Abstract
Muscle contraction stimulates thin fibre muscle afferents and evokes a reflex increase in blood pressure. In heart failure (HF) this reflex is accentuated. Of note, superoxide and other reactive oxygen species are increased in HF. In this report, we tested the hypothesis that excess superoxide contributes to the exaggerated muscle reflex in HF. HF was induced in rats by coronary artery ligation. Electrically induced 30 s hindlimb muscle contraction in decerebrate rats with myocardial infarction (MI) (left ventricular fractional shortening (FS) = 24 ± 1%; n= 15) evoked larger (P < 0.05) increases in mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) as compared to control rats (FS = 47 ± 1%; n= 14). In the MI rats, the pressor and RSNA responses to contraction were reduced by intra-arterial injection into the hindlimb circulation of tempol (10 mg), a superoxide dismutase mimetic (ΔMAP: 22 ± 2 vs. 11 ± 1 mmHg; ∫ΔRSNA: 1032 ± 204 vs. 431 ± 73 arbitrary units (a.u.); before vs. after tempol; P < 0.05). Tempol also attenuated the RSNA response to 1 min intermittent (1–4 s stimulation to relaxation) bouts of static contraction in the MI rats (116 ± 17 vs. 72 ± 11 a.u.; P < 0.05; n= 16). In the control rats, tempol had no effect on these responses. These results suggest that excess superoxide in HF sensitizes mechanically sensitive muscle afferents engaged during contraction. We hypothesize that oxidative stress contributes to the exaggerated muscle reflex in HF.
Introduction
During exercise, the sympathetic nervous system is activated (Mark et al. 1985). A reflex originating in contracting skeletal muscle contributes to the sympathoexcitation seen during exercise (Coote et al. 1971; McCloskey & Mitchell, 1972). This muscle reflex is evoked as thin fibre muscle afferents are stimulated by mechanical deformation of the afferents’ receptive fields (muscle mechanoreflex) as well as by metabolic by-products (muscle metaboreflex) during contraction. In turn, cardiovascular circuits in the brain are activated and sympathetic nervous system activity is increased. Group III and IV muscle afferents are thought to be predominantly mechanically and metabolically sensitive afferents, respectively (Kaufman et al. 1983; Kaufman & Hayes, 2002).
During exercise in heart failure (HF), augmented sympathoexcitation and sympathetically mediated excess peripheral vasoconstriction are observed (Zelis et al. 1974; LeJemtel et al. 1986; Silber et al. 1998; Middlekauff et al. 2000, 2001; Momen et al. 2004; Murai et al. 2009). These factors contribute to exercise intolerance of patients with HF (Wilson & Mancini, 1993). Data collected from patients and animal models suggest that end-organ responses to muscle reflex engagement are altered in HF (Middlekauff et al. 2000, 2001; Sinoway & Li, 2005; Smith et al. 2006). In patients with HF, muscle sympathetic nerve activity (MSNA) rises with exercise but falls towards baseline during post-exercise ischaemia (Sterns et al. 1991). Interestingly, MSNA in response to muscle stretch (muscle mechanoreflex activation) is augmented in HF patients (Middlekauff et al. 2004), suggesting that the muscle mechanoreflex is accentuated in HF. Experiments performed in decerebrate rats with HF after myocardial infarction (MI) showed that increases in blood pressure as well as sympathetic nerve activities in response to muscle contraction or stretch are augmented in HF (Smith et al. 2003, 2005a,b; Li et al. 2004; Koba et al. 2008). Further, compared with control rats, HF rats displayed an attenuated pressor response to capsaicin administered intra-arterially into the hindlimb circulation (Li et al. 2004; Smith et al. 2005b), a manoeuvre that selectively excites group IV afferent neurons (Kaufman et al. 1982). These animal and human studies in total suggest that, in HF, the muscle mechanoreceptors are sensitized whereas the muscle metaboreceptors are desensitized. As a consequence, the reflex originating in contracting muscle is exaggerated in this disease. The mechanisms responsible for the exaggerated mechanoreflex in HF are poorly understood.
In HF, oxidative stress is increased (Keith et al. 1998; Bauersachs et al. 1999). Excessive superoxide and other reactive oxygen species in skeletal muscle are linked to peripheral hypoperfusion as a consequence of low cardiac output and peripheral endothelial dysfunction (Thomas et al. 2001; Tsutsui et al. 2001). Superoxide may contribute to the exaggerated muscle reflex in HF. Previous studies have suggested that superoxide functions in the neural process for encoding peripheral stimuli. It was reported that tempol, a compound that mimics the enzymatic activity of superoxide dismutase (SOD) (Wilcox & Pearlman, 2008), alleviated mechanical hyperalgesia seen after injection of capsaicin in rats (Lee et al. 2007). In mice lacking Nox1, a superoxide-generating NAD(P)H oxidase, thermal and mechanical hyperalgesia was attenuated (Ibi et al. 2008). Finally, a recent report by Wang et al. (2009) showed that tempol reduced a pressor response to muscle contraction in decerebrate healthy rats. Based on these previous findings, we hypothesized that excess superoxide in HF sensitizes muscle afferents engaged during contraction, thereby contributing to the exaggerated muscle reflex. To test this hypothesis, we used the decerebrate rat preparation, and examined renal sympathetic nerve and cardiovascular responses to hindlimb muscle contraction before and after intra-arterial injection of tempol into the hindlimb circulation. Then, we compared the effect of tempol on these responses between healthy controls and rats with HF induced by MI. We employed two contraction protocols; 30 s continuous static contraction and 1 min intermittent (1–4 s stimulation to relaxation) static contraction. Continuous contraction stimulates both mechanically and metabolically sensitive muscle afferents whereas a short period (1 s) of contraction during the intermittent contracting protocol predominantly stimulates mechanosensitive afferents (Victor et al. 1989; Kaufman & Hayes, 2002; Koba et al. 2007, 2008).
Methods
All procedures outlined in this report complied with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care Committee of this institution. A total of 42 male Sprague–Dawley rats were used in the experiments of this report. The rats were housed in standard rodent cages and regulated on a 12 h light–12 h dark schedule. Food and water were made available ad libitum.
Coronary artery ligation and echocardiography
Coronary artery ligation surgery was performed as described previously (Li et al. 2004; Koba et al. 2008). Rats were anaesthetized with a mixture of isoflurane (< 4%) and oxygen, intubated, and artificially ventilated with a respirator (model 683, Harvard). An incision between the fourth and fifth ribs was made, and the left ventricular wall was exposed through a thoracotomy. The left coronary artery was then ligated.
More than 7 weeks after the ligation surgery, transthoracic echocardiography (Sequoia C256, Acuson/Siemens) was performed to assess left ventricular function (Table 1). Rats with left anterior descending coronary artery ligation had an average left ventricular fractional shortening of 24 ± 1%, which was significantly less than that measured for healthy controls (47 ± 1%). Moreover, left ventricular end-diastolic and end-systolic dimensions were significantly increased in the ligated rats (Table 1), indicating the development of a left ventricular dilatation.
Table 1.
Morphometric and echocardiographic characteristics of rats used for experiments where tempol was injected intra-arterially are shown
| Control | MI | |
|---|---|---|
| n | 15 | 19 |
| Body weight (g) | 526 ± 10 | 551 ± 8 |
| Heart weight (mg) | 1.51 ± 0.08 | 2.03 ± 0.06* |
| LVEDP (mmHg) | 4 ± 1 | 11 ± 1* |
| LVDD (mm) | 0.89 ± 0.02 | 1.05 ± 0.03* |
| LVSD (mm) | 0.47 ± 0.02 | 0.80 ± 0.02* |
| FS (%) | 47 ± 1 | 24 ± 1* |
Values are means ±s.e.m.*P < 0.05 vs. controls detected by unpaired t test. n, number of animals; LVEDP, left ventricular end-diastolic pressure; LVDD, left ventricular end-diastolic diameter; LVSD, left ventricular end-systolic diameter; FS, fractional shortening (= 100 × (LVDD − LVSD)/LVDD).
General preparations
After echocardiography, the MI rats as well as the age- and body weight-matched controls were anaesthetized with a mixture of isoflurane (< 4%) and oxygen. The trachea was cannulated, and the lungs were artificially ventilated (683, Harvard). The left jugular vein and common carotid artery were cannulated to administer drugs and to record arterial pressure (AP), respectively. The arterial catheter was attached to a pressure transducer (P23XL, BD). Two needle electrodes were placed on the chest to measure the electrocardiogram (ECG). The ECG signals were amplified with an AC amplifier (P511, Grass Instruments). Heart rate (HR) was calculated beat to beat with detection of the time between successive R waves in the ECG. Arterial pH was monitored with a pH meter (B-212, Horiba), and maintained within normal limits (pH = 7.4) by an intravenous infusion of a sodium bicarbonate solution (8.4%). Rectal body temperature was monitored with a digital thermometer (V912F, Vicks), and adequately maintained at 37.5–38.5°C with an external heating lamp. To measure renal sympathetic nerve activity (RSNA), a bipolar electrode made of a Teflon-insulated stainless steel wire (790600, A-M Systems) was connected to the renal nerve directed to the left kidney. The surgical procedure for rat RSNA measurements is described in detail in our previous reports (Koba et al. 2006, 2007, 2008; Gao et al. 2008). The RSNA signal was amplified with an AC amplifier (P511, Grass Instruments) with a band-pass low frequency filter of 100 Hz and a high frequency filter of 3 kHz, and made audible. A catheter (PE-10, 10 cm) was inserted into the right femoral artery, and then threaded into the right common iliac artery. The catheter tip was stabilized at the junction of the iliac arteries. A reversible ligature was placed around the left common iliac vein. The left Achilles’ tendon and left triceps surae muscles were isolated by cutting the calcaneus bone and dissecting the tendon and muscles free from the connective tissue that attached to the tibia. The hindlimb was fixed in space with a patellar precision clamp to prevent limb movement. The tension developed by the triceps surae muscles was measured with a force transducer (FT03, Grass Instruments) connected to the Achilles’ tendon. The rats were held in a stereotaxic apparatus (900LS, David Kopf Instruments).
A laminectomy exposed the lower lumbar portions of the spinal cord (L2–L6). The meningial layers surrounding the cord were cut and reflected laterally. Two nerve bundles obtained from L4 and L5 ventral roots were carefully isolated and sectioned. The peripheral cut ends of the roots were placed on an insulated bipolar electrode. The exposed neural tissue was immersed in mineral oil and maintained at a temperature of 37°C.
A decerebration was performed at the mid-collicular level by coronal section of the brain and aspiration of all neural tissues rostral to the section and cortical tissues covering the cerebellum (Koba et al. 2006, 2007, 2008). To minimize cerebral haemorrhage, the intact right carotid artery was ligated prior to the decerebration, and cotton balls soaked with normal saline (0.9% NaCl) were placed on the exposed surfaces of the brain. Then, gas anaesthesia was discontinued. A recovery period of at least 90 min was allowed before the experimental protocol was begun.
Experimental protocol
The decerebrate rats were mechanically ventilated (tidal volume 5.5–6.0 ml kg−1 and frequency 70 min−1). Contraction of the left hindlimb muscles was induced by excitation of ventral roots with constant current electrical stimulation (2 × motor threshold (MT), 0.1 ms, 40 Hz). The minimum current intensity necessary to induce muscle twitch served as MT. We used two forms of contraction; 30 s continuous static contraction and intermittent (1–4 s stimulation to relaxation) static contraction for 1 min. When both manoeuvres were tested in the same rat, 15 min was allowed between each manoeuvre. Subsequently, tempol (10 mg dissolved in 0.2 ml normal saline) was injected slowly over a 2 min period through the catheter placed in the right common iliac artery. Immediately prior to the injection of tempol, the reversible ligature around the left common iliac vein was tightened for 10 min to trap the injectate in the hindlimb circulation. During the 2 min injection, a string placed around the base of the tail was tightened to prevent the drug from entering the tail circulatory system. After 10 min, the ligature around the common iliac vein was loosened. The two forms of contractions were repeated at 15–30 min after trapping tempol. In two rats, Evans blue dye was injected with this procedure and labelled tissues within the left hindlimb but not tissues elsewhere, suggesting that this procedure trapped injectate within the hindlimb circulation and did not allow the chemical injected to reach into the systemic circulation. Thus, the injected tempol entered the left hindlimb circulation via the left common iliac artery, and was trapped within its vasculature.
In a subset of six normal healthy rats, we also examined the effect of tempol (10 mg) intravenously infused via the left jugular vein on RSNA and cardiovascular responses to 30 s continuous static contraction. Twenty to 25 min after intravenous administration of tempol, 30 s continuous contraction was repeated. This protocol was conducted to test if tempol administered systemically can influence reflex responses by processes not limited to changes in hindlimb levels of oxidative stress.
At the end of data collection, neuromuscular transmission in the rats was blocked with an intravenous infusion of pancuronium bromide (0.25 mg). The ventral roots were then continuously (30 s) stimulated at 2 × MT intensity. This procedure was conducted to confirm that the observed responses to contraction were not due to either current spread to the spinal cord or to direct stimulation of group III or IV primary afferents. Stimulation after neuromuscular blockade did not change the RSNA, AP or HR. After all of the experiments were conducted, the renal nerve was cut between the electrode and the neural axis in order to measure the background noise of RSNA. A polyethylene catheter was inserted into the right carotid artery and threaded into the left ventricle to measure left ventricular end-diastolic pressure. At the conclusion of the experiment, the rats were humanely killed with an intravenous infusion of sodium pentobarbital (75 mg kg−1) followed by an intravenous infusion of potassium chloride (2 mol l−1, 1 ml).
Data acquisition and statistical analyses
All measured variables were displayed continuously on a computer monitor and stored on a hard disk through an analog–digital interface (Powerlab/8s, ADInstruments). Mean AP (MAP) and HR were obtained beat-by-beat. MAP and HR were then averaged over every 1 s after re-sampling at 1 kHz. Data sets of 1 s averaged MAP and HR as well as tension developed within the triceps surae muscles were analysed.
RSNA was analysed as reported previously (Koba et al. 2006, 2007, 2008; Gao et al. 2008). We obtained full-wave rectified signals of RSNA as well as the background noise signals. The noise component was subtracted from the rectified RSNA. Then, the RSNA values were integrated over every 1 s. To quantify RSNA in response to continuous static contraction, basal values were obtained by taking mean values for 30 s of baseline prior to the manoeuvre, and considered as 100% by evaluating the mean. Then, relative changes from baseline were evaluated every 1 s (Koba et al. 2006; Gao et al. 2008). To quantify RSNA in response to a short period (1 s) of contraction during the intermittent contracting protocol, additional procedures were conducted, as reported previously (Koba et al. 2007, 2008). Moving averages of RSNA were obtained over 50 ms intervals. Relative changes from baseline were averaged over 100 ms intervals. The RSNA responses to 12 periods of muscle contraction were then superimposed on one another and averaged. After this normalization, the data obtained from each rat were used to examine the mean results (Koba et al. 2007, 2008).
The data are expressed as means ±s.e.m. Baseline data were obtained from the averaged values for 30 s immediately before muscle contraction. Peak values for tension, AP and HR were detected from 1 s averaged data. Tension–time index (TTI), an index of developed muscle tension during contraction, was calculated by integrating developed tension (integrated total tension minus integrated baseline tension prior to the manoeuvre) during the contraction period (Koba et al. 2007, 2008). The integrated ΔRSNA were calculated by integrating the increases in RSNA due to contraction. To assess significant differences, the data were analysed with a paired (within the same animal) or unpaired (MI vs. control) t test, and one-way (vs. basal values) or two-way (drug effect × rat group) repeated ANOVA followed by appropriate post hoc tests. As post hoc tests, Dunnett's (following one-way repeated ANOVA) and Tukey's (following two-way repeated ANOVA) methods were employed. The level of significance was set at P < 0.05.
Results
Continuous static contraction
In 14 control rats and 15 MI rats, we tested the effect of tempol (10 mg) injected into the arterial supply of the left hindlimb on RSNA and cardiovascular responses to 30 s continuous static contraction of the left hindlimb muscles. Baseline MAP and HR were not affected by tempol injection in both control (92 ± 3 mmHg and 393 ± 13 beats min−1 before tempol; 88 ± 3 mmHg and 378 ± 12 beats min−1 after tempol) and MI groups (87 ± 3 mmHg and 396 ± 10 beats min−1 before tempol; 85 ± 4 mmHg and 384 ± 10 beats min−1 after tempol). Tempol also had no effect on baseline RSNA (signal-to-noise ratio; control: 4.8 ± 0.7 and 4.3 ± 0.5 before and after tempol; MI: 5.0 ± 0.6 and 4.9 ± 0.6 before and after tempol). Continuous static contraction significantly increased AP, HR and RSNA (Figs 1–3), as reported previously (Smith et al. 2003, 2005a,b; Koba et al. 2006). Before tempol injection, the pressor and RSNA responses to continuous contraction in the MI rats were significantly larger than those seen in the healthy controls, despite the fact that triceps surae muscle tension was not significantly different (Fig. 3).
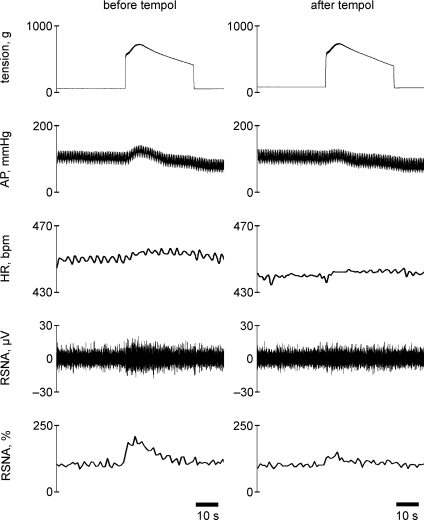
Figure 1.
Representative recordings from one myocardial infarction (MI) rat (fractional shortening = 17%) of tension developed within the left triceps surae muscles, arterial pressure (AP), heart rate (HR) (bpm = beats min−1), renal sympathetic nerve activity (RSNA), and the relative changes in RSNA during 30 s continuous contraction of the left hindlimb muscles before (left) and after (right) intra-arterial injection of tempol (10 mg) into the hindlimb circulation. Note that RSNA increased rapidly towards peak values from the onset of muscle tension development, and that the elevated RSNA gradually decreased towards baseline level.
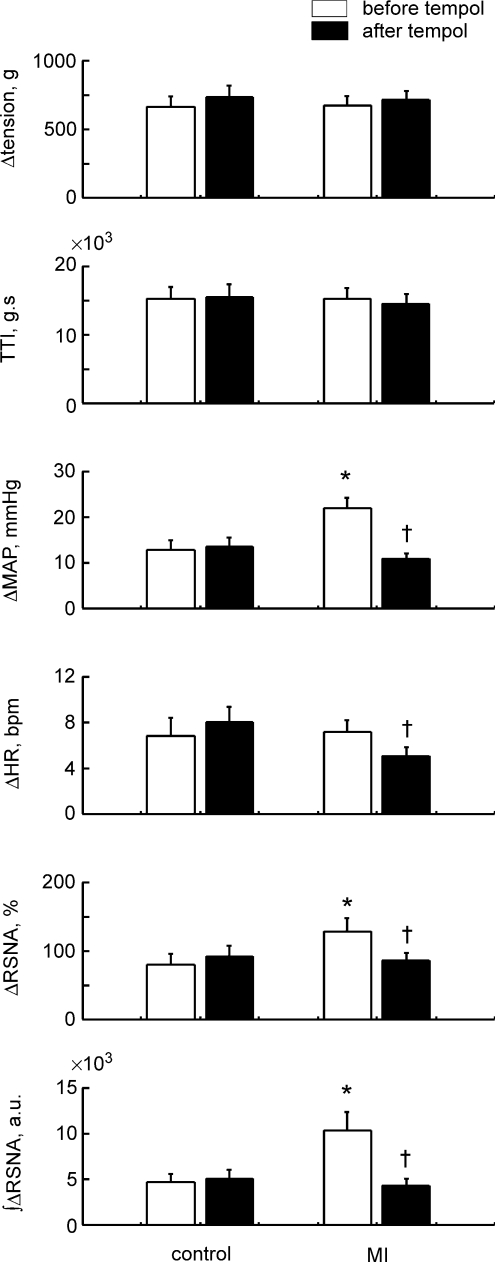
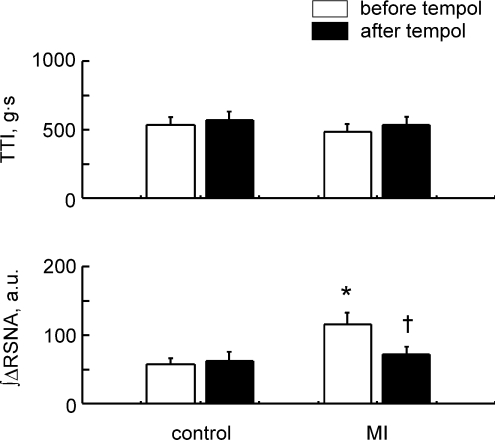
Figure 3.
Comparisons of Δtension, tension–time index (TTI), ΔMAP, ΔHR, ΔRSNA and the integrated ΔRSNA during 30 s continuous static contraction before and after injection of tempol in 14 healthy controls and 15 MI rats. Δvalues are the peak increases seen during 30 s contraction. TTI and the integrated ΔRSNA are the 30 s integrated values. Values are means ±s.e.m.*P < 0.05 vs. healthy controls. †P < 0.05 vs. before tempol injection. Significant differences were detected by Tukey's post hoc test following two-way repeated ANOVA.
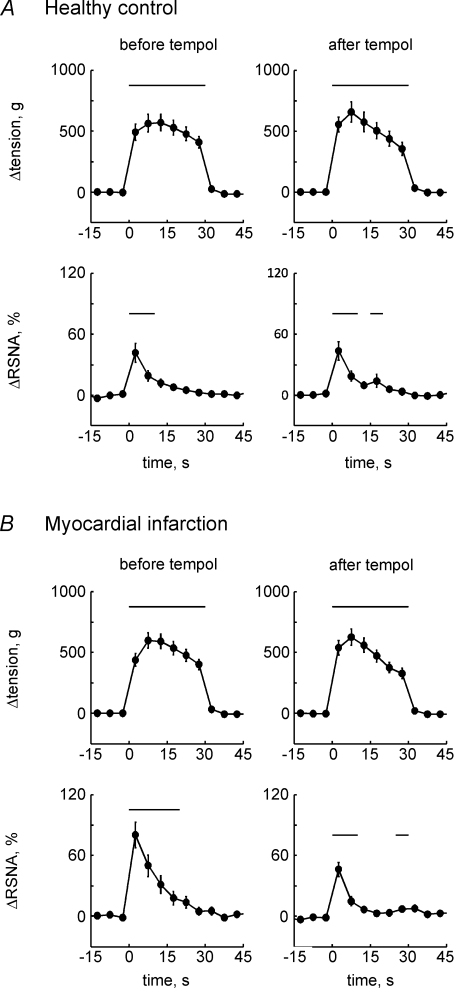
Five-second-averaged changes from baseline in muscle tension and RSNA during continuous static contraction before and after injection of tempol in healthy controls (n= 14, A) and MI rats (n= 15, B). Values are means ±s.e.m. Baseline data were obtained from the averaged values for 30 s immediately before contraction. Horizontal bars indicate significant (P < 0.05) differences from baseline, detected by Dunnett's post hoc test following one-way repeated ANOVA.
In the controls, responses of MAP, HR and RSNA to contraction were not affected by tempol injected intra-arterially (Fig. 3). On the other hand, in the MI rats, these cardiovascular and RSNA responses were significantly reduced by tempol (Fig. 3). The responses after tempol injection in the MI rats were at the equivalent level to those seen in the control rats before or after tempol injection (Fig. 3).
Intermittent static contraction
In 13 control rats and 16 MI rats, we tested the effect of tempol (10 mg) injected intra-arterially on RSNA and cardiovascular responses to 1 min intermittent (1–4 s stimulation to relaxation) static contraction. Baseline MAP and HR were not affected by tempol injection in both control (88 ± 4 mmHg and 390 ± 12 beats min−1 before tempol; 89 ± 4 mmHg and 385 ± 10 beats min−1 after tempol) and MI groups (91 ± 3 mmHg and 383 ± 9 beats min−1 before tempol; 88 ± 3 mmHg and 376 ± 9 beats min−1 after tempol). Tempol also had no effect on baseline RSNA (signal-to-noise ratio; control: 4.5 ± 0.7 and 4.0 ± 0.4 before and after tempol; MI: 5.2 ± 0.5 and 4.9 ± 0.5 before and after tempol). During the 1 min of intermittent contraction, RSNA response was synchronized to the increase in tension (Fig. 4). In both control rats and the MI rats, intermittent contraction did not significantly affect the average MAP because this contraction protocol induced variable patterns in the blood pressure response across rats. The characteristics of the sympathetic activity and blood pressure dynamics seen during intermittent contraction were consistent with previous reports in cats and rats (Victor et al. 1989; Koba et al. 2007, 2008). HR was slightly, but significantly, elevated from baseline by 1 min intermittent contraction (e.g. +5 ± 1 beats min−1 in the MI rats before tempol injection). Before tempol injection, the integrated RSNA response to intermittent contraction in the MI rats was significantly larger than that in controls at equivalent levels of developed muscle tension (Fig. 5). In the controls, the RSNA response to contraction was not affected by tempol injected intra-arterially (Fig. 5). On the other hand, in the MI rats, the integrated RSNA in response to contraction was significantly reduced by tempol (Fig. 5). The RSNA response after tempol injection in the MI rats was at the equivalent level to that in the controls before or after tempol injection (Fig. 5). Tempol did not have significant effects on AP or HR changes during contraction in either the controls or MI rats.
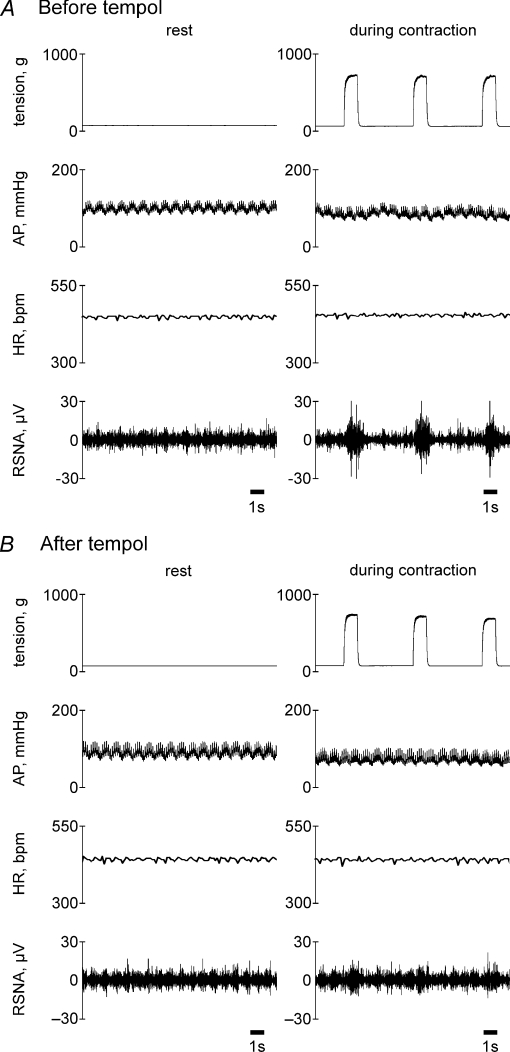
Figure 4.
Representative recordings from one MI rat of muscle tension, AP, HR and RSNA during intermittent (1–4 s stimulation to relaxation) static contraction before (A) and after (B) intra-arterial injection of tempol into the hindlimb circulation. Eighth, ninth and tenth of 12 repeated contractions are presented in this figure. RSNA responded synchronously as tension was developed. This MI rat, the same as the one shown in Fig. 1, displayed a tendency for the AP to decrease during contraction.
Figure 5.
Comparison of TTI and the integrated ΔRSNA in response to intermittent static contraction before and after injection of tempol in 13 healthy controls and 16 MI rats. Values are means ±s.e.m.*P < 0.05 vs. healthy controls. †P < 0.05 vs. before tempol injection. Significant differences were detected by Tukey's post hoc test following two-way repeated ANOVA.
Responses to continuous static contraction after intravenous infusion of tempol
In a subset of six normal healthy rats (513 ± 22 g of body weight), we tested the effect of tempol (10 mg) injected intravenously via the left jugular vein on RSNA and cardiovascular responses to 30 s continuous static contraction. Tempol had no effect on baseline MAP, HR or RSNA, but reduced RSNA and cardiovascular responses to continuous contraction at 20–25 min after injection (Table 2).
Table 2.
Baseline values and responses to 30 s continuous static contraction before and after intravenous infusion of 10 mg of tempol via the left jugular vein are shown (n= 6)
| Before | After | |
|---|---|---|
| Baseline values | ||
| MAP (mmHg) | 87 ± 6 | 90 ± 7 |
| HR (beats min−1) | 371 ± 21 | 354 ± 19 |
| Signal-to-noise ratio for RSNA | 4.0 ± 0.5 | 3.7 ± 0.3 |
| Responses to contraction | ||
| Δtensionpeak (g) | 623 ± 67 | 616 ± 86 |
| TTI (g s) | 143 ± 16 (×102) | 130 ± 23 (×102) |
| ΔMAPpeak (mmHg) | 15 ± 3 | 8 ± 3* |
| ΔHRpeak (beats min−1) | 8 ± 2 | 6 ± 2* |
| ΔRSNApeak (%) | 88 ± 14 | 59 ± 18* |
| ∫ΔRSNA (a.u.) | 628 ± 93 | 367 ± 112* |
Values are means ±s.e.m.*P < 0.05 vs. before tempol detected by paired t test.
Discussion
We found that RSNA and presssor responses to continuous contraction and the RSNA response to intermittent contraction were significantly larger in the MI rats than those seen in the healthy control rats. We also found that intra-arterial injection of tempol, a membrane-permeable SOD mimetic, into the hindlimb circulation reduced the RSNA, AP and HR responses to continuous contraction as well as the RSNA response to intermittent contraction in rats with HF. On the other hand, in the healthy rats, tempol injected intra-arterially did not mediate the RSNA and cardiovascular responses to either continuous or intermittent static contraction. These data suggest that superoxide in skeletal muscle plays a role in exaggerating the muscle reflex in HF.
We found that the RSNA and pressor responses to 30 s continuous contraction were larger in rats with HF than in healthy rats. Our findings are consistent with previous ones by Smith et al. (2003), showing a larger pressor response to continuous contraction in rats with HF. Additional studies by Smith et al. (2005a,b); and from our laboratory (Li et al. 2004; Koba et al. 2008), as well as human studies (Sterns et al. 1991; Middlekauff et al. 2000, 2001, 2004; Momen et al. 2004) have suggested that the enhanced muscle reflex in HF was due to an increased engagement of mechanically sensitive muscle afferents during contraction; metabolically sensitive afferents appeared to play no role. Moreover, prior work demonstrated that RSNA increases in response to repeated 1 s bouts of contraction were predominantly mediated by mechanosensitive muscle afferents (Victor et al. 1989; Koba et al. 2007, 2008). In our report, we showed that the RSNA response to repeated 1 s bouts of contraction was greater than the response seen in healthy controls. Since the RSNA response to intermittent contraction as well as RSNA and pressor responses to continuous contraction were reduced by tempol injected intra-arterially in HF rats, we believe that excess superoxide in skeletal muscle of HF sensitizes mechanically sensitive muscle afferents engaged during contraction. We further suggest that this sensitization helps exaggerate the sympathetic nerve component of the muscle reflex in HF.
Reactive oxygen species are formed by incomplete reduction of molecular oxygen. In HF, superoxide production is exaggerated (Keith et al. 1998; Bauersachs et al. 1999; Thomas et al. 2001; Tsutsui et al. 2001) as a result of excess activation of reactive oxygen species-generating pathways (mitochondrial oxidases, membrane-bound oxidoreductases such as NAD(P)H oxidase, extracellular and cytosolic xanthine oxidases, and so on) and impaired activity of the radical scavenger enzymes such as SOD and catalase (Kojda & Harrison, 1999; Linke et al. 2005). In this disease, muscle contraction probably accelerates the generation of superoxide within the muscle. Muscle contraction is known to increase reactive oxygen species (Reid & Durham, 2002) and to produce muscle metabolites such as cyclooxygenase products and bradykinin within the muscle (Kaufman & Hayes, 2002). Cyclooxygenase activation and bradykinin production are also a mechanistic source for superoxide generation (Holland et al. 1990; Kulkarni & Armstead, 2000). During exercise in HF, release of cyclooxygenase products and bradykinin from active muscle is greater than that seen in healthy controls (Scott et al. 2002). Therefore, during contraction in HF, cyclooxygenase activation and bradykinin release in the muscle probably contribute to excess superoxide generation, thereby enhancing the muscle reflex. Further, hydrogen peroxide, the second product obtained via spontaneous or enzymatically catalysed dismutation from superoxide, has been shown to synthesize prostaglandin E2 (Laurent & Ardaillou, 1986). There may be a positive feedback loop in which hydrogen peroxide generation leads to prostaglandin E2 production during contraction.
Mechanisms by which excess superoxide sensitizes mechanosensitive muscle afferents in HF need to be determined. Previous literature provides some potential clues for how oxidative stress may sensitize mechanoreceptors. For example, previous work suggests that superoxide can inhibit the activity of voltage-gated K+ channels in a variety of cells including neural cells (Duprat et al. 1995; Liu & Gutterman, 2002; Takeuchi & Yoshii, 2008). Specifically, sensory neurons express voltage-gated K+ currents which play an integral role in the regulation of spike repolarization, interspike intervals and burst adaptation (Gold et al. 1996; Rasband et al. 2001). Inhibition of voltage-gated K+ current with 4-aminopyridine (4-AP) enhances the amplitude and duration of action potentials of neurons (Bostock et al. 1981; Calabresi et al. 1990). Further, Shokoji et al. (2004) have shown that RSNA of anaesthetized rats was increased by direct administration on postganglionic efferents of diethyldithio-carbamic acid, a SOD inhibitor, and that this RSNA increase was prevented by 4-AP administered on the renal efferents. Finally, in isolated human ventricular myocytes from patients with HF, prolongation of the action potential (Gwathmey et al. 1987) and a reduction in densities of the inward rectifier K+ current and of the transient outward K+ current (Beuckelmann et al. 1993) were reported. Based on these findings, we speculate that superoxide leads to altered function of voltage-gated K+ channels located on muscle afferents in HF, thereby enhancing the increase in discharge of muscle afferents in response to contraction. In addition, calcium-activated K+ (BKCa) channels, whose currents on sensory neurons suppress a subsequent action potential and prolong the refractory period (Scholz et al. 1998), have also been reported to be directly inhibited by superoxide in cerebral arteries of insulin resistance rats (Erdös et al. 2004). Dysfunction of BKCa channels by excess superoxide may also contribute to the exaggerated muscle reflex in HF.
ATP-sensitive K+ (KATP) channels may also play a role in sensing painful stimuli (Kawano et al. 2009a,b;), and cerebral vascular KATP channels have been reported to be inhibited by superoxide (Armstead, 1997). However, other studies showed that KATP channels on ventricular cells are excited by superoxide (Tokube et al. 1996). Thus it is unclear if superoxide-induced sensitization of sensory neurons is due to effects on KATP channels.
Wang et al. (2009) found that 10 mg kg−1 of tempol injected intra-arterially reduced the pressor response to continuous contraction in healthy rats. However, we did not find an effect of tempol in healthy rats even though our dose of tempol was twice as large as that used by Wang et al. We speculate that, in the study by Wang et al., the injected tempol affected not only peripheral afferents but central sites such as brain and spinal cord. We base this supposition on the fact that they did not attempt to trap the injectate within the hindlimb circulation. In the present report, we confirmed that, with Evans blue dye, our procedure trapped the injectate within the hindlimb circulation and did not allow a large percentage of the injected chemical to reach the systemic circulation. We further found that 10 mg of tempol infused systemically via the jugular vein reduced RSNA and pressor responses to 30 s continuous contraction in normal rats. These data suggest that, in healthy rats, tempol is capable of reducing the muscle reflex responses by an effect on central cardiovascular pathways rather than through an effect on peripheral muscle afferents.
In this report, we did not directly measure reactive oxygen species. However, a number of other reports have shown that superoxide production is increased in peripheral tissue including skeletal muscle of HF (Bauersachs et al. 1999; Thomas et al. 2001; Tsutsui et al. 2001) and that tempol reduces superoxide production (Shokoji et al. 2003; Wang et al. 2009).
The exaggerated muscle reflex activity and the mechanoreflex overactivity previously reported in HF are considered contributors to excess sympathoexcitation during exercise (Sinoway & Li, 2005; Smith et al. 2006). Increased sympathetic activation is a hallmark of HF. Nevertheless, excess sympathetic activation is also considered a compensatory mechanism to support circulatory function. Our findings suggest that in the future antioxidant treatment may hold therapeutic potential for those with severe HF. Whether this approach will reduce sympathoexcitation during exercise in HF remains to be determined.
In conclusion, we have shown that tempol injected intra-arterially reduced muscle reflex regulation of sympathetic nerve activity in HF. We suggest that excess superoxide in skeletal muscle of HF sensitizes mechanically sensitive muscle afferents engaged during contraction compared with healthy individuals, thereby contributing to the exaggerated muscle reflex in this disease.
Acknowledgments
We thank Dr Marc Kaufman for scientific input, Ms Valarie Kehoe for assistance with coronary artery ligation surgery, and Ms Jennifer Stoner for secretarial assistance. This work was supported by American Heart Association Beginning Grant-in-Aid 0865416D (S.K.).
Glossary
Abbreviations
- AP
arterial pressure
- FS
fractional shortening
- HF
heart failure
- HR
heart rate
- MAP
mean arterial pressure
- MI
myocardial infarction
- RSNA
renal sympathetic nerve activity
- SOD
superoxide dismutase
Author contributions
S.K. and L.I.S. designed research. Z.G. and S.K. performed echocardiography, and Z.G. analyzed echocardiography data. S.K. performed experiments and analyzed data. S.K. and L.I.S drafted the manuscript, and all of the authors approved the paper. Experiments were performed in the laboratory of S.K.
References
- Armstead WM. Brain injury impairs ATP-sensitive K+ channel function in piglet cerebral arteries. Stroke. 1997;28:2273–2279. doi: 10.1161/01.str.28.11.2273. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation. 1999;100:292–298. doi: 10.1161/01.cir.100.3.292. [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Näbauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- Bostock H, Sears TA, Sherratt The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol. 1981;313:301–315. doi: 10.1113/jphysiol.1981.sp013666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons. II. An in vitro analysis. J Neurophysiol. 1990;63:663–675. doi: 10.1152/jn.1990.63.4.663. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Guillemare E, Romey G, Fink M, Lesage F, Lazdunski M, Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A. 1995:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös B, Simandle SA, Snipes JA, Miller AW, Busija DW. Potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke. 2004;35:964–969. doi: 10.1161/01.STR.0000119753.05670.F1. [DOI] [PubMed] [Google Scholar]
- Gao Z, Koba S, Sinoway L, Li J. 20-HETE increases renal sympathetic nerve activity via activation of chemically and mechanically sensitive muscle afferents. J Physiol. 2008;586:2581–2591. doi: 10.1113/jphysiol.2008.150730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Holland JA, Pritchard KA, Pappolla MA, Wolin MS, Rogers NJ, Stermerman MB. Bradykinin induces superoxide anion release from human endothelial cells. J Cell Physiol. 1990;143:21–25. doi: 10.1002/jcp.1041430104. [DOI] [PubMed] [Google Scholar]
- Ibi M, Matsumoto K, Shiba D, Katsuyama M, Iwata K, Kakehi T, Nakagawa T, Sango T, Shirai Y, Yokoyama T, Kaneko S, Saito N, Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin afferent fibres with ending in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of group III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kawano T, Zoga V, McCallum JB, Wu H-E, Gemes G, Liang M-Y, Abram S, Kwok W-M, Hogan QH, Sarantopoulos CD. ATP-sensitive potassium currents in rat primary afferent neurons: biophysical, pharmacological properties, and alterations by painful nerve injury. Neuroscience. 2009a;162:431–443. doi: 10.1016/j.neuroscience.2009.04.076. [DOI] [PubMed] [Google Scholar]
- Kawano T, Zoga V, McCallum JB, Wu H-E, Gemes G, Liang M-Y, Abram S, Kwok W-M, Hogan QH, Sarantopoulos CD. Suppressed Ca2+/CaM/CaMKII-dependent KATP channel activity in primary afferent neurons mediates hyperalgesia after axotomy. Proc Natl Acad Sci U S A. 2009b;106:8725–8730. doi: 10.1073/pnas.0901815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- Koba S, Xing J, Sinoway LI, Li J. Differential sympathetic outflow elicited by active muscle. Am J Physiol Heart Circ Physiol. 2007;293:H2335–H2343. doi: 10.1152/ajpheart.00469.2007. [DOI] [PubMed] [Google Scholar]
- Koba S, Xing J, Sinoway LI, Li J. Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H311–H321. doi: 10.1152/ajpheart.00835.2007. [DOI] [PubMed] [Google Scholar]
- Koba S, Yoshida T, Hayashi N. Renal sympathetic and circulatory responses to activation of the exercise pressor reflex in rats. Exp Physiol. 2006;91:111–119. doi: 10.1113/expphysiol.2005.031666. [DOI] [PubMed] [Google Scholar]
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Kulkarni M, Armstead WM. Superoxide generation links nociceptin/orphanin FQ (NOC/oFQ) release to impaired N-methyl-d-aspartate cerebrovasodilation after brain injury. Stroke. 2000;31:1990–1996. doi: 10.1161/01.str.31.8.1990. [DOI] [PubMed] [Google Scholar]
- Laurent B, Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol Renal Physiol. 1986;251:F765–F776. doi: 10.1152/ajprenal.1986.251.5.F765. [DOI] [PubMed] [Google Scholar]
- Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeJemtel TH, Maskin CS, Lucido D, Chadwick BJ. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation. 1986;74:245–251. doi: 10.1161/01.cir.74.2.245. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Mobius-Winkler S, Schubert A, Schuler G, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol. 2002;29:305–311. doi: 10.1046/j.1440-1681.2002.03649.x. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–H1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation. 2000;101:784–789. doi: 10.1161/01.cir.101.7.784. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal muscle mechanoreflex control of renal vasoconstriction in heart failure. J Appl Physiol. 2001;90:1714–1719. doi: 10.1152/jappl.2001.90.5.1714. [DOI] [PubMed] [Google Scholar]
- Momen A, Bower D, Bohmer J, Kunselman AR, Leuenberger UA, Sinoway LI. Renal blood flow in heart failure patients during exercise. Am J Physiol Heart Circ Physiol. 2004;287:H2834–H2839. doi: 10.1152/ajpheart.00394.2004. [DOI] [PubMed] [Google Scholar]
- Murai H, Takamura M, Maruyama M, Nakano M, Ikeda T, Kobayashi D, Otowa K, Ootsuji H, Okajima M, Furusho H, Takata S, Kaneko S. Altered firing pattern of single-unit muscle sympathetic nerve activity during handgrip exercise in chronic heart failure. J Physiol. 2009;587:2613–2622. doi: 10.1113/jphysiol.2009.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Durham WJ. Generation of reactive oxygen and nitrogen species in contracting skeletal muscle: potential impact on aging. Ann N Y Acad Sci. 2002;959:108–116. doi: 10.1111/j.1749-6632.2002.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Scholz A, Grub M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol. 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJS, Piepoli MF. Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilator control. Circulation. 2002;106:214–220. doi: 10.1161/01.cir.0000021603.36744.5e. [DOI] [PubMed] [Google Scholar]
- Shokoji T, Fujisawa Y, Kimura S, Rahman M, Kiyomoto H, Matsubara K, Moriwaki K, Aki Y, Miyatake A, Kohno M, Abe Y, Nishiyama A. Effects of local administrations of tempol and diethyldithio-carbamic on peripheral nerve activity. Hypertension. 2004;44:236–243. doi: 10.1161/01.HYP.0000136393.26777.63. [DOI] [PubMed] [Google Scholar]
- Shokoji T, Nishiyama A, Fujisawa Y, Hitomi H, Kiyomoto H, Takahashi N, Kimura S, Kohno M, Abe Y. Renal sympathetic nerve responses to Tempol in spontaneously hypertensive rats. Hypertension. 2003;41:266–273. doi: 10.1161/01.hyp.0000049621.85474.cf. [DOI] [PubMed] [Google Scholar]
- Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol. 1998;84:1551–1559. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mammen PPA, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation. 2003;108:1126–1132. doi: 10.1161/01.CIR.0000084538.40542.56. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Naseem H, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005a;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Mitchell JH, Mammen PPA, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005b;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor responses are attenuated in heart failure. Circulation. 1991;84:2034–2039. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Yoshii K. Superoxide modifies AMPA receptors and voltage-gated K+ channels of mouse hippocampal neurons. Brain Res. 2008;1236:49–56. doi: 10.1016/j.brainres.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res. 2001;88:816–823. doi: 10.1161/hh0801.089341. [DOI] [PubMed] [Google Scholar]
- Tokube K, Kiyosue T, Arita M. Openings of cardiac KATP channel by oxygen free radicals produced by xanthine oxidase reaction. Am J Physiol Heart Circ Physiol. 1996;271:H478–H489. doi: 10.1152/ajpheart.1996.271.2.H478. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Ide T, Hayashidani S, Suematsu N, Shiomi T, Wen J, Nakamura Ki, Ichikawa K, Utsumi H, Takeshita A. Enhanced generation of reactive oxygen species in the limb skeletal muscles from a murine infarct model of heart failure. Circulation. 2001;104:134–136. doi: 10.1161/01.cir.104.2.134. [DOI] [PubMed] [Google Scholar]
- Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic nerve activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res. 1989;64:592–599. doi: 10.1161/01.res.64.3.592. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W. NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol. 2009;107:450–459. doi: 10.1152/japplphysiol.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Mancini DM. Factors contributing to the exercise limitation of heart failure. J Am Coll Cardiol. 1993;22:93A–98A. doi: 10.1016/0735-1097(93)90469-h. [DOI] [PubMed] [Google Scholar]
- Zelis R, Longhurst J, Capone RJ, Mason DT. A comparison of regional blood flow and oxygen utilization during dynamic forearm exercise in normal subjects and patients with congestive heart failure. Circulation. 1974;50:137–143. doi: 10.1161/01.cir.50.1.137. [DOI] [PubMed] [Google Scholar]