Abstract
We aimed to determine whether exercise-induced elevations in systemic concentration of testosterone, growth hormone (GH) and insulin-like growth factor-1 (IGF-1) enhanced post-exercise myofibrillar protein synthesis (MPS) and phosphorylation of signalling proteins important in regulating mRNA translation. Eight young men (20 ± 1.1 years, BMI = 26 ± 3.5 kg m−2) completed two exercise protocols designed to maintain basal hormone concentrations (low hormone, LH) or elicit increases in endogenous hormones (high hormone, HH). In the LH protocol, participants performed a bout of unilateral resistance exercise with the elbow flexors. The HH protocol consisted of the same elbow flexor exercise with the contralateral arm followed immediately by high-volume leg resistance exercise. Participants consumed 25 g of protein after arm exercise to maximize MPS. Muscle biopsies and blood samples were taken as appropriate. There were no changes in serum testosterone, GH or IGF-1 after the LH protocol, whereas there were marked elevations after HH (testosterone, P < 0.001; GH, P < 0.001; IGF-1, P < 0.05). Exercise stimulated a rise in MPS in the biceps brachii (rest = 0.040 ± 0.007, LH = 0.071 ± 0.008, HH = 0.064 ± 0.014% h−1; P < 0.05) with no effect of elevated hormones (P= 0.72). Phosphorylation of the 70 kDa S6 protein kinase (p70S6K) also increased post-exercise (P < 0.05) with no differences between conditions. We conclude that the transient increases in endogenous purportedly anabolic hormones do not enhance fed-state anabolic signalling or MPS following resistance exercise. Local mechanisms are likely to be of predominant importance for the post-exercise increase in MPS.
Introduction
Resistance exercise can acutely increase serum concentrations of hormones such as testosterone, growth hormone (GH), and insulin-like growth factor-1 (IGF-1). The magnitude of this elevation is dependent on the intensity (Kraemer et al. 1990), volume (Gotshalk et al. 1997), rest interval (Kraemer et al. 1987) and exercising muscle mass (Kraemer & Ratamess, 2005) of the resistance exercise bout. Simply, exercising a large muscle mass at a moderate–high intensity and with high volume and short rest intervals leads to greater rises in GH, IGF-1 and testosterone. Given the known influence of anabolic hormones during development (Mauras, 2001), it has been suggested that the acute response of the neuroendocrine system to resistance exercise is of primary importance in remodelling skeletal muscle proteins and ultimately in regulating muscle hypertrophy (Kraemer & Ratamess, 2005). However, even though the hormonal responses to resistance exercise programme variables have been well characterized (Kraemer et al. 1990), the influence of exercise-induced hormone response to muscle protein synthesis has not yet been directly quantified.
Muscle protein synthesis, in particular the synthesis of the myofibrillar protein fraction (Moore et al. 2009b), is elevated in response to resistance exercise (Phillips et al. 1997). Repeated bouts of resistance exercise, especially when combined with high quality protein ingestion, result in small net accretions in muscle protein that summate over time to produce muscle hypertrophy (Phillips, 2004; Rennie et al. 2004). Importantly, the acute muscle protein synthetic response to resistance exercise is often predictive of adaptations to chronic resistance training (Hartman et al. 2007; Wilkinson et al. 2007). The activation (indicated by phosphorylation) of intracellular signalling proteins involved in the phases of mRNA translation initiation and elongation, while not necessarily linearly related to muscle protein synthesis (Greenhaff et al. 2008), is thought to be the primary site of regulation (Kimball et al. 2002); this appears to be especially relevant for the exercise-induced activation of p70S6K (Terzis et al. 2008). The Janus kinase (JAK)–signal transducers and activator of transcription (STAT) pathway mediates signal transduction of GH-regulated genes (Nielsen et al. 2008); it is not known whether these proteins are responsive to exercise-induced increases in GH.
Therefore, our aim was to test the hypothesis that a greater exercise-induced hormonal response would enhance MPS and the phosphorylation of key intracellular signalling proteins involved in translation initiation following an acute bout of resistance exercise. We used a unilateral protocol and manipulated the amount of active muscle mass to create both a low hormone (LH) condition using arm only exercise and a high hormone (HH) condition using arm and leg exercise within the same subject. This methodology probably represents the optimal design for assessing the physiological response to endogenous hormonal stimuli within a single individual. Thus we are measuring the stimulus posed by relatively transient resistance exercise-induced hormone elevations to MPS in contrast to the stimulus posed by persistant supraphysiological hormone levels that can be achieved through exogenous administration. We also performed the study in the fed state since this is the condition in which muscle protein balance is positive (Biolo et al. 1997) and when any elevated concentrations of endogenous hormones would be most likely to affect signalling and MPS.
Methods
Subjects
Eight healthy young men (20.0 ± 1.1 years, 1.79 ± 0.03 m, 84.1 ± 4.1 kg; means ±s.e.m.) volunteered to participate in the study after being informed of the procedures and potential risks involved in the investigation. Subjects were recreationally active with no formal weight-lifting experience or regular weight-lifting activity over the last year. The protocol was approved by the Research Ethics Board of Hamilton Health Sciences and McMaster University and was written in accordance with standards set by the Declaration of Helsinki.
Experimental design
Participants completed two infusion trials on separate days. In one trial, participants performed single arm (unilateral) cable ‘preacher’ curl exercises aimed exclusively at activating the biceps muscle. In the other trial, participants performed the same single arm exercise with their contralateral arm, followed immediately by a bout of high volume intense heavy leg exercise using short rest intervals designed to elicit a large increase in systemic hormones. Trial order and arm dominance were taken into account and trials were performed in a randomized counter-balanced fashion. Participants underwent strength testing at least 1 week in advance of the first infusion trial to determine an appropriate load for each exercise (e.g. 10 repetition maximum (10RM)). Exercise with arm alone consisted of four sets of 10 repetitions at a load that was ∼95% of their 10RM such that voluntary failure occurred during the final set. To elicit a large hormonal response, the same arm exercise was performed in the contralateral arm followed by five sets of 10 repetitions of leg press at ∼90% of 10RM and three sets of 12 repetitions of leg extension/leg curl ‘supersets’ (1 set of each back-to-back with no rest between sets). Between-set rest intervals for arm exercise and leg exercise were 120 s and 60 s, respectively. In pilot work from our lab we determined that the volume and intensity of the leg workout elicited a large increase in GH, IGF-1 and testosterone that was comparable to other studies
Infusion protocol
On the trial day, participants reported to the lab after an overnight fast and having refrained from any strenuous physical activity for at least 3 days. A 20-gauge plastic catheter was inserted into an antecubital vein of the non-exercising arm and a baseline blood sample was obtained. Following the start of a primed constant infusion of l-[ring-13C6]phenylalanine (prime: 2 μmol kg−1; 0.05 μmol kg−1 min−1), participants began their exercise protocol. Due to the differing durations of the two protocols (∼10 min for LH and ∼30 min for HH), participants completing the combined protocol began exercising immediately following the start of their isotope infusion whereas participants completing the arm only protocol sat quietly for 20 min while being infused and then began their exercise. Immediately following the completion of the exercise protocol, participants had a second catheter inserted into an antecubital vein of the exercised arm that was used to sample blood for the remainder of the trial. Participants were weighed on the morning of each experiment trial and infusion rates were adjusted accordingly to maintain an infusion rate at 0.05 μmol l-[ring-13C6]phenylalanine kg−1 min−1. Following exercise, the participants rested comfortably on a bed for the remainder of the infusion.
All participants ingested a bolus dose of 25 g of whey protein after arm exercise in both trials that was enriched to 6% with tracer to minimize disturbances in isotope equilibrium. The whey protein drink served as a typical post-workout protein supplement and provided essential amino acids as the substrate for the muscle protein synthetic response to exercise. Blood samples were processed as previously described (Moore et al. 2009a). A muscle biopsy (∼80 mg) was taken from the biceps brachii of each arm 240 min post-exercise. Muscle biopsies were performed with a Bergström needle that was custom-modified for manual suction under local anaesthesia (2% xylocaine). Biopsy samples were blotted and freed of any visible fat and connective tissue, frozen in liquid nitrogen (within ∼20 s of being taken from the muscle) and stored at −80°C until further analysis.
Analyses
Blood samples were analysed for serum cortisol, testosterone, GH and IGF-1 at the Core Laboratory of McMaster University Medical Centre. All intra-assay coefficients of variation for these hormones were below 5% and all assays included standards and daily quality assurance procedures. Blood amino acids were analysed by HPLC as previously described (Moore et al. 2005). Plasma insulin was measured using a commercially available immunoassay kit (ALPCO Diagnostics, Salem, NH, USA) and blood glucose was measured using a standard spectrophotometric kit (Stanbio Laboratory, Boerne, TX, USA). Lactate was measured on neutralized, deproteinized whole blood using an enzymatic–colorimetric assay kit (Pointe Scientific, Inc., Canton, MI, USA).
As described previously (Moore et al. 2009b), approximately 20 mg of wet muscle was used to isolate free intracellular amino acids and the mixed-muscle protein bound fraction. A separate piece of muscle (∼30 mg) was used to isolate, hydrolyse, purify, derivatize and analyse the myofibrillar protein fraction.
Western blots
Western blots for JAK2 and STAT3 proteins were conducted as follows. Muscle was homogenized with a glass pestle on ice in buffer (20 mm Tris-HCl, 1 mm Na3VO4, 50 mm NaF, 40 mmβ-glycerolphosphate, 20 mm sodium pyrphosphate, 0.5% Triton X-100, 2 complete mini Roche protease inhibitor tabs, pH 7.2). Protein content was determined by the Bradford assay (Thermo Fisher Scientific, Rockford, IL, USA). Equal amounts of protein (75 μg) were separated on a 7.5% gel and transferred to a polyvinyl difluoride (PVDF) membrane (Millipore, Etobicoke, Canada). Membranes were blocked in 5% BSA and incubated with primary antibody (p-JAK2 Tyr1007/1008 1: 500, p-STAT3 Tyr705 1: 1000; Cell Signaling, Danvers, MA, USA) overnight at 4°C. After washing, membranes were incubated with secondary antibody (goat anti-rabbit HRP, 1: 50,000; Abcam Inc., Cambridge, MA, USA). Phosphorylated protein levels were detected with ECL (SuperSignal West Dura; Thermo Fisher Scientific) and Alpha Innotech FluorChem SP (Alpha Innotech Corp., San Leandro, CA, USA). Membranes were stripped with Restore Western Blot Stripping Buffer (Thermo Fisher Scientific), re-probed with primary antibody (JAK2 D2E12 1: 500, STAT3 1: 1000) overnight and incubated with secondary antibody. Total protein detection was conducted as phosphorylated protein detection and quantified using AlphaEase FC Software, v. 5.0.2 (Alpha Innotech Corp.). The ratio of phosphorylated to total protein levels is presented. Western blotting of the remaining proteins was conducted as follows. Muscle samples were homogenized in ice-cold extraction buffer (10 μl mg−1) containing 50 mm Tris-HCl (pH 7.4), 0.1% Triton X-100, 1 mm EDTA, 1 mm EGTA, 0.1% 2-mercaptoethanol, 10 mmβ-glycerophosphate, 50 mm NaF, 0.5 mm activated sodium orthovanadate (all chemicals from Sigma-Aldrich, Poole, UK) and a complete protease inhibitor cocktail tablet (Roche, West Sussex, UK). Homogenates were rotated on a vibrating platform for 10 min at 4°C, centrifuged at 10 000 g for 10 min at 4°C, before recovery of supernatants representing sarcoplasmic fractions. Bradford assays were used to determine sarcoplasmic protein concentrations after which samples were standardized to 1 mg ml−1 by dilution with Laemmli loading buffer in order to measure relative phosphorylated protein concentrations of elongation factor eEF2 Thr56, p70S6K Thr389, eukaryotic initiation factor 4E binding protein 1 (4EBP1) Thr37/46, acetyl-CoA carboxylase β (ACC-β) Ser729, Akt Ser473, proline-rich Akt substrate of 40 kilodaltons (PRAS40) Ser246 (New England Biolabs, Ipswich, MA, USA), and α-actin (Sigma-Aldrich). Samples were mixed and heated at 95°C for 7 min before 15 μg of protein per lane was loaded onto Criterion XT Bis-Tris 12% SDS-PAGE gels (Bio-Rad, Hemel Hempstead, UK) for electrophoresis at 200 V for ∼60 min. Gels were equilibrated in transfer buffer (25 mm Tris, 192 mm glycine, 10% methanol) for 30 min before proteins were electroblotted onto 0.2 μm PVDF membranes (Bio-Rad) at 100 V for 30 min. After blocking with 5% low-fat milk in TBS-T (Tris-buffered saline and 0.1% Tween-20, both Sigma-Aldrich) for 1 h, membranes were rotated overnight with primary antibody against the aforementioned targets at a concentration of 1: 2000 at 4°C. Membranes were washed (3 × 5 min) with TBS-T and incubated for 60 min at room temperature with horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (New England Biolabs, Ipswich, MA, USA) before further washing (3 × 5 min) with TBS-T and incubation for 5 min with enhanced chemiluminescence (ECL) reagents (Immunstar kit; Bio-Rad). Blots were imaged and quantified by assessing peak density after ensuring bands were within the linear range of detection using the Chemidoc XRS system (Bio-Rad). Phosphorylation of signalling proteins was corrected for loading to α-actin.
Calculations
The rate of myofibrillar protein synthesis was calculated using the standard precursor–product method:
where E2b and E1b are the bound protein enrichments from muscle at time 2 (E2b) and plasma proteins or the previous muscle biopsy at time 1 (i.e. baseline; E1b), and thus their difference is the change in bound protein enrichment between two time points; Eic is the intracellular phenylalanine enrichment; and t is the tracer incorporation time. Since the number of biopsies we could ethically take from the relatively small biceps brachii muscle was limited and the basal enrichment of the heavy [ring-13C6]phenylalanine isotope in body proteins of ‘tracer naive’ subjects would have been undetectable, we used a mixed plasma protein fraction as the baseline enrichment from a pre-infusion blood sample in the first trial. In doing so we made an assumption that these subjects would have an m+6 phenylalanine enrichment of virtually zero and that would be equivalent in muscle, enrichment equal to that of the tracer and blood; this is an assumption that we have confirmed in our laboratory. The enrichment obtained from the pool of all plasma proteins therefore represents a basal measure of isotopic enrichment for m+6 from which the enriched measurement can be taken. The muscle protein enrichment from the resting arm in the first trial (which would then become the exercised arm in the subsequent trial) was subsequently used as the baseline enrichment in the second trial. We have used this approach previously and shown it to be robust against taking a subsequent biopsy in the second trial for baseline enrichment (Tang et al. 2007).
Statistics
This study was a within-subject repeated measures design. Blood analytes were analysed using two factor (time × condition) repeated measures analysis of variance (ANOVA) statistics. MPS and signalling protein data were analysed using one factor (condition) repeated measures ANOVA statistics. Tukey's honestly significant different post hoc test was used to determine differences between individual values if a significant main effect or interaction was detected in the ANOVA (P < 0.05). Values are expressed as means ±s.e.m.
Results
Blood amino acid concentrations
There was a main effect for time for branched-chain amino acids (BCAA) and essential amino acids (EAA) (P < 0.001). Protein ingestion 60 min post-exercise stimulated a rise in EAA and BCAA concentrations that were elevated at 90 min, peaked at 120 min and returned to basal levels by 240 min post-exercise. There were no differences between conditions. Table 1 shows the average BCAA and EAA concentrations from the two trials.
Table 1.
Plasma amino acid, glucose and insulin concentrations
| Time (min) | Pre | 0 | 15 | 30 | 60 | 90 | 120 | 180 | 240 |
|---|---|---|---|---|---|---|---|---|---|
| Glucose (mm) | 4.8 ± 0.1 | 4.9 ± 0.2 | 4.8 ± 0.2 | 4.9 ± 0.3 | 4.8 ± 0.2 | 4.4 ± 0.3 | 4.5 ± 0.1 | 4.7 ± 0.1 | 4.6 ± 0.2 |
| Insulin (μIU ml−1) | 6.1 ± 0.6a | 8.5 ± 0.8a | 8.0 ± 0.5a | 7.4 ± 0.6a | 6.4 ± 0.4a | 20.0 ± 2.7b | 15.9 ± 1.4b | 6.4 ± 0.7a | 4.8 ± 0.6a |
| ∑EAA (μm) | 568 ± 102a | 578 ± 92a | 536 ± 78a | 579 ± 106a | 525 ± 59a | 838 ± 115bc | 963 ± 142bd | 799 ± 84bc | 658 ± 63ac |
| ∑BCAA (μm) | 316 ± 55ae | 304 ± 50a | 285 ± 44a | 317 ± 54ae | 262 ± 30a | 476 ± 60bd | 584 ± 80bc | 444 ± 46de | 368 ± 35ae |
Glucose and insulin: there was a main effect for time and condition for insulin, but no significant interaction; therefore values are means ±s.e.m. across LH and HH conditions. Means with different letters are significantly different from each other (P < 0.001). Amino Acids: there was no significant difference between conditions; therefore values are means ±s.e.m (in μm) for average essential amino acid (EAA) and branched-chain amino acid (BCAA) concentrations across LH and HH conditions. There was a main effect for time for EAA and BCAA (P < 0.001) where means with different letters are significantly different from each other (P < 0.05). Note: 25 g of protein were consumed at 60 min.
Blood glucose and plasma insulin
Fasted blood glucose was 4.5 ± 0.1 mm at rest and did not differ between conditions (Table 1). Fasted plasma insulin concentration was 6.1 ± 0.4 μIU ml−1 and increased by ∼230% 90 min post-exercise and returned to resting values by 180 min. There were significant main effects for condition (HH > LH, P < 0.05) and time (P < 0.001), but the interaction did not reach significance (P > 0.05).
Serum testosterone, cortisol, growth hormone, insulin-like growth factor-1 and blood lactate
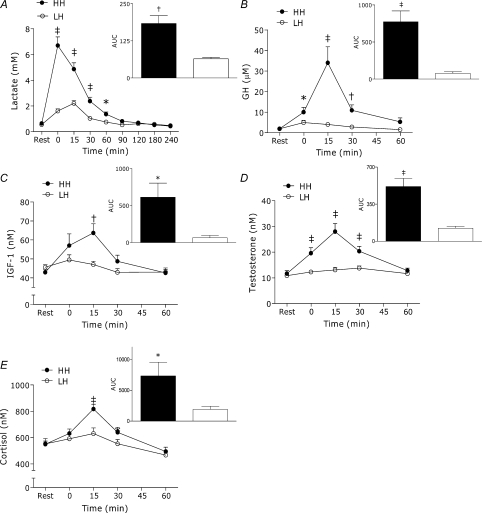
The HH condition produced a large rise in blood lactate concentration (∼9-fold increase) that was significantly greater than LH (P < 0.001) for 60 min post-exercise (Fig. 1A). The LH condition elicited low GH and IGF-1 responses that were not different from baseline whereas the HH condition produced significantly greater GH and IGF-1 (GH: ∼8-fold greater at peak, P < 0.001, Fig. 1B; IGF-1: ∼1.3-fold greater at peak, P < 0.05, Fig. 1C). Testosterone and cortisol concentrations did not change from baseline following LH, but were significantly elevated after HH (main effects for condition: testosterone, P < 0.001, Fig. 1D; cortisol, P < 0.05, Fig. 1E).
Figure 1. Whole blood lactate (A) and serum GH (B), IGF-1 (C) testosterone (D) and cortisol (E) concentrations at rest and after LH and HH exercise protocols.
Inset: net area under the curve (rest = 0); filled bars – HH, open bars – LH. HH significantly greater than LH for corresponding time points and for AUC, *P < 0.05, †P < 0.01, ‡P < 0.001. Values are means ±s.e.m.
Plasma and muscle intracellular free phenylalanine enrichment
Resting, LH and HH precursor phenylalanine enrichments were as follows: 3.8 ± 0.4, 3.7 ± 0.4, 4.1 ± 0.3 (tracer/tracee), respectively; P > 0.05. Plasma enrichments at 60, 120 and 180 min were 6.1 ± 0.2, 6.1 ± 0.2 and 6.7 ± 0.2, respectively. Linear regression analysis indicated that the slopes of the plasma enrichments were not significantly different from zero (P > 0.05), suggesting that isotopic plateau was achieved and that the use of the steady-state precursor product equation is appropriate.
Myofibrillar protein synthesis
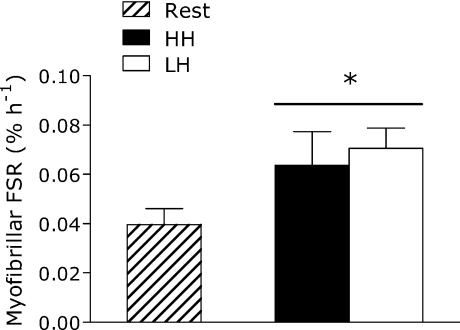
Elbow flexor exercise elevated MPS by 78% (LH) and 61% (HH) (P < 0.05) with no difference between conditions (P= 0.72) (Fig. 2). Similar results were obtained for mixed muscle protein synthesis (see online Supplemental Material). There was no effect of trial order on MPS (P > 0.05). There was also no difference in resting MPS between conditions (P > 0.05).
Figure 2. Rate of myofibrillar protein synthesis in the fed state at rest and following LH and HH exercise protocols.
*Significantly different from rest, P < 0.05. Values are means ±s.e.m.
Signalling proteins
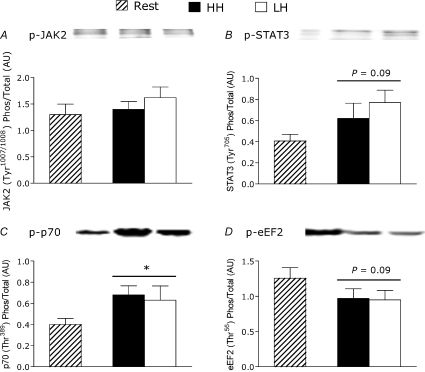
JAK2 phosphorylation was not affected by exercise or by elevated hormone concentrations induced by the HH condition (Fig. 3A). There was a trend toward increased STAT3 phosphorylation in response to exercise (P= 0.09), but with no additive effect of elevated hormones (Fig. 3B). Phosphorylation of p70S6K increased (P < 0.05, Fig. 3C), and there was a trend toward decreased phosphorylation of eEF2 (P= 0.09, Fig. 3D), in response to exercise but there were no differences between conditions. The phosphorylation status of ACC-β, PRAS40, Akt and 4EBP1 was not different from rest after either exercise protocol or from each other (see Supplemental Material).
Figure 3. Phosphorylated to total protein ratio of JAK2 (A), STAT3 (B), p70S6K (C) and eEF2 (D) at rest and after LH and HH exercise protocols.
*Significantly different from rest, P < 0.05. Values are means ±s.e.m.
Discussion
We report here that, despite being exposed to substantial differences in purportedly anabolic hormones such as testosterone, GH, and IGF-1, the rate of MPS in identically exercised muscles was not different. These data demonstrate that local factors are paramount in determining not only the signalling pathway activation but also the response of MPS. Furthermore, our results indicate that increases in MPS are able to occur without increases in systemic anabolic hormone concentrations and are not enhanced by the acute elevation that can follow resistance exercise; this finding is in agreement with previous work from our lab showing that increases in circulating hormones are not necessary for hypertrophy (Wilkinson et al. 2006). If our results are broadly applicable and generally accepted models of protein accretion in humans are correct (Phillips, 2004; Rennie et al. 2004), then our results also bring into question the posited role of anabolic hormones, which are important during growth and development, in the process of muscular hypertrophy in adults (Kraemer & Ratamess, 2005). However, we do not know if or how the hormonal elevations may have affected muscle protein balance as a whole since we did not measure muscle protein breakdown.
The present study was conducted in the fed state. Participants consumed a whey protein supplement 1 h after exercise in an attempt to provide a substrate for any potential divergent signalling or synthetic responses initiated by the two different hormone environments. It is possible that the synthetic response from the protein dominated any potential subtle differences in MPS. In our minds, especially from an applied standpoint, this only emphasizes the importance of consuming a high-quality protein after resistance exercise rather than designing exercise regimes around the exercise-induced hormone response.
Arm exercise was selected to precede leg exercise so that there would be no possible confounding influence of central fatigue (i.e. participants would be able to exert maximal effort during arm exercise in both conditions without any residual fatigue from the leg exercises). We can only speculate, but we do not anticipate that our results would have changed significantly had leg exercise preceded the arm exercise unless the muscle was somehow responsive to hormones at some critical threshold during contraction or immediately thereafter (presumably, the hormones would have peaked near the end of the arm exercise or closer to the zero time point). Recently, we measured the hormone responses to the same exercise protocols used in the present study before and after 15 weeks of training (unpublished data). While the responses between conditions were very different (like the present study), the responses within each condition across time were very similar (i.e. a divergent hormone profile was maintained). We are not aware of any data that show that training alters the synthetic sensitivity of the skeletal muscle to exercise-induced hormonal increases.
If hormones were driving increments in protein synthesis, we should expect to see an association between the magnitude of the exercise-induced hormone response and the acute MPS or signalling responses. In contrast, we found no enhancement of MPS or phosphorylation of key signalling proteins. Our data suggest that exercise-induced local mechanisms, rather than increases in serum hormone availability, are the activators of STAT3, p70S6K and eEF2 signalling and the subsequent acute elevation of MPS. Two studies, one in rodents (Baar & Esser, 1999) and the other in humans (Terzis et al. 2008), report that hypertrophy is strongly related to increases in phosphorylation of p70S6K; this is in agreement with the notion that signal activation initiates an increase in muscle protein synthesis which may lead to hypertrophy with training. The extent of p70S6K phosphorylation is also related to the extent of MPS 1–2 h after resistance exercise in young individuals (Kumar et al. 2009).
The underlying mechanisms affecting increments in hormone concentration were not measured in the present study, but the differential hormone response may be due to differences in hormone production/release (Kraemer, 2000), clearance (Kraemer, 2000) as well as shifts in plasma volume (Ploutz-Snyder et al. 1995). The ∼9-fold increase in lactate in HH provides indirect support for the stimulatory action of lactate on GH (Godfrey et al. 2003) and testosterone (Lu et al. 1997).
In summary, transient resistance exercise-induced increases in endogenous purportedly anabolic hormones did not enhance anabolic signalling or the acute (4 h) post-exercise MPS response in the fed state. Post-exercise increases in these hormones cannot be used as proxy markers for hypertrophic potential in human skeletal muscle. In contrast, local mechanisms appear to be predominant in the acute post-exercise MPS response.
Acknowledgments
We thank Todd Prior and Tracy Rerecich for technical assistance and our participants for their time and effort. This work was supported by a Natural Science and Engineering Research Council of Canada grant to S.M.P. D.W.D.W., J.E.T. and D.R.M. are CIHR Canada Graduate Scholarship Award recipients and all authors acknowledge those sources of support during the conduct of this research. Also, UK BBSRC (BB/X510697/1 BB/O516779/1) and European Union EXGENESIS program to M.J.R.
Glossary
Abbreviations
- 10RM
10 repetition maximum
- GH
growth hormone
- IGF-1
insulin-like growth factor-1
- MPS
myofibrillar protein synthesis
Author contributions
D.W.D.W., G.W.K., D.R.M., J.E.T. and S.M.P. planned the study; D.W.D.W., G.W.K., N.A.B., J.P.P., S.K.B. and S.M.P. collected data; D.W.D.W., G.W.K., D.R.M., P.A., N.A.B., J.P.P., M.D. and S.M.P. analysed data; D.W.D.W., G.W.K., D.R.M., N.A.B., G.P., M.J.R., S.K.B. and S.M.P. wrote and edited the manuscript. M.J.R. and S.M.P. raised funds. None of the authors have a financial or personal conflict of interest to declare.
Supplemental material
Supplementary Figure 1. Rate of mixed muscle protein synthesis in the fed state at rest and following Arm and Arm+Legs exercise protocols. *Significantly different from rest, P < 0.01. Values are means ± SE.
Supplementary Figure 2. Phosphorylated to total protein ratio of Akt (A), PRAS40 (B), 4EBP1 (C) and ACC-beta (D) at rest and after Arm and Arm+Legs exercise protocols.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Godfrey RJ, Madgwick Z, Whyte GP. The exercise-induced growth hormone response in athletes. Sports Med. 2003;33:599–613. doi: 10.2165/00007256-200333080-00005. [DOI] [PubMed] [Google Scholar]
- Gotshalk LA, Loebel CC, Nindl BC, Putukian M, Sebastianelli WJ, Newton RU, Hakkinen K, Kraemer WJ. Hormonal responses of multiset versus single-set heavy-resistance exercise protocols. Can J Appl Physiol. 1997;22:244–255. doi: 10.1139/h97-016. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signalling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS. Invited review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002;93:1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ. Endocrine responses to resistance exercise. In: Baechle TR, Earle RW, editors. Essentials of Strength and Conditioning. Champaign, IL: Human Kinetics; 2000. pp. 91–114. [Google Scholar]
- Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, Mello R, Frykman P, McCurry D, Fleck SJ. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol. 1990;69:1442–1450. doi: 10.1152/jappl.1990.69.4.1442. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Noble BJ, Clark MJ, Culver BW. Physiologic responses to heavy-resistance exercise with very short rest periods. Int J Sports Med. 1987;8:247–252. doi: 10.1055/s-2008-1025663. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35:339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SS, Lau CP, Tung YF, Huang SW, Chen YH, Shih HC, Tsai SC, Lu CC, Wang SW, Chen JJ, Chien EJ, Chien CH, Wang PS. Lactate and the effects of exercise on testosterone secretion: evidence for the involvement of a cAMP-mediated mechanism. Med Sci Sports Exerc. 1997;29:1048–1054. doi: 10.1097/00005768-199708000-00010. [DOI] [PubMed] [Google Scholar]
- Mauras N. Growth hormone and sex steroids. Interactions in puberty. Endocrinol Metab Clin North Am. 2001;30:529–544. doi: 10.1016/s0889-8529(05)70200-0. [DOI] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009a;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009b;587:897–904. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C, Gormsen LC, Jessen N, Pedersen SB, Moller N, Lund S, Jorgensen JO. Growth hormone signalling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab. 2008;93:2842–2850. doi: 10.1210/jc.2007-2414. [DOI] [PubMed] [Google Scholar]
- Phillips SM. Protein requirements and supplementation in strength sports. Nutrition. 2004;20:689–695. doi: 10.1016/j.nut.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Ploutz-Snyder LL, Convertino VA, Dudley GA. Resistance exercise-induced fluid shifts: change in active muscle size and plasma volume. Am J Physiol Regul Integr Comp Physiol. 1995;269:R536–R543. doi: 10.1152/ajpregu.1995.269.3.R536. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007;32:1132–1138. doi: 10.1139/H07-076. [DOI] [PubMed] [Google Scholar]
- Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol. 2008;102:145–152. doi: 10.1007/s00421-007-0564-y. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, Grant EJ, Correia CE, Phillips SM. Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Eur J Appl Physiol. 2006;98:546–555. doi: 10.1007/s00421-006-0300-z. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.