Abstract
Nucleotide-binding oligomerization domain 2 (Nod2) is required for sensing of intracellular bacteria and subsequent inflammatory responses. Unexpectedly, new evidence suggests that Nod2 influences T helper cell signaling, proliferation and differentiation and effector responses against Toxoplasma gondii.
The early immune response to most pathogens is triggered by pattern-recognition receptors (PRRs), including Toll-like receptors (TLR) and nucleotide binding and oligomerization domain (Nod)-like receptors (NLRs). TLRs can stimulate antigen-presenting cells, thus indirectly allowing the effective induction of T cell immunity. Many TLRs induce activation of the canonical (IKKα-IKKβ-IKKγ–dependent) NF-κB pathway through the adaptor MyD88 and the serine/threonine kinase RIP2 (Fig. 1). Most early studies assumed that any requirement for TLRs and MyD88 in immunity reflected their roles in innate cells, but this view has been modified by recent data demonstrating that MyD88 can directly control T cell function, either through TLRs now known to be expressed on T cells or through IL-1R family members that can also engage MyD88 (refs. 1,2). In this issue of Nature Immunology, Shaw et al.3 now suggest that this type of innate cell–T cell dual activity is also shown by Nod2, an intracellular PRR. Nod2 can stimulate epithelial cells, macrophages and dendritic cells by sensing muramyl dipeptide (MDP), a component of bacterial peptidoglycan, and can also trigger activation of RIP2 and the canonical NF-κB pathway in these cells4. Shaw et al.3 challenge the current dogma that Nod2 is dedicated for innate immune recognition of bacterial motifs by showing that Nod2 can provide a T cell–intrinsic signal that does not rely on MDP recognition.
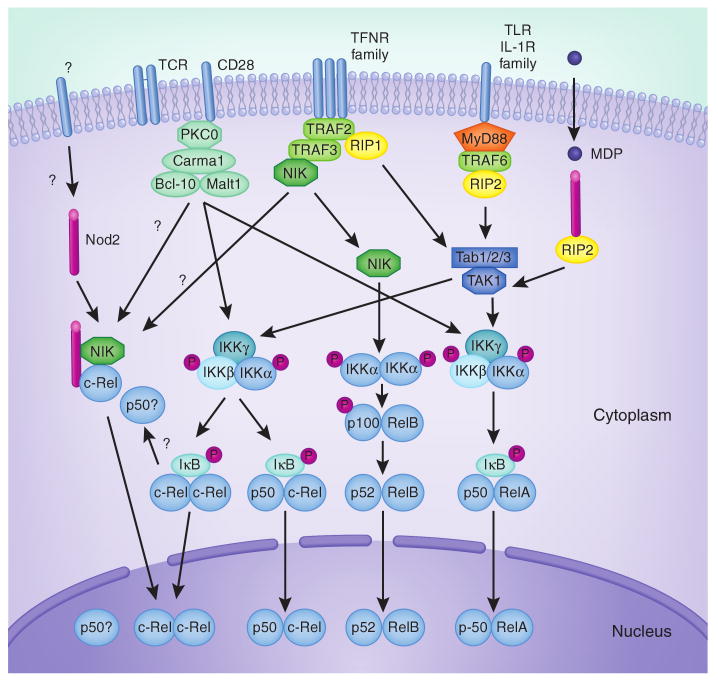
Figure 1.
Possible cross-talk between Nod2, TCR, Cd28, TLR and TNFR family members in regulating NF-κB. In non-T cells, muramyl dipeptide (MDP) can initiate a signaling cascade that requires Nod2 and RIP2, and results in nuclear accumulation of p50 and RelA as a result of Tab-TAK1-mediated activation of the IKKα-IKKβ-IKKγ complex and subsequent degradation of IκB. This pathway is also activated by TLR and IL-1R family members through MyD88, TRAF6 and RIP2, and by TNFR family members through TRAF2 and RIP1. whereas TLR, IL-1R and TNFR members can promote p50-RelA activation in T cells, it is not clear whether MDP, through Nod2, can also affect this pathway in T cells or whether T cells can take up MDP through specific transporters or phagocytosis. Shaw et al.3 propose that Nod2, independent of MDP recognition, influences T cell activity by binding NIK and c-Rel and by promoting c-Rel nuclear accumulation (left). whether c-Rel is activated as a homodimer or as a heterodimer with p50 was not investigated. It is unclear whether this putative NIK–c-Rel–Nod2 complex is downstream of the TCR, CD28, cytokine receptors or other receptors, and whether it is associated with other molecules. Cooperation between the TCR and CD28 in T cells can also promote p50-RelA nuclear translocation through PKCθ-Carma1-Bcl10-Malt1. TCR, CD28, TNFRs and TLRs can also trigger nuclear translocation of c-Rel (in the form of c-Rel–c-Rel or p50–c-Rel dimers) by targeting IκB. TNFR family members, by recruiting TRAF3 and thus inhibiting TRAF3-mediated degradation of NIK, can also activate the IKKα-dependent noncanonical NF-κB pathway leading to p52-RelB nuclear translocation. This may also promote cross-talk with Nod2 by stabilizing and freeing NIK to allow formation of the NIK–c-Rel–Nod2 complex.
The initial focus that led to this conclusion was the finding that Nod2-deficient animals succumb to T. gondii infection. Through a series of experiments with this pathogen, Shaw et al.3 revealed a specific defect in the ability of T cells from Nod2-deficient mice to produce interferon (IFN)-γ, raising the possibility that Nod2 might exert T cell–intrinsic functions. Consistent with this idea, upon T. gondii challenge, wild-type CD4+ T cells differentiated into T helper type-1 (TH1) effector cells in a Nod2−/− host, whereas Nod2−/− CD4+ T cells failed to differentiate into TH1 cells in a Nod2-sufficient environment.
Does Nod2 then have a more general role in T cell function? Shaw et al.3 found that Nod2 is required for lymphopenia-induced homeostatic T cell proliferation, a response driven by T cell antigen receptor (TCR)–peptide-MHC interactions and cytokines that signal through the common γ-chain. Nod2−/− donor CD4+ T cell populations, if transferred alone, expand normally in a lymphopenic host. However, when transferred with wild-type T cells, Nod2−/− CD4+ T cells show impaired population expansion. The authors also used a mouse model of colitis whereby the transfer of naive CD4+ T cells into Rag1−/− mice leads to gut inflammation. Recipients reconstituted with Nod2−/− T cells remained healthy and did not succumb to wasting disease.
These results support the idea that, in some situations, Nod2 expressed in T cells can affect T cell activity independently of sensing a bacterial product. In support of this argument, Shaw et al.3 also found that in the apparent absence of any Nod2 ligand, Nod2-deficient CD4+ TCR-transgenic T cells exposed to antigen in vitro produced less interleukin (IL)-2 and showed impaired differentiation into TH1 or TH2 cells. Addition of exogenous IL-2 restored the defective TH cell differentiation, leading the authors to speculate that the primary requirement for Nod2 is to regulate IL-2 secretion. In light of the T. gondii studies, this might be a reasonable conclusion as Il2−/− mice succumb to this pathogen in a time frame similar to that reported for Nod2−/− mice5. However, it is not clear that defective production of IL-2 by Nod2−/− T cells can explain the defects in homeostatic proliferation, as competition for cytokines and a requirement for Nod2 in common γ-chain cytokine receptor signaling is just as feasible. In the colitis experiments, Shaw et al.3 found reduced IFN-γ production by Nod2−/− CD4+ T cells, but this might also be unrelated to a lack of IL-2 production. IL-2 produced by CD4+ T cells can induce peripheral conversion of CD25– T cells into CD25+Foxp3+ inducible regulatory (iTreg) cells, leading one to predict that if the primary defect of Nod2−/− T cells in this situation was in producing IL-2, a more severe disease would ensue as a result of impaired iTreg cell generation.
The present model of Nod-mediated signaling in non-T cells holds that after detection of MDP, Nod2 recruits RIP2, leading to a common pathway shared by TLRs, the IL-1R family and members of the tumor necrosis factor receptor (TNFR) superfamily, wherein Tab-TAK1-mediated activation of the IKKα-IKKβ-IKKγ complex promotes nuclear translocation of the NF-κB subunits p50 and RelA (ref. 4) (Fig. 1). In line with a ligand-independent activity of Nod2 in T cells, Shaw et al.3 found that mice lacking RIP2 were resistant to T. gondii infection; these findings also agree with previous reports that T cell proliferation and differentiation are normal in the absence of RIP2 (ref. 6). In contrast, overexpression systems and reporter assays indicate that the function of Nod2 in T cells may be related to its ability to interact with the NF-κB family member c-Rel, as well as with NF-κB–inducing kinase (NIK), a downstream target of TNFR family members and a trigger of the noncanonical (IKKα-dependent) NF-κB pathway and nuclear translocation of p52–RelB heterodimers. T cell production of IL-2 in response to TCR and CD28 costimulation can depend on c-Rel, which can interact with NF-κB binding sites as well as the CD28-response element (CD28RE) in the proximal Il2 promoter region7,8. Additionally, it has been proposed that the ability of c-Rel to interact with CD28RE can be regulated by NIK9. Certainly, the in vitro data from Shaw et al.3 support the notion of a cooperative action of c-Rel and NIK in promoting Il2 transcription, and the notion that this can be boosted by Nod2. This conclusion also agrees with their finding of weaker nuclear accumulation of c-Rel in the absence of Nod2. In contrast, they found no effect of Nod2 deficiency on nuclear accumulation of RelA, suggesting that Nod2 does not affect the canonical NF-κB pathway, which induces nuclear translocation of p50– RelA heterodimers, in T cells.
However, several caveats warrant further investigation. First, how strongly c-Rel or NIK, compared to p50–RelA, contribute to IL-2 production by T cells in most physiological conditions is unknown. Additionally, although initial reports of NIK-mutant T cells from mice with alymphoplasia (aly/aly) suggested a global defect in IL-2 secretion, later reports found that the primary requirement for NIK was in memory T cells, not naive T cells10. Assuming that Shaw et al.3 analyzed naive TCR-transgenic Nod2-deficient T cells, a requirement for a NIK–c-Rel–Nod2 complex may not explain the defect. Another potential issue is with the availability of NIK to participate in such a complex. The current view, from studies of the TNFR family, is that NIK is continuously degraded by TRAF3 until TRAF3 is recruited and itself degraded by TNFR engagement11. Thus, for NIK to complex with Nod2 and c-Rel, this might require prior binding of a TNFR ligand to a T cell, and it is not clear whether any TNFR-ligand interactions were occurring in the experimental systems examined by Shaw et al.3. Lastly, whether this complex is physiologically induced in T cells was not determined and needs to be investigated; whether complex formation is the result of signaling from the TCR, CD28 and/or cytokine receptors also remains to be determined. Shaw et al.3 hypothesized that a Nod2–c-Rel–NIK complex might be downstream of CD28, but CD28 is not required for protection against T. gondii12.
The findings of Shaw et al.3 in many ways raise more questions than they provide answers. It is an intriguing study that will attract the attention of those focused on the requirement of Nod2 in host defense, as well as those interested in understanding the molecular regulation of T cell activation. The idea of a T cell–intrinsic role for Nod2 will undoubtedly generate much debate. A recent report assessed the role of Nod2 in models of graft-versus-host disease and found no apparent role in T cells. In particular, a mixed lymphocyte reaction with Nod2-deficient CD4+ and CD8+ T cells did not mimic the defective responses of Nod2-deficient TCR-transgenic T cells when responding to antigen13. Obvious differences between this study and Shaw et al.3 will spur further research into the influence of Nod2 in other T cell–driven immune responses. The subcellular localization, trafficking and expression of Nod2 in T cells remains poorly defined. Therefore, when and where Nod2 will and will not be active in an intrinsic signaling role in T cells needs to be determined. It is also not clear whether exogenous Nod2 ligands can stimulate T cells, and this issue is of obvious interest. Furthermore, it will be important to define the targets up- and downstream of Nod2 in T cells and to compare their cross-talk and roles with those involved in signaling from TLRs and TNFRs, which have already been proven key for NF-κB-dependent pathways.
Contributor Information
Shahram Salek-Ardakani, Email: ssalek@liai.org.
Michael Croft, Email: mick@liai.org.
References
- 1.Gelman AE, et al. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Y, et al. J Immunol. 2009;182:6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw MH, et al. Nat Immunol. 2009;10:1267–1274. doi: 10.1038/ni.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Shaw MH, Kim YG, Nunez G. Annu Rev Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 5.Villegas EN, Lieberman LA, Carding SR, Hunter CA. Infect Immun. 2002;70:4757–4761. doi: 10.1128/IAI.70.9.4757-4761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall HT, et al. Eur J Immunol. 2008;38:64–72. doi: 10.1002/eji.200737393. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh P, Tan TH, Rice NR, Sica A, Young HA. Proc Natl Acad Sci USA. 1993;90:1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Vig M, Lyons J, Van Parijs L, Beg AA. J Exp Med. 2003;197:861–874. doi: 10.1084/jem.20021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Valdepeñas C, Martin AG, Ramakrishnan P, Wallach D, Fresno M. J Immunol. 2006;176:4666–4674. doi: 10.4049/jimmunol.176.8.4666. [DOI] [PubMed] [Google Scholar]
- 10.Ishimaru N, Kishimoto H, Hayashi Y, Sprent J. Nat Immunol. 2006;7:763–772. doi: 10.1038/ni1351. [DOI] [PubMed] [Google Scholar]
- 11.Vallabhapurapu S, et al. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reichmann G, Villegas EN, Craig L, Peach R, Hunter CA. J Immunol. 1999;163:3354–3362. [PubMed] [Google Scholar]
- 13.Penack O, et al. J Exp Med. 2009;206:2101–2110. doi: 10.1084/jem.20090623. [DOI] [PMC free article] [PubMed] [Google Scholar]