Abstract
We present a rapid and highly efficient method to form microstructure of poly(ethylene glycol) (PEG)-based acrylates by microwave-induced thermal crosslinking. PEG-based polymeric microstructures such as polymer microarrays and microwells were fabricated on 3-(trimethoxysilyl)propyl methacrylate (TMSPMA)-coated glass slides that were placed on top of a silicon wafer. In comparison to ultraviolet (UV) irradiation curing, microwave-induced thermal crosslinking could be completed within 10 s, without thermal degradation or oxygen inhibition in the presence of ambient oxygen. Furthermore, the activation of surviving free radical impurities by microwave-induced heating enabled crosslinking even without an exogenous radical initiator (e.g., 2,2′-azoisobutyronitrile (AIBN)). This approach can be beneficial for fabricating various PEG-based microstructures for high-throughput screening assays, cell-based biosensors, and biomedical microdevices.
Keywords: crosslinking, FT-IR, high-throughput screening, sensors, synthesis
Introduction
Polymeric microstructures play an important role in various biomedical applications such as drug delivery,[1] tissue engineering[2] and cell-based high-throughput screening.[3] In particular, poly(ethylene glycol) (PEG)-based polymers have been widely used for the synthesis of various polymeric microstructures due to their resistance to protein and cell adhesion, non-toxicity, non-immunogenicity, and blood compatibility.[4] In most cases, structurally diverse PEG-based acrylates have been crosslinked by using ultraviolet (UV)-irradiated free radical polymerization processes.[5] Free radical photopolymerization for curing PEG-based acrylates in air is often influenced by oxygen inhibition,[6] which causes undercuring, tacky surface properties, short polymer kinetic chain length, and slow polymerization rates.[7] To overcome the oxygen inhibition, there have been attempts to consume oxygen by using high photoinitiator concentrations and light intensities, as well as by using reactors containing inert gases;[8] however, efficient reduction of oxygen inhibition still remains a challenge.
An alternative to photocrosslinking is controlled/living radical polymerizations, including atom transfer radical polymerization (ATRP), nitroxide-mediated polymerization (NMP), and reversible addition-fragmentation chain transfer (RAFT) polymerization.[9] While these controlled mechanisms have recently attracted the attention of many research groups, there is concern about the drawbacks of these technologies, such as metal impurities and slow polymerization rates.
Microwave heating has been previously used in drug discovery,[10] organic and polymer chemistry,[11] micro-fluidic [12] and tissue engineering. In particular, microwave-assisted free radical polymerization has been widely exploited to accelerate free radical polymerization process.[13] The main advantages of this approach are short reaction times, high yields and less side reactions. Despite these advantages, the use of microwave heating has not been adapted for patterning of polymers in comparison to other methods such as nitrogen-blanketed UV-irradiated polymerization,[8] redox-initiated polymerization[14] and plasma polymerization.[15] Therefore, the development of a technique with reduction of oxygen inhibition and rapid crosslinking may be beneficial in the synthesis of micro-patterned polymeric structures.
In early studies, household domestic microwave ovens were often used for organic synthesis using solvent. Although research grade microwave reactors are more reliable and reproducible, modified household microwave ovens are still used in various laboratories for free radical polymerizations due to the relatively high cost of the microwave reactor. To overcome the limitations of using household microwave ovens, solvent-free systems[16] have been used for microwave-assisted organic synthesis to enable a clean, efficient and economical reaction.[17] Moreover, a dry reaction technique avoids solvents with low boiling points, making the use of a household microwave oven safe.[18] Furthermore, since the microwave irradiation activates a semiconductor,[19] it can be used to rapidly heat a silicon wafer up to 1 000 °C (125 °C ·s−1) to induce rapid crosslinking of the polymer.[20]
Results and Discussion
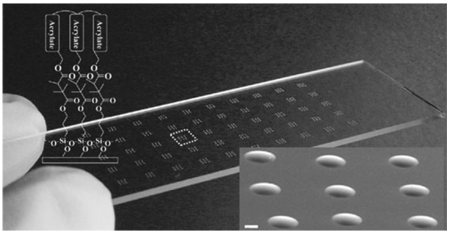
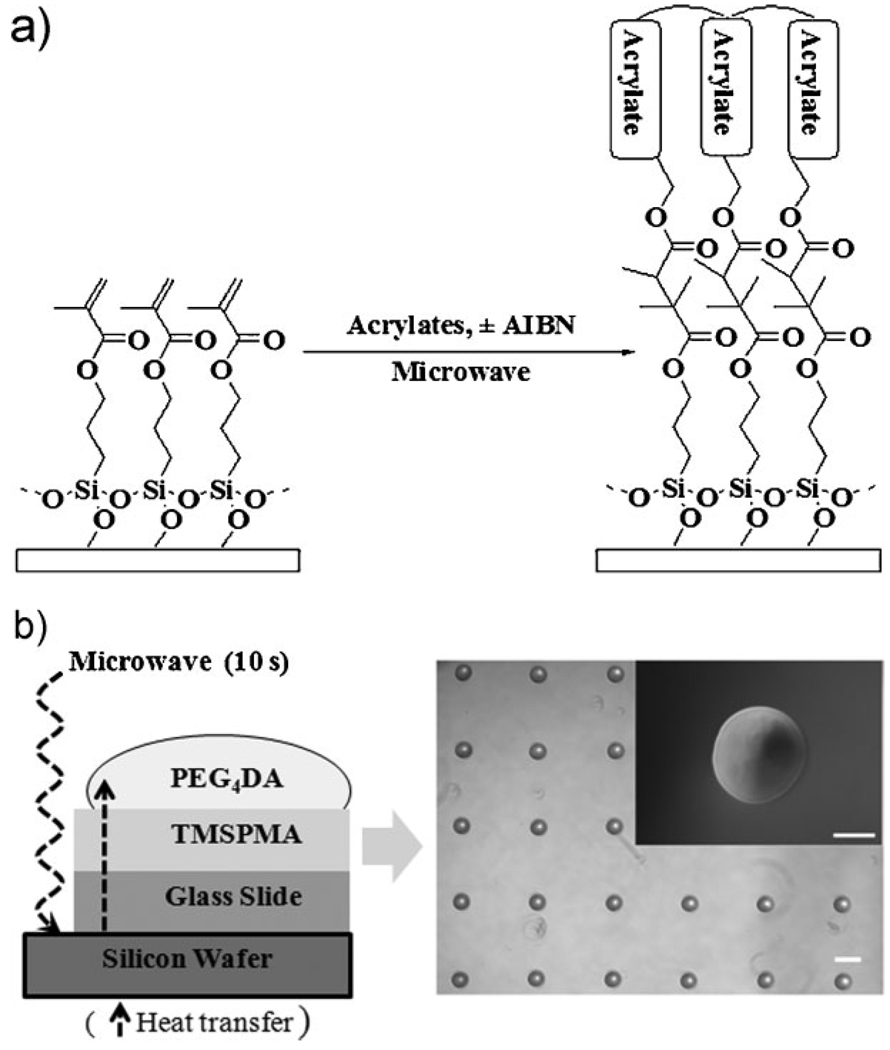
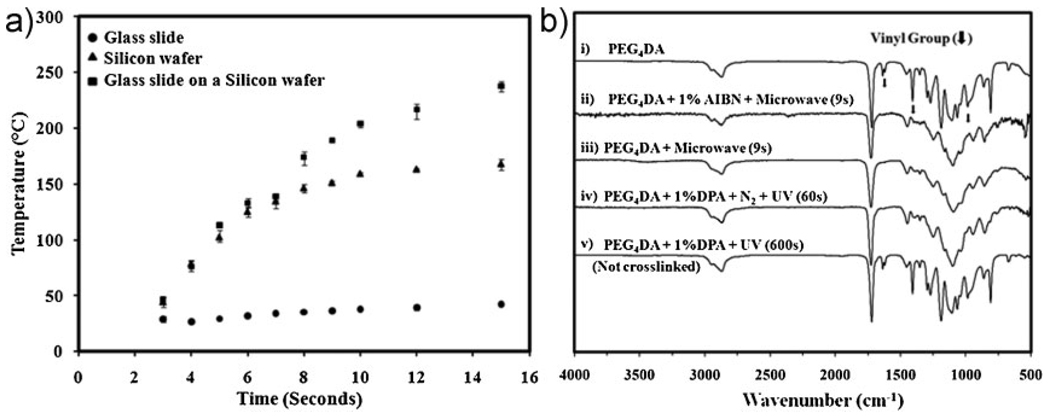
In this paper, we developed an approach to crosslink acrylated polymers to generate microstructures by using microwave-induced heating. This was achieved by using the scheme shown in Figure 1. Crosslinked poly(ethylene glycol)4 diacrylate (PEG4DA) microspots were fabricated on 3-(trimethoxysilyl)propyl methacrylate (TMSPMA)-coated glass slides that were placed on top of a silicon wafer by using microwave-induced thermal crosslinking with 1% 2,2′-azosisobutyronitrile (AIBN) for 10 s in the presence of ambient oxygen (Figure 1 and also see the Supporting Information (S.I.) Experimental). Microscopic and scanning electron microscope (SEM) images show that microwave-induced heating can form homogeneous microspots (Figure 1(b) and see S.I. Figure S1). To enhance the energy transfer from microwaves to the acrylated monomers, the substrate was placed on a silicon wafer. As shown in the microwave-induced heating profiles in Figure 2(a), glass slides on the top of the silicon wafer were heated up to 204 °C within 10 s in comparison to glass slides (38 °C in 10 s) without the silicon wafer. The glass slide on silicon wafer became warmer than the neat silicon wafer due to a loss of heat from neat silicon wafer in air during measurement of heat. Thus, as the microwave irradiation rapidly heats the silicon wafer,[20] heat is transferred from silicon wafer to glass slide. Moreover, a rotating plate of a microwave oven allowed samples to heat in a uniform and homogeneous fashion. During microwave-induced heating, PEG4DA crosslinking was observed between 7 s (139 °C) and 10 s (204 °C). In contrast, the acrylated precursors on glass slides without the silicon wafer were not completely polymerized, presumably due to lower temperatures. Interestingly, the time required to crosslink PEG4DA using conventional thermal heating (190 °C) was longer (30 s) (see S.I. Figure S2), suggesting that heat transfer may be enhanced in microwave-induced heating.
Figure 1.
Polymerization of acrylate by using microwave-induced heating. (a) Crosslinking of acrylate by microwave-induced heating. (b) Scheme of microwave-induced heating technique and phase contrast image of a crosslinked PEG4DA after microwave-induced heating with 1% AIBN for 10 s in the presence of ambient oxygen. Scale bars are 100 µm (top inset) and 300 µm.
Figure 2.
(a) Microwave-induced heating profiles of a glass slide (circle), a silicon wafer (triangle), and a glass slide on a silicon wafer (rectangular), respectively. (b) FTIR spectra before and after crosslinking: (i) Uncrosslinked PEG4DA monomer before microwave-induced heating; (ii) Crosslinked PEG4DA polymer after microwave-induced heating with 1% AIBN for 9 s; (iii) Crosslinked PEG4DA under microwave-induced heating without 1% AIBN for 9 s; (iv) Crosslinked PEG4DA under UV irradiation (20 mW · cm−2) with 1% DPA for 60 s in the presence of nitrogen; (v) Uncrosslinked PEG4DA under UV irradiation with 1% DPA for 600 s in the presence of ambient oxygen.
In comparison to the microwave-induced crosslinking technique, polymerization of PEG4DA containing 1% (w/v) 2,2-dimethoxy-2-phenyl acetophenone(DPA)photoinitiator was incomplete under UV irradiation (20mW · cm−2), even after 600 s of exposure. This may be due to the oxygen inhibition effect (see S.I. Figure S3) that oxygen retards the polymerization reaction as it reacts with the free radicals that propagate the reaction.[6] Therefore, it may be that the microwave-induced heating can generate significantly more free radicals that overcome the oxygen inhibitory effect. This may also eliminate the need for inert gases (e.g., argon and nitrogen gases) and increase the reaction speeds.
To assess the chemical properties of the crosslinked microstructures, Fourier transform infrared (FT-IR) spectroscopy spectra of microwave- and UV-irradiated crosslinked polymers were analyzed. As shown in Figure 2(b), the FT-IR spectrum of uncrosslinked PEG4DA monomer (Figure 2(b) (i)) before microwave-induced heating was significantly different than the crosslinked PEG4DA macromer structures (Figure 2(b) (ii)). The spectrum of the uncrosslinked PEG4DA monomer shows acrylic vinyl group peaks at 1 619cm−1 (C=C), 1 407cm−1 (=CH2), and 984 cm−1 (Figure 2(b) (i)).[21] These characteristic vinyl group peaks disappear in the spectra of the crosslinked PEG4DA (Figure 2(b) (ii)). Moreover, the shift of the carbonyl group from 1 720 cm−1 (C=O of monomer) to 1 727 cm−1 (C=O of polymer) is shown in Figure 2(b) (i)and 2(b) (ii), respectively. The disappearance of the vinyl group and shift of the carbonyl group implied that PEG4DA monomers were crosslinked by using microwave-induced heating with 1% AIBN for 9 s in the presence of ambient oxygen. Similarly, as shown in Figure 2(b) (iii), the spectrum of the PEG4DA crosslinked by using microwave-induced heating without 1% AIBN also does not have the characteristic peaks. Both of the crosslinked polymers’ spectra show an ester functional group at 1 724 cm−1 (C=O stretching), 1 248 cm−1 (C–O stretching), 1 162 cm−1 (C–C stretching) and an ether functional group between 1 096 and 1 046 cm−1 (C–O–C asymmetric and C–O–C symmetric stretching).[22] These bands strongly indicate that the microwave-induced polymerization of PEG4DA monomer took place under ambient oxygen without thermal degradation of ester and ether functionalities. The spectrum of the PEG4DA crosslinked with 1% DPA by using UV irradiation curing under nitrogen atmosphere for 60 s is shown in Figure 2(b) (iv), further supporting that microwave-induced heating could be used to crosslink PEG4DA polymer under ambient oxygen. However, the same UV-irradiated polymerization was incomplete under ambient oxygen for 600 s (Figure 2(b) (v)). Therefore, in comparison to UV-induced crosslinking, the crosslinking of PEG4DA monomer in the presence of ambient oxygen by using the microwave-induced heating technique is highly efficient.
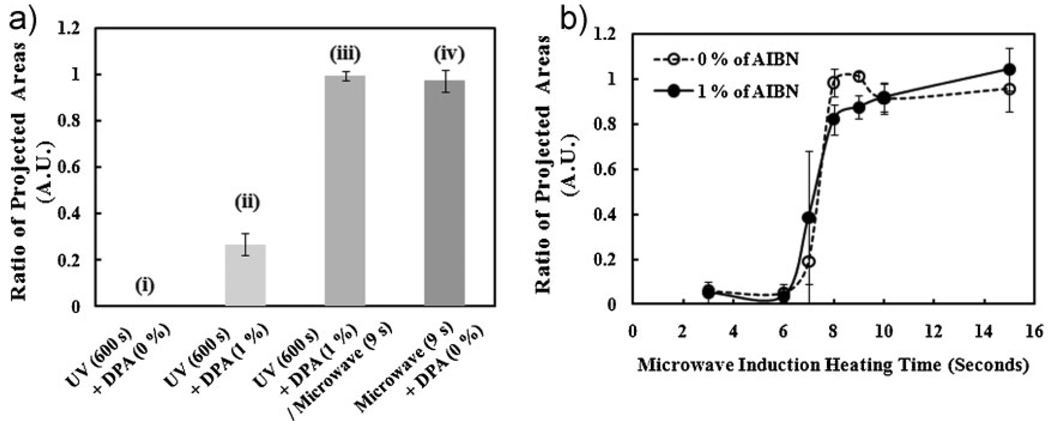
To directly compare the microwave-induced crosslinking with the UV-irradiated crosslinking, polymerizations were conducted under four different experimental conditions: (i) UV irradiation curing without DPA photoinitiator for 600 s; (ii) UV irradiation curing with 1% DPA for 600 s; (iii) UV irradiation curing with 1% DPA for 600 s and then microwave-induced crosslinking for 9 s; (iv) microwave-induced crosslinking for 9 s (Figure 3(a)). We selected these conditions to compare and quantify UV-irradiated and microwave-induced polymerization with and without the addition of exogenous DPA. To further compare the crosslinking methods, we used a method to quantify the amount of polymer that remained on the substrate after polymerization and washing steps. This was determined by using the ratio of the projected microstructure areas (RPA) which can be defined as the ratio of the area of a printed polymer spot (PA0) to the area of the crosslinked polymer spot (PA1) after washing (see S.I. Figure S4(a)). This ratio was calculated by quantifying the phase contrast images using the Image J software (see S.I. Figure S4(b)).[23] As shown in Figure 3(a) (i) and Figure 3(a) (ii), UV-induced crosslinking required the addition of a free radical source. On the other hand, crosslinking under microwave irradiation rapidly progressed without an additional radical source at atmospheric condition. Furthermore, the time required for crosslinking was significantly reduced. Interestingly, we found that a removal of the radical initiator did not inhibit the crosslinking reaction, providing an additional benefit for the use of microwave-assisted crosslinking.
Figure 3.
Polymerization of PEG4DA under four different crosslinking conditions: (i) UV irradiation (20 mW · cm−2) without DPA for 600 s; (ii) UV irradiation with 1% DPA for 600 s; (iii) UV irradiation with 1% DPA for 600 s and then exposure to microwaves for 9 s; (iv) microwave-induced heating for 9 s. (a) Quantification of ratio of the projected areas. (b) Analysis of ratio of projected areas of PEG4DA crosslinked by microwave-induced heating with and without 1% AIBN.
To further characterize the ability of the crosslinking reaction without an exogenous source of initiator, we characterized the microwave-induced crosslinking of PEG4DA with and without 1% w/v AIBN thermal initiator (Figure 3(b)). Recently, an initiator-free controlled polymerization under microwave irradiation was shown for polymerization of methyl methacrylate (MMA) at high temperatures.[24] In our experiments, both polymerizations with and without AIBN under microwave irradiation were completed within 10 s. This result suggests that PEG4DA monomer stocks may contain impurities which initiate the reaction. In general, conventional PEGDAs and PEGDMAs are stored with various retarders such as hydroquinone (HQ), hydroquinone monomethyl ether (MEHQ), and butylated hydroxytoluene (BHT).[25] This is because increase of propagation rate by free radicals would result in unwanted polymerization.[26] As shown in Figure 3(a), we compared polymerisation performances between microwave-induced thermal curing and UV irradiation curing using the PEG4DA monomer. In our experiments, we found that the PEG4DA monomer were not polymerized during UV irradiation in air. In contrast, the microwave-induced heating induced rapid polymerization under the same condition (Figure 3(a) (i) and (iv)). These results indicate that microwave-induced crosslinking can be used to form microstructures without an additional radical source in the presence of radical impurities and retarders in air. Interestingly, the degree of polymerizations were similar for both cases, suggesting that the microwave-assisted polymerization was carried out regardless of the AIBN thermal initiator for a low volume associated with each printed polymer spot. The mechanism of the microwave-assisted PEGDA thermal crosslinking may be due to the thermal polymerization initiated by free radical species such as the radical impurities, peroxides, and oxygen plasma.
To further analyze this mechanism as well as to verify the reproducibility of the microwave-induced polymerization, we fabricated microwell structures by using a micromolding technique. First, we created microwells of PEG4DA with and without AIBN (see S.I. Figure S5). The polymerizations with and without AIBN could be used to fabricate PEG4DA microwells in 12 s and 9 s, respectively. Therefore, the polymerization rate for creating microwell structures was enhanced by radical flux from the decomposition of AIBN. These results imply that for a smaller reaction volume, e.g., a spoton a microarray, an addition of AIBN will not enhance polymerization rate, while for larger reaction volumes an addition of AIBN will enhance the reaction rate.
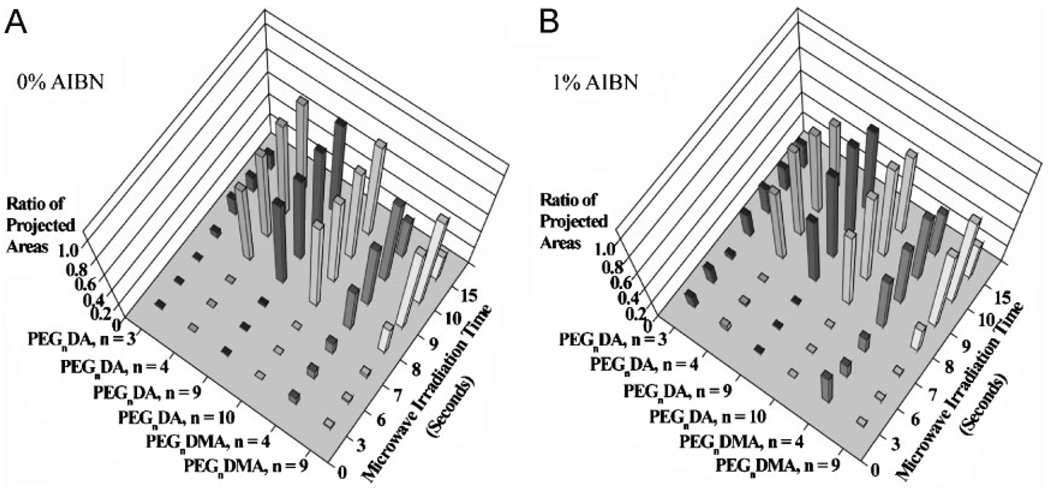
To validate that microwave-induced crosslinking can be used with a wide range of acrylated precursors, we crosslinked microarrays containing a range of PEG-based diacrylates and dimethacrylates, using the microwave-induced heating technique (Figure 4 and see S.I. Figure S6). All the acrylate monomers with and without 1% AIBN were consecutively printed by a robotic dispenser on the TMSPMA-coated glass slides, followed by microwave-induced crosslinking and quantitative analysis of the ratio of the projected areas. All acrylate monomers with and without 1% AIBN started to be cured in 6 s and then were completely crosslinked in 9 s. We observed that after the printing, the area of the printed spots of PEG4DA was larger than that of PEG10DA. This is due to different levels of hydrophobicity of the acrylated monomers. Spot size can be controlled by adjusting hydrophobicity. On the other hand, the area of the polymerized PEG3DA spot was reduced compared to that of the printed spot. This instability is caused by evaporation or shrinkage of the acrylate polymer under microwave irradiation.[8] Similar trends were also observed at larger exposure times. Therefore, the polymerization was optimized in 9 s for minimizing instability. To precisely manipulate the polymerization in a controlled manner,[27] future work will focus on synthesis of polymer microarrays using a microwave instrument containing an individual power controller and single-mode microwave reactor.
Figure 4.
Analysis of crosslinked PEG-based acrylate polymers by using microwave-induced heating. Ratio of projected areas for various PEGnDA (n = 3, 4, 9, 10) and PEGnDMA (n = 4, 9) microarrays with (a) 0% and (b) 1% AIBN.
Conclusion
In this paper, we developed a microwave-induced crosslinking technique that enabled rapid and highly efficient polymerization of PEG-based acrylate monomers in the presence ofambient oxygen. In comparison to conventional UV-irradiated polymerization and thermal curing techniques, there are three main advantages of polymerization using this microwave-induced heating: (i) a short reaction time (<10 s); (ii) no need for an additional radical source (e.g., AIBN); (iii) minimal oxygen inhibition in the presence of ambient oxygen. Furthermore, the results of FTIR spectra and the ratio of projected areas showed that the microwave-induced crosslinking did not cause thermal degradation. The results also imply that this technique is potentially beneficial to directly synthesize homogeneous PEG-based polymer arrays and microwells without prepolymerizations, solvents, and exogenous initiators. Therefore, this approach can be useful for various chemical and biological applications such as high-throughput screening of polymer libraries, cell-based biosensors, and biomedical microdevices.
Supplementary Material
Acknowledgements
This paper was supported by the National Institutes of Health (EB007249; DE019024; HL092836), the US Army Core of Engineers and the Charles Stark Draper Laboratory. S. H. Lee was partially supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-357-C00063).
Footnotes
Supporting information for this article is available at the bottom of the article’s abstract page, which can be accessed from the journal’s homepage at http://www.mrc-journal.de, or from the author.
Contributor Information
Seung Hwan Lee, Center for Biomedical Engineering, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Won Gu Lee, Center for Biomedical Engineering, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Bong Geun Chung, Center for Biomedical Engineering, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Jae Hong Park, Center for Biomedical Engineering, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Ali Khademhosseini, Center for Biomedical Engineering, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
References
- 1.Grayson ACR, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Nature Mater. 2003;2:767. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 2.Peters A, Brey DM, Burdick JA. Tissue Eng. 2009 doi: 10.1089/ten.TEB.2009.0049. doi:10.1089/ten.TEB.2009.0049. [DOI] [PubMed] [Google Scholar]
- 3.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Proc. Natl. Acad. Sci. USA. 2006;103:2480. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirelli N, Lutolf MP, Napoli A, Hubbell JA. Rev. Mol. Biotech. 2002;90:3. doi: 10.1016/s1389-0352(01)00057-5. [DOI] [PubMed] [Google Scholar]
- 5.Nie ZZ, Kumacheva E. Nature Mater. 2008;7:277. doi: 10.1038/nmat2109. [DOI] [PubMed] [Google Scholar]
- 6.Decker C. Macromol. Rapid Commun. 2002;23:1067. [Google Scholar]
- 7.Jeong HE, Kwak R, Kim JK, Suh KY. Small. 2008;4:1913. doi: 10.1002/smll.200800151. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DG, Levenberg S, Langer R. Nature Biotech. 2004;22:863. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 9.Zetterlund PB, Kagawa Y, Okubo M. Chem. Rev. 2008;108:3747. doi: 10.1021/cr800242x. [DOI] [PubMed] [Google Scholar]
- 10.Kappe CO, Dallinger D. Nature Rev. Drug Disc. 2006;5:51. doi: 10.1038/nrd1926. [DOI] [PubMed] [Google Scholar]
- 11.Hoogenboom R, Schubert US. Macromol. Rapid Commun. 2007;28:368. [Google Scholar]
- 12.Issadore D, Humphry KJ, Brown KA, Sandberg L, Weitz D, Westervelt RM. Lab Chip. 2009 doi: 10.1039/b822357b. DOI: 10.1039/b822357b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dallinger D, Kappe CO. Chem. Rev. 2007;107:2563. doi: 10.1021/cr0509410. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Liberski A, Khan F, Diaz-Mochon J, Bradley M. Chem. Commun. 2008:1317. doi: 10.1039/b717932d. [DOI] [PubMed] [Google Scholar]
- 15.Bouaidat S, Berendsen C, Thomsen P, Petersen SG, Wolff A, Jonsmann J. Lab Chip. 2004;4:632. doi: 10.1039/b406285j. [DOI] [PubMed] [Google Scholar]
- 16.Varma RS. Green Chem. 1999:43. [Google Scholar]
- 17.Lew A, Krutzik PO, Hart ME, Chamberlin AR. J. Comb. Chem. 2002;4:95. doi: 10.1021/cc010048o. [DOI] [PubMed] [Google Scholar]
- 18.Loupy A, Petit A, Hamelin J, Texier-Boullet F, Jacquault P, Mathe D. Synthesis. 1998:1213. [Google Scholar]
- 19.Kniep R. Angew. Chem., Int. Ed. 1993;32:1411. [Google Scholar]
- 20.Thompson K, Booske JH, Gianchandani YB, Cooper RF. IEEE Elec. Dev. Lett. 2002;23:127. [Google Scholar]
- 21.Zhao Z, Li Z, Xia Q, Xi H, Lin Y. Eur. Poly. J. 2008;44:1217. [Google Scholar]
- 22.Pfluger CA, Carrier RL, Sun B, Ziemer KS, Burkey DD. Macromol. Rapid Commun. 2009;30:126. doi: 10.1002/marc.200800647. [DOI] [PubMed] [Google Scholar]
- 23.Sezgin M, Sankur B. J. Electr. Imaging. 2004;13:146. [Google Scholar]
- 24.Paulus RM, Becer CR, Hoogenboom R, Schubert US. Aust. J. Chem. 2009;62:254. [Google Scholar]
- 25.Cutie SS, Henton DE, Powell C, Reim RE, Smith PB, Staples TL. J. Appl. Poly. Sci. 1997:64. [Google Scholar]
- 26.Nathan A, Bolikal D, Vyavahare N, Zalipsky S, Kohn J. Macromolecules. 1992;25:4476. [Google Scholar]
- 27.Zhang H, Hoogenboom R, Meier M, Schubert U. Meas. Sci. Technol. 2005;16:203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.