Abstract
The spatial resolution of conventional transcranial direct current stimulation (tDCS) is considered to be relatively diffuse owing to skull dispersion. However, here we show that electric fields may be clustered at distinct gyri/sulci sites due to details in tissue architecture/conductivity notably cerebrospinal fluid (CSF). We calculated the cortical electric field/current density magnitude induced during tDCS using a high spatial resolution (1 mm3) MRI-derived finite element human head model; cortical gyri/sulci were resolved. The spatial focality of conventional rectangular-pad (7 × 5 cm2) and the ring (4 × 1) electrode configurations were compared. The rectangular-pad configuration resulted in diffuse (un-focal) modulation, with discrete clusters of electric field magnitude maxima. Peak induced electric field magnitude was not observed directly underneath the pads, but at an intermediate lobe. The 4 × 1 ring resulted in enhanced spatial focality, with peak induced electric field magnitude at the sulcus and adjacent gyri directly underneath the active electrode. Cortical structures may be focally targeted using ring configurations. Anatomically accurate high resolution MRI-based forward-models may guide the ‘rational’ clinical design and optimization of tDCS.
Keywords: tDCS, focality, Finite Element Modeling, MRI Human Head Model, TES
Introduction
Conventional transcranial direct current stimulation (tDCS) involves weak direct currents (260 µA – 2 mA) applied to the scalp via sponge-based rectangular pads (nominally 25 - 35 cm2) (1–4). tDCS modulates cortical function and has been applied to facilitate learning, alter behavioral performance, and improve impaired brain function (5–13).
A pivotal factor for tDCS efficacy and safety is the spatial extent of induced cortical electric fields/current density. tDCS is considered to be poorly focused using rectangular-pad electrode configurations (14–17). The spatial focality of induced cortical electric field (EF)/current densities has been proposed to increase using reduced electrode sizes (16–18), appropriate placement of electrodes (15,17,19), and ring electrode configurations (17). However, the precise role of complex tissue-compartment morphology in influencing the flow of currents during tDCS has not been systematically addressed, including potential discrete cortical “hotspots” (clustering) of induced electric fields (20–22).
During any transcranial current stimulation modality, the current which reaches the cortex is significantly altered from the applied scalp current due to intermediate tissue properties. A portion of the injected current is shunted across the scalp. The portion which crosses into the skull is then conducted by cerebrospinal fluid (CSF). Through the highly conductive CSF network, current can eventually cross into the brain. In the case of DC stimulation, induced cortical currents / electric fields have been shown to modulate the firing properties of neurons and ‘condition’ neuronal excitability (23–27). There is a general perception that the low conductivity of skull places a severe limit on the spatial focality of brain modulation.
One objective of this paper was to compare the focality of ‘conventional’ 7 × 5 cm2 rectangular-pad stimulation with the 4 × 1 ring electrode configuration using a high resolution MRI-based finite element model of the human head. Spherical-based (17,21) and MRI-derived (22,28) head models have previously been used to calculate tDCS electric fields. In this study, we incorporated gyri/sulci specificity by developing a model with 1 mm3 resolution. Induced cortical electric fields were used to predict relative spatial focality and the influence of tissue geometry/conductivity. We report that tDCS modulation maps are fundamentally influenced by detailed cortical architecture and consider limitations on transcranial stimulation focality
Methods
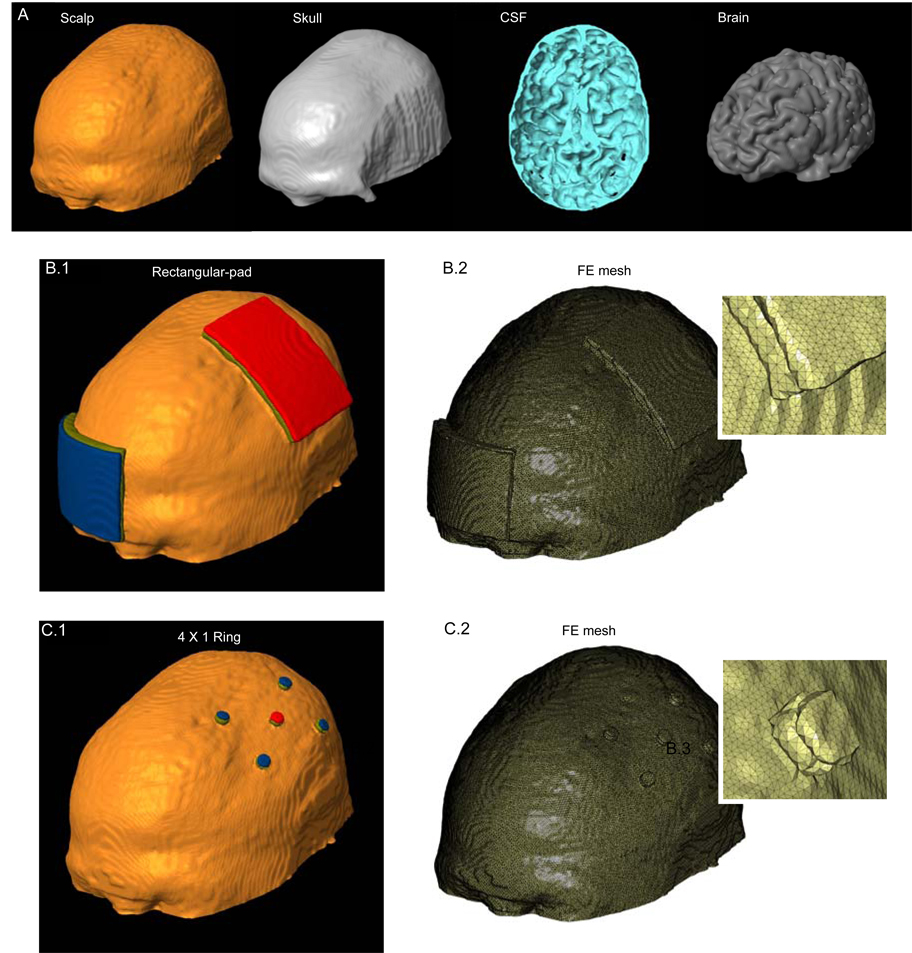
Volume conductor models were created with the same resolution (1 mm3) as the MRI data used to derive them. Raw 3T MRI scans were contrast enhanced and noise filtered. The head was segmented into compartments representing the brain tissue, cerebrospinal fluid (CSF), skull, muscle, fatty tissue, eyes, blood vessels and the scalp respectively (Figure 1 A; SIMPLEWARE Ltd., Exeter, UK). The stimulation rectangular pads and discs were imported as CAD models (see below). The volumetric mesh was generated (minimum quality factor > 0.5) from the segmented data and eventually exported to COMSOL Multiphysics 3.4 (COMSOL Inc., MA, USA). The resulting mesh comprised >10,000,000 tetrahedral elements (>15,000,000 degrees of freedom).
Figure 1.
Finite element (FE) model of the conventional 7 × 5 cm2 rectangular-pad and 4 × 1 ring configurations. (A) Segmented compartments in the following order: Scalp, Skull, CSF and Brain. (B.1) FE model of the conventional rectangular-pad configuration and corresponding FE mesh (B.2). (C.1) FE model of the 4 × 1 ring electrode configuration and corresponding FE mesh (C.2). The two insets show the zoomed mesh images, highlighting finer detail. ‘Red’: Anode electrode; ‘Blue’: Cathode electrode(s); ‘Olive green’: sponge/gel.
The electrical properties of the tissues were assigned representative isotropic average values (in S/m): brain: 0.2; CSF: 1.65; skull: 0.01; scalp: 0.465 (29–36). The muscle, fatty tissue, eyes and blood vessel compartments were assigned the conductivity of scalp tissue.
We modeled two electrode configurations:
‘Rectangular-pad’ (Figure 1B): Two pads (7 × 5 cm2) were placed at sites commonly used for tDCS of the primary motor cortex, with the “active” (anode) electrode over the left motor cortex and the “return” (cathode) electrode at the forehead above the contralateral orbita. Typically, the rectangular sponges are soaked in saline for conventional tDCS application and the abutting electrode is energized. The sponge was therefore assigned the conductivity of saline: 1.4 S/m.
‘4 × 1 ring’ (Figure 1C): In order to practically implement the concentric-ring configuration (17), we approximated a ring using four “return” (cathode) disc electrodes arranged in a circular fashion around an “active” (anode) center electrode. The active electrode is placed over the motor cortex (coinciding with the center of the active pad used for rectangular-pad stimulation) and surrounded by four return electrodes (each at a disc center to disc center distance of 3 cm from the active electrode). The disc electrodes had a 4 mm radius. The 4 × 1 ring electrode system was implemented by passing current through disc electrodes into the scalp using a customized tDCS gel (CCNY-4) with conductivity: 0.3 S/m.
All electrodes had a thickness of ~ 1 mm and were modeled as conductors with the conductivity of copper: 5.8 × 107 S/m. The thickness of the CCNY-4 gel was ~ 2 mm while the thickness of the sponge varied from 1 to 2.5 mm (thickness changed with scalp curvature in order to maintain continuous contact).
The Laplace equation ▽.(σ▽V) = 0 (V: potential; σ: conductivity) was solved and the boundary conditions used were (1) inward current flow = Jn (normal current density) applied to the exposed surface of the anode electrode, (2) ground applied to the exposed surface of the cathode electrode(s) and (3) all other external surfaces treated as insulated. Current densities corresponding to 1 mA total current for the rectangular-pad configuration and 2 mA total current for the 4 × 1 ring configuration were respectively applied. These currents resulted in similar peak cortical induced EF magnitude for each of the configurations. The finite element method (FEM) model was implemented using COMSOL. The linear system solver of conjugate gradients was used with a relative tolerance of 1 × 10−6.
‘Surface-magnitude’ plots were generated by plotting the magnitude of EF on the surface of brain tissue. In addition, ‘Cross-section magnitude’ plots were generated by plotting the EF magnitude on coronal slices (17). Because the conductivity of brain is uniform, these same plots also represent induced current density profiles (where the actual current density values can be scaled using: J = σ.E).
In this study, the induced brain electric field magnitude was assumed to correlate with the degree of brain “modulation”. The consideration of the electric field (as opposed to the classical activating function) may be appropriate if: 1) tDCS induced electric fields are uniform at the scale of a neuron; and 2) neuronal modulation may be directly correlated with uniform electric field magnitude (17, 26, 37–41). Our electric field magnitude modulation maps do not consider any specific neuronal orientation (and target non-oriented structures) (17, 42).
Results
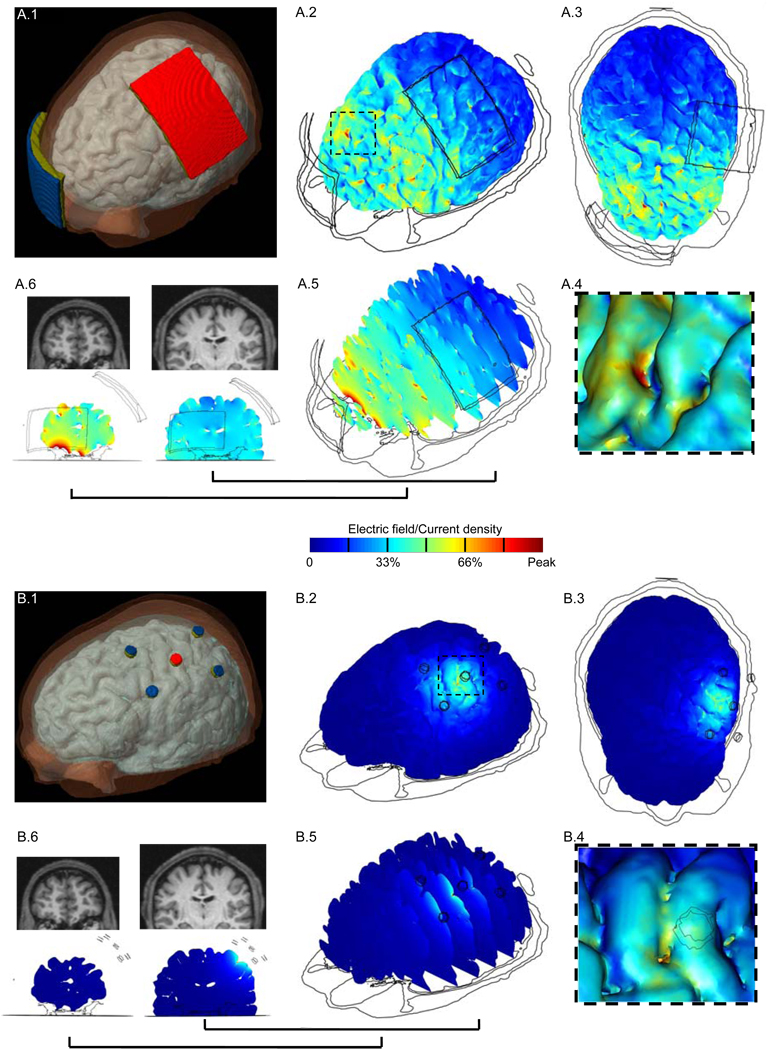
For the 7 × 5 cm2 rectangular-pad and the 4 × 1 ring configuration models (Figure 1B and Figure 1C), we calculated the induced electric field/current density magnitude in the brain. The surface-magnitude/cross-section magnitude EF plots for each of the configurations allow a direct comparison of relative cortical surface and depth focality (Figure 2).
Figure 2.
Brain modulation during tDCS using conventional rectangular-pad (A.1) and the 4 × 1 ring electrode configuration (B.1). ‘Red’: Anode electrode; ‘Blue’: Cathode electrode(s); ‘Olive green’: sponge/gel. For each configuration, we calculated the induced cortical electric field (EF) magnitude. (A.2, B.2) Surface-magnitude plots of EF along the brain surface, same view as (A.1, B.1). The dashed region is expanded in inset. (A.3, B.3) Top view of the brain showing the induced surface-magnitude EF. The insets (A.4) and (B.4) show the zoomed surface-magnitude EF plots. These images are obtained with “lighting” on [COMSOL Multiphysics] to highlight gyri/sulci modulation. (A.5, B.5) Cross-section magnitude EF plotted on a series of successive cortical slices, same view as (A.1, B.1). The cross-section magnitude EF plots for two slices and their corresponding MRI scans are shown in (A.6), (B.6). One slice is chosen directly from underneath the rectangular-pad/active electrode of 4 × 1 ring configuration and another from the prefrontal area of the brain. The same slices are shown for each of the configurations. All plots are normalized between zero and the peak positive cortical EF magnitude: conventional rectangular-pad peak = 0.67 V/m (or 0.13 A/m2 current density peak); 4 × 1 ring peak = 0.44 V/m. (Note that for the rectangular-pad, the EF magnitude peak is observed between the pads and not underneath).
Rectangular-pad stimulation with 7 × 5 cm2 pads results in widespread diffuse (un-focal) modulation over the entire cortical surface owing to the large size and separation of pads (Figure 2A.2). 1 mA injected through the pads results in 0.67 V/m peak cortical EF magnitude (encompassed in dashed region of Figure 2A.2; expanded in Figure 2A.4) in the walls of a frontal lobe gyrus. The peak cortical EF magnitude of 0.67 V/m corresponds to 0.13 A/m2 peak cortical current density magnitude. The local EF magnitude peak directly underneath the pads was 0.45 V/m. The minimum cortical induced EF magnitude in the entire frontal area of the brain was ~ 0.16 V/m (see coronal slice, Figure 2A.6) and ~ 0.06 V/m in the occipital area.
The presence of distinct clusters of EF magnitude maxima (Figure 2A.2) was influenced by regions of reduced skull thickness, which may provide preferential current pathways of current crossing into the CSF (see also Supplementary Figure), as well as channels of high-conductivity CSF bounded by brain and skull. For example, a particular gyrus may have high induced EF magnitude due to wide pockets of CSF on either side acting as a current “sink” (36) as well as reduced CSF thickness over that gyri crown acting as a “funnel”.
The 4 × 1 ring configuration leads to a significant increase in spatial focality (Figure 2B.2). Peak electric field magnitude is observed in the gyri and the intermediate sulcus directly underneath the active stimulation electrode (see coronal slice, Figure 2B.6). The region of modulation is generally constrained between the active electrode and the four return electrodes with a 0.44 V/m EF magnitude peak (encompassed in dashed region of Figure 2B.2; expanded in Figure 2B.4). Thus, using the 4 × 1 ring configuration, 2 mA resulted in a comparable peak EF magnitude in the motor cortex as 1 mA rectangular-pad (7 × 5 cm2) stimulation. However, for the 4 × 1 ring configuration, there was no cortical modulation (< 0.01 V/m) in the frontal regions, on the contralateral (right) motor regions of the brain, or on the occipital lobe of the brain. The overall spatial profile of brain modulation was strongly influenced by tissue inhomogeneity, notably due to CSF (Supplementary Figure).
Discussion
The translation of stimulation models to clinical applications requires reproducing application-appropriate anatomical features (43–50). The incorporation of gyri/ sulci specificity in our 3D human head model can guide rational tDCS design and optimization (42, 51).
The overall current flow due to any transcranial electric stimulation is complex and is influenced by a convergence of factors including: 1) electrode size/geometry and separation-distance, with related scalp shunting (17); 2) skull thickness, presence of sutures and eye cavities; 3) channels of high conductivity CSF enclosing/and perfusing the underlying cortex; 4) convoluted brain surface morphology; and 5) differences in tissue conductivities at boundaries between tissue compartments.
tDCS using conventional ‘large’ rectangular pads resulted in diffuse (un-focal) cortical modulation. Moreover, the complex geometry of the brain and regional differences in conductance cause local non-uniformities of current density through the CSF (e.g. “sinks” and “funnels”) that are reflected in clustering of brain electric field magnitude at distinct sites. Indeed, some imaging and physiological studies suggest diffuse brain modulation and clustering of regional cerebral blood flow/EF, independent of anatomical connections (20,52). Separate imaging, TMS mapping studies and, clinical studies indicate some level of functional spatial selectivity (1, 5, 7–9); which may be explained by FE models with more specific modulation maps (17) including non-linearities and thresholding.
Whereas our electric-field magnitude modulation maps assume no particular neuronal geometry or waveform dependent biophysical transduction mechanisms, direction-specific modulation maps incorporating cellular orientation (radial versus tangential) (17) and electric field derivative (classical activating function) (53,54) have been applied elsewhere (43,44,47). In cases of pulsed or AC stimulation waveforms, the appropriate modulation map (parameter/function that determines degree of brain “modulation”) may differ (20, 55, 56).
Any FE human head model is limited by the accuracy of tissue dimensions and conductivity values incorporated (inhomogeneity and anisotropy). The present study investigated the distribution of tDCS induced currents in brain using a highly detailed anatomical model. The high MRI scan resolution (1 mm3) allows accurate segmentation of individual tissue compartments. Consequently the precise 3D model rendered, captures anatomical detail such as cortical folding (Figure 2.A.4). Finally the precise FE mesh generated (> 10 million elements), allows accurate computation of induced fields. The importance of incorporating gyri/sulci specificity is highlighted by the observance of clustering of brain modulation during conventional tDCS (see also Supplementary Figure). Our results also suggest that individual variability would affect the magnitude and spatial extent of cortical electric fields. For example, young children have vascularized fontanels, there are differences in the degree/timing of suture closing amongst adults (36), and elderly subjects have larger fractional CSF volumes (57).
Our results indicate that the focality of clinical tDCS application can be significantly enhanced by the 4 × 1 ring configuration. Additionally, since the peak induced cortical electric field magnitude is similar to the rectangular-pad stimulation; the 4 × 1 ring results in more targeted brain modulation (hence potentially, a safer electrode configuration). The cortical current density of 0.09 A/m2 induced underneath the pads by rectangular pad (1 mA) and 4 × 1 ring (2 mA) configurations is more than two orders of magnitude away from the threshold for histopathologically observed tDCS brain damage in a rat model (58). We emphasize that using the 4 × 1 ring configuration, the more surface current needed does not lead to more peak induced cortical EF magnitude, but reflects shunting across the scalp (without crossing into the brain).
For skin safety, the increased scalp current associated with 4 × 1 ring can be offset by increasing the separation distance between stimulation electrodes, but at the cost of stimulation focality (17). Importantly when using conventional sponge electrodes, the current density at the scalp is in fact concentrated at the sponge edges and thus exceeds the average current density (injected current / electrode surface area) (21,22). Moreover, electrode materials and design are as pivotal to comfort as is average current density (59–61); using appropriate hardware (electrode adapters, stimulation gels), 39.8 A/m2 current density may be applied without pain (62). Because of the above issues, pain perception for skin should be addressed in a clinical study (63). Thus electrode designs that mitigate skin irritation should be developed along with electrode configurations that enhance spatial focality.
The quasi-static field approximation implies conservation and linearity of EF solution. Thus, our EF magnitude ‘spatial profile’ results can be extrapolated to other transcranial current stimulation modalities (e.g. suprathreshold transcranial electrical stimulation, electroconvulsive therapy, transcranial alternating current stimulation, transcranial random noise stimulation, and cranial electrotherapy stimulation) (20,55,56,64,65) where the 4 × 1 ring configuration may be used to focally target cortical structures.
The ‘transparency’ of the skull to magnetic stimulation has led to the development of specialized coils for focused transcranial stimulation (50,66–69). There is a general perception that the low conductivity of skull places a severe limit on spatial focality of electrical stimulation. The results of this modeling study support the further development of transcranial current stimulation technology for focal stimulation.
Supplementary Material
Influence of tissue properties on the spatial profiles of electric field/current density magnitudes induced by stimulation with rectangular-pad (A) and 4 × 1 ring (B) electrode configurations. First column: Simulation results with inhomogeneous electrical conductivities. Second column: Simulation results using the same head model but with σCSF = σbrain = 0.2 S/m. Third column: Simulation results with homogeneous tissue conductivity (σ = 0.2 S/m). Rows A.1 and B.1: Cortical surface-magnitude electric fields. Rows A.2 and B.2: Induced current density magnitude in both CSF and brain on a single cross sectional slice (same slice as in Figure 2 of manuscript). Rows A.3 and B.3: Cortical Cross-section magnitude electric fields. For the rectangular-pad stimulation, sites of high current densities in CSF generally correspond with regions of high induced cortical electric field magnitudes which are reflected as distinct clusters of modulation (see A.2a and A.3a). Note that A.1a and B.1a are same as Figure 2A.2 and 2B.2 in the manuscript. When the brain and CSF compartments have same conductivity, the region of peak induced electric fields roughly encompasses the entire cortical surface between the pads (A.1b). In addition, distinct clustering of electric fields is not observed owing to similar electrical properties and absence of convoluted cortical morphology (A.2b and A.3b). The cortical surface comprising the peak induced EF magnitudes demonstrates regions of reduced skull thickness (A.1b). Two distinct peaks of modulation beneath the two corresponding rectangular pads are observed when homogeneous conductivities are considered (A.1c). For the 4 × 1 ring configuration, the region of peak cortical electric field magnitude corresponds to sites of high induced current densities in CSF (B.3a and B.2a) similar to rectangular-pad simulation. For the homogeneous case and when the brain and CSF compartments have same conductivity, region of peak induced electric fields roughly encompasses the cortical surface between the active and return electrodes (B.1b and B.1c). Note that for the cross-sectional plots (Rows: A.2,A.3,B.2,B.3) the maximum has been scaled for clarity as indicated.
Acknowledgements
Ross Cotton of SIMPLEWARE Ltd. and Konrad Juethner of COMSOL Inc. The authors also wish to thank Pejman Sehatpour at Nathan Kline Institute; Thomas Radman, Fortunato Battaglia, and Lucas Parra of the City College of New York. This work was supported by NIH (41341-23, 41595-00), Andy Grove Foundation, and PSC-CUNY grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall L, Molle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neurosci. 2005;6:23. doi: 10.1186/1471-2202-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer MB, Mattu U, Graffman J, et al. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 4.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 5.Nitsche MA, Schauenburg A, Lang N, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 6.Uy J, Ridding MC. Increased cortical excitability induced by transcranial DC and peripheral nerve stimulation. J Meurosci Methods. 2003;127:193–197. doi: 10.1016/s0165-0270(03)00142-0. [DOI] [PubMed] [Google Scholar]
- 7.Kincses TZ, Antal A, Nitsche MA, Bartfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia. 2004;42:113–117. doi: 10.1016/s0028-3932(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 8.Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci. 2004;45(2):702–707. doi: 10.1167/iovs.03-0688. [DOI] [PubMed] [Google Scholar]
- 9.Boggio PS, Bermpohl F, Vergara AO, et al. Go-no-go task performance improvement after anodal transcranial DC stimulation of the left dorsolateral prefrontal cortex in major depression. J Affect Disord. 2006;101:91–98. doi: 10.1016/j.jad.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Liebetanz D, Klinker F, Hering D, et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47:1216–1224. doi: 10.1111/j.1528-1167.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 11.Boggio PS, Ferrucci R, Rigonatti SP, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249:31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 12.Fregni F, Boggio PS, Lima MC, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Boggio PS, Khoury LP, Martins DC, et al. Temporal cortex DC stimulation enhances performance on a visual recognition memory task in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2008 doi: 10.1136/jnnp.2007.141853. Epub. [DOI] [PubMed] [Google Scholar]
- 14.Rossini PM, Marciani MG, Caamia M, Roma V, Zarola F. Nervous propagation along ‘central’ motor pathways in intact man: characteristics of motor responses to ‘bifocal’ and ‘unifocal’ spine and scalp non-invasive stimulation. Electroencephalogr Clin Neurophysiol. 1985;61:272–286. doi: 10.1016/0013-4694(85)91094-6. [DOI] [PubMed] [Google Scholar]
- 15.Nathan SS, Sinha SR, Gordon B, Lesser RP, Thakor NV. Determination of current density distributions generated by electrical stimulation of the human cerebral cortex. Electroencephalogr Clin Neurophysiol. 1993;86:183–192. doi: 10.1016/0013-4694(93)90006-h. [DOI] [PubMed] [Google Scholar]
- 16.Nitsche MA, Doemkes S, Karakose T, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 17.Datta A, Elwassif M, Battaglia F, Bikson M. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng. 2008;5:163–174. doi: 10.1088/1741-2560/5/2/007. [DOI] [PubMed] [Google Scholar]
- 18.Kwon YH, Ko MH, Ahn SH, et al. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett. 2008;435:56–59. doi: 10.1016/j.neulet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Borckardt JJ, Linder KJ, Ricci R, et al. Focal electrically administered therapy: device parameter effects on stimulus perception in humans. J ECT. 2008 doi: 10.1097/YCT.0b013e318183c6a4. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai R, Chaieb L, Antal A, Walsh V, Paulus W. Frequency-dependent electrical stimulation of the visual cortex. Curr Biol. 2008;18(23):1839–1843. doi: 10.1016/j.cub.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117:1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Wagner T, Fregni F, Fecteau S, et al. Transcranial direct current stimulation: a computer based human model study. Neuroimage. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Purpura DP, McMurty JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- 24.Bindman LJ, Lippold OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gartside IB. Mechanisms of sustained increases of firing rate of neurons in the rat cerebral cortex after polarization: role of protein synthesis. Nature. 1968;220:383–384. doi: 10.1038/220383a0. [DOI] [PubMed] [Google Scholar]
- 26.Bikson M, Inoue M, Akiyama H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radman T, Su Y, An JH, Parra LC, Bikson M. Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J Neurosci. 2007;27:3030–3036. doi: 10.1523/JNEUROSCI.0095-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oostendorp TF, Hengeveld YA, Wolters CH, et al. Modeling Transcranial DC Stimulation; Conf Proc IEEE Eng Med Biol Soc; 20–25 Aug; 2008. pp. 4226–4229. [DOI] [PubMed] [Google Scholar]
- 29.Geddes LA, Baker LE. The specific resistance of biological material-a compendium of data for the biomedical engineer and physiologist. Med Biol Eng. 1967;5:271–293. doi: 10.1007/BF02474537. [DOI] [PubMed] [Google Scholar]
- 30.Akhtari M, Bryant HC, Mamelak AN, et al. Conductivities of a three-layer live human skull. Brain Topogr. 2002;14(3):151–167. doi: 10.1023/a:1014590923185. [DOI] [PubMed] [Google Scholar]
- 31.Ranck JB. Specific impedance of rabbit cerebral cortex. Exp Neurol. 1963;7:144–152. doi: 10.1016/s0014-4886(63)80005-9. [DOI] [PubMed] [Google Scholar]
- 32.Baumann SB, Wozny DR, Kelly SK, Meno FM. The electrical conductivity of human cerebrospinal fluid at body temperature. IEEE Trans Biomed Eng. 1997;44(3):220–223. doi: 10.1109/10.554770. [DOI] [PubMed] [Google Scholar]
- 33.Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol. 1996;41(11):2231–2249. doi: 10.1088/0031-9155/41/11/001. [DOI] [PubMed] [Google Scholar]
- 34.Hauesien J, Ramon C, Eiselt M, Brauer H, Nowak H. Influence of tissue resistivities on neuromagnetic fields and electric potentials studied with a finite element model of the head. IEEE Trans Biomed Eng. 1997;44(8):727–735. doi: 10.1109/10.605429. [DOI] [PubMed] [Google Scholar]
- 35.Nadeem M, Thorlin T, Gandhi OP, Persson M. Computation of electric and magnetic stimulation in human head using the 3-D impedance method. IEEE Trans Biomed Eng. 2003;50:900–907. doi: 10.1109/TBME.2003.813548. [DOI] [PubMed] [Google Scholar]
- 36.Holdefer RN, Sadleir R, Russell MJ. Predicted current densities in the brain using transcranial electrical stimulation. Clin Neurophysiol. 2006;117:1388–1397. doi: 10.1016/j.clinph.2006.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tranchina D, Nicholson C. A model for the polarization of neurons by extrinsically applied electric fields. J Biophys. 1986;50:1139–1156. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagarajan SS, Durand DM, Warman EN. Effects of induced electric fields on finite neuronal structures: a simulation study. IEEE Trans Biomed Eng. 1993;40:1175–1188. doi: 10.1109/10.245636. [DOI] [PubMed] [Google Scholar]
- 39.Amassian VE, Eberle L, Maccabee PJ, Cracco RQ. Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fibre bending in excitation. Electroencephalogr Clin Neurophysiol. 1992;85:291–301. doi: 10.1016/0168-5597(92)90105-k. [DOI] [PubMed] [Google Scholar]
- 40.Roth BJ. Mechanisms for electrical stimulation of excitable tissue. Crit Rev Biomed Eng. 1994;22:253–305. [PubMed] [Google Scholar]
- 41.Chan CY, Nicholson C. Modulation by applied electric fields of purkinje and stellate cell activity in the isolated turtle-cerebellum. J Physiol. 1986;371:89–114. doi: 10.1113/jphysiol.1986.sp015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bikson M, Bulow P, Stiller JW, et al. Transcranial direct current stimulation for major depression: a general system for quantifying transcranial electrotherapy dosage. Curr Treat Options Neurol. 2008;10(5):377–385. doi: 10.1007/s11940-008-0040-y. [DOI] [PubMed] [Google Scholar]
- 43.Johnson MD, McIntyre CC. Quantifying the neural elements activated and inhibited by global pallidus deep brain stimulation. 2008;100(5):2549–2563. doi: 10.1152/jn.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wongsarnpigoon A, Grill WM. Computational modeling of epidural cortical stimulation. J Neural Eng. 2008;5(4):443–454. doi: 10.1088/1741-2560/5/4/009. [DOI] [PubMed] [Google Scholar]
- 45.Im CH, Jung HH, Choi JD, Lee SY, Jung KY. Determination of optimal electrode positions for transcranial direct current stimulation (tDCS) Phys Med Biol. 2008;53:219–225. doi: 10.1088/0031-9155/53/11/N03. [DOI] [PubMed] [Google Scholar]
- 46.Manola L, Holsheimer J, Veltink P, Buitenweg JR. Anodal vs cathodal stimulation of motor cortex: a modeling study. Clin Neurophysiol. 2007;118:464–474. doi: 10.1016/j.clinph.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34(2):661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998;36(8):531–540. doi: 10.1038/sj.sc.3100717. [DOI] [PubMed] [Google Scholar]
- 49.De Lucia M, Parker GJM, Embleton K, Newton JM, Walsh V. Diffusion tensor MRI-based estimation of the influence of brain tissue anisotropy on the effects of transcranial magnetic stimulation. Neuroimage. 2007;36:1159–1170. doi: 10.1016/j.neuroimage.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 50.Deng ZD, Peterchev AV, Lisanby SH. Coil design considerations for deep-brain transcranial magnetic stimulation (dTMS); Conf Proc IEEE Eng Med Biol Soc; 20–25 Aug; 2008. pp. 5675–5679. [DOI] [PubMed] [Google Scholar]
- 51.Bikson M, Radman T, Datta A. Rational modulation of neuronal processing with applied electric fields; Conf Proc IEEE Eng Med Biol Soc; 2006. pp. 1616–1619. [DOI] [PubMed] [Google Scholar]
- 52.Lang N, Siebner HR, Ward NS, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rattay F. Analysis of models for external stimulation of axons. IEEE Trans Biomed Eng. 1986;33:974–977. doi: 10.1109/TBME.1986.325670. [DOI] [PubMed] [Google Scholar]
- 54.Plonsey R, Barr RC. Electric field stimulation of excitable tissue. IEEE Trans Biomed Eng. 1995;42:329–336. doi: 10.1109/10.376126. [DOI] [PubMed] [Google Scholar]
- 55.Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high frequency random noise stimulation. J Neurosci. 2008;28(53) doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lisanby SH. Electroconvulsive therapy for depression. N Eng J Med. 2007;357:1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- 57.Murphy DG, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B. Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol. 1992;49(8):839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- 58.Liebetanz D, Koch R, Mayenfels S, et al. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol. 2009 doi: 10.1016/j.clinph.2009.01.022. “In Press”. [DOI] [PubMed] [Google Scholar]
- 59.Dundas JE, Thickbroom GW, Mastaglia FL. Perception of comfort during transcranial direct current stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clin Neurophysiol. 2007;118:1166–1170. doi: 10.1016/j.clinph.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Palm U, Keeser D, Schiller C, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS) Brain Stimulation. 2008;1:386–387. doi: 10.1016/j.brs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 62.Prausnitz MR. The effects of electric current applied to skin: A review for transdermal drug delivery. Advanced Drug Delivery Reviews. 1996;18:395–425. [Google Scholar]
- 63.Arana AB, Borckardt JJ, Ricci R, et al. Focal electrical stimulation as a sham control for repetitive transcranial magnetic stimulation: does it truly mimic the cutaneous sensation and pain of active prefrontal repetitive transcranial magnetic stimulation? Brain Stimulation. 2008;1:44–51. doi: 10.1016/j.brs.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. ‘Threshold-level’ multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88:457–470. doi: 10.3171/jns.1998.88.3.0457. [DOI] [PubMed] [Google Scholar]
- 65.Schroeder MJ, Barr RE. Quantitative analysis of the electroencephalogram during cranial electrotherapy stimulation. Clin Neurophysiol. 2001;112:2075–2083. doi: 10.1016/s1388-2457(01)00657-5. [DOI] [PubMed] [Google Scholar]
- 66.Cohen LG, Roth BJ, Nilsson J, et al. Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalogr Clin Neurophysiol. 1990;75:350–357. doi: 10.1016/0013-4694(90)90113-x. [DOI] [PubMed] [Google Scholar]
- 67.Ren C, Tarjan PP, Popovic DB. A novel electric design for electromagnetic stimulation - The slinky coil. IEEE Trans Biomed Eng. 1995;42:918–925. doi: 10.1109/10.412658. [DOI] [PubMed] [Google Scholar]
- 68.Ruohonen J, Ilmoniemi RJ. Focusing and targeting brain stimulation using multiple coils. Med Biol Eng Comput. 1998;36:297–301. doi: 10.1007/BF02522474. [DOI] [PubMed] [Google Scholar]
- 69.Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. 2002;19:361–370. doi: 10.1097/00004691-200208000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Influence of tissue properties on the spatial profiles of electric field/current density magnitudes induced by stimulation with rectangular-pad (A) and 4 × 1 ring (B) electrode configurations. First column: Simulation results with inhomogeneous electrical conductivities. Second column: Simulation results using the same head model but with σCSF = σbrain = 0.2 S/m. Third column: Simulation results with homogeneous tissue conductivity (σ = 0.2 S/m). Rows A.1 and B.1: Cortical surface-magnitude electric fields. Rows A.2 and B.2: Induced current density magnitude in both CSF and brain on a single cross sectional slice (same slice as in Figure 2 of manuscript). Rows A.3 and B.3: Cortical Cross-section magnitude electric fields. For the rectangular-pad stimulation, sites of high current densities in CSF generally correspond with regions of high induced cortical electric field magnitudes which are reflected as distinct clusters of modulation (see A.2a and A.3a). Note that A.1a and B.1a are same as Figure 2A.2 and 2B.2 in the manuscript. When the brain and CSF compartments have same conductivity, the region of peak induced electric fields roughly encompasses the entire cortical surface between the pads (A.1b). In addition, distinct clustering of electric fields is not observed owing to similar electrical properties and absence of convoluted cortical morphology (A.2b and A.3b). The cortical surface comprising the peak induced EF magnitudes demonstrates regions of reduced skull thickness (A.1b). Two distinct peaks of modulation beneath the two corresponding rectangular pads are observed when homogeneous conductivities are considered (A.1c). For the 4 × 1 ring configuration, the region of peak cortical electric field magnitude corresponds to sites of high induced current densities in CSF (B.3a and B.2a) similar to rectangular-pad simulation. For the homogeneous case and when the brain and CSF compartments have same conductivity, region of peak induced electric fields roughly encompasses the cortical surface between the active and return electrodes (B.1b and B.1c). Note that for the cross-sectional plots (Rows: A.2,A.3,B.2,B.3) the maximum has been scaled for clarity as indicated.