Abstract
The formation of immune complexes results in activation of the innate immune system and subsequent induction of host inflammatory responses. In particular, the binding of IgG immune complexes to Fcγ receptors (FcγR) on monocytes triggers potent inflammatory responses leading to tissue injury in disease. We investigated whether activation of monocytes via FcγRs induced cell differentiation imparting specific inflammatory functions of the innate immune response. hIgG alone induced monocytes to differentiate into cells with an immature DC (iDC) phenotype, including upregulation of CD1b, CD80, CD86 and CD206. Differentiation into CD1b+ iDC was dependent upon activation via CD64 (FcγRI) and induction of GM-CSF. The hIgG-differentiated iDC were phenotypically different than GM-CSF-derived iDC at the same level of CD1b expression, with higher cell surface CD86, but lower MHC class II, CD32, CD206 and CD14. Finally, in comparison to GM-CSF derived iDC, IgG-differentiated iDC were more efficient in activating T cells in both autologous and allogeneic mixed lymphocyte reactions, but less efficient at presenting microbial antigen to T cells. Therefore, the ability of immune complexes to activate FcγRI on monocytes triggering subsequent differentiation into specialized iDC with the capacity to expand autoreactive T cells likely contributes to the pathogenesis of immune complex mediated tissue injury.
Introduction
The formation of immune complexes triggers a series of inflammatory responses that contribute to the pathophysiology of autoimmune diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (1–3). One mechanism by which immune complexes trigger inflammatory responses is by their ability to interact with receptors for the Fc portion of the immunoglobulin (Ig). In particular immune complexes composed of IgGs crosslink FcγRs on cells of the monocyte/macrophage lineage (4). The cross-linking of FcγRs triggers phagocytosis of opsonized foreign bodies, antibody-dependent cell-mediated cytotoxicity (ADCC) and production of various proinflammatory cytokines and chemokines (5,6).
In humans, three different classes of FcγRs have been described: FcγR I (CD64), FcγR II (CD32) and FcγR III (CD16). These FcγRs differ in cell distribution, function and affinity for IgG isotype (4). CD64 is a high affinity receptor (Kd=10−8 M for monomeric IgG) whereas CD32 and CD16 exhibit low affinity for monomeric IgG, with Kd for monomeric IgG ranging from 10−5 to 10−7 M (4). CD64 is exclusively expressed on myeloid cells, including monocytes, macrophages and IFN-γ activated granulocytes. The cross-linking of CD64 on human monocytes results in the production of several proinflammatory cytokines such as TNF-α, IL-6 and GM-CSF (7–9).
Cells of the monocyte lineage can be activated by various receptors and cytokines to differentiate into specialized functional cellular subsets of the innate immune system including dendritic cells (DC), specialized antigen presenting cells (APC) that play a crucial rolls in the regulation of innate responses and in the initiation of adaptive immunity (10,11). We have previously shown that activation of specific TLRs triggers the rapid differentiation of monocytes into two distinct populations of cells, CD1b+ DC that are potent antigen presenting cells, and CD209+ macrophages that are highly phagocytic (12). CD1b+ DC accumulate at the site of infection and have been shown to correlate with the resistance against the pathogen in leprosy (12, 13). In addition to treatment of monocytes with TLR ligands, CD1b+ DC differentiation is induced by low dose treatment with GM-CSF alone (0.2–10 U/ml) as well as a combination of GM-CSF and IL-4. The presence of CD209 on GM-CSF/IL-4-derived DC but not circulating or tissue DC suggests that the derivation of CD209− DC with GM-CSF is more physiologic relevant (12). Given that FcγRs are expressed on monocytes, and given the ability of monocytes to differentiate into DC, we hypothesized that FcγR activation of monocytes could trigger differentiation into DC, cells of the innate immune system with the ability to instruct the adaptive immune response in a manner that could contribute to autoimmune disease.
Materials and Methods
Ligands and antigens
We purchased TLR2/1 ligand: Mycobacterium tuberculosis 19 kD lipopeptide (EMC Microcollections). M. tuberculosis extracts were prepared by probe sonication as previously described (24) and provided by Dr. John Belisle (Colorado State University, Fort Collins, CO). The GroES protein was provided by Dr. Patrick Brennan through a contract with the National Institute of Allergy and Infectious Disease, contract N01-AI-25469, “Leprosy Research Support”. The GroES peptide (25) was synthesized by SynPep.
Plate-bound human IgG
Human IgG derived from healthy donor pooled sera (Equitech-Bio, Inc., Kerrville, TX) was reconstituted in various concentration (0.2–125µg/ml) into endotoxin free phosphate buffered saline (Invitrogen, Carlsbad, CA), and incubated in the plastic culture plate overnight in 4°C prior to monocyte culture. Coated plastic was washed twice with PBS before monocyte culture.
Monocyte differentiation
We obtained whole blood from healthy donors (UCLA Institutional Review Board #92-10-591-33) with informed consent. PBMC were purified by Ficoll-Hypaque gradient centrifugation (Ficoll-Paque; Pharmacia Biotech AB). Peripheral blood monocytes were isolated from PBMC thought Percoll (Amersham Biosciences, Uppsala, Sweden) density gradient centrifugation technique. Unless otherwise stated, monocyte cultures were performed at 37°C in RPMI 1640 supplemented with glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml) and 10% heat-inactivated FCS (OMEGA Scientific, CA). We stimulated monocytes with media, immobilized hIgG, soluble hIgG (0.04–125 µg/ml), rGM-CSF (0.2–10 U/ml), or the M. tb 19 kD lipopeptide (10 µg/ml) for 2 or 3 days. DCs were differentiated with rGM-CSF and matured with lipopeptide as described (26).
To study the CD16+ and CD64+ subsets of peripheral monocytes, we first isolated monocytes as above and then an anti-CD16-MACS beads (Miltenyi Biotech) was added for 20 min at 4°C. Flow-though was designated as the CD64+ monocytes. Remaining cells were designated as the CD16+ cells. Both cells were enriched twice prior to stimulation.
FcγR, GM-CSF blocking assays
Enriched monocytes as above were pre-incubated with 20 mg/ml of 3G8 (anti-CD16, BioLegend), AT10 (anti-CD32, AbD Serotec), 10.1 (anti-CD64, AbD Serotec), 107.3 (mIgG1k isotype control for anti-FcγR mAbs, BD Biosciences), BVD2-23B6 (anti-GM-CSF, BD Biosciences) or R35-95 (Rat IgG2a isotype for anti-GM-CSF mAbs, BD Biosciences) and placed on ice for 20 min before starting culture in hIgG-coated culture well.
Flow cytometric analysis
The following mAbs were used for flow cytometry studies: BCD1b3.1 (anti-CD1b (27), mIgG1k), 4A76 (anti-CD1b, Beckman Coulter, mIgG2a), HI149 (anti-CD1a, BD Bioscience), AD5-8E7 (anti-CD1c, Miltenyl Biotec), L243 (anti-HLA-DR, BD Biosciences), MEM-233 (anti-CD80, Caltag Laboratories), 2331(FUN-1) (anti-CD86, BD Bioscience), HB14 (anti-CD40, Caltag Laboratories), GHI/61 (anti-CD163, BD Biosciences), 19.2 (anti-CD206), DCN46 (anti-CD209, BD Biosciences), TüK4 (anti-CD14, Caltag Laboratories), 3G8 (anti-CD16, Caltag Laboratories), FLI8.26 (anti-CD32, BD Biosciences), 10.1 (anti-CD64, BD Biosciences), M5E2 (anti-CD14, BD Biosciences), HB15e (anti-CD83, BD Biosciences), SCO6 (anti-CDw116, Immunotech), and appropriate isotype control (Caltag Laboratories, Sigma-Aldrich and BD Biosciences).
GM-CSF Real-time PCR
Monocytes were stimulated with media, plate-bound hIgG (0.04–25 µg/ml in coating solution) or 19 kD lipopeptide for 3 hour. We isolated RNA and synthesized cDNA as described (25). GM-CSF primer were as follows: forward, 5’-GCCTCACCAAGCTCAAGGG-3’; reverse, 5’-GGAGGGCAGTGCTGTTTGTAG-3’. Reactions used Sybr Green PCR Master Mix (BioRad). The relative quantities of GM-CSF per sample and normalization were calculated as described (25).
T cell assays
For mixed-lymphocyte reaction, FcγR-activated and rGM-CSF induced CD1b+ cells were stimulated as above, harvested, washed, irradiated and plated. We obtained T cell from unmatched donors for allogeneic MLRs or form identical donors for autologous MLRs with Rosette Sep T cell enrichment cocktail (StemCell Technologies). Media or T cells were added. We measured IFN-γ, TNF-α, IL-4, and IL-17 levels by ELISA (BD Biosciences and Invitrogen). Proliferation was measured as described (28). For MHC II studies, monocytes were differentiated and irradiated as above. Cells were cultured with MHC II-restricted T-cells and the GroES protein or GroES peptide as previously described (12).
Results
FcγR activation induces monocyte differentiation
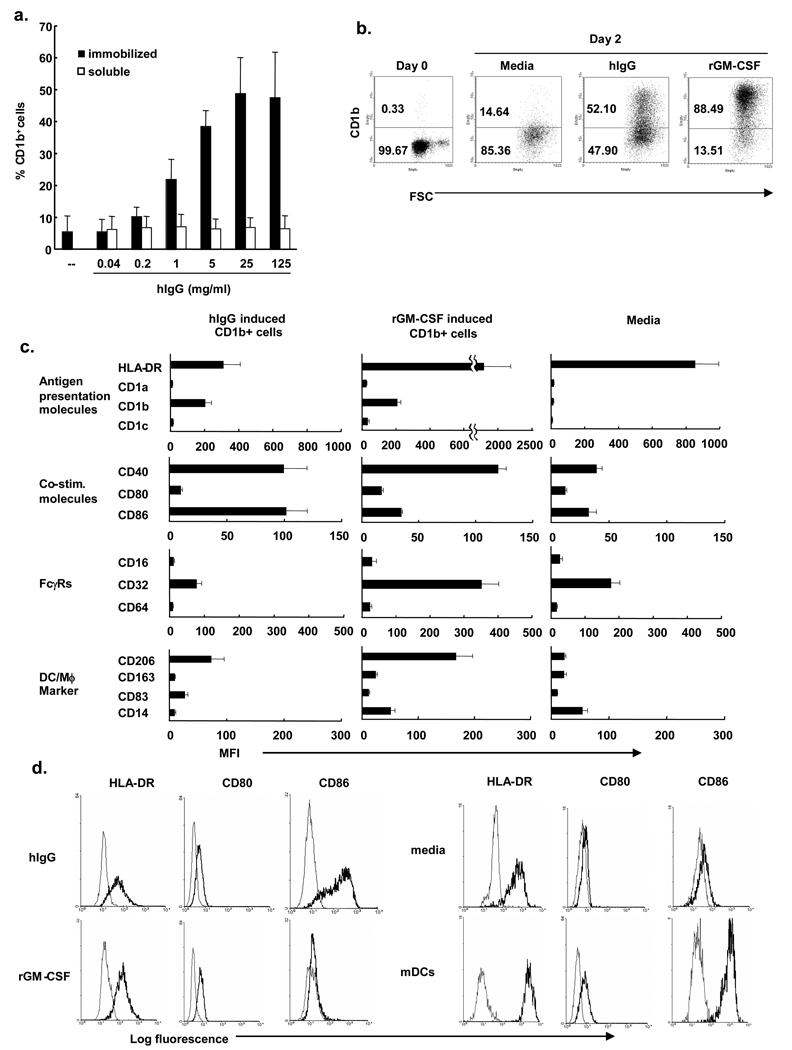
Signaling through FcγR is known to inhibit the differentiation and maturation of cytokine-derived DC (14). Here, the ability of FcγR stimulation to directly induce monocyte differentiation was investigated using plate-immobilized human IgG (hIgG) as a model for immune complex activation of FcγR on monocytes (6,8). Human peripheral blood monocytes were cultured in various concentrations (0.04–125 µg/ml) of immobilized human serum IgG. On day two, cells were harvested and the expression of the DC marker CD1b was measured by flow cytometry. Immobilized IgG induced CD1b expression in a dose-dependent manner (Figure 1a). In contrast, soluble human IgG did not induce CD1b expression on monocytes. Previously we demonstrated that rGM-CSF treatment of monocytes for two days induced differentiation into immature DC as characterized by morphology, phenotype including CD1b and function (12,15). The frequency of CD1b+ cells was comparable for hIgG vs. rGM-CSF, although the mean fluorescence intensity was greater for rGM-CSF derived cells (Figure 1b).
Figure 1. FcγR activation by immobilized human IgG induces CD1b+ immature dendritic cell differentiation.
(a) Monocytes were stimulated with various concentrations of immobilized IgG (hIgG), soluble human IgG (0.04–125 µg/ml) or recombinant GM-CSF (10 U/ml). Two days later the percent of CD1b+ cells was determined using flow cytometry. Data represent mean percent CD1b expression +/− SEM from 3 independent experiments. (b) Monocytes were stimulated with hIgG (25 µg/ml), rGM-CSF (10 U/ml) or left unstimulated and levels of CD1b were measured by flow cytometry. Dot plots are representative of three independent experiments. (c) Human monocytes were stimulated with hIgG (25 µg/ml), rGM-CSF (0.2 U/ml) or media. After two days, expression of cell surface markers were examined by flow cytometry, gating on CD1b+ cells (for hIgG and GM-CSF stimulation). Data are shown as mean fluorescence intensity (MFI) of at least three independent experiments +/− s.e.m. (d) Representative histograms for HLA-DR, CD80, and CD86 are shown to illustrate the differentiation stage of the dendritic cells.
Therefore, it was logical to compare the phenotype of CD1b+ cells derived by FcγR activation vs. culture with rGM-CSF. We compared expression levels of various surface molecules in CD1b+ cells treated for two days with hIgG (25 µg/ml) or rGM-CSF (0.2 U/ml) to normalize to the level of CD1b expression (Figure 1c and d). The expression patterns of cell surface markers for both activation via FcγR and rGM-CSF were consistent with an immature DC-like phenotype, including high levels of MHC class II, CD40, CD86 (B7.2, co-stimulatory molecule) as well as low expression of CD16 (FcγRIII), CD64 (FcγRI), CD14 (TLR co-receptor) and CD83. Yet there were also clear differences. The levels of CD86 were found to be higher on FcγR activated CD1b+ cells. Meanwhile, levels of MHC class II, CD32 (FcγRII), CD206 (mannose receptor) and CD14 were lower on FcγR activated cells. In comparison to mature DCs, HLA-DR, CD80, CD86 (Figure 1d) and CD83 (data not shown) expression was lower in the hIgG- and rGM-CSF-differentiated cells. Together, these data suggest that DC derived by activation with hIgG vs. rGM-CSF are immature and have distinct phenotypes.
FcγR activation by hIgG induces GM-CSF transcription in monocytes
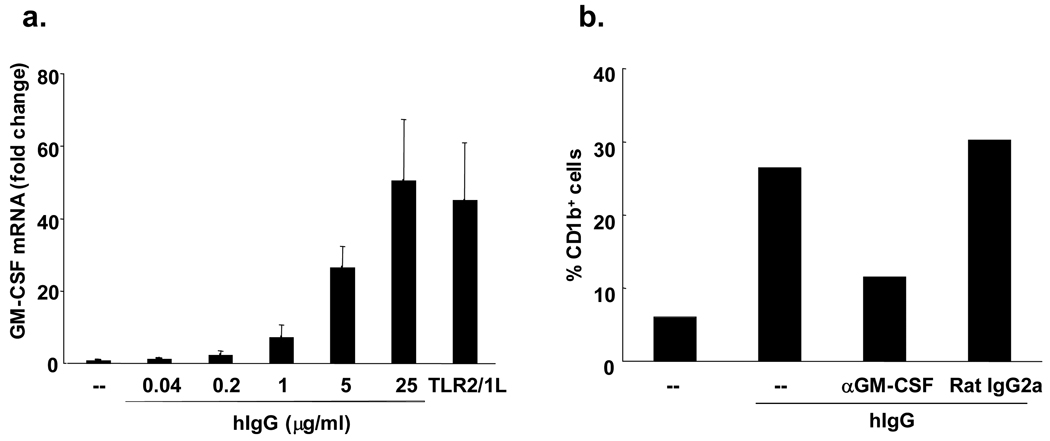
To assess whether GM-CSF mediates the FcγR induction of CD1b on monocytes, we first evaluated whether FcγR activation could trigger the induction of GM-CSF mRNA. Peripheral blood monocytes were stimulated with hIgG and levels of GM-CSF mRNA were determined by qPCR. hIgG induced GM-CSF mRNA in a dose-dependent manner and to levels similar to that seen with TLR2/1L activation (50-fold v 45-fold) (Figure 2a). FcγR stimulated monocytes also secreted the pro-inflammatory cytokines TNF-α, IL-6 and IL-1β, whereas treatment of monocytes with rGM-CSF did not (data not shown).
Figure 2. FcγR activation by hIgG induces GM-CSF transcription in monocytes.
(a) Monocytes were stimulated with hIgG (0.04 –25 µg/ml) or TLR 2/1 ligand (10 µg/ml) for three hours. Cells were harvested and qPCR was performed. Fold changes of GM-CSF mRNA are shown +/− SEM from three independent experiments. (b) Monocytes were stimulated with hIgG (0.2 µg/ml) in the presence or absence of anti-GM-CSF mAb or Rat IgG2a as an isotype control. Two days later, the percent of CD1b+ cells was measured by flow cytometry. Data are representative of two independent experiments.
In order to determine whether GM-CSF mediated the induction of CD1b on FcγR activated monocytes, we stimulated monocytes with hIgG in the presence or absence of a GM-CSF blocking antibody or isotype control. Monocytes stimulated with hIgG in the presence of the anti-GM-CSF blocking antibody expressed approximately 60% lower levels of CD1b compared to monocytes cultured with isotype control or hIgG alone (Figure 2b). These data demonstrate that activation of monocytes by hIgG triggers the induction of GM-CSF, required for optimal differentiation into CD1b+ iDC.
Surface expression of FcγRs on peripheral blood monocyte populations
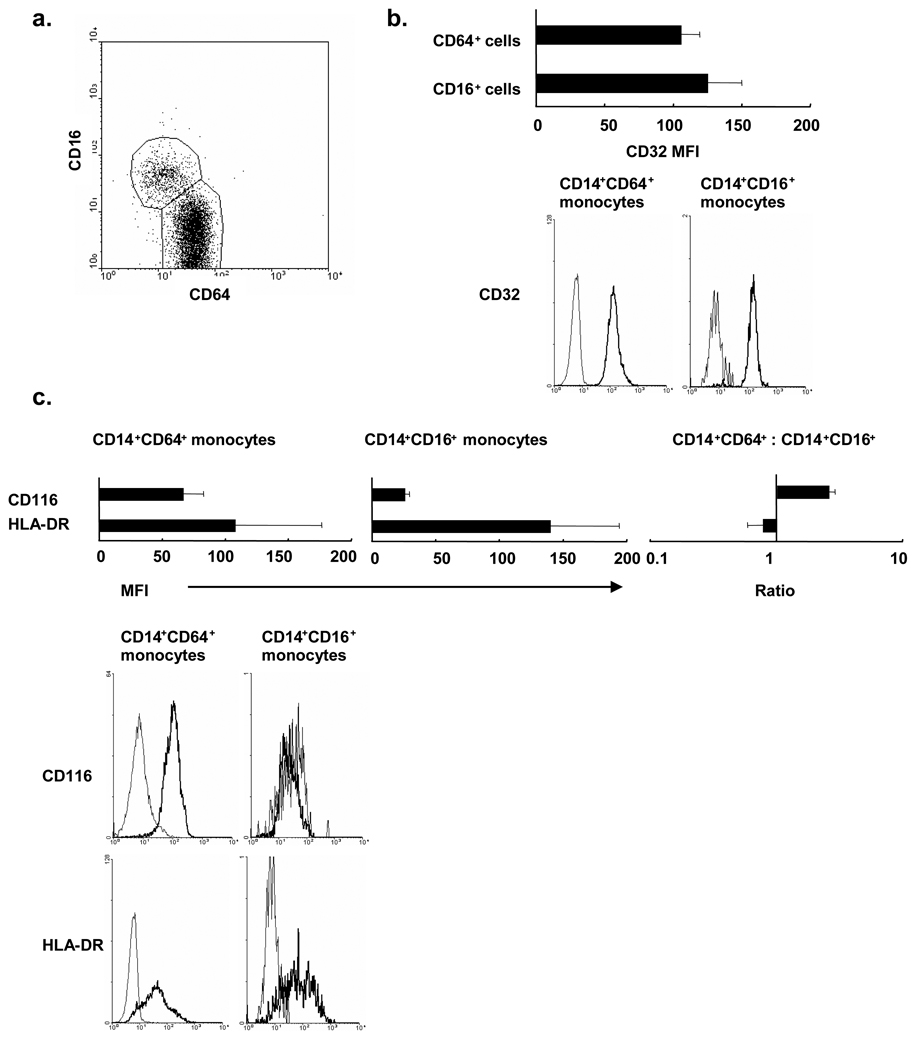
Previous studies have indicated that human peripheral blood monocytes consist of 2 major subsets: CD14dimCD16+CD64 monocytes and CD14bright CD16−CD64+monocytes. In order to evaluate which subsets of monocytes are the precursors of CD1b cells, we examined surface expression level of FcγRs, HLA-DR and GM-CSF receptor. Peripheral blood monocytes from healthy donors were labeled with CD14, CD16 and CD64 concurrently with CD32, CD116 (alpha chain of GM-CSF receptor), and various DC markers. As described previously, CD14+ monocytes were separated into the CD16+CD64− subset, a relatively small population, and the CD16−CD64+ subset, a relatively larger population (Figure 3a). Both subsets expressed comparable level of CD32 and HLA-DR (Figure 3b). Interestingly, the CD16− CD64+ subset expressed higher levels of CD116 compared to CD16+CD64− subset (Figure 3c). CD1a and CD1b expression was not detected in either subset. MHC class I and co-stimulatory molecules (CD40, CD80 and CD86) were comparably expressed (data not shown). Given the higher expression level of GM-CSF receptors, these data suggest that CD16−CD64+ subset of blood monocytes are more likely the CD1b+ cell precursors.
Figure 3. FcγR expression on human peripheral monocyte subsets.
(a) Peripheral human monocytes were labeled with monoclonal antibodies specific for CD14, CD16, and CD64. The dot plot represents CD16 and CD64 expression by CD14 positive monocytes. (b, c) CD14+CD16+ subset and CD14+CD64+ subset were labeled with CD32, HLA-DR or CD116. Data are shown as mean fluorescence intensity (MFI) of three independent experiments +/− s.e.m. (left and center) in addition to representative plots from a single donor.
hIgG triggers differentiation via CD64
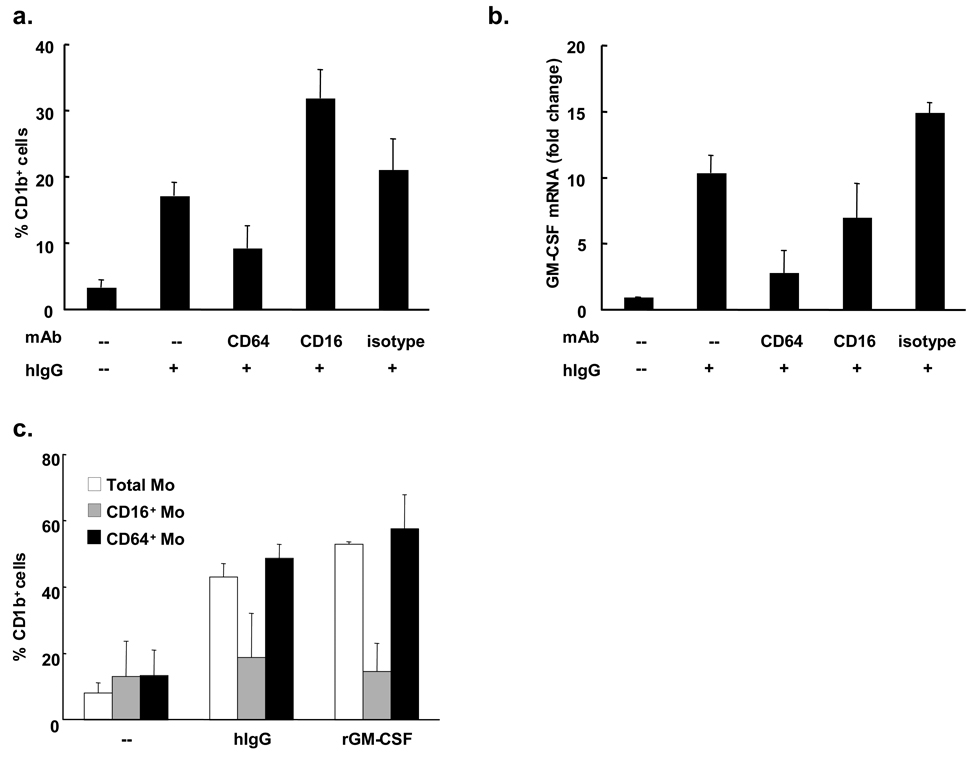
To further determine which subset of human peripheral blood monocytes is the likely precursor of CD1b+ iDC, mAbs which block the interaction of FcγRs and Fc portion of IgGs were utilized. At first, the effects of anti-FcγRs blocking mAbs on the CD1b surface expression levels were studied. Monocytes were stimulated with hIgG in the presence or absence of anti-FcγRs mAbs or isotype control. After two days, hIgG stimulated monocytes cultured with anti-CD64 mAb expressed 46% lower levels of CD1b while an anti-CD16 mAb or isotype control antibody did not inhibit CD1b expression (Figure 4a).
Figure 4. hIgG-triggered differentiation of CD1b+ cells is dependent on CD64.
(a) Peripheral monocytes were stimulated with hIgG (0.2 µg/ml) in the presence or absence of the indicated blocking monoclonal antibodies (10 µg/ml) or isotype control. After two days cells were labeled with anti-CD1b monoclonal antibody and flow cytometry was performed (n=2). (b) Monocytes were stimulated with hIgG (25 µg/ml) in presence or absence of indicated blocking monoclonal antibodies (10 µg/ml) or isotype control. RNA was collected after three hours. Levels of GM-CSF mRNA were measured by qPCR (n=2). (c) Monocytes were separated into CD16+ and CD16− populations using anti-CD16 mAb conjugated magnetic beads. CD16+, CD16− or total monocytes were stimulated with hIgG (25 µg/ml) or recombinant GM-CSF (0.2 U/ml) for two days. The percent of CD1b+ cells was determined using flow cytometry (n=2).
Secondly, the effect of blocking CD64 was investigated in terms of GM-CSF mRNA expression. FcγR stimulation and CD64 blocking were performed as described above. After three hours of culture with hIgG, monocyte GM-CSF mRNA expression was inhibited by both the anti-CD64 mAb (73%) and anti-CD16 mAb (32%) but not the isotype control (Figure 4b). Although the anti-CD32 mAb also suppressed both GM-CSF mRNA and CD1b+ expression following FcγR activation, the extent of blocking was less than CD64 blocking (data not shown).
To determine whether the CD16−CD64+ subset of CD14+ monocytes was the precursor population for CD1b induction, magnetically separated monocyte subsets were stimulated with hIgG. Activation by either hIgG or rGM-CSF induced CD1b expression on both peripheral blood monocytes and the CD16−CD64+ subset, whereas induction of CD1b expression on the CD16+CD64− was similar to the unstimulated control (Figure 4c). In conclusion, hIgG activates the CD64 FcγR on CD16−CD64+ monocytes, leading to the upregulation of GM-CSF, and subsequent differentiation into CD1b+ iDC.
FcγR-differentiated CD1b+ immature dendritic cells activate T cells
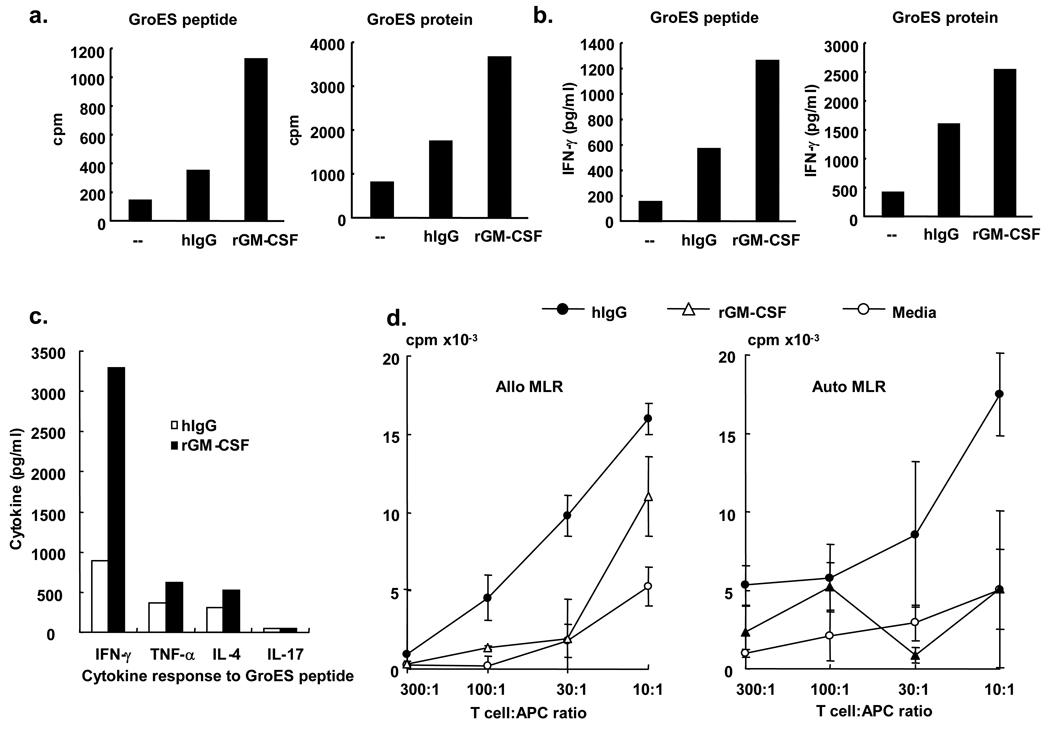
Given the iDC-like phenotype of both FcγR activated and rGM-CSF induced CD1b+ cells, we investigated the capacity of these CD1b+ cells to activate T cells. The ability of iDC derived by FcγR activation vs. rGM-CSF were tested for their ability to present antigen to a MHC class II-restricted T cell clone 9-14B GroES, which recognize a peptide from the Mycobacterium leprae 10kDa GroES. Both CD1b+ iDC populations triggered T cell proliferation and cytokine production, including IFN-γ, TNF-α, and IL-4, however the iDC derived by culture with rGM-CSF were more potent for antigen presentation to an MHC class II-restricted T cell clone (Figure 5a–c). In contrast, in the mixed-lymphocyte reaction to measure allogeneic responses, FcγR activated CD1b+ iDC were more potent than rGM-CSF induced CD1b+ iDC, inducing between about two- and five- fold greater T cell proliferation across a range of T cell/antigen-presenting cell ratios (Figure 5d). In addition, the iDC populations were evaluated for their ability to activate an autologous MLR by adding different concentrations of T cells from the same donor as the iDC. Again, the iDC derived from FcRγ activation were more potent than those derived using rGM-CSF in activating autologous MLR (Figure 5d). Differences in the magnitude of the auto-MLR vs. the allo-MLR may be related to the difference sources of APC and T cells. These data suggest that iDC derived from activation via FcRγ vs. GM-CSF are functionally distinct, with the FcRγ differentiated iDC more potent in triggering allogeneic/autologous T cell responses.
Figure 5. FcγR-differentiated CD1b+ immature dendritic cells activate T cells.
For antigen presentation studies, monocytes were differentiated with either media, hIgG or GM-CSF as above. Cells were cultured with MHC class II-restricted T-cells (1 × 104) and the GroES protein or GroES peptide. We then measured T cell proliferation (a) and cytokine production (b and c). Data are reported from triplicate values and are representative of two independent experiments. (d) For the mixed-lymphocyte reaction, hIgG and rGM-CSF differentiated CD1b+ cells were obtained as above. We obtained T cells from an unmatched donor for the allogeneic MLR or from the identical donor for autologous MLRs using Rosette Sep T cell enrichment cocktail (StemCell Technologies). The T cell:APC ratio varied between 10:1 to 300:1. Proliferation was measured by the thymidine incorporation assay. Data are reported as triplicate values (mean± sem) and are representative of three independent experiments.
Discussion
The ability of immune complexes to trigger FcRs on cells of the innate immune system results in inflammatory responses that contributes to tissue injury in disease. In this study, we demonstrated that FcγR activation of human peripheral blood monocytes induces differentiation into CD1b+ iDC. The mechanism of iDC differentiation was dependent on CD64 (FcRγI) and upregulation of GM-CSF. A precursor monocyte population was identified as a CD14+CD64+ subset, expressing high levels of GM-CSF receptor, and could be separately activated by hIgG to differentiate into iDC. The iDC induced by FcγR activation vs. GM-CSF were phenotypically and functionally distinct: FcγR-activated iDC were more efficient at activating autoreactive and allogeneic T cells as compared to GM-CSF-derived iDC, whereas the latter were more efficient at presenting microbial antigens.
The ability of the innate immune response to instruct the adaptive immune response is largely initiated and modulated by DC (16). Previous studies have focused on the regulation of DC differentiation and maturation by individual and combinations of cytokines (14). In the process of immune complex-related autoimmune disease or inflammation, DCs also play important roles (17,18). However, the direct relationships and mechanisms between FcγR cross-linking and differentiation of monocytes into DC have not been well understood, and has largely been investigated in terms of regulating cytokine-derived DCs. The present study provides evidence that FcγR cross-linking alone triggers differentiation of monocytes into iDC by, and these iDC stimulate autologous T cell proliferation. Our findings suggest that the presence of immune complexes may be sufficient for initiating autoimmune manifestations in immune complex disease. Further studies using immune complexes isolated from serum are required for additional insight into the pathogenesis of these diseases.
Although the combination of GM-CSF and IL-4 result in the generation of potent monocyte-derived DC that are useful for immunotherapy, we have established a model of immature DC by culture with GM-CSF alone (12,15). The morphology, cell surface phenotype and enhanced antigen presenting function of the GM-CSF stimulated monocytes show that these cells are consistent with iDC previously demonstrated (12,19) and correlate with the cell surface phenotype observed in vivo in human lymphoid tissue and leprosy skin lesion (12). In this study, we demonstrated that the GM-CSF is essential for CD1b+ iDC differentiation from FcγR-activated monocytes by showing that FcγR activated monocytes up-regulated GM-CSF and addition of anti-GM-CSF monoclonal Ab suppressed CD1b+ DC differentiation. GM-CSF is thought to participate in autoimmune disease, with mRNA and protein detected in inflamed tissues of autoimmune disease patients (20). However, the relative potencies of FcγR activation vs. GM-CSF in inducing CD1b expression were different. The rGM-CSF requirement to induce equivalent expression level of CD1b molecule with maximum FcγR activation (25 µg/ml in coating solution) was one-fifth to one-50th of that previously used (0.2 U/ml). Similarly, the GM-CSF concentrations observed in normal human serums or synovial fluids are very low or undetectable (21). Even in synovial fluids from RA patients GM-CSF concentration measured by ELISA were much lower than the 0.2 U/ml (equivalent of about 35 ng/ml of GM-CSF) (20). In addition, the two iDC populations express different phenotypes, and importantly functional capacities, with the FcγR-differentiated iDC more efficient at presentation to autologous and allogeneic T cells. These data indicate that although FcγR-induced iDC differentiation is GM-CSF dependent on GM-CSF, additional signaling or cytokine pathways must contribute to their differentiation into a unique subset of iDC.
The contribution of FcγRs in the pathogenesis of autoimmune diseases have been studied in the context of reciprocal regulation of immune responses via the pair of CD32a, which has an ITAM-motif in its cytoplasmic domain, and CD32b, which has an ITIM-motif (22,23). The role of CD64 (FcγR) in immune complex and autoimmune disease is less well understand, even though it has the highest affinity for IgG, 10–100-fold higher as compared to CD32 and CD16 (18). Here, we demonstrate that CD64 is the major FcγR contributing to CD1b+ iDC differentiation by hIgG, by finding that anti-CD64 blocking monoclonal Abs inhibited differentiation. Moreover, the magnetically selected CD64+CD14+ subpopulation of naive peripheral monocytes differentiated into CD1b+ iDC not the CD16+CD14+ monocytes, suggesting that CD14+CD64+ monocytes are the precursor of CD1b+ DC. The higher levels of GM-CSF receptor in CD64+CD14+ monocytes support this idea. Although the expression levels of CD32 were considerably higher than CD64, the effect observed by blocking CD32 was always of minor relevance compared with anti-CD64. The effect of FcγRI activation on differentiation may be specific to the stage of development. A recent report indicated that CD64 cross-linking during monocyte stimulation by GM-CSF and IL-4, resulted in monocyte differentiation into DC with less differentiated phenotypes and impaired APC functions compare to the cytokine combination alone (14). In conclusion, the ability of FcγR-activated naive monocytes to differentiate into CD1b+ iDCs with the potential to promote autoreactive T cell responses provides a possible mechanism of immune complex related autoimmune diseases and inflammation.
Acknowledgments
We thank the University of California-Los Angeles Flow Cytometry Core Laboratory for use of their facilities.
This work was supported in part by grants from the Heiser Program for Research in Leprosy and Tuberculosis of the New York Community Trust, the Japan Antibiotics Research Association Pfizer Infectious Diseases Fund 2005, and the National Institutes of Health (AR40312, A122553)
References
- 1.Lorenz HM, Herrmann M, Kalden JR. The pathogenesis of autoimmune diseases. Scand.J.Clin.Lab Invest Suppl. 2001;235:16–26. doi: 10.1080/003655101753352004. [DOI] [PubMed] [Google Scholar]
- 2.Schifferli JA, Taylor RP. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989;35:993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt RE, Gessner JE. Fc receptors and their interaction with complement in autoimmunity. Immunol.Lett. 2005;100:56–67. doi: 10.1016/j.imlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Ravetch JV, Bolland S. IgG Fc receptors. Annu.Rev.Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 5.Berger S, Ballo H, Stutte HJ. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes: a network of pro- and anti-inflammatory cytokines dependent on the antigen:antibody ratio. Eur.J.Immunol. 1996;26:1297–1301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- 6.Marsh CB, Gadek JE, Kindt GC, Moore SA, Wewers MD. Monocyte Fc gamma receptor cross-linking induces IL-8 production. J.Immunol. 1995;155:3161–3167. [PubMed] [Google Scholar]
- 7.Debets JM, Van de Winkel JG, Ceuppens JL, Dieteren IE, Buurman WA. Cross-linking of both Fc gamma RI and Fc gamma RII induces secretion of tumor necrosis factor by human monocytes, requiring high affinity Fc-Fc gamma R interactions. Functional activation of Fc gamma RII by treatment with proteases or neuraminidase. J.Immunol. 1990;144:1304–1310. [PubMed] [Google Scholar]
- 8.Krutmann J, Kirnbauer R, Kock A, Schwarz T, Schopf E, May LT, Sehgal PB, Luger TA. Cross-linking Fc receptors on monocytes triggers IL-6 production. Role in anti-CD3-induced T cell activation. J.Immunol. 1990;145:1337–1342. [PubMed] [Google Scholar]
- 9.Herrmann F, De VS, Brach M, Riedel D, Lindemann A, Mertelsmann R. Secretion of granulocyte-macrophage colony-stimulating factor by human blood monocytes is stimulated by engagement of Fc gamma receptors type I by solid-phase immunoglobulins requiring high-affinity Fc-Fc gamma receptor type I interactions. Eur.J.Immunol. 1992;22:1681–1685. doi: 10.1002/eji.1830220703. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu.Rev.Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 11.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 12.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat.Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sieling PA, Jullien D, Dahlem M, Tedder TF, Rea TH, Modlin RL, Porcelli SA. CD1 expression by dendritic cells in human leprosy lesions: Correlation with effective host immunity. J Immunol. 1999;162:1851–1858. [PubMed] [Google Scholar]
- 14.Laborde EA, Vanzulli S, Beigier-Bompadre M, Isturiz MA, Ruggiero RA, Fourcade MG, Catalan Pellet AC, Sozzani S, Vulcano M. Immune complexes inhibit differentiation, maturation, and function of human monocyte-derived dendritic cells. J.Immunol. 2007;179:673–681. doi: 10.4049/jimmunol.179.1.673. [DOI] [PubMed] [Google Scholar]
- 15.Lee DJ, Sieling PA, Ochoa MT, Krutzik SR, Guo B, Hernandez M, Rea TH, Cheng G, Colonna M, Modlin RL. LILRA2 activation inhibits dendritic cell differentiation and antigen presentation to T cells. J Immunol. 2007;179:8128–8136. doi: 10.4049/jimmunol.179.12.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 17.Wenink MH, van den Berg WB, van Riel PL, Radstake TR. Fc gamma receptor mediated modulation of dendritic cells as a potential strategy in the battle against rheumatoid arthritis. Neth.J.Med. 2006;64:103–108. [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat.Rev.Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell AL, Magill MK, McKane WR, Kirk F, Irvine AE. Measurement of colony-stimulating factors in synovial fluid: potential clinical value. Rheumatol.Int. 1995;14:177–182. doi: 10.1007/BF00262295. [DOI] [PubMed] [Google Scholar]
- 21.Abdelaal MA, Hashim IA, Zawawi TH, Felimban SK, Sobhi EM, Jeje O, Oni GA. Circadian rhythm of granulocyte-macrophage colony-stimulating factor in normal subjects and neutropenic hospitalised patients. Ir.J.Med.Sci. 2000;169:55–57. doi: 10.1007/BF03170487. [DOI] [PubMed] [Google Scholar]
- 22.Banki Z, Kacani L, Mullauer B, Wilflingseder D, Obermoser G, Niederegger H, Schennach H, Sprinzl GM, Sepp N, Erdei A, Dierich MP, Stoiber H. Cross-linking of CD32 induces maturation of human monocyte-derived dendritic cells via NF-kappa B signaling pathway. J.Immunol. 2003;170:3963–3970. doi: 10.4049/jimmunol.170.8.3963. [DOI] [PubMed] [Google Scholar]
- 23.Su K, Yang H, Li X, Li X, Gibson AW, Cafardi JM, Zhou T, Edberg JC, Kimberly RP. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J.Immunol. 2007;178:3272–3280. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckman EM, Melian A, Behar SM, Sieling PA, Chatterjee D, Furlong ST, Matsumoto R, Rosat JP, Modlin RL, Porcelli SA. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J.Immunol. 1996;157:2795–2803. [PubMed] [Google Scholar]
- 25.Kim J, Sette A, Rodda S, Southwood S, Sieling PA, Mehra V, Ohmen JD, Oliveros JL, Appella E, Higashimoto Y, Rea TH, Bloom BR, Modlin RL. Determinants of T cell reactivity to the Mycobacterium leprae GroES homologue. J.Immunol. 1997;159:335–343. [PubMed] [Google Scholar]
- 26.Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via TLR2. J Immunol. 2000;166:2444–2450. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 27.Behar SM, Porcelli SA, Beckman EM, Brenner MB. A pathway of costimulation that prevents anergy in CD28- T cells: B7-independent costimulation of CD1-restricted T cells. J.Exp.Med. 1995;182:2007–2018. doi: 10.1084/jem.182.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Soriano T, Brenner MB, Kronenberg M, Brennan PJ, Modlin RL. CD1-restricted T cell recognition of microbial lipoglycans. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]