Abstract
Hypoxia is well known to reduce the body temperature (Tb) of mammals, although the neural origins of this response remain uncertain. Short-term hypoxic exposure causes a reduction in the lower critical temperature of the thermal neutral zone and a reduction in whole body thermal conductance of rodents, providing indirect support that hypoxia lowers Tb in a regulated manner. In this study, we examined directly the potential for changes in central thermosensitivity to evoke the hypoxic metabolic response by heating and cooling the preoptic area of the hypothalamus (the area which integrates thermoreceptor input and regulates thermoeffector outputs) using chronic, indwelling thermodes in ground squirrels during normoxia and hypoxia (7, 10 and 12% O2). We found that the threshold hypothalamic temperature for the metabolic response to cooling (Tth) of ∼38°C in normoxia was proportionately reduced in hypoxia (down to 28–31°C at 7% O2) and that the metabolic thermosensitivity (α; the change in metabolic rate for any given change in hypothalamic temperature below the lower critical temperature) was comparatively reduced by 5 to 9 times. This provides strong support for the hypothesis that the fall in temperature that occurs during hypoxia is the result of a reduction in the activation of thermogenic mechanisms. The decrease in the central thermosensitivity in hypoxia, however, appears to be a critical factor in the alteration of mammalian Tb. We suggest, therefore, that an altered central thermosensitivity may provide a proximate explanation of how low oxygen and similar stressors reduce normal fluctuations in Tb (i.e. circadian), in addition to the depression in regulated Tb.
Introduction
Hypoxia can manifest as changes in environmental oxygen, which occur naturally in some burrow-dwelling animals (Birchard et al. 1984; Kuhnen, 1986), or as hypoxaemia associated with circulatory disorders, particularly those related to septicemic or endotoxic shock (Kozak, 1997; Romanovsky et al. 2005; Kozak et al. 2006). Both forms of oxygen limitation reduce metabolism and body temperature (Tb) in many small mammals (Frappell et al. 1992; Mortola, 1993), primates (Tattersall et al. 2002) including humans (Kottke et al. 1948), and a host of ectothermic vertebrates (Wood, 1991; Wood & Gonzales, 1996). This reversible metabolic suppression, however, is also accompanied by a behavioural selection of lower temperatures (Gordon & Fogelson, 1991; Brown et al. 2005), an increase in behavioural responses that facilitate heat loss (e.g. adoption of postures conducive to heat loss; Mortola & Feher, 1998) and physiological responses that preferentially ‘dump’ core heat to the periphery (Tattersall & Milsom, 2003). Extensive evidence suggests that the reduction in Tb results from neurophysiological control rather than a failure of thermoeffectors. For example, non-shivering thermogenesis in rats is inhibited by hypoxic stimulation of arterial chemoreceptors, mediated via brainstem mechanisms involving the NTS, and possibly the reticular thalamic nucleus (RTN) (Madden & Morrison, 2005). Shivering has also been demonstrated to be rapidly and reversibly reduced in hypoxic mammals (Barros et al. 2001). These thermoregulatory adjustments, however, occur without evidence of anaerobic metabolism or oxygen limitation in the tissues, per se (Frappell et al. 1991; Rohlicek et al. 1998). Combined, this evidence points to neural mechanisms being strongly involved in temperature regulation during hypoxic conditions.
Thus, the lowering of Tb in hypoxia appears to result from a suite of mechanisms that cause a decrease in thermoeffector activity and lead to a decline in Tb. This has not, however, been definitively proven or tested centrally. Indeed, as a result of the diverse and indirect approaches to this question, the hypoxic thermoregulatory response has been referred to by assorted names: regulated hypothermia (Gordon, 1983), hypoxia-induced torpor (Hayden & Lindberg, 1970), hypoxic hypometabolism (Mortola, 1993; Rohlicek et al. 1998), hypoxia-induced hypothermia (Wood & Gonzales, 1996; Branco et al. 1997; Fabris et al. 1999), hypoxia-induced anapyrexia (Steiner & Branco, 2002; Steiner et al. 2002), and the hypoxic metabolic response (Barros et al. 2001; Tattersall & Milsom, 2003). Since evidence to date suggests functional thermoregulatory control in hypoxia, any use of the prefix ‘hypo’ to describe this decline in Tb is misleading since it implies that Tb falls uncontrollably (IUPS Thermal Commission, 2001).
Since the central nervous system (CNS) Tb regulator has been observed to exhibit similar characteristics in euthermic and hibernatory states, hibernation may offer insight into the question of whether central mechanisms are responsible for the hypoxic thermoregulatory response. The preoptic area of the anterior hypothalamus of mammals contains thermosensitive neurons that respond to cooling and warming of hypothalamus temperature (Th), effecting physiological changes that act in a feedback fashion to reverse the change in Th (Heller & Hammel, 1972; Heller & Colliver, 1974; Heller & Glotzbach, 1977). Cooling this region results in a proportional increase in metabolic heat production and a peripheral vasoconstriction. Warming this region produces the opposite response (decreased heat production and increased heat loss). In fact, the threshold for activation of thermogenic defences (Tth) can be explored at the same time as hypothalamic thermosensitivity (i.e. sensitivity of the CNS Tb regulator), where the latter value should be an estimate of the intrinsic temperature-related output of thermosensitive hypothalamic neurons. To examine the nature of the central control of Tb, Th can be manipulated, producing responses that adhere to the following control equation (Hammel, 1968):
| (1) |
where R is heat production, R0 is basal heat production, and the incremental change in heat production above basal levels ([R−R0]≥ 0) is proportional to the product of thermosensitivity (α) and the difference between hypothalamic temperature (Th) and a threshold temperature (Tth). This product is the effective error signal with which the CNS regulator operates, producing heat production responses that are related to α (provided Th < Tth). The farther that Tth is from the actual Th, the larger the error signal, and thus, the larger corrective heat production required.
The usefulness of this technique in assessing thermoregulatory responses is its ability to examine the central control of Tb in a closed loop system by ‘opening the feedback loop’ that influences Tb (Hammel, 1968). By manipulating only Th, numerous processes and stimuli have been established that alter Tth and α (Heller & Hammel, 1972; Hammel et al. 1973; Glotzbach & Heller, 1975; Florant & Heller, 1977; Florant et al. 1978; Sakaguchi et al. 1979). Low ambient temperatures raise Tth by stimulating cutaneous cold thermoreceptors which relay information to the preoptic area (Glotzbach & Heller, 1975); thus, the threshold Th for activation of thermogenesis is higher at low ambient temperatures, ensuring that any deviation in brain temperature is met with compensatory heat production. Neural output from the preoptic area is also state dependent (Sakaguchi et al. 1979; Glotzbach & Heller, 1984); the normal facilitation (high α and Tth) that occurs during wakefulness is reduced during slow-wave sleep (SWS), leading to a reduction in Tth and α. In REM sleep, no facilitation occurs, and no discernible hypothalamic thermosensitivity can be measured; thus, there are no thresholds that elicit either thermogenesis during cooling, or respiratory panting during warming (Parmeggiani et al. 1973; Glotzbach & Heller, 1976, 1984). Finally, exercise and infectious agents that cause fever raise Tth for both heat production and heat loss pathways (Hammel et al. 1973). Whether alterations in blood or cerebral fluid oxygen levels alter the integration of thermal signals by the CNS regulator of Tb is unknown; however, the idea of an adjustable Tth with respect to hypoxia is compatible with the models for the CNS regulation of Tb (Kozak, 1997; Cabanac, 2006; Kozak et al. 2006). The question of whether Tth is reduced by hypoxia, however, has only ever been indirectly assessed from whole animal cooling experiments; squirrels and other rodents actively recruit thermogenesis in hypoxia, but at reduced levels, and Tb is not as tightly defended, varying, instead with ambient temperature (Dupréet al. 1988; Barros et al. 2001). Thus, from these kinds of experiments, it is uncertain whether hypoxia alters the capacity to generate body heat under low temperature conditions, alters heat loss and heat production pathways differently, or reflects an underlying central mechanism.
Ground squirrels and other hibernating mammals have served as model animals for elucidating underlying thermoregulatory mechanisms common to mammalian Tb control (Hammel et al. 1973; Heller et al. 1974; Florant & Heller, 1977; Larkin & Heller, 1996). Not only do they exhibit a profound reduction in Tb and metabolism in hypoxia (Barros et al. 2001) and a high degree of hypoxia tolerance (Drew et al. 2007; Levesque & Tattersall, 2009), they also possess a very high α (Heller, 1978, 1979), a profound, cold-activated thermogenesis from brown adipose tissue (Milner et al. 1989), and have been shown to progressively lower Tth as they enter into hibernation (Heller et al. 1977). Thus, ground squirrels are a powerful model for exploring questions concerning the hypoxic response and thermoregulatory control. We describe, in this study, experiments designed to probe the changes in central Tth, the intrinsic thermosensitivity (α) of heat production, and associated cardiorespiratory indicators during the hypoxia-induced periods of reduced Tb. The specific objectives were: (i) to assess the hypothalamic thermosensitivity and threshold temperature (Tth) for metabolic heat production; (ii) to assess the integration of relevant ventilatory and cardiovascular parameters with thermoregulatory adjustments; and (iii) to determine the characteristics of the CNS temperature regulator operating under hypoxic conditions. By fulfilling these objectives, we anticipate addressing whether the threshold for activation of hypothalamic heat production defences (Tth) decreases in hypoxia and whether the thermosensitivity of the central regulator of Tb is reduced by hypoxia, and thereby determine how the CNS exerts regulatory influences on Tb in hypoxia.
Methods
Ethical approval
All experiments were approved by the University of British Columbia Animal Care and Use Committee and conformed to Canadian Council for Animal Care guidelines, and to the principles and policies outlined by Drummond (2009).
Animals
Golden-mantled ground squirrels (N= 7; average mass 250 ± 18 g) and Columbian ground squirrels (N= 7; average mass 496 ± 62 g) were caught from wild populations in Redding, CA, USA and Manning Park, British Columbia, Canada. All squirrels were housed at 20°C and studied during the months of July and August, when they were normothermic. No squirrel underwent torpor during the period of experimentation, as verified by daily observations. Animals were supplied ad libitum with standard rat chow, supplemented with sunflower seeds and fruit. Both male and female golden-mantled squirrels (GMGS) were examined, and only female Columbian ground squirrels (CGS) were studied. In GMGS, sex did not exert a significant effect and, thus, data were pooled for both sexes.
Surgeries and thermode implantation
Squirrels were anaesthetized with 65 mg kg−1 sodium pentobarbital (i.p.), and throughout surgeries maintained within an appropriate surgical plane using supplemental injections as required. Water-perfused metal thermodes were implanted bilaterally (2 mm from midline) in the medial preoptic area of the hypothalamus as described in Heller & Hammel (1972), allowing for the manipulation of Th. Th was measured using a 30 gauge thermocouple wire that was placed down a one-ended re-entrant tube (26 gauge needle with a welded end) located half-way between the two thermodes and 1.5 mm caudal. The placement of the thermodes and re-entrant tube was determined using established skull suture line morphologies (Heller & Hammel, 1972; Heller & Colliver, 1974; Heller et al. 1974). Using the nose bar of the stereotaxic apparatus set +5 mm provided the appropriate correction factor to ensure that skull morphologies, lambda and bregma were within the same horizontal plane (Harris & Milsom, 2001), allowing the use of rat brain coordinates (Paxinos & Watson, 1997). The stereotaxic coordinates used for GMGS were 9.5 mm rostral to EBZ and 1.5 mm lateral to midline, and those for CGS were 11 mm rostral to EBZ and 1.5 mm lateral to midline. In both species, the thermodes were implanted to a depth of 9 mm. As Heller & Hammel (1972) found, depth rather than rostral–caudal or lateral orientation had the greatest influence on evoking metabolic changes. Placing thermodes only 8 mm deep did not evoke significant changes in metabolic rate with hypothalamic cooling, whereas thermodes placed 1 mm rostral or caudally (in separate instances) evoked similar increments in metabolic rate with cooling. Since the total distance from the top of the cranium to the base was between 9 and 10 mm, 9 mm depth was chosen to minimise damage to the neural structures of interest.
After placing the thermode assembly, four bone screws were fixed bilaterally into the occipital and parietal bones. Dental cement and dental acrylic were used to fuse the plexiglass thermode assembly to the bone screws. Proper placement of the thermodes and re-entrant tube was verified initially by perfusing the thermodes with cool water which evoked strong and rapid shivering in awake animals, and later in animals post mortem, through brain dissections. Thermode placement was verified to be directly above the optic chiasm through visual examination of the brain of animals that were humanely killed with an overdose of sodium pentobarbital. Electroencephalogram (EEG) leads were attached to two of the bone screws, allowing for contralateral cortical EEG recordings (in the GMGS only). Due to limited input channels on the data acquisition system and electrical interference that resulted from multiple temperature probes, EEG measurements were only conducted in the GMGS, where Tb was not measured. Electrocardiogram (ECG) and electromyogram (EMG) leads were implanted subcutaneously in the pectoral and neck muscles, respectively, for the assessment of heart rate and shivering as described previously (Barros et al. 2001; Zimmer & Milsom, 2001). A thermocouple wire housed within a small, sealed cannula was implanted into the peritoneal cavity and was passed subcutaneously back to the thermode assembly where the copper and constantan poles were left available as contact points for recording core Tb. Unfortunately, the core temperature thermocouples were prone to malfunction not long after implantation, providing limited data on core temperature. Th was necessarily the best estimate of core temperature during periods when water was not perfusing through the thermode assemblies. Following surgeries, squirrels were allowed to recover for 12 h in a warm environment (24–28°C) while being monitored for vital signs. Following recovery, squirrels were returned to their cages, and not studied until fully healed (approximately 3 weeks following surgery). Immediately following surgery, squirrels were provided with an antibiotic (Baytril, 5–10 mg kg−1, i.m.), in addition to daily applications of a topical xylocaine/clove oil mixture to the wound site. By the time of experimentation, wounds were fully healed, and no animal exhibited any signs of fever.
Data acquisition
All parameters (O2, CO2, differential pressure, Tb, Th, EMG, ECG and EEG) were recorded at a sampling rate of 200 Hz using an 8-channel Windaq data acquisition system (Data Instruments, Akron, OH, USA). Due to a limitation in number of input channels, EEG was recorded only in GMGS in place of Tb, whereas Tb was recorded in CGS in place of EEG. Temperatures were recorded using thermocouple meters (Model BAT-12, Physitemp Instruments). ECG and EMG voltages were first passed through an amplifier (Model 7P511L, Grass Instruments). ECG values were low-pass (LP) and high-pass (HP) filtered (50% filters: LP, 30 Hz; HP, 0.1 kHz), providing appropriate resolution of the QRS complex for peak determination. EMG voltages were low-pass filtered (50% filter LP, 300 Hz) prior to integration and analysis. EMG data were integrated over 5 s (∫EMG), and the average ∫EMG values during periods of interest obtained and expressed as percentages relative to resting, non-disturbed values. EEG voltages were low-pass and high-pass filtered (50% filters: LP, 1 Hz; HP, 30 kHz), prior to being processed. EEG values were only examined during stable periods of normoxia and hypoxia. Multiple (approximately 10 per squirrel per O2 level) 2 min epochs of EEG voltages were fourier transformed, producing power spectra where total power (PEEG; area under the power spectrum, units: V2 s−1) was compared between normoxia and hypoxia, as well as the ratio of power within the 2–4 Hz band versus the 13.5 to 20 Hz band (P2-4 Hz:P13.5-20Hz), as an indicator of slow:fast EEG activity. Due to electrical interference, accurate EEG measures during Th manipulation were not practical. In addition to the 2 h transition periods into hypoxia (see experimental protocol below for details), the periods during which Th was manipulated were exported to custom spreadsheets for peak detection (fR and fH), as well as 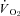 ,
, 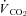 , VTI and ∫EMG. Average values during the stable portion of the manipulation period were obtained.
, VTI and ∫EMG. Average values during the stable portion of the manipulation period were obtained.
Metabolic rate determination
Metabolic rates (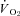 and
and 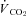 ) were determined using flow-through respirometry as described in Tattersall & Milsom (2003). Briefly, squirrels were placed in 0.5 l plexiglass chambers with incurrent gas flow set to 1000–1500 ml min−1 (depending on the squirrel size). Gases (7, 10, 12 or 21% O2) were mixed and flow controlled using a gas mixing flow meter (Cameron GF-3/MP). A subsample (200 ml min−1) of the excurrent gas from the respirometer was scrubbed of water vapour and CO2 and analysed for O2 content (Beckman OM-11 Oxygen analyser), while another subsample (200 ml min−1) of gas was scrubbed of water vapour and analysed for CO2 content (Beckman LB-2 CO2 analyser).
) were determined using flow-through respirometry as described in Tattersall & Milsom (2003). Briefly, squirrels were placed in 0.5 l plexiglass chambers with incurrent gas flow set to 1000–1500 ml min−1 (depending on the squirrel size). Gases (7, 10, 12 or 21% O2) were mixed and flow controlled using a gas mixing flow meter (Cameron GF-3/MP). A subsample (200 ml min−1) of the excurrent gas from the respirometer was scrubbed of water vapour and CO2 and analysed for O2 content (Beckman OM-11 Oxygen analyser), while another subsample (200 ml min−1) of gas was scrubbed of water vapour and analysed for CO2 content (Beckman LB-2 CO2 analyser). 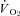 and
and 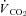 were subsequently calculated using equations from Withers (1977), and reported at STPD (ml kg−1 h−1). The respiratory exchange ratio (RER) was determined from
were subsequently calculated using equations from Withers (1977), and reported at STPD (ml kg−1 h−1). The respiratory exchange ratio (RER) was determined from 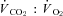 .
.
Cardiovascular and ventilatory parameters
Heart rate (fH), tidal volume (VTI; ml kg−1), breathing frequency (fR), minute ventilation (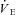 ; ml kg−1 h−1) and air convection requirement (
; ml kg−1 h−1) and air convection requirement (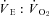 ) were determined as outlined in Barros et al. (2001). Peak detection for the determination of interbeat interval (IBI) and fH (60 × IBI−1; min−1) was determined offline using custom peak detection spreadsheets. Ventilation was assessed with whole body plethysmography using a flow-through system (Tattersall et al. 2002), consisting of two identical 0.5 l chambers. One chamber served as the respirometer for the animal (see above), while the other as the pressure reference chamber. Gas flows to each chamber were set to be balanced at 1500 ml min−1 each by producing a negligible pressure-differential between them (total flow of mixed gas was 3000 ml min−1). The pressure signal was measured using a differential pressure transducer (Model DP103-8, Validyne Engineering, Northridge, CA, USA), amplified (Model 7P122E, low-level direct current amplifier, Grass Instruments), and recorded to the computer data acquisition system. The plethysmograph was calibrated using pulse injections of air (3 ml), as well as confirmed with dynamic pressure signals generated as described by McArthur & Milsom (1991). The time constant of the pressure signal was >4 s, producing negligible filtering of the pressure amplitude. All ventilatory variables (
) were determined as outlined in Barros et al. (2001). Peak detection for the determination of interbeat interval (IBI) and fH (60 × IBI−1; min−1) was determined offline using custom peak detection spreadsheets. Ventilation was assessed with whole body plethysmography using a flow-through system (Tattersall et al. 2002), consisting of two identical 0.5 l chambers. One chamber served as the respirometer for the animal (see above), while the other as the pressure reference chamber. Gas flows to each chamber were set to be balanced at 1500 ml min−1 each by producing a negligible pressure-differential between them (total flow of mixed gas was 3000 ml min−1). The pressure signal was measured using a differential pressure transducer (Model DP103-8, Validyne Engineering, Northridge, CA, USA), amplified (Model 7P122E, low-level direct current amplifier, Grass Instruments), and recorded to the computer data acquisition system. The plethysmograph was calibrated using pulse injections of air (3 ml), as well as confirmed with dynamic pressure signals generated as described by McArthur & Milsom (1991). The time constant of the pressure signal was >4 s, producing negligible filtering of the pressure amplitude. All ventilatory variables (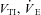 ) are reported at BTPS (ml kg−1 and ml kg−1 h−1, respectively).
) are reported at BTPS (ml kg−1 and ml kg−1 h−1, respectively).
Experimental protocol
GMGS were studied at 21 and 7% O2, whereas CGS were studied at 21, 12, 10 and 7% O2; therefore, most experimental results presented are from CGS, with reference to GMGS made where appropriate. Protocols for both species were identical, except for the additional oxygen levels in CGS. At the beginning of the experimental period, an individual squirrel was placed into the respirometer, which was housed within a temperature-controlled environmental chamber held at 20–22°C throughout all experiments. Squirrels were unrestrained and allowed 30–60 min to habituate to the respirometer before altering oxygen levels or manipulating Th (the last 10 min of this initial period served as the control, non-manipulated values). Th was altered above and below resting (non-manipulated) values for 10 min intervals. Inbetween manipulations, squirrels were given 10 min to recover before a change in Th was induced, long enough for values to return to resting levels. In normoxia, Th was manipulated from a resting value of ∼38°C down to ∼33°C and up to ∼43°C (exact values were measured for each manipulation). Squirrels were left in normoxia (21% O2) for 2 h, during which time Th was lowered or raised. After completing changes to Th, squirrels were exposed to hypoxic gases for at least 150 min to allow Th and physiological parameters to reach a new, lowered value. Only one level of hypoxia was examined per day. After steady state values had been reached (2 h was sufficient; see Fig. 1), Th was manipulated at temperatures above and below the hypoxic Th value, as in normoxia. For 12% O2, Th was manipulated from ∼31 to ∼41°C, for 10% O2, from ∼29 to ∼41°C, and at 7% O2, from ∼26 to ∼40°C; the lower extreme values were chosen because they generated maximum heat production responses. Since each animal was examined over a period of 3 days, multiple periods of normoxia were possible during the start of each experiment, allowing for completion of Th manipulations in normoxia. During all periods of Th manipulation, the following parameters were measured (average of the values between the 2nd and 8th minute of manipulation): O2 consumption (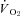 ), CO2 production (
), CO2 production (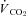 ), tidal volume (VTI), breathing frequency (fR), total ventilation (
), tidal volume (VTI), breathing frequency (fR), total ventilation (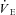 ), heart rate (fH) and integrated neck muscle electromyographic activity (∫EMG). Due to variability in electrode placement and quality of the electrical signal, ∫EMG was expressed as percentage of the steady state values. During Th manipulations, squirrels were periodically observed to ensure that recording occurred during waking states.
), heart rate (fH) and integrated neck muscle electromyographic activity (∫EMG). Due to variability in electrode placement and quality of the electrical signal, ∫EMG was expressed as percentage of the steady state values. During Th manipulations, squirrels were periodically observed to ensure that recording occurred during waking states.
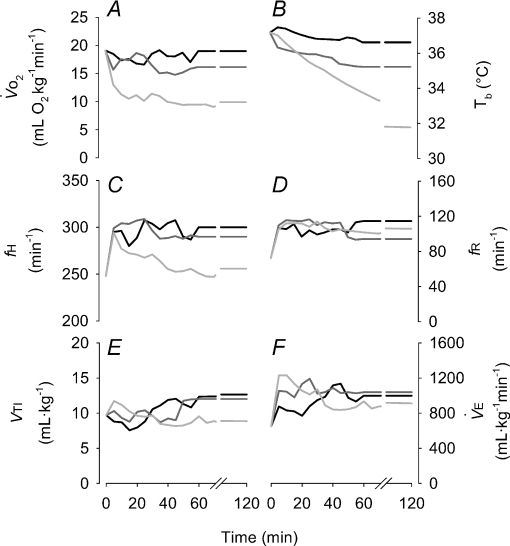
Figure 1. Time course (average values; error bars not shown for visual clarity) of the pertinent physiological factors under exposure to different levels of oxygen in the Columbian ground squirrel, demonstrating steady state values were achieved by the second hour of exposure.
Black lines indicate 12% O2, dark grey indicate 10% O2 and light grey indicate 7% O2. Shown are 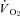 (A), Tb (B), heart rate (C), breathing frequency (D), tidal volume (E) and minute ventilation (F).
(A), Tb (B), heart rate (C), breathing frequency (D), tidal volume (E) and minute ventilation (F).
Data analysis
Steady state values for all physiological parameters were determined after 2 h at each O2 level. For the Th manipulation results, all physiological parameters were measured as the average values during the 2nd to 8th minute of all periods of Th manipulation to ensure suitable time for physiological equilibration. At least six, and usually up to nine separate 10 min manipulations at different Th values were obtained at each oxygen level. Subsequently, slopes (analogous to α) of the various physiological parameters versus manipulated Th (e.g. ml O2 g−1 h−1°C−1 for 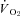 ) were determined from each animal both below (cool slope) and above (warm slope) the steady state Th, to explore the thermosensitivity of physiological parameters above and below the steady state Th. Slopes were chosen not necessarily because a linear relationship was expected in all cases, but to simplify the analysis for comparison to previous research (Heller et al. 1974; Florant et al. 1978) and to readily incorporate the slopes into subsequent statistics. Cases where the slopes were zero at one level of oxygen, but positive or negative in another, would still reveal evoked physiological changes associated with Th changes. After calculating the slopes, Tth for metabolic heat production was determined using eqn (1) as described by Heller et al. (1974) for each animal and at each level of oxygen. One additional parameter was examined: the ‘heat gain ratio’ (Cormareche-Leydier et al. 1985). Immediately following every 10 min Th manipulation (ΔTh; Th,before−Th,during manipulation), a change in Tb (ΔTb=Tb,before−Tb,after manipulation) manifested from the altered balance between metabolic heat production and heat loss. Thus, we examined the ratio of ΔTb:ΔTh as an indicator of how much Tb changed for a given Th stimulus, across the different oxygen treatments, using a one-way repeated measures (rm) ANOVA. This ratio provided an indication of the effectiveness of hypothalamic manipulation to manifest a change in heat load, as well as information on extrahypothalamic feedback. Steady state physiological parameters, cooling and warming slopes from Th manipulations, and Tth were examined statistically using a one-way rmANOVA, with O2 level as the factor. Post hoc multiple comparisons were made using a Holm–Sidak test compared against the normoxic value. All statistical tests were considered significant at an α of 0.05. Data are presented as mean ±s.d., except for graphical clarity where s.e.m. is utilised.
) were determined from each animal both below (cool slope) and above (warm slope) the steady state Th, to explore the thermosensitivity of physiological parameters above and below the steady state Th. Slopes were chosen not necessarily because a linear relationship was expected in all cases, but to simplify the analysis for comparison to previous research (Heller et al. 1974; Florant et al. 1978) and to readily incorporate the slopes into subsequent statistics. Cases where the slopes were zero at one level of oxygen, but positive or negative in another, would still reveal evoked physiological changes associated with Th changes. After calculating the slopes, Tth for metabolic heat production was determined using eqn (1) as described by Heller et al. (1974) for each animal and at each level of oxygen. One additional parameter was examined: the ‘heat gain ratio’ (Cormareche-Leydier et al. 1985). Immediately following every 10 min Th manipulation (ΔTh; Th,before−Th,during manipulation), a change in Tb (ΔTb=Tb,before−Tb,after manipulation) manifested from the altered balance between metabolic heat production and heat loss. Thus, we examined the ratio of ΔTb:ΔTh as an indicator of how much Tb changed for a given Th stimulus, across the different oxygen treatments, using a one-way repeated measures (rm) ANOVA. This ratio provided an indication of the effectiveness of hypothalamic manipulation to manifest a change in heat load, as well as information on extrahypothalamic feedback. Steady state physiological parameters, cooling and warming slopes from Th manipulations, and Tth were examined statistically using a one-way rmANOVA, with O2 level as the factor. Post hoc multiple comparisons were made using a Holm–Sidak test compared against the normoxic value. All statistical tests were considered significant at an α of 0.05. Data are presented as mean ±s.d., except for graphical clarity where s.e.m. is utilised.
Results
Throughout, results are presented primarily for CGS, with data from GMGS provided in accompanying tables and figures for comparative purposes. Results where species differences occur or where data from only one species was collected are highlighted below.
Metabolic and cardiorespiratory responses to hypoxia
All physiological parameters reached steady state values within 1–2 h of exposure to hypoxia (Fig. 1). Tb and Th were strongly dependent on oxygen level (12, 10 and 7% significantly lower than 21% O2); however, only 7% O2 led to significant reductions in 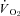 and
and 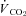 (Tables 1 and 2). Steady state RER values were not influenced by inspired oxygen. Compared to normoxia, steady state fH values exhibited a stimulation at 12 and 10% O2, which did not persist at 7% O2 (Table 2). Steady state VTI values were not affected by oxygen, but in combination with an increase in fR at all levels of hypoxia,
(Tables 1 and 2). Steady state RER values were not influenced by inspired oxygen. Compared to normoxia, steady state fH values exhibited a stimulation at 12 and 10% O2, which did not persist at 7% O2 (Table 2). Steady state VTI values were not affected by oxygen, but in combination with an increase in fR at all levels of hypoxia,  was significantly higher at 12 and 10% O2.
was significantly higher at 12 and 10% O2. 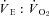 was progressively higher with increasing degree of hypoxia.
was progressively higher with increasing degree of hypoxia.
Table 1.
Cardio-respiratory parameters in normoxic (21% O2) and hypoxic (7% O2) golden-mantled ground squirrels during steady state conditions1
| Variable | 21% O2 | 7% O2 |
|---|---|---|
| Th (°C) | 38.0 ± 0.6 | 30.8 ± 1.6 ‡ |
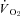 (ml O2 kg−1 min−1) (ml O2 kg−1 min−1) |
27.5 ± 7.2 | 19.8 ± 2.1‡ |
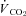 (ml CO2 kg−1 min−1) (ml CO2 kg−1 min−1) |
23.5 ± 5.7 | 17.4 ± 4.7† |
| RER (ml CO2 (ml O2)−1) | 0.86 ± 0.08 | 0.87 ± 0.11 |
| fH (beats min−1) | 342 ± 28 | 284 ± 35† |
| VTI (ml kg−1) | 8.71 ± 3.62 | 16.4 ± 2.4† |
| fR (beats min−1) | 150 ± 53 | 121 ± 22 |
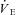 (ml kg−1 min−1) (ml kg−1 min−1) |
1150 ± 260 | 1960 ± 330‡ |
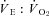 (ml O2 ml−1) (ml O2 ml−1) |
43.9 ± 12.6 | 101 ± 24‡ |
| PEEG (V2 s−1) | 11581 ± 6064 | 13728 ± 12443 |
| P2-4Hz:P13.5-20Hz | 2.44 ± 1.44 | 4.75 ± 2.53* |
Values are mean ±s.d. 1Steady state refers to measurements obtained after 2 h. RER, respiratory exchange ratio.
Significantly different from normoxic values (P < 0.05).
Significantly different from normoxic values (P < 0.01).
Significantly different from normoxic values (P < 0.001).
Table 2.
Cardio-respiratory parameters in normoxic (21% O2) and hypoxic (12, 10 and 7% O2) Columbian ground squirrels during steady state conditions1
| Variable | 21% O2 | 12% O2 | 10% O2 | 7% O2 |
|---|---|---|---|---|
| Th (°C) | 37.7 ± 0.4 | 36.8 ± 0.4† | 35.4 ± 0.9‡ | 31.9 ± 0.6‡ |
| Tb (°C) | 37.5 ± 0.6 | 36.7 ± 0.3* | 35.1 ± 1.0‡ | 31.4 ± 0.6‡ |
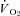 (ml O2 kg−1 min−1) (ml O2 kg−1 min−1) |
19.1 ± 2.6 | 17.7 ± 2.0 | 16.0 ± 1.6 | 8.80 ± 2.60‡ |
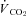 (ml CO2 kg−1 min−1) (ml CO2 kg−1 min−1) |
17.2 ± 2.4 | 17.5 ± 2.8 | 15.7 ± 1.9 | 7.91 ± 2.46‡ |
| RER (ml CO2 (ml O2)−1) | 0.90 ± 0.04 | 0.99 ± 0.06 | 0.99 ± 0.13 | 0.93 ± 0.15 |
| fH (beats min−1) | 248 ± 28 | 300 ± 12† | 295 ± 31† | 251 ± 14 |
| VTI (ml kg−1) | 9.70 ± 3.53 | 11.8 ± 4.2 | 12.5 ± 5.6 | 8.87 ± 3.0 |
| fR (beats min−1) | 72.5 ± 15.1 | 103 ± 30* | 99.3 ± 37.9* | 103 ± 7* |
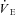 (ml kg−1 min−1) (ml kg−1 min−1) |
650 ± 168 | 1130 ± 384† | 1080 ± 298† | 897 ± 270 |
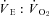 (ml O2 ml−1) (ml O2 ml−1) |
34.0 ± 7.4 | 62.2 ± 16.1† | 67.4 ± 17.3‡ | 103 ± 14‡ |
Values are mean ±s.d. 1Steady state refers to measurements obtained after 2 h.
Significantly different from normoxic values (P < 0.05).
Significantly different from normoxic values (P < 0.01).
Significantly different from normoxic values (P < 0.001).
Temperatures during normoxic–hypoxic transitions
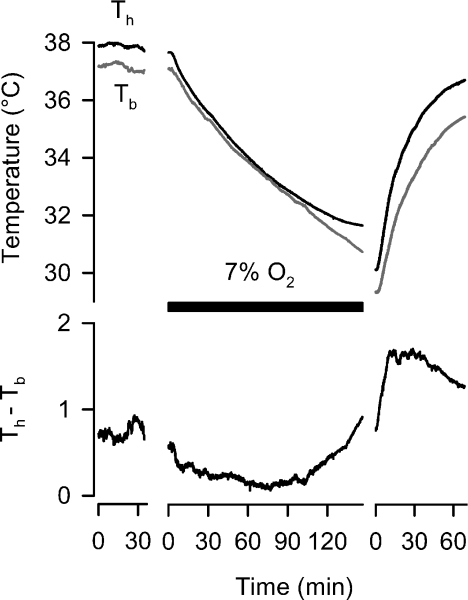
In CGS, we were able to record both Th and Tb simultaneously during entry and recovery from 7% O2. The rate of change for Tb was 0.058°C min−1 during hypoxia, and 0.2°C min−1 during re-warming from hypoxia. Typically, Th was higher than Tb; however, this differential diminished during hypoxia, until such a time as Th reached a steady state value after 2 h (Fig. 2). During re-warming, Th rose prior to Tb, creating an initial, large differential between Th and Tb until Th approached 36°C, after which the rate of Th increase diminished.
Figure 2. Time course (average values, top trace) of hypothalamic (Th) and Tb from Columbian ground squirrels during entry and recovery from 7% O2 (black bar).
Normally, Th is elevated slightly above Tb, although this difference (bottom trace) undergoes dynamic changes, decreasing during the initial exposure to hypoxia, and subsequently increasing during re-oxygenation.
EEG responses to hypoxia in golden-mantled ground squirrels
Cortical EEG measurements were made in GMGS during their transition to 7% O2. Power spectra of EEG traces revealed that high frequency components declined in hypoxia, while low frequency components rose compared to normoxia (the ratio of low to high frequency components was significantly affected by oxygen levels; Table 1). Overall power, however, did not change between normoxia and hypoxia.
Responses to Th manipulation
Only data where proportionality constants were significantly different from zero in normoxia were reported and analysed. Warming the hypothalamus did not produce proportionality constants significantly different from zero.
Metabolic responses to induced Th changes
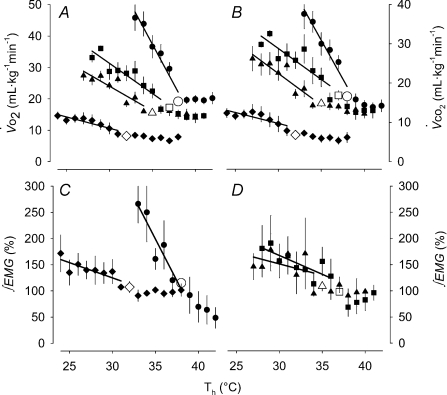
Brief, 10 min manipulations of Th led to reversible changes in metabolic parameters in squirrels under normoxic and hypoxic conditions (Fig. 3). Hypothalamic cooling led to a proportional change in 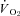 in both CGS and GMGS at all levels of oxygen tested (Table 3; Fig. 4). Hypoxia had strong influences on the proportionality constants for
in both CGS and GMGS at all levels of oxygen tested (Table 3; Fig. 4). Hypoxia had strong influences on the proportionality constants for 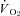 and
and 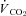 (Table 3), with all levels of hypoxia (7, 10 and 12%) showing a significant attenuation of the metabolic response to hypothalamic manipulation (Fig. 4). Cooling the hypothalamus was accompanied by activation of shivering thermogenesis (Fig. 5). In accord with changes in
(Table 3), with all levels of hypoxia (7, 10 and 12%) showing a significant attenuation of the metabolic response to hypothalamic manipulation (Fig. 4). Cooling the hypothalamus was accompanied by activation of shivering thermogenesis (Fig. 5). In accord with changes in 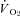 , hypothalamic cooling led to proportional changes in the activation of nuchal muscle EMG. These proportional changes (Table 3) were significantly attenuated by hypoxic exposure compared to normoxia (Fig. 4, Table 3). Return to normoxic conditions was met with rapid initiation of shivering and increments in metabolic rate (Fig. 6), suggesting a rapid return to normoxic thermoregulatory patterns. In one case, however, we transiently suppressed this post-hypoxic shivering by warming the hypothalamus to 40°C (Fig. 6).
, hypothalamic cooling led to proportional changes in the activation of nuchal muscle EMG. These proportional changes (Table 3) were significantly attenuated by hypoxic exposure compared to normoxia (Fig. 4, Table 3). Return to normoxic conditions was met with rapid initiation of shivering and increments in metabolic rate (Fig. 6), suggesting a rapid return to normoxic thermoregulatory patterns. In one case, however, we transiently suppressed this post-hypoxic shivering by warming the hypothalamus to 40°C (Fig. 6).
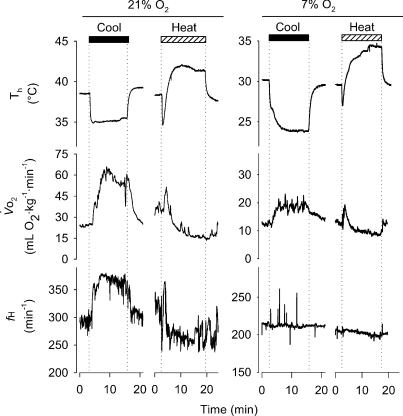
Figure 3. Sample traces of the influence of Th manipulation on metabolic heat production (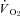 ) and heart rate (fH) under normoxia (left traces) and hypoxia (7% O2; right traces) in Columbian ground squirrels.
) and heart rate (fH) under normoxia (left traces) and hypoxia (7% O2; right traces) in Columbian ground squirrels.
Traces depict raw data from one squirrel before, during and after 10 min periods of artificial manipulations of Th below (black bars) and above (hatched bars) normal values. Different scales for normoxic and hypoxic values are shown to demonstrate the influence of Th manipulation under both oxygen conditions.
Table 3.
Slopes resulting from Th cooling trials in normoxic (21% O2) and hypoxic (12,10 and 7% O2) golden-mantled ground squirrels (GMGS) and Columbian ground squirrels (CGS)
| GMGS |
CGS |
|||||
|---|---|---|---|---|---|---|
| 21% O2 | 7% O2 | 21% O2 | 12% O2 | 10% O2 | 7% O2 | |
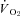 |
−12.1 ± 1.9 | −1.58 ± 0.71‡ | −6.01 ± 1.51 | −2.70 ± 0.78‡ | −2.03 ± 0.42‡ | −1.10 ± 0.52‡ |
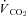 |
−9.64 ± 1.86 | −0.954 ± 0.331‡ | −4.62 ± 0.84 | −2.46 ± 0.86‡ | −1.79 ± 0.31‡ | −0.986 ± 0.40‡ |
| fH | −21.6 ± 15.3 | −1.38 ± 1.99* | −20.4 ± 2.80 | −9.90 ± 3.96‡ | −7.70 ± 4.46‡ | −0.932 ± 3.15‡ |
| ∫EMG | −81.0 ± 45.7 | −19.0 ± 22.0* | −32.4 ± 23.2 | −12.0 ± 12.0† | −11.9 ± 9.3† | −4.12 ± 5.70† |
| VTI | −1.62 ± 0.76 | −0.132 ± 0.342† | −0.566 ± 0.604 | −0.148 ± 0.303 | 0.126 ± 0.694 | −0.504 ± 0.259 |
| fR | 1.47 ± 9.62 | −3.70 ± 1.60 | −5.48 ± 4.76 | −4.58 ± 3.99 | −5.29 ± 1.41 | −1.64 ± 1.67* |
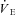 |
−331 ± 85 | −90.0 ± 56.3‡ | −111 ± 61.2 | −69.9 ± 78.9 | −63.2 ± 56.9 | −64.2 ± 39.4 |
All values are mean ±s.d., expressed in units °C−1 (i.e. per change in Th) – negative values indicate that cooling Th caused that parameter to rise.
Significantly different from normoxic values (P < 0.05).
Significantly different from normoxic values (P < 0.01).
Significantly different from normoxic values (P < 0.001).
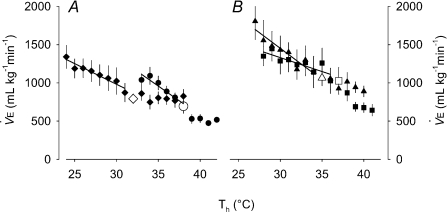
Figure 4. Summary (mean ±s.e.m.) metabolic heat production values from the Th manipulation experiments in Columbian ground squirrels.
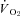 and
and 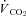 are shown in A and B, respectively, for all levels of inspired O2, whereas EMG responses are split into C (21 and 7% O2) and D (12 and 10% O2) for visual clarity. Open symbols represent the steady state, non-manipulated values, whereas filled symbols represent values measured during the 10 min Th manipulation trials. For all graphs (A–D), circles refer to 21%, squares refer to 12, triangles refer to 10 and diamonds refer to 7% inspired O2. Regression lines are plotted to demonstrate the degree of proportionality resulting from hypothalamic cooling.
are shown in A and B, respectively, for all levels of inspired O2, whereas EMG responses are split into C (21 and 7% O2) and D (12 and 10% O2) for visual clarity. Open symbols represent the steady state, non-manipulated values, whereas filled symbols represent values measured during the 10 min Th manipulation trials. For all graphs (A–D), circles refer to 21%, squares refer to 12, triangles refer to 10 and diamonds refer to 7% inspired O2. Regression lines are plotted to demonstrate the degree of proportionality resulting from hypothalamic cooling.
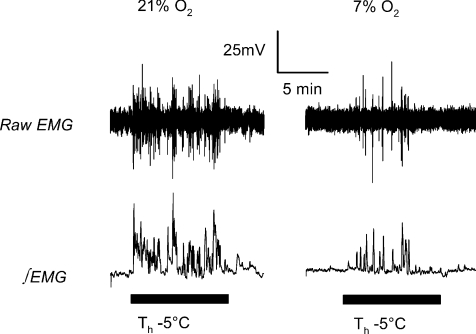
Figure 5.
Sample traces of raw EMG voltage (top) and integrated EMG voltage (∫EMG; bottom) during 10 min periods (black bars) of Th manipulation 5°C below steady state values in normoxia (left) and 7% O2 (right) in a Columbian ground squirrel.
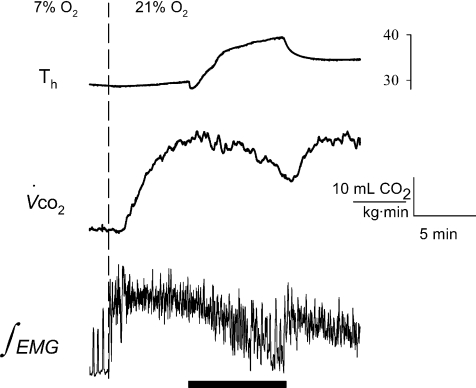
Figure 6. Sample trace of a Columbian ground squirrel re-warming following exposure to 7% O2, demonstrating a rapid onset of shivering thermogenesis with normoxia.
During re-warming, Th (top trace) was transiently warmed to 40°C (black bar), which was the minimum temperature required to offset the post-hypoxic rise in metabolism (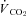 , middle trace), which can be readily observed by the rapid, transient changes in neck muscle electromyography (bottom trace).
, middle trace), which can be readily observed by the rapid, transient changes in neck muscle electromyography (bottom trace).
Tb responses to induced Th changes
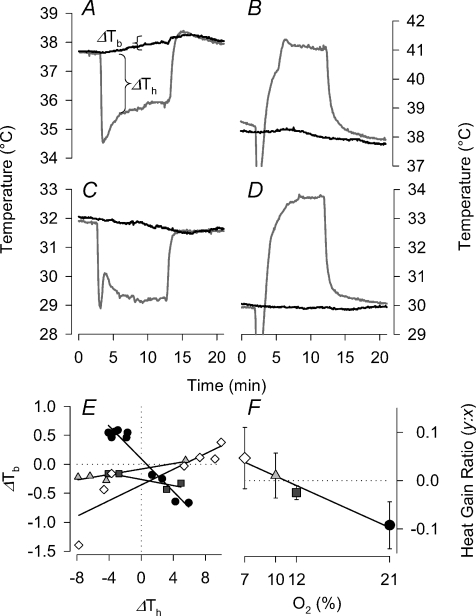
In addition to metabolic changes, manipulation of Th also led to changes in Tb itself (Fig. 7A–D). Indeed, in normoxic squirrels, warming the hypothalamus led to a decline in Tb, whereas cooling raised Tb. The relationship between changes in Th and Tb manifested in a linear ‘heat gain ratio’ in normoxic squirrels of approximately 0.1 (Fig. 7E). Exposure to hypoxia, however, abolished the heat gain ratio, such that warming or cooling Th produced little change in Tb (Fig. 7E and F). The effect of oxygen on the heat gain ratio was significant (F3,11= 17.0; P= 0.00019), with 7 and 10% O2 values higher than normoxia, but not significantly different from zero (t5= 1.8 and 0.44; P= 0.07 and 0.31, respectively). Heat gain ratios for 21 and 12% O2 were significantly different from zero (t5= 4.7 and 4.2; P= 0.003 and 0.004, respectively).
Figure 7. Change in Th (ΔTh is the difference between manipulated and unmanipulated Th) over 10 min periods of manipulations induce changes in Tb (ΔTb=Tb after manipulation −Tb before manipulation) in Columbian ground squirrels.
Sample traces of manipulations from normoxic (A and B) and hypoxic (7% O2; C and D) ground squirrels during Th cooling (A and C) and Th warming (B and D). Sample results of multiple Th manipulations from one squirrel are shown in E. Average slope (ratio of ΔTb:ΔTh) values from all Columbian ground squirrels combined are shown in F. In both E and F, the symbol fills refer to inspired oxygen level: 21% (black circles), 12% (dark grey squares), 10% (light grey triangles) and 7% O2 (white circles).
Cardiorespiratory responses to Th cooling and warming
Cardiovascular changes in response to hypothalamic manipulations closely mirrored metabolic changes (Fig. 8). Heart rate rose with hypothalamic cooling in normoxic squirrels. Furthermore, the proportionality constants of fH for hypothalamic cooling were significantly influenced by hypoxia (Table 3; Fig. 8A and B); all levels of hypoxia significantly attenuated the influence of cooling on the fH response.
Figure 8. Summary (mean ±s.e.m.) cardiovascular values from the Th manipulation experiments in Columbian ground squirrels.
fH is shown in A (21 and 7% O2) and B (12 and 10% O2). Open symbols represent the steady state, non-manipulated values, whereas filled symbols represent values measured during the 10 min Th manipulation trials. Circles refer to 21%, squares refer to 12%, triangles refer to 10% and diamonds refer to 7% inspired O2. Regression lines are plotted to demonstrate the degree of proportionality resulting from hypothalamic cooling.
Ventilatory responses during hypothalamic manipulations were more complex than metabolic and cardiovascular responses. Generally, hypothalamic cooling induced increases in VTI; however, the proportionality constants were not affected by hypoxia (Table 3), except in GMGS where 7% O2 attenuated the influence of Th cooling. fR, on the other hand, only manifested a significant influence on the proportionality constant for Th cooling in CGS at 7% O2, where the change in fR with Th was significantly diminished compared to normoxia. 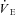 was affected by hypothalamic cooling, exhibiting proportionality constants different from zero in all cases (Fig. 9); however, oxygen did not significantly influence the proportionality constants in CGS. In GMGS, on the other hand, 7% O2 significantly attenuated the ventilatory changes associated with Th cooling (Table 3).
was affected by hypothalamic cooling, exhibiting proportionality constants different from zero in all cases (Fig. 9); however, oxygen did not significantly influence the proportionality constants in CGS. In GMGS, on the other hand, 7% O2 significantly attenuated the ventilatory changes associated with Th cooling (Table 3).
Figure 9. Summary (mean ±s.e.m.) minute ventilation (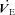 ) values from the Th manipulation experiments in Columbian ground squirrels.
) values from the Th manipulation experiments in Columbian ground squirrels.
 is shown in A (21 and 7% O2) and B (12 and 10% O2), for visual clarity. Open symbols represent the steady state, non-manipulated values, whereas filled symbols represent values measured during the 10 min Th manipulation trials. Circles refer to 21%, squares refer to 12%, triangles refer to 10% and diamonds refer to 7% inspired O2. Regression lines are plotted to demonstrate the degree of proportionality resulting from hypothalamic cooling.
is shown in A (21 and 7% O2) and B (12 and 10% O2), for visual clarity. Open symbols represent the steady state, non-manipulated values, whereas filled symbols represent values measured during the 10 min Th manipulation trials. Circles refer to 21%, squares refer to 12%, triangles refer to 10% and diamonds refer to 7% inspired O2. Regression lines are plotted to demonstrate the degree of proportionality resulting from hypothalamic cooling.
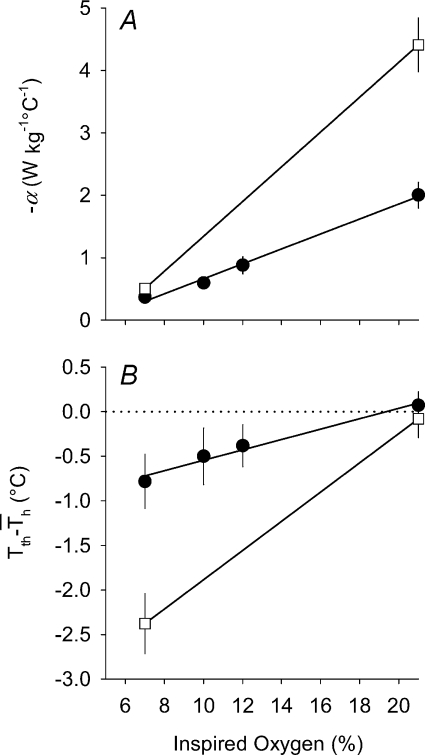
α and Tth
α values for metabolic heat production (calculated from 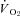 values) are reported in Table 4. It was evident that α was linearly related to the level of inspired oxygen in CGS (Fig. 10A; r= 0.91; F1,20= 91; P < 0.00001), whereas Tth demonstrated a more non-linear response, with larger declines in Tth occurring at the lowest levels of oxygen (Table 4). However, the difference between Tth and steady state Th (
values) are reported in Table 4. It was evident that α was linearly related to the level of inspired oxygen in CGS (Fig. 10A; r= 0.91; F1,20= 91; P < 0.00001), whereas Tth demonstrated a more non-linear response, with larger declines in Tth occurring at the lowest levels of oxygen (Table 4). However, the difference between Tth and steady state Th (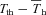 ; where
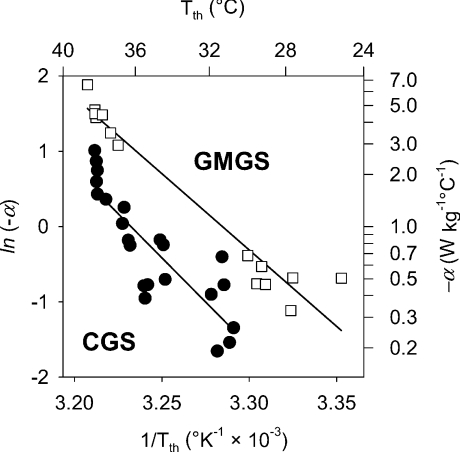
; where 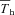 is the Th in the absence of thermal manipulation) decreased linearly with hypoxia, becoming statistically significant from normoxic values at 10 and 7% O2 (Table 4, Fig. 10B; r= 0.51; F1,20= 7.1; P= 0.014). Correlations between α and Tth were evident from slopes derived from Arrhenius plots of ln (α)vs.T−1th (Fig. 11), yielding estimates of the activation energy, which were subsequently converted into Q10 values. In CGS, the Q10 for the relationship between α and Tth was 12.0, and in GMGS the Q10 was 9.78.
is the Th in the absence of thermal manipulation) decreased linearly with hypoxia, becoming statistically significant from normoxic values at 10 and 7% O2 (Table 4, Fig. 10B; r= 0.51; F1,20= 7.1; P= 0.014). Correlations between α and Tth were evident from slopes derived from Arrhenius plots of ln (α)vs.T−1th (Fig. 11), yielding estimates of the activation energy, which were subsequently converted into Q10 values. In CGS, the Q10 for the relationship between α and Tth was 12.0, and in GMGS the Q10 was 9.78.
Table 4.
Parameters (mean ±s.d.) related to metabolic heat production resulting from hypothalamic thermal manipulations in ground squirrels
| GMGS |
CGS |
|||||
|---|---|---|---|---|---|---|
| 21% O2 | 7% O2 | 21% O2 | 12% O2 | 10% O2 | 7% O2 | |
| α(W kg−1°C−1) | −4.41 ± 1.14 | −0.506 ± 0.110‡ | −2.00 ± 0.50 | −0.879 ± 0.31‡ | −0.594 ± 0.204‡ | −0.367 ± 0.182‡ |
| Tth(°C) | 37.9 ± 0.6 | 28.4 ± 0.8‡ | 38.1 ± 0.2 | 36.3 ± 0.5‡ | 34.9 ± 0.5‡ | 31.3 ± 0.3‡ |
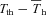 |
−0.080 ± 0.56 | −2.38 ± 0.893‡ | 0.070 ± 0.38 | −0.38 ± 0.52 | −0.50 ± 0.71† | −0.78 ± 0.68‡ |
Significantly different from normoxic values (P < 0.01).
Significantly different from normoxic values (P < 0.001).
Figure 10. Mean (±s.e.m.) values for A, hypothalamic thermosensitivity (α) and B, 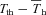 (difference between Tth and steady state Th;
(difference between Tth and steady state Th;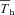 ) during normoxic and hypoxic exposure in Columbian ground squirrels (filled circles) and golden-mantled ground squirrels (open squares).
) during normoxic and hypoxic exposure in Columbian ground squirrels (filled circles) and golden-mantled ground squirrels (open squares).
α in CGS was strongly correlated (r= 0.91; F1,20= 91; P < 0.00001) with inspired oxygen, whereas 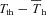 demonstrated a weaker, albeit significant correlation (r= 0.51; F1,20= 7.1; P= 0.014).
demonstrated a weaker, albeit significant correlation (r= 0.51; F1,20= 7.1; P= 0.014).
Figure 11. Correlation of hypothalamic thermosensitivity (α) and the threshold hypothalamic temperature (Tth) from individual animals required to elicit a rise in metabolic heat production in Columbian ground squirrels (CGS, filled circles) and golden-mantled ground squirrels (GMGS, open squares).
Arrhenius plots revealed Q10 values of 12 for CGS and 10 for GMGS, much higher than that predicted from simple temperature effects (Q10 2–3).
Discussion
This study examined the properties of the CNS Tb regulator that change in hypoxia. Central regulation of Tb remains operational in hypoxia, however, low oxygen progressively reduces Tth as well as suppressing metabolic thermosensitivity. Combined, this suggests that the hypoxic thermoregulatory response results from a regulated decline in body temperature, similar to that observed during sleep states, torpor, and hibernation.
Steady state responses to hypoxia
Acute, but prolonged (i.e. > 2 h) exposure to hypoxia leads to major adjustments in mammalian cardiorespiratory, metabolic and thermoregulatory homeostasis (Powell et al. 1998; Mortola & Frappell, 2000). The results of the present study are no exception (Fig. 1). The overall response to hypoxia that occurred in these two ground squirrel species was: (1) a progressive reduction in Tb which is accompanied by a reduction in metabolic heat production, (2) a ventilatory stimulation (increased 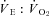 ), driven initially by an increase in heart rate, and later on by changes in tidal volume, and (3) a hypoxic tachycardia in mild hypoxia, that converted to a bradycardia as the metabolic requirements dropped in extreme hypoxia. The overall responses support the idea that hypoxia initiates a chemoreceptor-driven ventilatory stimulation and a decline in metabolic heat production accompanied by a fall in Tb. Ultimately, this cascade of events is geared toward the balance of oxygen supply and demand through changes in delivery (cardiorespiratory) and consumption (metabolic) pathways. This physiological response, often termed hypoxic-anapyrexia (Steiner & Branco, 2002; Steiner et al. 2002), was relatively stable throughout the experimental period, allowing for the manipulation of Th. In order to ascertain whether the hypoxic decline in Tb resulted from a regulated decrease, it was necessary to manipulate Th under these steady state conditions and observe whether concomitant changes in physiological responses reflected attempts to defend homeostatic states.
), driven initially by an increase in heart rate, and later on by changes in tidal volume, and (3) a hypoxic tachycardia in mild hypoxia, that converted to a bradycardia as the metabolic requirements dropped in extreme hypoxia. The overall responses support the idea that hypoxia initiates a chemoreceptor-driven ventilatory stimulation and a decline in metabolic heat production accompanied by a fall in Tb. Ultimately, this cascade of events is geared toward the balance of oxygen supply and demand through changes in delivery (cardiorespiratory) and consumption (metabolic) pathways. This physiological response, often termed hypoxic-anapyrexia (Steiner & Branco, 2002; Steiner et al. 2002), was relatively stable throughout the experimental period, allowing for the manipulation of Th. In order to ascertain whether the hypoxic decline in Tb resulted from a regulated decrease, it was necessary to manipulate Th under these steady state conditions and observe whether concomitant changes in physiological responses reflected attempts to defend homeostatic states.
Hypothalamic control of physiological responses in hypoxia
The most profound results from the hypothalamic manipulation trials were manifested in the metabolic changes. Cooling the hypothalamus led to activation of thermogenic mechanisms proportional to the degree of cooling. This has been observed in prior work on a range of mammals from mice, rats, cats, dogs and seals (Simon et al. 1986). The proportionality of the central mechanisms for heat production are thought to reflect the presence of cold-responsive neurons (or, more specifically, from inhibitory warm-sensitive neurons) within the CNS regulator, whose output is coupled to thermogenic activation in the periphery (Bligh, 1998). The metabolic thermosensitivity is reduced, but not fully eliminated by hypoxic exposure; α values decrease 5.4- and 8.7-fold, respectively in CGS and GMGS comparing 21 to 7% O2. Shivering can still be recruited in hypoxia by hypothalamic cooling, albeit at reduced levels (Fig. 5). This alone demonstrates that the hypoxic decline in Tb is supported by thermogenic mechanisms acting with reduced amplitude (Barros et al. 2001). For the most part, the proportionality constants for cooling in other physiological parameters (e.g. fH, fR) also exhibited similar declines in hypoxia. We did not record major physiological changes that reflected regulatory responses to hypothalamic warming (in normoxia or hypoxia); ideally, however, these experiments need to be conducted in a species that exhibits profound thermolytic responses to hypothalamic heating (Parmeggiani et al. 1973) to resolve this question. An important caveat from this study, however, is that other thermoeffectors may not exhibit similar thresholds for activation in hypoxia, since recent evidence points toward multiple, independent temperature sensors and pathways within the brain responsible for activating heat loss, heat production and temperature-seeking behaviour (Nagashima et al. 2000).
Hypoxic thermoregulatory threshold and thermosensitivity
The ultimate goal of this study was to shed light on whether hypoxia induces a decrease in hypothalamic ‘set-point’ for Tb regulation, as hypothesised by Wood (1991). The term ‘set-point’, however, has produced much debate, confusion and controversy, and recently inspired abandonment (Romanovsky, 2004; Cabanac, 2006). Part of the debate stems from different uses of the term (Cabanac, 2006), as well as from an expectation that a stable ‘reference’ neuron must exist that maintains a constant firing rate across a range of brain temperatures. Hammel's early neuron network model (Hammel, 1968, 1972), however, did not require constancy in a reference neuron, but simply a network of interacting neurons with differing thermal sensitivities (Bligh, 1998; Cabanac, 2006). In fact, simple changes in the pattern of neuronal firing (see Hori et al. 1987, 1988; Koga et al. 1987) would help explain how the hypothalamic threshold actually varies; changes in physiological state, such as fever, exercise, sleep and hibernation, may alter the inherent thermosensitivities of the neurons within the network, and thereby alter the balance point (Romanovsky, 2004, 2007) that represents the regulated Tb. We have used the term Tth similarly to Heller & Hammel (1972), as a threshold temperature that activates/deactivates the intrinsic thermosensitivity of the central Tb regulator.
Indeed, one of the major results from the current study is that the Tth is reset progressively to lower levels by hypoxic exposure. The notion that Tb is down-regulated has been a prevailing hypothesis in the literature on hypoxic anapyrexia (Kozak, 1997; Barros et al. 2001; Tattersall & Milsom, 2003). However, to date, the most compelling evidence for this assertion has come from work done on ectotherms, which actively select lower environmental temperatures in hypoxia (Dupré & Wood, 1988; Wood & Malvin, 1993; Tattersall & Boutilier, 1997; Bicego et al. 2006; Cadena & Tattersall, 2009). An active preference for lower Tb requires neurophysiological coordination, and thus strongly implies that the central thermoregulatory mechanisms are reset, since effectors for Tb control include behavioural as well as physiological mechanisms. Lowered temperature preferences have also been observed in endotherms (Gordon & Fogelson, 1991; Wood, 1995; Gordon, 1997) as well as a wide range of animals (Wood & Gonzales, 1996), suggesting an adaptive value to lowering Tb during hypoxic stress. Therefore, if hypoxia down-regulated Tb, then it should follow that correlates of energy metabolism, thermoregulation and heat loss would operate in a coordinated fashion to facilitate this drop in Tb. Further indirect evidence for hypoxia eliciting apparent declines in regulated Tb are found in cats and rabbits where artificially raising Tb back to normothermic temperatures elicits a heat stress or relative hyperthermic responses (Rohlicek et al. 1996; Seifert et al. 2006). Furthermore, changes to the thermosensitivity of hypothalamic neurons (both warm- and cold-responsive neurons) do occur (Koga et al. 1987; Tamaki & Nakayama, 1987) in hypoxia, suggesting that the hypoxic effects on α may be a direct effect on neuronal activity in the hypothalamus, as has been implied for osmotic and cardiovascular influences on Tb (Hori et al. 1987, 1988).
It is also possible, however, that the decline in α in hypoxia is a passive consequence of the drop in Tb itself, due to inherent thermal effects on neuronal firing rates (Heller & Colliver, 1974; Heller et al. 1974). We can address this by examining the temperature sensitivity for α; the relationship between α and Tth yielded Q10 values ranging from 10 to 12, substantially higher than that expected from simple temperature effects, as well as higher than that observed in hibernation (Florant & Heller, 1977; Florant et al. 1978), where Tth has been well established to be co-ordinately lowered. Indeed, the likely explanation is that the central thermosensitivity changes in hypoxia result from a direct suppressive effect on thermosensitive neuronal activity (or an alteration in the balance of excitatory and inhibitory inputs), rather than α values passively following Tb. One consequence of a suppression of neuronal activity is that the hypoxic decline in Tb produces a more variable thermoregulatory pattern due to the reduced gain or sensitivity of the CNS Tb regulator. Precedence for this is observed in ectothermic studies showing a decreased precision of regulated Tb in hypoxia (Cadena & Tattersall, 2009), as well as studies in mammals that demonstrate that the hypoxic decline in Tb is more variable and also strongly dependent on ambient temperature (Barros et al. 2001; Bishop et al. 2001; Levesque & Tattersall, 2009). Indeed, one interesting result from the present study is the remarkable similarity between the changes in α and Tth and those observed during different sleep states in rodents. During slow wave sleep (SWS), α and Tth are partially reduced, whereas during REM sleep, Tth and α are entirely absent (Glotzbach & Heller, 1975, 1976; Sakaguchi et al. 1979), which has led to the notion that REM sleep is essentially a period where thermoregulatory defences are abandoned. Hypoxia appears to produce a qualitatively similar state to SWS; coincidentally, hypoxia also produces alterations in EEG patterns, which show a relative increase in slow wave activity. These similar patterns suggest parallel mechanisms between SWS and hypoxia, although whether hypoxia influences the hypothalamic regulator via similar processes is unknown.
Under resting, normoxic conditions, feedback from extrahypothalamic warming would appear to be minor (Heller et al. 1974) since Tb continues to rise linearly during Th cooling without abatement. Indeed, ground squirrels exhibit little inhibitory extra-hypothalamic feedback during euthermia and hibernation (Heller & Hammel, 1972). In hypoxia, the relative amount of extrahypothalamic sensitivity (assessed by the heat gain ratio) trended toward zero, since cooling Th, which augments heat production, does not evoke the same rise in Tb as it did in normoxia. This may actually be the result of a sustained peripheral vasodilatation in hypoxia (Tattersall & Milsom, 2003), allowing the centrally derived heat to be driven from the animals, or from the involvement of peripheral chemoreceptor activation acting via medullary pathways producing a sustained reduction in sympathetic nervous activation of thermogenesis (Madden & Morrison, 2005). Combined with the low hypothalamic thermosensitivity, this may explain why hypoxia also produces an ambient-temperature-dependent Tb in addition to lowering Tth (Barros et al. 2001). Similarly, we observed that 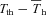 was directly related to oxygen level, indicating that the threshold for heat activation is actually below the apparent steady state
was directly related to oxygen level, indicating that the threshold for heat activation is actually below the apparent steady state 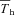 value in hypoxia. Thus, despite an achievement of steady state physiological responses to hypoxia, Tth drops below and in advance of Th, similar to what is observed during entrance into hibernation (Heller et al. 1977). Furthermore, we also observed instances in hypoxic squirrels where Th did not return to previous values after completion of Th manipulation (Fig. 7), reinforcing that the threshold to activate thermogenesis in hypoxia is very often well below that of Th.
value in hypoxia. Thus, despite an achievement of steady state physiological responses to hypoxia, Tth drops below and in advance of Th, similar to what is observed during entrance into hibernation (Heller et al. 1977). Furthermore, we also observed instances in hypoxic squirrels where Th did not return to previous values after completion of Th manipulation (Fig. 7), reinforcing that the threshold to activate thermogenesis in hypoxia is very often well below that of Th.
Interestingly, upon initial exposure to hypoxia, Th−Tb, which is normally positive, decreases, implying that the stimulus to regulate an elevated Th is diminished. After 2 h of exposure to hypoxia, however, this difference begins to rise, driven by a stabilisation of Th (Fig. 2). This suggests that Th is being regulated in hypoxia; upon re-oxygenation, the Th−Tb difference rapidly rises, for, perhaps two reasons. Firstly, Tth for thermogenesis would instantly return to normal values (as witnessed by the near-instantaneous activation of shivering upon returning oxygen levels to 21%; Fig. 6), and given the small size of the brain relative to its high blood flow, maximal thermogenesis would raise Th prior to Tb. It is also possible that the greater difference between Th and Tb at this point is due to selective brain warming, which is consistent with how many hibernators re-warm from torpor; an elevated thoracic and head temperature is achieved prior to re-warming the rest of the body, achieved via sympathetically mediated constriction in the periphery (Osborne et al. 2005).
Universality of the hypoxic thermoregulatory response?
Although the present study demonstrated a robust decline in Tb, interestingly, acute hypoxia does not exert profound changes in Tb or 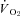 in larger mammals (>2 kg; Frappell et al. 1992), which may be due to a size-limited inability to cool significantly, akin to the size constraints of deep hibernation observed in small mammals (Geiser, 1998). Given, however, that the lowering of Tb is a nearly ubiquitous response to hypoxia in the animal kingdom, large mammals may still exhibit the appropriate control mechanisms for reducing Tb in hypoxia, but are simply incapable of doing so due to size restrictions. Equally, however, the slight decline in Tb that is observed in large mammals in hypoxia may be due to scaling influences on hypothalamic thermosensitivity. Mammalian hypothalamic thermosensitivity scales negatively (exponent =−0.37) with body mass (Heller, 1978), suggesting a diminishing importance of central neuronal thermosensitivity in maintaining Tb in large animals. If hypoxia reduces primarily central thermosensitivity, with minimal effects on peripheral thermosensation, this may explain the profound reduction in Tb of small mammals. Further examination of central and peripheral thermosensitivities in hypoxic states is warranted to test this hypothesis.
in larger mammals (>2 kg; Frappell et al. 1992), which may be due to a size-limited inability to cool significantly, akin to the size constraints of deep hibernation observed in small mammals (Geiser, 1998). Given, however, that the lowering of Tb is a nearly ubiquitous response to hypoxia in the animal kingdom, large mammals may still exhibit the appropriate control mechanisms for reducing Tb in hypoxia, but are simply incapable of doing so due to size restrictions. Equally, however, the slight decline in Tb that is observed in large mammals in hypoxia may be due to scaling influences on hypothalamic thermosensitivity. Mammalian hypothalamic thermosensitivity scales negatively (exponent =−0.37) with body mass (Heller, 1978), suggesting a diminishing importance of central neuronal thermosensitivity in maintaining Tb in large animals. If hypoxia reduces primarily central thermosensitivity, with minimal effects on peripheral thermosensation, this may explain the profound reduction in Tb of small mammals. Further examination of central and peripheral thermosensitivities in hypoxic states is warranted to test this hypothesis.
Conclusions and perspectives
The present study illustrates that informative features of how the CNS Tb regulator operates can be elucidated from simple changes in inspired oxygen. This approach has shed light on a fundamental homeostatic mechanism (temperature regulation), demonstrating that a change in oxygen, which could be manifested in a number of ways (e.g. alteration in brain blood flow, airway occlusion/asphyxia, sleep apnoea, as well as naturally hypoxic states), produces a rheostatic adjustment in homeostasis (Mrosovsky, 1990). Indeed, Tb regulation is lowered from ∼38°C in normoxia to ∼30°C in hypoxia. Central thermoregulatory control, however, is still intact in hypoxia, although the thresholds for changes in metabolism and shivering are reduced in magnitude. The degree of central thermosensitivity is drastically reduced, in a manner similar, but more profound, to that observed during SWS. Thus, although hypoxia produces a decrease in the regulated Tb, this begs the question about whether the term ‘hypoxia-induced anapyrexia’ (Steiner & Branco, 2002; Steiner et al. 2002) is entirely appropriate to apply to a state that reduces both the regulated temperature and the sensitivity (α) of the regulator itself. Equally, however, hypoxia does not produce a state where thermoregulatory responses are completely abandoned, arguing against ‘hypothermia’ being an appropriate description of the hypoxic thermoregulatory response.
Acknowledgments
We would like to acknowledge Beth Zimmer and Michael Harris for assistance with surgeries, Colin Sanders for assistance with care of the animals, and the staff of Manning Park, British Columbia for assistance with ground squirrel collection. The research was funded by the Natural Sciences and Engineering Research Council of Canada.
Glossary
Abbreviations
- α
metabolic thermosensitivity during hypothalamic cooling
- CGS
Columbian ground squirrel
- CNS
central nervous system
- EBZ
ear-bar zero (stereotactic terminology)
- fH
heart rate
- fR
breathing frequency
- GMGS
golden-mantled ground squirrel
- NTS
nucleus tractus solitarii
- RER
respiratory exchange ratio (whole animal respiratory quotient)
- RTN
reticular thalamic nucleus
- Tb
body temperature
- Th
hypothalamic temperature
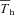
steady state Th, measured without thermal manipulation
- Tth
threshold Th below which metabolic heat production is activated above basal levels during hypothalamic cooling
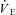
rate of ventilatory air exchange (minute ventilation)
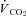
rate of carbon dioxide production
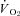
rate of oxygen consumption
- VTI
tidal volume of air exchanged with each breath
Author contributions
Conception and design: G.J.T. and W.K.M. Data collection: G.J.T. Analysis and interpretation: G.J.T. Article drafting, revisions and final approval: G.J.T. and W.K.M.
References
- Barros RC, Zimmer ME, Branco LG, Milsom WK. Hypoxic metabolic response of the golden-mantled ground squirrel. J Appl Physiol. 2001;91:603–612. doi: 10.1152/jappl.2001.91.2.603. [DOI] [PubMed] [Google Scholar]
- Bicego KC, Barros RC, Branco LG. Physiology of temperature regulation: comparative aspects. Comp Biochem Phys A Mol Integr Physiol. 2006;147:616–639. doi: 10.1016/j.cbpa.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Birchard GF, Kilgore DL, Jr, Boggs DF. Respiratory gas concentrations and temperatures within burrows of three species of burrow-nesting birds. Wilson Bull. 1984;96:451–456. [Google Scholar]
- Bishop B, Silva G, Krasney J, Nakano H, Roberts A, Farkas G, Rifkin D, Shucard D. Ambient temperature modulates hypoxic-induced changes in rat body temperature and activity differentially. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1190–R1196. doi: 10.1152/ajpregu.2001.280.4.R1190. [DOI] [PubMed] [Google Scholar]
- Bligh J. Mammalian homeothermy: an integrative thesis. J Therm Biol. 1998;23:143–258. [Google Scholar]
- Branco LG, Carnio EC, Barros RC. Role of the nitric oxide pathway in hypoxia-induced hypothermia of rats. Am J Physiol Regul Integr Comp Physiol. 1997;273:R967–R971. doi: 10.1152/ajpregu.1997.273.3.R967. [DOI] [PubMed] [Google Scholar]
- Brown JW, Whitehurst ME, Gordon CJ, Carroll RG. Thermoregulatory set point decreases after hemorrhage in rats. Shock. 2005;23:239–242. [PubMed] [Google Scholar]
- Cabanac M. Adjustable set point: to honor Harold T. Hammel. J Appl Physiol. 2006;100:1338–1346. doi: 10.1152/japplphysiol.01021.2005. [DOI] [PubMed] [Google Scholar]
- Cadena V, Tattersall GJ. Decreased precision contributes to the hypoxic thermoregulatory response in lizards. J Exp Biol. 2009;212:137–144. doi: 10.1242/jeb.023531. [DOI] [PubMed] [Google Scholar]
- Cormareche-Leydier M, Shimada SG, Stitt JT. Hypothalamic thermosensitivity in capsaicin-desensitized rats. J Physiol. 1985;363:227–236. doi: 10.1113/jphysiol.1985.sp015706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem. 2007;102:1713–1726. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré RK, Romero AM, Wood SC. Thermoregulation and metabolism in hypoxic animals. Adv Exp Med Biol. 1988;227:347–351. doi: 10.1007/978-1-4684-5481-9_32. [DOI] [PubMed] [Google Scholar]
- Dupré RK, Wood SC. Behavioural temperature regulation by aquatic ectotherms during hypoxia. Can J Zool. 1988;66:2649–2652. [Google Scholar]
- Fabris G, Anselmo-Franci JA, Branco LG. Role of nitric oxide in hypoxia-induced hyperventilation and hypothermia: participation of the locus coeruleus. Braz J Med Biol Res. 1999;32:1389–1398. doi: 10.1590/s0100-879x1999001100009. [DOI] [PubMed] [Google Scholar]
- Florant GL, Heller HC. CNS regulation of body temperature in euthermic and hibernating marmots (Marmota flaviventris) Am J Physiol Regul Integr Comp Physiol. 1977;232:R203–R208. doi: 10.1152/ajpregu.1977.232.5.R203. [DOI] [PubMed] [Google Scholar]
- Florant GL, Turner BM, Heller HC. Temperature regulation during wakefulness, sleep, and hibernation in marmots. Am J Physiol Regul Integr Comp Physiol. 1978;235:R82–R88. doi: 10.1152/ajpregu.1978.235.1.R82. [DOI] [PubMed] [Google Scholar]
- Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol. 1992;262:R1040–R1046. doi: 10.1152/ajpregu.1992.262.6.R1040. [DOI] [PubMed] [Google Scholar]
- Frappell P, Saiki C, Mortola JP. Metabolism during normoxia, hypoxia and recovery in the newborn kitten. Respir Physiol. 1991;86:115–124. doi: 10.1016/0034-5687(91)90043-i. [DOI] [PubMed] [Google Scholar]
- Geiser F. Evolution of daily torpor and hibernation in birds and mammals: importance of body size. Clin Exp Pharmacol Physiol. 1998;25:736–739. doi: 10.1111/j.1440-1681.1998.tb02287.x. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC. CNS regulation of metabolic rate in the kangaroo rat Dipodomys ingens. Am J Physiol. 1975;228:1880–1886. doi: 10.1152/ajplegacy.1975.228.6.1880. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC. Central nervous regulation of body temperature during sleep. Science. 1976;194:537–539. doi: 10.1126/science.973138. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC. Changes in the thermal characteristics of hypothalamic neurons during sleep and wakefulness. Brain Res. 1984;309:17–26. doi: 10.1016/0006-8993(84)91006-0. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. A review of terms for regulated vs. forced, neurochemical-induced changes in body temperature. Life Sci. 1983;32:1285–1295. doi: 10.1016/0024-3205(83)90802-0. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. The role of behavioural thermoregulation as a thermoeffector during prolonged hypoxia in the rat. J Thermal Biol. 1997;22:315–324. [Google Scholar]
- Gordon CJ, Fogelson L. Comparative effects of hypoxia on behavioural thermoregulation in rats, hamsters, and mice. Am J Physiol Regul Integr Comp Physiol. 1991;260:R120–R125. doi: 10.1152/ajpregu.1991.260.1.R120. [DOI] [PubMed] [Google Scholar]
- Hammel HT. Regulation of internal body temperature. Annu Rev Physiol. 1968;30:641–710. doi: 10.1146/annurev.ph.30.030168.003233. [DOI] [PubMed] [Google Scholar]
- Hammel HT. The set-point in temperature regulation: analogy or reality. In: Bligh J, Moore RE, editors. Essays on Temperature Regulation. Amsterdam: North Holland; 1972. pp. 121–137. [Google Scholar]
- Hammel HT, Heller HC, Sharp FR. Probing the rostral brainstem of anaesthetized, unanaesthetized, and exercising dogs and of hibernating and euthermic ground squirrels. Fed Proc. 1973;32:1588–1597. [PubMed] [Google Scholar]
- Harris MB, Milsom WK. The influence of NMDA receptor-mediated processes on breathing pattern in ground squirrels. Respir Physiol. 2001;125:181–197. doi: 10.1016/s0034-5687(00)00219-x. [DOI] [PubMed] [Google Scholar]
- Hayden P, Lindberg RG. Hypoxia-induced torpor in pocket mice (genus: Perognathus) Comp Biochem Physiol. 1970;33:167–179. doi: 10.1016/0010-406x(70)90492-5. [DOI] [PubMed] [Google Scholar]
- Heller HC. Hypothalamic thermosensitivity in mammals. Experientia Suppl. 1978;32:267–276. doi: 10.1007/978-3-0348-5559-4_30. [DOI] [PubMed] [Google Scholar]
- Heller HC. Hibernation – neural aspects. Annu Rev Physiol. 1979;41:305–321. doi: 10.1146/annurev.ph.41.030179.001513. [DOI] [PubMed] [Google Scholar]
- Heller HC, Colliver GW. CNS regulation of body temperature during hibernation. Am J Physiol. 1974;227:583–589. doi: 10.1152/ajplegacy.1974.227.3.583. [DOI] [PubMed] [Google Scholar]
- Heller HC, Colliver GW, Anand P. CNS regulation of body temperature in euthermic hibernators. Am J Physiol. 1974;227:576–582. doi: 10.1152/ajplegacy.1974.227.3.576. [DOI] [PubMed] [Google Scholar]
- Heller HC, Colliver GW, Beard J. Thermoregulation during entrance into hibernation. Pflugers Arch. 1977;369:55–59. doi: 10.1007/BF00580810. [DOI] [PubMed] [Google Scholar]
- Heller HC, Glotzbach SF. Thermoregulation during sleep and hibernation. In: Robertshaw D, editor. Environmental Physiology II. Baltimore: University Park Press; 1977. [PubMed] [Google Scholar]
- Heller HC, Hammel HT. CNS control of body temperature during hibernation. Comp Biochem Physiol A Comp Physiol. 1972;41:349–359. doi: 10.1016/0300-9629(72)90066-7. [DOI] [PubMed] [Google Scholar]
- Hori T, Kiyohara T, Nakashima T, Shibata M, Koga H. Multimodal responses of preoptic and anterior hypothalamic neurons to thermal and nonthermal homeostatic parameters. Can J Physiol Pharmacol. 1987;65:1290–1298. doi: 10.1139/y87-205. [DOI] [PubMed] [Google Scholar]
- Hori T, Nakashima T, Koga H, Kiyohara T, Inoue T. Convergence of thermal, osmotic and cardiovascular signals on preoptic and anterior hypothalamic neurons in the rat. Brain Res Bull. 1988;20:879–885. doi: 10.1016/0361-9230(88)90105-0. [DOI] [PubMed] [Google Scholar]
- IUPS Thermal Commission. Glossary of terms for thermal physiology – Third edition. Jpn J Physiol. 2001;51:245–280. [Google Scholar]
- Koga H, Hori T, Kiyohara T, Nakashima T. Responses of preoptic thermosensitive neurons to changes in blood pressure. Brain Res Bull. 1987;18:749–755. doi: 10.1016/0361-9230(87)90210-3. [DOI] [PubMed] [Google Scholar]
- Kottke FJ, Phalen JS, Taylor CB, Visscher MB, Evans GT. Effect of hypoxia upon temperature regulation of mice, dogs, and man. Am J Physiol. 1948;153:10–15. doi: 10.1152/ajplegacy.1948.153.1.10. [DOI] [PubMed] [Google Scholar]
- Kozak W. Regulated decreases in body temperature. In: Mackowiak PA, editor. Fever: Basic Mechanisms and Management. 2nd edn. Philadelphia: Lippincott-Raven Publishers; 1997. p. 506. [Google Scholar]
- Kozak W, Wrotek S, Walentynowicz K. Hypoxia-induced sickness behaviour. J Physiol Pharmacol. 2006;57:35–50. [PubMed] [Google Scholar]
- Kuhnen G. O2 and CO2 concentrations in burrows of euthermic and hibernating golden hamsters. Comp Biochem Physiol A Comp Physiol. 1986;84:517–522. doi: 10.1016/0300-9629(86)90359-2. [DOI] [PubMed] [Google Scholar]
- Larkin JE, Heller HC. Temperature sensitivity of sleep homeostasis during hibernation in the golden-mantled ground squirrel. Am J Physiol Regul Integr Comp Physiol. 1996;270:R777–R784. doi: 10.1152/ajpregu.1996.270.4.R777. [DOI] [PubMed] [Google Scholar]
- Levesque DL, Tattersall GJ. Seasonal changes in thermoregulatory responses to hypoxia in the Eastern chipmunk (Tamias striatus) J Exp Biol. 2009;212:1801–1810. doi: 10.1242/jeb.027094. [DOI] [PubMed] [Google Scholar]
- McArthur MD, Milsom WK. Ventilation and respiratory sensitivity of euthermic columbian and golden-mantled ground squirrels (Spermophilus columbianus and Spermophilus lateralis) during the summer and winter. Physiol Biochem Zool. 1991;64:921–939. [Google Scholar]
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol. 2005;566:559–573. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner RE, Wang LC, Trayhurn P. Brown fat thermogenesis during hibernation and arousal in Richardson's ground squirrel. Am J Physiol Regul Integr Comp Physiol. 1989;256:R42–R48. doi: 10.1152/ajpregu.1989.256.1.R42. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Hypoxic hypometabolism in mammals. News Physiol Sci. 1993;8:79–82. [Google Scholar]
- Mortola JP, Feher C. Hypoxia inhibits cold-induced huddling in rat pups. Respir Physiol. 1998;113:213–222. doi: 10.1016/s0034-5687(98)00056-5. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Frappell PB. Ventilatory responses to changes in temperature in mammals and other vertebrates. Annu Rev Physiol. 2000;62:847–874. doi: 10.1146/annurev.physiol.62.1.847. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Rheostasis: The Physiology of Change. New York: Oxford Univ. Press; 1990. [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Osborne PG, Sato J, Shuke N, Hashimoto M. Sympathetic alpha-adrenergic regulation of blood flow and volume in hamsters arousing from hibernation. Am J Physiol Regul Integr Comp Physiol. 2005;289:R554–R562. doi: 10.1152/ajpregu.00004.2005. [DOI] [PubMed] [Google Scholar]
- Parmeggiani PL, Franzini C, Lenzi P, Zamboni G. Threshold of respiratory responses to preoptic heating during sleep in freely moving cats. Brain Res. 1973;52:189–201. doi: 10.1016/0006-8993(73)90658-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain, in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Rohlicek CV, Saiki C, Matsuoka T, Mortola JP. Cardiovascular warming and respiratory consequences of body warming during hypoxia in conscious newborn cats. Pediatr Res. 1996;40:1–5. doi: 10.1203/00006450-199607000-00001. [DOI] [PubMed] [Google Scholar]
- Rohlicek CV, Saiki C, Matsuoka T, Mortola JP. Oxygen transport in conscious newborn dogs during hypoxic hypometabolism. J Appl Physiol. 1998;84:763–768. doi: 10.1152/jappl.1998.84.3.763. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Do fever and anapyrexia exist? Analysis of set-point definitions. Am J Physiol Regul Integr Comp Physiol. 2004;287:R992–R995. doi: 10.1152/ajpregu.00068.2004. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Glotzbach SF, Heller HC. Influence of hypothalamic and ambient temperatures on sleep in kangaroo rats. Am J Physiol Regul Integr Comp Physiol. 1979;237:R80–R88. doi: 10.1152/ajpregu.1979.237.1.R80. [DOI] [PubMed] [Google Scholar]
- Seifert EL, Sant Anna GM, Rohlicek CV. Effect of body warming on regional blood flow distribution in conscious hypoxic one-month-old rabbits. Biol Neonate. 2006;90:104–112. doi: 10.1159/000092069. [DOI] [PubMed] [Google Scholar]
- Simon E, Pierau FK, Taylor DC. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev. 1986;66:235–300. doi: 10.1152/physrev.1986.66.2.235. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Branco LG. Hypoxia-induced anapyrexia: implications and putative mediators. Annu Rev Physiol. 2002;64:263–288. doi: 10.1146/annurev.physiol.64.081501.155856. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Rocha MJA, Branco LGS. A neurochemical mechanism for hypoxia-induced anapyrexia. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1412–R1422. doi: 10.1152/ajpregu.00328.2002. [DOI] [PubMed] [Google Scholar]
- Tamaki Y, Nakayama T. Effects of air constituents on thermosensitivities of preoptic neurons: hypoxia versus hypercapnia. Pflugers Arch. 1987;409:1–6. doi: 10.1007/BF00584743. [DOI] [PubMed] [Google Scholar]
- Tattersall GJ, Blank JL, Wood SC. Ventilatory and metabolic responses to hypoxia in the smallest simian primate, the pygmy marmoset. J Appl Physiol. 2002;92:202–210. doi: 10.1152/japplphysiol.00500.2001. [DOI] [PubMed] [Google Scholar]
- Tattersall GJ, Boutilier RG. Balancing hypoxia and hypothermia in cold-submerged frogs. J Exp Biol. 1997;200:1031–1038. doi: 10.1242/jeb.200.6.1031. [DOI] [PubMed] [Google Scholar]
- Tattersall GJ, Milsom WK. Transient peripheral warming accompanies the hypoxic metabolic response in the golden-mantled ground squirrel. J Exp Biol. 2003;206:33–42. doi: 10.1242/jeb.00057. [DOI] [PubMed] [Google Scholar]
- Withers PC. Measurement of Vo2, Vco2, and evaporative water loss with a flow-through mask. J Appl Physiol. 1977;42:120–123. doi: 10.1152/jappl.1977.42.1.120. [DOI] [PubMed] [Google Scholar]
- Wood SC. Interactions between hypoxia and hypothermia. Annu Rev Physiol. 1991;53:71–85. doi: 10.1146/annurev.ph.53.030191.000443. [DOI] [PubMed] [Google Scholar]
- Wood SC. Oxygen as a modulator of body temperature. Braz J Med Biol Res. 1995;28:1249–1256. [PubMed] [Google Scholar]
- Wood SC, Gonzales R. Hypothermia in hypoxic animals: Mechanisms, mediators, and functional significance. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:37–43. doi: 10.1016/0305-0491(95)02045-4. [DOI] [PubMed] [Google Scholar]
- Wood SC, Malvin GM. The Vertebrate Gas Transport Cascade Adaptations to Environment and Mode of Life. Boca Raton: CRC Press Inc.; 1993. Behavioural hypothermia of toads: an adaptive stress response; pp. 194–199. [Google Scholar]
- Zimmer MB, Milsom WK. Effects of changing ambient temperature on metabolic, heart, and ventilation rates during steady state hibernation in golden-mantled ground squirrels (Spermophilus lateralis) Physiol Biochem Zool. 2001;74:714–723. doi: 10.1086/322930. [DOI] [PubMed] [Google Scholar]