B), but caffeine again increased the amplitude of Ca2+ release within each release junction by lowering the [Ca2+]SR level of release termination. At a given initial [Ca2+]SR, there was junction-to-junction variability in the absolute [Ca2+]SR level where release terminated, which can be seen by comparing profiles a and b at high [Ca2+]SR (Fig. 3B). However, as [Ca2+]SR was varied, the termination level for release remained constant within an individual release junction. This tendency was also observed in the presence of caffeine, albeit at an overall lower [Ca2+]SR level. Summary data from 15 individual release junctions (5 cells) indicate that caffeine lowered the [Ca2+]SR termination level for release during ECC by 21 ± 2% (P < 0.05). Therefore, based on these measurements it appears that RyR sensitization with caffeine increases release junction recruitment and synchronizes SR Ca2+ release, and also increases fractional release within the individual release junction.
Caffeine lowers the [Ca2+]SR level of Ca2+ spark termination
Intra-SR Ca2+ measurements from individual release junctions during ECC must be interpreted with caution due to the signal summation from the many release junctions that fire and the rapid diffusion of Ca2+ within the SR network. To circumvent this issue and further examine the effects of caffeine on elementary Ca2+ release events from individual release junctions, we monitored spontaneous Ca2+ sparks and their corresponding Ca2+ blinks in permeabilized myocytes. This approach has the experimental advantage that single elementary Ca2+ release events are monitored in a precisely controlled cytosolic environment. As shown in Fig. 4A, application of 250 μm caffeine caused an immediate increase in Ca2+ spark frequency (by 80 ± 9.3%, N= 6) consistent with caffeine increasing the sensitivity of RyR release clusters to open at a given [Ca2+]SR (Lukyanenko et al. 2001). More importantly, in contrast to Ca2+ blinks under control conditions which terminated at a [Ca2+]SR level of 60.9 ± 2.6% (n= 16) of initial [Ca2+]SR, caffeine caused the Ca2+ blink termination level to decrease to 53.5 ± 3.0% (n= 16, P < 0.05, Fig. 4A and C), or by 12%, similar to the effect observed in experiments during ECC in intact cells (Fig. 3). Figure 4B presents the changes in spatiotemporal properties of Ca2+ sparks and Ca2+ blinks immediately after caffeine addition at similar [Ca2+]SR. Caffeine increased the amplitude of both Ca2+ sparks and blinks, but only increased the spatial width and duration of the Ca2+ sparks. We next examined if the shift in [Ca2+]SR termination level was dependent on initial [Ca2+]SR preceding the spark or [Ca2+] in the dyadic cleft during the spark. Caffeine caused a gradual decrease in resting [Ca2+]SR, which allowed for monitoring of Ca2+ release events at various initial [Ca2+]SR. To obtain [Ca2+]SR-matched data points in the absence of RyR stimulation we added the SERCA inhibitor thapsigargin (TG, 10 μm), which blocked SR Ca2+ uptake and lowered [Ca2+]SR. Ca2+ in the dyadic cleft can be reasonably approximated from release flux during a spark (Sipido & Wier, 1991; Song et al. 1998; Zima et al. 2008b), which progressively decreased as the gradient for Ca2+ release diminished at low [Ca2+]SR. Figure 4C and D compares [Ca2+]SR and peak release flux with Ca2+ blink termination level both under control conditions and in the presence of caffeine. As previously reported (Zima et al. 2008b), under control conditions and during SERCA inhibition, the Ca2+ blink termination level was independent of initial [Ca2+]SR (Fig. 4C) or release flux (Fig. 4D). In the presence of caffeine, Ca2+ spark release flux was initially much greater than that of control Ca2+ sparks. As [Ca2+]SR progressively decreased due to the increase in Ca2+ spark frequency, release flux also progressively declined (to near the initial control values). However, Ca2+ blinks still terminated at the ∼53%[Ca2+]SR level (Fig. 4D). This suggests that caffeine simply resets the Ca2+ blink termination level to a new [Ca2+]SR, an effect that is independent of initial [Ca2+]SR or [Ca2+]i in the dyadic cleft.
The [Ca2+]SR level for Ca2+ spark termination is lowered during heart failure
The results using acute application of caffeine clearly show that RyR sensitization increases fractional SR Ca2+ release through effects on both release activation and release termination. Therefore, we hypothesized that similar effects may occur in heart failure, where myocytes exhibit an increase in fractional SR Ca2+ release at low pacing frequencies when SR Ca2+ load is experimentally matched (Shannon et al. 2003b; Guo et al. 2007). To test how SR Ca2+ release is altered during heart failure, we monitored [Ca2+]i and [Ca2+]SR in myocytes isolated from rabbits that had developed heart failure (Pogwizd, 1995). Similar to previous reports using this model (Shannon et al. 2003b; Guo et al. 2007), when diastolic [Ca2+]SR was matched between normal (non-failing) and heart failure myocytes, fractional SR Ca2+ release was higher in the heart failure myocytes (Fig. 5A and B). We next measured Ca2+ sparks and blinks in permeabilized myocytes. This experimental strategy is advantageous as the L-type Ca2+ channel trigger for CICR may be altered during heart failure, either directly (Schroder et al. 1998) or due to changes in T-tubule architecture and coupling to SR Ca2+ release junctions (Gomez et al. 1997; He et al. 2001; Song et al. 2006; Lyon et al. 2009). In permeabilized heart failure myocytes, resting SR Ca2+ load and Ca2+ spark frequency were decreased compared to non-failing myocytes (Fig. 5D and F) while Ca2+ spark amplitude was similar between the two groups (Fig. 5E). However, it is problematic to directly compare these results due to the direct effects of [Ca2+]SR on Ca2+ spark frequency and amplitude (Satoh et al. 1998; Lukyanenko et al. 2001). To appropriately compare Ca2+ spark frequency and amplitude, we matched [Ca2+]SR in non-failing cells to their failing counterparts using SERCA inhibition with thapsigargin. When SR Ca2+ load was matched, both Ca2+ spark frequency and amplitude were markedly increased in failing myocytes (Fig. 5C–E). The increase in Ca2+ spark frequency suggests an increased sensitivity to release activation. We next examined if the increase in Ca2+ spark amplitude associated with a change in the [Ca2+]SR level for release termination. Similar to results with caffeine, the [Ca2+]SR termination level for Ca2+ sparks was decreased in heart failure (Fig. 6). As summarized in Fig. 6B, resting SR Ca2+ load was decreased in failing myocytes, and the [Ca2+]SR termination level of Ca2+ sparks was lower in failing (53.0 ± 1.9%, n= 11) than in non-failing myocytes (60.1 ± 0.9%, n = 82, P < 0.05). This suggests that the modifications that occur in heart failure sensitize RyRs and increase fractional SR Ca2+ release by decreasing the [Ca2+]SR level where release terminates. Collectively, these data show that RyR sensitization produces effects on both release activation and release termination, and each of these mechanisms may be altered during the progression of heart failure.
Discussion
The RyR is the principal SR Ca2+ release channel in the heart, and as such has become an experimental and potential therapeutic target to improve contractility in cardiac disease. However, as Eisner and colleagues have shown in rat ventricular myocytes, RyR sensitization with caffeine produces only transient positive inotropic effects, and does not lead to a sustained increase in SR Ca2+ release (Trafford et al. 1998). This phenomenon is explained by the initial increase in SR Ca2+ release shifting the cellular Ca2+ flux balance toward Ca2+ extrusion, causing eventual unloading of the SR and a decrease in the Ca2+ transient amplitude back to control levels. Our data from rabbit ventricular myocytes showed that SR Ca2+ loss was particularly pronounced, which led to a decrease in steady-state SR Ca2+ release (Fig. 1). The experimental differences between rat (‘autoregulation’ and return to steady-state release) and rabbit (marked SR Ca2+ loss and decrease in steady-state release) are likely to be due to the differences in expression of SERCA and NCX between these species (Bassani et al. 1994). Compared with rat, rabbit has less SERCA but increased NCX expression, which functionally will translate into less Ca2+ re-uptake into the SR via SERCA, more Ca2+ extrusion from the cell via NCX, and greater net cellular Ca2+ loss.
While RyR sensitization with caffeine did not produce a sustained increase in steady-state SR Ca2+ release, it became clear that when initial [Ca2+]SR was matched, caffeine caused a large increase in fractional SR Ca2+ release (Fig. 2D). At a given [Ca2+]SR, we observed two distinct functional mechanisms by which caffeine increased the amount of global Ca2+ release. First, RyRs were sensitized to CICR, the percentage of Ca2+ release units that opened during ECC was increased, and global Ca2+ release became highly synchronized (Fig. 3). This sensitization to release activation was also observed here, or in previous investigations, as an increase in spark frequency (Satoh et al. 1998; Lukyanenko et al. 2001) and Ca2+ wave occurrence (Lukyanenko et al. 1999). In addition, the increased sensitivity of RyRs in the presence of caffeine was further manifest in our experiments as an increase in Ca2+ spark flux at a given [Ca2+]SR (Fig. 4D). This observation can be explained by several mechanisms, including the opening of more RyR channels within a release cluster or by synchronizing the opening of the RyR channels in the cluster to achieve more coordinated release.
In addition to the effect of caffeine on Ca2+ release activation, we found a second mechanism that explains the increase in global fractional release. At each release junction that opens during ECC, more Ca2+ is released due to a decrease in the [Ca2+]SR termination level. This was observed at individual release junctions in response to electrical stimulation (Fig. 3) as well as during spontaneous Ca2+ sparks (Fig. 4). Like Ca2+ spark termination under control conditions, the Ca2+ spark [Ca2+]SR termination level in the presence of caffeine was independent of Ca2+ spark release flux, which shows that it was independent of [Ca2+]i in the dyadic cleft. These data, as well as results from other studies (Sobie et al. 2002; Terentyev et al. 2002; Hinch, 2004), suggest that [Ca2+]i-dependent inactivation of the RyR is not the principal mechanism by which Ca2+ release terminates in cardiac myocytes. The precise mechanism by which caffeine lowers the [Ca2+]SR termination level for release remains unclear, although it most likely reflects alterations in RyR channel or cluster gating by [Ca2+]SR, as the set termination threshold observed under control conditions was simply ‘reset’ to a lower level in the presence of caffeine. Increasing evidence points to a luminal Ca2+ sensor on the RyR (Sitsapesan & Williams, 1994; Gyorke & Gyorke, 1998), and it has been suggested that caffeine exerts its effects on the RyR exclusively via altering luminal [Ca2+]SR-dependent gating (Kong et al. 2008). Thus, changes in the [Ca2+]SR-dependent gating mechanism would adequately explain the effects of caffeine on both the increased sensitivity for release activation and the decrease in the [Ca2+]SR level for release termination.
The chronic modifications that occur in heart failure produce effects which are very similar to those caused by caffeine application. Clearly this type of comparison must be made with caution, as caffeine sensitizes RyRs acutely as a channel agonist while the RyR alterations that occur in heart failure progress over time, likely as post-translational modifications. These modifications remain highly controversial (Bers et al. 2003; Marks, 2003), with current evidence suggesting a role for protein kinase A (Marx et al. 2000), calmodulin-dependent protein kinase II (Ai et al. 2005), or reduction–oxidation processes (Terentyev et al. 2008a). Nevertheless, despite these caveats, important conceptual information can be gained by comparing the functional effects of caffeine with those observed in heart failure. Numerous Ca2+ transport systems are altered during the progression of heart failure, including changes in expression and/or function of the L-Type Ca2+ channel, NCX, SERCA, and the RyR (for review see Bers, 2006). Therefore, measurements of Ca2+ sparks in permeabilized myocytes are particularly useful for evaluating SR Ca2+ release, as the influence of the sarcolemma is eliminated, [Ca2+]i and soluble components of the intracellular milieu are controlled, and [Ca2+]SR is measured and controlled experimentally. SERCA activity does not contribute to the amplitude of the Ca2+ spark or blink (Zima et al. 2008b). Therefore, once SR Ca2+ content is experimentally matched, changes in Ca2+ sparks specifically reflect alterations in RyR release cluster function. Ca2+ sparks from failing myocytes exhibited alterations in both release activation and release termination, effects which were similar to those observed following RyR sensitization with caffeine. Ca2+ spark frequency, an index of basal sensitivity to release activation, was higher in failing than non-failing myocytes when SR Ca2+ load was matched (Fig. 5D) (Kubalova et al. 2005; Song et al. 2005; Lyon et al. 2009). Additionally, the [Ca2+]SR level where Ca2+ sparks terminated was lower in heart failure myocytes (Fig. 6), an observation also seen in canine heart failure (Kubalova et al. 2005). In the intact cellular environment, the increase in Ca2+ spark frequency is assumed to be the primary contributing factor to the elevated diastolic SR Ca2+ leak in heart failure (Shannon et al. 2003b). However, at a given Ca2+ spark frequency, decreasing the [Ca2+]SR termination level for release will further enhance SR Ca2+ loss. Increasing fractional release during systole will also increase total SR Ca2+ loss, as is clearly evident by the effects of acute caffeine application (Fig. 1). This effect will be particularly pronounced when powerful sarcolemmal Ca2+ extrusion mechanisms exist, such as in species with high expression of NCX (Bassani et al. 1994) or when NCX is upregulated during heart failure (Pogwizd et al. 1999). Together, these alterations explain the rather paradoxical association of increased fractional SR Ca2+ release with decreased total SR Ca2+ release in the failing heart.
Our data functionally separate the mechanisms of release activation and release termination. Implicit in the logic of this investigation is that these functional observations correspond to distinct RyR gating mechanisms, which is still unclear. If release activation and release termination are distinct mechanisms, the key remaining question is whether they can be regulated separately. Although the decrease in [Ca2+]SR termination level is to some extent counterproductive as it contributes to loss of [Ca2+]SR, it is undoubtedly a key means by which systolic fractional SR Ca2+ release is enhanced at the lower [Ca2+]SR. Thus, one may speculate that decreasing the termination level for release without concomitant effects on release activation would be an ideal positive inotropic intervention, as systolic fractional SR Ca2+ release would be enhanced without substantial diastolic SR Ca2+ leak.
In summary, we find that RyR sensitization has multiple effects on global SR Ca2+ release. First, it alters RyR activation, increases recruitment of release junctions and synchronizes release during ECC. Furthermore, at each individual release junction, RyR sensitization lowers the [Ca2+]SR level of release termination and increases the amplitude of SR Ca2+ release. Alteration of the [Ca2+]SR termination level for release is therefore likely to serve as an additional mechanism by which ECC can be tuned to match the physiological needs of the heart.
Acknowledgments
This work was supported by National Institutes of Health Grants P01HL80101 and R01HL62231 (to L.A.B.) and F32HL090211 (to T.L.D.). The authors would like to thank the laboratory of Dr Steven M. Pogwizd for providing rabbit heart failure myocytes. The authors would also like to thank Dr Elisa Bovo, Stephen Shonts and Demetrio Santiago for assistance with myocyte isolation, and Drs Andreas Rinne and Vyacheslav Shkryl for critical reading of the manuscript.
Glossary
Abbreviations
- CICR
calcium-induced calcium release
- ECC
excitation–contraction coupling
- FDHM
full-duration half-maximum
- FWHM,
full-width half-maximum
- HF
heart failure
- NCX
Na+/Ca2+ exchanger
- RyR
ryanodine receptor
- SERCA
sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum
- TG
thapsigargin
Author contributions
All authors contributed to the conception and design of the study, analysis and interpretation of data, and writing of the article. All authors have approved the version to be published. T.L.D. and A.V.Z. performed the experimental work.
References
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Gyorke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J. 2007;93:4083–4092. doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation–Contraction Coupling and Cardiac Contractile Force. Dordrecht: Kluwer Academic Publishers; 2001. [Google Scholar]
- Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93:487–490. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- Brochet DX, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signalling. Proc Natl Acad Sci U S A. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Domeier TL, Zima AV, Blatter LA. Ryanodine receptor sensitization alters local and global sarcoplasmic reticulum calcium release termination threshold in rabbit ventricular myocytes. Biophys J. 2009;96:276a. [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Choi HS, Diaz ME, O’Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- George CH. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res. 2008;77:302–314. doi: 10.1093/cvr/cvm006. [DOI] [PubMed] [Google Scholar]
- Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- Guo T, Ai X, Shannon TR, Pogwizd SM, Bers DM. Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ Res. 2007;101:802–810. doi: 10.1161/CIRCRESAHA.107.152140. [DOI] [PubMed] [Google Scholar]
- Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- Hinch R. A mathematical analysis of the generation and termination of calcium sparks. Biophys J. 2004;86:1293–1307. doi: 10.1016/S0006-3495(04)74203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobai IA, O’Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 2001;103:1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SR. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J. 2008;414:441–452. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Gyorke S. Abnormal intrastore calcium signalling in chronic heart failure. Proc Natl Acad Sci U S A. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner M, Erdmann E, Beuckelmann DJ. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol. 1998;30:743–749. doi: 10.1006/jmcc.1997.0626. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Subramanian S, Gyorke I, Wiesner TF, Gyorke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J Physiol. 1999;518:173–186. doi: 10.1111/j.1469-7793.1999.0173r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Gyorke S. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci U S A. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR. A guide for the perplexed: towards an understanding of the molecular basis of heart failure. Circulation. 2003;107:1456–1459. doi: 10.1161/01.cir.0000059745.95643.83. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, Ohkusa T, Ikeda Y, Kobayashi S, Ikemoto N, Matsuzaki M. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation. 2005;111:3400–3410. doi: 10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007;293:C1073–1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034–1048. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Satoh H, Katoh H, Velez P, Fill M, Bers DM. Bay K 8644 increases resting Ca2+ spark frequency in ferret ventricular myocytes independent of Ca influx: contrast with caffeine and ryanodine effects. Circ Res. 1998;83:1192–1204. doi: 10.1161/01.res.83.12.1192. [DOI] [PubMed] [Google Scholar]
- Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RH, Weil J, Herzig S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003a;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003b;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Wier WG. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation–contraction coupling. J Physiol. 1991;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by luminal Ca2+ J Membr Biol. 1994;137:215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- Sobie EA, Dilly KW, dos Santos Cruz J, Lederer WJ, Jafri MS. Termination of cardiac Ca2+ sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LS, Pi Y, Kim SJ, Yatani A, Guatimosim S, Kudej RK, Zhang Q, Cheng H, Hittinger L, Ghaleh B, Vatner DE, Lederer WJ, Vatner SF. Paradoxical cellular Ca2+ signalling in severe but compensated canine left ventricular hypertrophy. Circ Res. 2005;97:457–464. doi: 10.1161/01.RES.0000179722.79295.d4. [DOI] [PubMed] [Google Scholar]
- Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking ‘Ca2+ spikes’ in rat cardiac myocytes. J Physiol. 1998;512:677–691. doi: 10.1111/j.1469-7793.1998.677bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD, Cheng H. Putting out the fire: what terminates calcium-induced calcium release in cardiac muscle? Cell Calcium. 2004;35:591–601. doi: 10.1016/j.ceca.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008a;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Kubalova Z, Valle G, Nori A, Vedamoorthyrao S, Terentyeva R, Viatchenko-Karpinski S, Bers DM, Williams SC, Volpe P, Gyorke S. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys J. 2008b;95:2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Diaz ME, Eisner DA. Stimulation of Ca-induced Ca release only transiently increases the systolic Ca transient: measurements of Ca fluxes and sarcoplasmic reticulum Ca. Cardiovasc Res. 1998;37:710–717. doi: 10.1016/s0008-6363(97)00266-6. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, Van Der Nagel R, Morales R, Sun J, Cheng Z, Deng SX, de Windt LJ, Landry DW, Marks AR. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci U S A. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Picht E, Bers DM, Blatter LA. Sarcoplasmic reticulum Ca2+ depletion contributes to termination of cardiac myocyte Ca2+ sparks. Biophys J. 2007;92:343a. [Google Scholar]
- Zima AV, Picht E, Bers DM, Blatter LA. Partial inhibition of sarcoplasmic reticulum Ca release evokes long-lasting Ca release events in ventricular myocytes: role of luminal Ca in termination of Ca release. Biophys J. 2008a;94:1867–1879. doi: 10.1529/biophysj.107.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res. 2008b;103:e105–115. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]