Abstract
In vitro, morphogens such as transforming growth factor (TGF)-β can up-and down-regulate cell growth at low and high concentrations respectively, i.e. they behave like hormetic agents. The hormetic morphogen theory of curvature proposes that in vivo tissue gradients of such morphogens secreted by source cells determine the fate of cells within their gradient fields (field cells) and that morphogen-induced amplitude modulation of field cell mitochondrial adenosine triphosphate (ATP) generation controls field cell growth along the morphogen gradients: At the high concentration end of gradients, field cell ATP generation and field cell growth is reduced. With declining concentrations along the rest of the gradients field cell ATP and growth is progressively less reduced until an equidyne point is reached, beyond which ATP generation and growth gradually increases. Thus, the differential growth rates along the gradients curve the tissue. Apoptosis at very high morphogen concentrations enables lumen and cavity formation of tubular, spherical, cystic, domed, and other curved biological structures. The morphogen concentration, the gradient slope and the hormesis responses of field cells determine the curvature of such structures during developmental morphogenesis, tissue remodeling and repair of injury. Aberrant hormetic morphogen signaling is associated with developmental abnormalities, vascular diseases, and tumor formation.
Keywords: Curvature, morphogenesis, hormesis, hormetic morphogen, gradients, development, atherosclerosis, cancer
INTRODUCTION
Morphogens such as transforming growth factor beta (TGF-β) in mammals and auxin in plants can down- and up-regulate basic growth rates at high and low concentrations respectively, i.e. they can induce hormetic growth responses. The hormetic morphogen theory of curvature presented in this paper proposes that gradients of hormetic morphogens can 1) modulate production of adenosine triphosphate (ATP) by cells within their fields (field cells); 2) form curved structures such as vessel walls by inhibiting cell growth at high and stimulating cell growth at low morphogen concentrations; 3) induce formation of tubular lumens and cavities of cyst-like structures via apoptosis and cell disintegration at very high morphogen concentrations; 4) induce hormetic morphogenetic fields that determine the morphology of nodular, tubular, and cyst-like and other curved structures in biology. The theory also proposes that the morphogenetic effects can be derailed by inflammatory mediators. Thus, it suggests a link between chronic inflammation and the abnormal morphogenesis in atherogenesis and carcinogenesis.
Stated briefly, the theory proposes that development, maintenance, remodeling, and repair of curved biological structures occur via gene-to-phenotype cell positional information transfer in the form of morphogenetic fields induced by tissue hormetic morphogen concentration-gradients. The morphogens are released by morphogen source cells, and the morphogen gradients induce tissue curvature by modulating spatial and temporal growth of field cells via modulation of their ATP generation. Details of the theory, evidence in its support, and applications to developmental morphogenesis, developmental malformations and carcinogenesis are outlined below.
HORMETIC MORPHOGEN GRADIENTS
Morphogens of the TGF-beta (TGF-β) family and the Wingless and Hedgehog families can form concentration gradients in tissues (Kerszberg and Wolpert 1998; Mehlen et al. 2005). The TGF beta family members TGF-betas, bone morphogenetic proteins (BMPs), activin, and nodals, anti-Müllerian hormone and their receptors and the Wnt superfamilies and their receptors play important roles during mammalian developmental morphogenesis, tissue remodeling, and carcinogenesis (Jones et al. 1996; McDowell et al. 1997; Barker et al. 2000; Edlund et al. 2005 ; Orvis et al. 2008; Fosslien 2008; Wu and Hill 2009).
To explain the principles of the hormetic morphogen theory of curvature, this paper focuses on the morphogenetic effects of gradients of hormetic morphogens such as TGF-β and retinoic acid in mammals and auxin in plants. In vivo, TGF-β is often secreted in an inactive form and activated by secreted activators, whereas the active form is usually employed for in vitro studies of the effects of TGF-β.
In vitro, dose-dependent bimodal or biphasic effects of TGF-beta are characteristic of hormesis; a biological dose-response observed across scientific disciplines such as in toxicology, cancer biology, behavioral pharmacology, preconditioning, medicine, plant biology and in algae (Calabrese and Baldwin 1998; Calabrese 2005; Cedergreen et al. 2006; Calabrese 2008a, 2008b; Mattson 2008; Calabrese and Blain 2009). Hence, morphogens such as TGF-β, retinoic acid, and auxin in plants that modulate growth rates in a bimodal or biphasic manner may be characterized as hormetic morphogens.
TGF-β signals through stimulatory and inhibitory pathways. The TGF-β signaling system thus appears to conform to the definition of a hormetic regulatory system introduced by Calabrese and Baldwin (2001) who suggested that responses to agonist concentration gradients of hormetic agents can form generalized hormetic regulatory systems. I believe that the theory presented in this paper provides plausible mechanisms for a role for hormetic regulatory systems in the morphogenesis of curved structures in biology.
CURVATURE
Concepts of the hormetic morphogen theory of curvature grew out of the interpretation of experimental data obtained by Lin Qiu, a graduate student in my laboratory, and others (see below). Adapting a Moss and Benditt (1975) leiomyoma model of proliferation of smooth muscle cells (SMCs) in atherosclerotic plaques, Qiu (1995) investigated the in vitro effects of various hormones and growth factors on cell lines derived from SMCs of human uteri and uterine leiomyomas (fibroids) and found that transforming growth factor betas (TGF-β1, TGF-β2, TGF-β3) stimulated and inhibited in vitro growth of uterine SMCs at low and high concentrations respectively; the growth modulation was related to log TGF-β concentration. Other investigators have reported similar dose-dependent biphasic growth responses to TGF-β by chondrocytes and fibroblasts (Battegay et al. 1990; McAnulty et al. 1997; Cordeiro et al. 2000) (Figure 1).
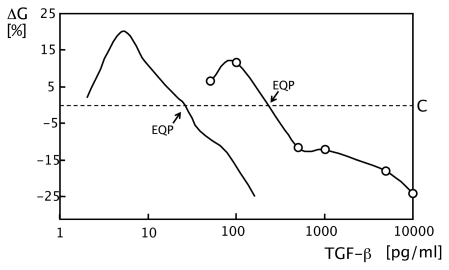
FIGURE 1.
In vitro hormesis: Graphs illustrating hormetic growth responses induced by transforming growth factor (TGF)-β. Low concentrations stimulate and high concentrations inhibit growth. Left curve illustrates an approximation of data published by McAnulty et al. (1997). Right curve, data from my laboratory (Qui 1995; Fosslien et al. 1997): Proliferation of smooth muscle cells (SMCs), average of in vitro triplicate measurements. Controls (C) represents the in vitro growth rates of corresponding non-supplemented cells. ΔG%: percent modulation of cell growth compared to controls. At the equidyne point (EQP), stimulation and inhibition of the growth rates by TGF-β are in balance and equal to the basic growth rates of control cells. For further details see text.
The TGF-β data obtained by Lin Qui showed only moderate (+/− 5–20%) biphasic growth-modulating effect induced by TGF-β. Nevertheless, I considered the effect significant for vascular morphogenesis because of 1) the bimodal nature of the effect, 2) the relatively limited thickness of blood vessel walls, 3) the finding that in vitro, the growth modulation expressed as a percentage of basic growth rates appeared to be independent of the basic growth rate of the different cell lines examined.
In 2002, I proposed that bimodal morphogen gradients could control the mural curvature and thereby the diameter and shape of tubular, cystic, domed, nodular and other curved structures in biology (Fosslien 2002). I suggested that diffusion/perfusion gradients of TGF-β can induce tissue curvature along the gradients, forming a concave curvature as seen from the high concentration end of the gradients. I refer to such morphogens as concave morphogens and to morphogens that form convex curvature as seen from high concentration ends of morphogen gradients as convex morphogens (Figure 2).
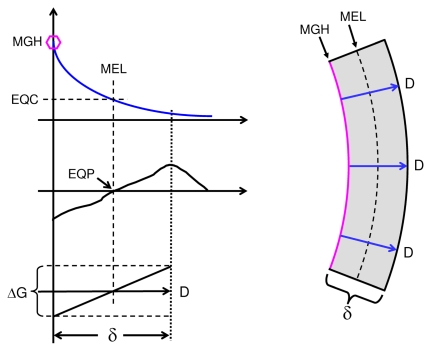
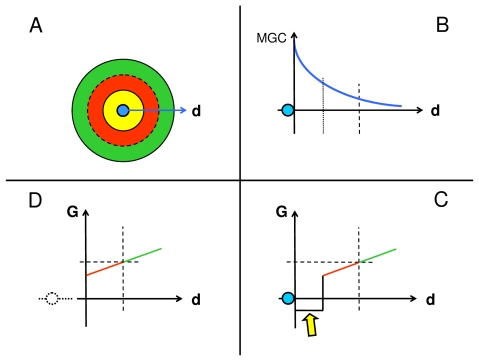
FIGURE 2.
Curvature formation by hermetic morphogen; single concentration gradient shown on top left, MGH (hexagon) indicates morphogen gradient head; left middle graph illustrates a theoretical hormetic response curve of field cells to the morphogen gradient (compare Figure 1); bottom left graph illustrates a linearized part of the induced growth gradient (ΔG) along the morphogen gradient. Right panel: concave curvature of mural section by radial gradients of the hormetic morphogen (blue arrows) that induce declining inhibition of mural cell growth from morphogen gradient heads up to the morphogen equidyne line (MEL), beyond which mural cell growth is gradually stimulated. EQC: Morphogen equidyne concentration; EQP: equidyne point of field cell hormesis curve. At a mural thickness (δ) of 0.5 mm and a 20% morphogen gradient-induced growth difference (compare Figure 1), the resulting curvature of the wall would result in a theoretical vessel internal diameter of 5 mm.
In blood vessel walls, the endothelium may be a source of the hormetic morphogen. Alternatively, in tubular and cystic structures, the luminal fluid may contain the morphogen. In certain cases, the source of the hormetic morphogen may not be local. For example, in tubular structures such as blood vessels, lymphatic vessels and ducts, a morphogen may be carried from a source location to a different location of gradient formation. In blood vessels, a hormetic morphogen may be released by cells circulating in the vessels. I shall therefore consider radial gradients such as those occurring in blood vessels walls as having heads and tails, the heads being located where the radial mural gradients start (in the following examples the endothelial side) and the tails representing the rest of the gradient path through the mural tissue (Figure 2).
MODULATION OF ATP PRODUCTION
I propose that radiating diffusion/perfusion gradients of exponentially declining concentration of a hormetic morphogen such as TGF-β can modulate field cell ATP generation along the gradients in a biphasic, hormetic concentration-dependent manner: The morphogenetic signal transmitted by the hormetic morphogen concentration gradients is converted to radiating linear ATP gradients that modulate field cell growth along the gradients. Along hormetic gradients of declining concentration at high but declining concentration, field cell ATP generation is progressively reduced less until the morphogen equidyne point (EQP) along the morphogen gradient is reached, beyond which ATP generation and growth gradually increases. At the EQP, the inhibitory and stimulatory forces are in balance, and the ATP production and growth rates of field cells at this point equal the growth rate of field cells unexposed to the morphogen. The morphogen concentration at this point is the morphogen equidyne concentration (MEC).
Thus, on each side of the equidyne point, there is a range of linear amplitude modulation of field cell ATP synthesis (Figure 3A). This differential generation of ATP creates radial ATP gradients that modulate cell proliferation in a corresponding manner with increasing distance from the gradient head, causing tissues to curve as cell proliferation is reduced at high concentrations close to the radial gradient heads and increases along the gradient tails. This is how tissue gradients of a hormetic morphogen can generate curvature (Figure 3B).
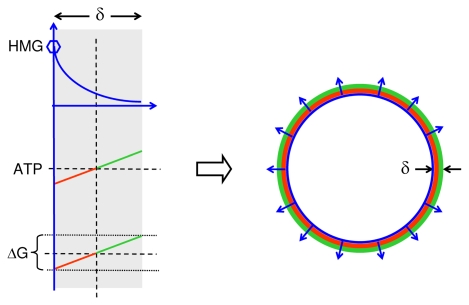
FIGURE 3A.
Tubulogenesis: Role of energetics in curvature formation of a tubular or cystic structure. Left panels illustrate different single mural gradients of a hormetic morphogen radiating through mural tissue with thickness δ. The hormetic morphogen gradient (top left) modulates field cell ATP generation thus forming a mural ATP gradient (middle left) that induces a mural growth gradient ΔG (bottom left) along the mural morphogen gradient. Right: The radial mural growth gradients curve the mural tissue. Tissue gradients of hormetic morphogens can thus induce concave tissue curvatures as seen from the high concentration end of morphogen gradients.
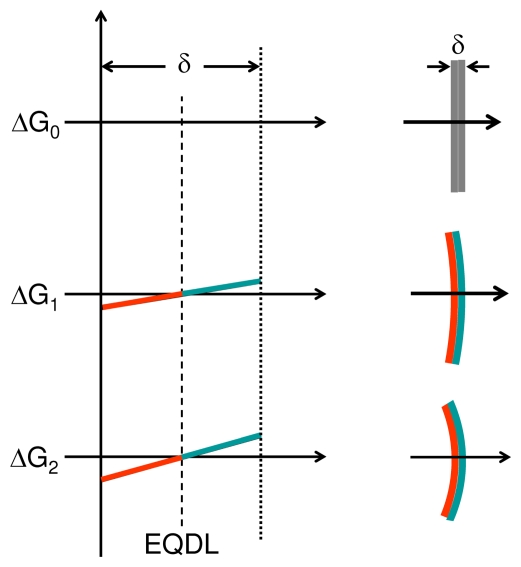
FIGURE 3B.
Growth gradient (G) regulation of curvature of tissue with thickness δ and at stable field cell hormesis: The morphogen concentration at the heads of the radial gradients and the slope of the gradients determine the radius of curvature and thereby the tube or cyst diameter. Top panel, ΔG0: no growth differential (left), no curvature (right); middle panel: ΔG1, mild growth differential, mild slope (left), mild curvature (right); bottom panel: ΔG2, large growth differential, steeper slope (left), more curvature (right). EQDL: equidyne line.
LUMEN FORMATION
The hormetic morphogen theory of curvature proposes that at very high concentrations, TGF-β and similar hormetic morphogens can form lumens and cavities via cell disintegration (necrosis and/or apoptosis) during morphogenesis of tubular biological structures such as blood vessels and cystic structures.
Experiments using retinoic acid support this concept. Retinoic acid is an essential developmental morphogen (Shroot 1990; Hernandez et al. 2007; Carpenedo et al. 2009) and an upstream regulator of TGF-β expression (Glick et al. 1989). Chuong et al.(1992) placed beads soaked in different concentrations of retinoic acid ranging from 0.01mg/ml to 1 mg/ml onto explants of avian skin and detected a clear zone of inhibition of feather buds around the beads starting at 0.05mg/ml. Moreover, the investigators reported that the area of inhibition around the beads appeared linear with retinoic acid concentrations between 0.05mg/ml and 3mg/ml. At 1mg/ml, buds just outside and adjacent to the clear zone showed altered morphology and orientation; however, no hormetic effects on growth were noted.
Upon close examination of the photographic records, it appears that buds located just outside the clear zones were smaller in size compared to enlarged buds located further away from the pellets, suggesting a hormetic effect of bud size by the diffusing morphogen. To illustrate this interpretation, I have outlined such a theoretical effect in Figure 4 as two concentric zones separated by a morphogen equidyne line and have additionally provided plots that illustrate the theoretic hormetic growth modulation along diffusion gradients of retinoic acid radiating from the pellets.
FIGURE 4.
Evidence-based and theoretical effects of hormetic morphogen radial gradients (blue arrow) diffusing from central pellet (blue) soaked in the morphogen. A: Very high concentrations of morphogen causes formation of a pellet-surrounding zone (yellow) of cell disintegration (apoptosis, necrosis), which is itself surrounded by a coaxial “mural” zone consisting of an inner zone of growth inhibition (red) and an outer zone of growth stimulation (green) separated by an equidyne line (dotted line). B: Graph illustrates the hormetic morphogen concentration along one radial (blue) gradient d (d=distance) shown in A; small circle illustrates the location of pellet. C. Graph of inhibition (red) and stimulation (green) of growth along concentrations gradient shown in B. Apoptosis/necrosis illustrated as negative (yellow arrow) growth (disintegration of cells). D: Graph of a single mural growth gradient.
Segura et al.(2002) found that apoptosis is a requirement for proper formation of new blood vessels. Using Matrigel assays and human umbilical vein endothelial cells (HUVECs), they showed that inhibition of apoptosis inhibited vessel formation. Inhibition of apoptosis resulted in defective vascular structures when vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) were employed to induce in vitro tubulogenesis in collagen gels. Fierlbeck et al.(2003) observed that in rats, glomerular capillaries are initially filled with endothelial cells; based upon in vivo and in vitro studies, the investigators proposed that central cells are subsequently removed by TGF-β1-dependent apoptosis to create the capillary lumen.
Taken together, these reports indicate that hormetic morphogen-induced central cell disintegration and/or apoptosis can enable lumen and cavity formation during hormetic morphogen-induced morphogenesis of tubular and cystic structures.
DISCUSSION
Morphogenetic fields
Morphogen gradients are basic forms of morphogenetic fields. The importance of morphogenetic fields is illustrated by effects of the embryonic microenvironment, which can restrain malignant behavior of malignant tumor cells. Malignant tumor cells resemble embryonic cells in their plasticity, and they can be forced to behave like normal cells when exposed to proper morphogenetic fields. For example, Illmensee and Mintz (1976) transplanted teratocarcinoma cells into normal mouse blastocysts and observed that the new environment reversed the cancerous properties of the transplanted cells. Kulesa et al.(2006) transplanted human malignant melanoma cells into chick embryonal tissue placing the transplanted malignant cells next to host pre-migratory neural crest cells, and observed that the tumor cells developed morphologies that resembled host neural crest cells. Morphogenetic fields can restrain cell behavior in plants and fish as well: in tobacco plants, crown gall teratoma cells participate in the morphogenesis of plants that appear normal, however, when grown in basic culture medium and thus lacking exposure to proper morphogenetic fields, the cells regain their malignant phenotype (Braun and Wood 1976), and invasive type malignant human melanoma cells transplanted into zebrafish embryos fail to form tumors (Lee et al. 2005).
These experimental findings support the morphogenetic concepts of the hormetic morphogen theory of curvature because they indicate that failure of formation and maintenance of morphogenetic fields may play a causative role in aberrant morphogenesis of developmental abnormalities and diseases such as atherosclerosis and cancer.
Energetics and morphogenesis
Child (1915) thought that energy metabolism gradients could explain embryonal morphogenesis, and that the orderly sequence of events in time and space during embryogenesis was due to differences in rate rather that in kind of metabolic reaction. Investigating cancer metabolism and viewing cancer morphogenesis as due to dedifferentiation, Warburg (1956) considered ‘combustion’ - mitochondrial respiration - a sine qua non for cell differentiation and morphogenesis of higher life forms.
Along similar lines of thought, in the hormetic morphogen theory of morphogenesis, I propose that hormetic modulation of growth rates and thereby curvature along their hormetic morphogen gradients is achieved by amplitude modulation of mitochondrial generation of ATP, the mitochondrial rate-limiting growth regulator in mammals, plants and algae. The major supply of cellular mitochondrial ATP depends upon the total number of active mitochondria in a cell, which depends upon the rates of mitochondrial synthesis and degradation, the fuel supply (food metabolites), the oxygen available to the mitochondria, and the structure and function of components of the mitochondrial energy conversion system.
In my paper on the role of mitochondrial energetics in cancer morphogenesis (Fosslien 2008), I reviewed experimental evidence which demonstrates that TGF-β can regulate mitochondrial ATP generation in several ways. For example, TGF-beta can regulate expression of mito-chondrial adenine nucleotide transporters (ANT1, ANT2) (Figure 5). In rodents, TGF-β can regulate ANT1 expression via TGF-β responsive elements present in the ANT1 promoter, but not in the ANT2 promoter as it lacks a TGF-β-responsive element (Law et al. 2004). However, TGF-β can signal via Smad proteins and Sp1; the latter interacts with inhibitory and stimulatory elements of the ANT2 promoter; TGF-β thus can up-regulate and down-regulate ANT2 expression in a concentration-dependent manner (Li et al. 1996; Zaid et al. 1999, 2001).
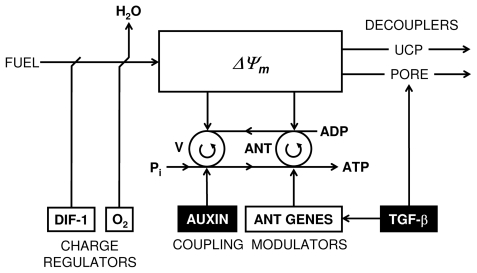
FIGURE 5.
Evidence-based examples of regulation of mitochondrial ATP synthesis by charge regulators (DIF-1 and O2) that can limit the amount of fuel-energy conversion to mitochondrial inner membrane potential (Δψm), or via amplitude modulation of coupling to ATP synthesis by hormetic morphogens (TGF-β and auxin), or by uncoupling of Δψm energy, either via opening of membrane pores by apoptotic concentrations of TGF-β or by TGF-β-induced increases in uncoupling protein (UCP) via cyclooxygenase induction (not shown). TGF-β can modulate ATP synthesis by regulating the expression of adenine nucleotide transporters (ANT1 and ANT2). Auxin (IAA) can modulate ADP phosphorylation (For a more complete analysis see Fosslien 2008).
Plant morphogenesis
Experimental evidence indicates that curvature formation in plants also involves hormetic modulation of mitochondrial energetics. As examples, the auxin indolacetic acid (IAA) can modulate plant mitochondrial respiration in a concentration-dependent, biphasic manner, stimulating respiration at low and inhibiting respiration at high concentration (Bonner 1933), i.e. IAA can behave like a hormetic agent. IAA can induce curvature in plants, and the degree of curvature is proportional to the log of IAA concentration (Bialek et al. 1983). In vitro, at low concentration (up to about 3 moles/L), IAA stimulates lengths of Avena coleoptiles, gradually stimulating less up to 10 moles/l, and with further increase in concentration progressively inhibiting up to 300 moles/L (Foster et al. 1952). Inhibitory IAA concentrations limit coleoptile section elongation. By comparison, within the stimulatory concentration range, the elongation rate was steady for up to 10 hours (Kefford and Bonner 1961).
It could be argued that the role of plant mitochondria in plant differential growth is quite different from mammalian morphogenesis because plant mitochondria harbor an alternative oxidase (AOX) system (Rasmusson et al. 2004). However, plant mitochondria share most basic features such as cytochrome-dependent respiration with mammalian mitochondria ( Millerd et al. 1951), and the mechanisms of mitochondrial involvement in auxin-induced curvature formation therefore appear relevant for elucidating the mechanisms of hormetic morphogen-induced curvature in biology.
Further credence to these interpretations is given by studies of morphogenesis in the cellular slime mold (slug, Dictyostelium discoideum) and the amoeba Caenorhabditis elegans. The studies document that morphogen regulation of mitochondrial energetics is involved in their embryogenesis. For example, gradients of differentiation factor-1 (DIF-1) regulate early embryonic morphogenesis in the slug (Brookman et al. 1987; Kay et al. 1993) and the amoeba (Ahringer 1995). However, unlike modulation of mitochondrial energetics by TGF-β, DIF-1 regulates mitochondrial ATP production via regulation of fuel import into mitochondria (Figure 5).
Shaulsky and Loomis (1995) found that the DIF-1 gene encodes a vital mitochondrial solute carrier protein and reported that with increasing concentrations above 0.1μM, DIF-1 progressively decreases the mitochondrial inner membrane potential (Δψm). They suggested that regulation of mitochondrial energetics by DIF-1 may be a primary means of establishing and maintaining proportions of cell types in the slug. The investigators speculated that regulation of energy metabolism may apply to the morphogenesis in other multicellular developing systems as well. Oey et al.(2005) identified the amoebal DIF-1 encoded protein as a mitochondrial carnitine acylcarnitine transporter. In humans, mutations of such transporter genes can cause mitochondrial transmembrane transport defects, which are associated with mitochondrial energy deficiencies that are involved in a number of diseases and if severe, lead to multiple congenital malformations (Longo et al. 2006; Palmieri 2008).
Malformations
The ability of TGF-β to modulate mitochondrial energetics implies that aberrant TGF-β signaling can cause secondary mitochondrial dysfunction and failure. Taken together with reports on association of primary mitochondrial dysfunction due to germline mutations of genes coding for mitochondrial proteins with development of benign and malignant tumors (Fosslien 2008), these findings indicate that modulation of mitochondrial energetics plays a essential role of in hormetic morphogen-induced morphogenesis.
Abnormal hormetic morphogen signaling and exogenous substances that interfere with mitochondrial energetics may lead to abnormal morphogenesis associated with developmental malformation. For example, use of certain non-steroidal anti-inflammatory drugs (NSAIDs) during pregnancy increases the risk of developmental malformations, i.e. these NSAIDs can act as teratogens. Several traditional NSAIDs and some cyclooxygenase (COX)-2-selective inhibitors can target mitochondria (Fosslien 1998), and cause mitochondrial dysfunction (Fosslien 2001a). In mitochondria isolated from rat hearts, aspirin inhibits alpha-ketoglutarate (KGDH) irreversibly, and salicylic acid inhibits the enzyme competitively, reducing electron transfer from the Krebs cycle to the respiratory chain and lowering ATP synthesis. In addition, salicylic acid can cause mitochondrial uncoupling, potentially reducing ATP further (Nulton-Persson et al. 2004). Indomethacin, diclofenac, and SC-236, a COX-2-selective inhibitor, can uncouple respiration and decrease ATP generation of isolated mitochondria and intact cells in vitro (Krause et al. 2003). COX inhibition can also increase levels of arachidonic acid, which can inhibit OXPHOS and increase generation of reactive oxygen species (Fosslien 2005). These in vitro NSAID effects may be dose-related, and since an individual’s over-the-counter NSAID use is often unclear, it is difficult to estimate the clinical risk of developmental malformation due to NSAID use.
By comparison, the association is clearer in animal models: Experimental lack of morphogenetic signaling either by lack of TGF-β3 or by inhibiting its signal transmission via drug induced mitochondrial dysfunction can cause lack of palate fusion: TGF-β3 −/− mouse embryos develop cleft palate and abnormal lungs and the mice die shortly after birth (Kaartinen 1995; Taya et al. 1999), and some NSAIDs also prevent mouse palate fusion: Of 5 common NSAIDs (diclofenac, indomethacin, mefenamic acid, naproxen, and sulindac) tested for their inhibitory effect on palate fusion in mice, sulindac was the most teratogenic, whereas indomethacin was the least teratogenic (Montenegro and Palomino 1990). Studies on isolated rat liver mitochondria and HepG2 cells revealed that the sulindac metabolite, sulindac sulfide, but not sulindac, can induce mitochondrial uncoupling, dissipate Δψm, deplete ATP production, and cause cell death (Leite et al. 2006).
These examples suggest that use of teratogenic NSAIDs should be avoided during pregnancy – a period of rapid morphogenesis - because they may interfere with mitochondrial function and thereby with developmental hormetic morphogen signaling.
Vascular development
The hormetic morphogen theory of curvature proposes that the curvature and thereby the diameter of blood vessels and other tubular structures, is mostly determined by the signaling of hormetic morphogen mural radial gradients and by the hormesis of vessel mural cells. This interpretation of vascular curvature control may help explain how blood vessels can adjust their morphology (mural curvature and thereby diameter) to blood flow requirements during intrauterine and post-natal development, adaptation, and repair.
In vivo, oxygen tension differs between the arterial and venous sides of the circulation. Reduced oxygen tension can limit the maintenance of mitochondrial Δψm. Thus, it seems reasonable to expect some differences in morphogen-induced amplitude modulation of mitochondrial ATP between the two sides of the vascular system, as it is likely that the hormesis of field cells are affected by oxygen tension of their environment. Thus, hormetic morphogen-induced regulation of vascular diameter may differ between the arterial and venous side of the circulation.
TGF-β can signal through stimulatory and inhibitory receptor-mediated pathways (Figure 6). Itoh et al.(2009) showed that TGF-β signaling via both its activin receptor-like kinase (ALK)1 (stimulatory) and ALK5 (inhibitory) pathways are indispensable for in vivo vasculogenesis. Wilcox and Derynck (1988) detected TGF-β1 mRNA in hematopoietic cells of capillaries and yolk sac blood islands as early as 9 days p.c., which suggests that the hematopoietic cells may serve as hormetic morphogen source cells for TGF-β1 during blood island development and that they are themselves resistant to inhibition or apoptosis by the morphogen they secrete, but that they induce apoptosis of immediately adjacent, non-hematopoietic cells, which then disintegrate and release their plasma, thus forming the initial blood plasma of blood islands. Once blood circulation has been established, endothelial cells may also serve as hormetic morphogen source cells. This view is supported by Hannan et al.(1988), who reported that cultured bovine vascular endothelium cells (aortic and capillary) synthesize and secret the inactive form of TGF-β, and by Carvalho et al.(2004) who reported that endothelial cells are major contributors of circulating TGF-β1.
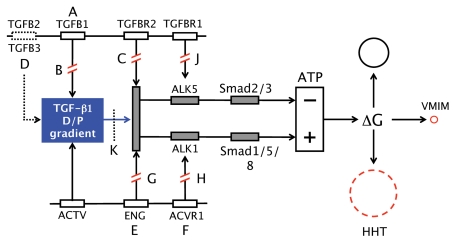
FIGURE 6.
Vascular pathologies: Evidence-based and theoretical diagram illustrating selected aberrations of parts of TGF-β1-gradient-induced curvature formation. TGF-β1, secreted by morphogen source cells and activated by activator (ACTV), form diffusion/perfusion (D/P) gradients that signal via receptor complexes consisting of TGF-β Type II receptors and endoglin (ENG) and then via inhibitory (top, ALK5, Smad2/3) and stimulatory (bottom, ALK2 and Smad1/5/8) pathways, reducing and stimulating mitochondrial synthesis of ATP respectively. Radial hormetic morphogen gradients up- and down-regulate field cell ATP synthesis. Amplitude modulation of field cell ATP along the gradients induces differential growth (ΔG) along the tissue gradients (growth gradients), which induces tissue curvature. HHT: hereditary hemorrhagic teleangiectasia; VMIM: vascular mimicry. For further explanation see text.
Too much or too little TGF-β signaling is associated with abnormal yolk sac morphogenesis. Transgenic overexpression of TGF-β1 in vascular smooth muscle cells (Figure 6, A) causes abnormal and dilated blood islands to form with the mice dying before E10.5 (Agah et al. 2000). Mice lacking TGF-β1 (Figure 6, B) or lacking Type II TGF-beta receptors (Figure 6, C) develop similar defects of yolk sac blood islands (Dickson 1995). Oshima et al.(1996) pointed out that lack of the Type II receptor is more lethal than lack of TGF-β1, and suggested that other TGF-β isoforms (TGF-β2, TGF-β3) may compensate for loss of TGF-β1 (Figure 6, D). Lack of the TGF-β receptor component endoglin also prevents proper vascular development: Germline mutation of endoglin (ENG) (Figure 6, E) or ALK1 (Figure 6, F) genes are associated with lack of SMC differentiation and hereditary hemorrhagic teleangiectasia (HHT), and complete lack of endoglin (Figure 6, G) or of the ALK1 gene (ACVR1) is lethal (Figure 6, H). ALK5 expression is required for mouse yolk sac morphogenesis (Figure 6, J) (Carvalho et al. 2004, 2007). Malignant melanomas can form very narrow vascular channels referred to as vasculogenic mimicry (VMIM) (Maniotis et al. 1999; Hess et al. 2007). Their etiology is unknown. However, if perfusion pressure is very low or lacking due to lack of circulation, their formation could possibly be caused by very short and steep TGF-β diffusion gradients, which would induce vascular channels with very high curvature and very small diameters.
These findings corroborate the model concepts of the hormetic morphogen theory of curvature, i.e. that biphasic effects of hormetic morphogen gradients such as TGF-β serve important functions during yolk sac vasculogenesis by establishing blood vessel curvature and thereby vessel diameter and help vessels adapt their curvatures to accommodate developmental and post-natal physiological changes in blood flow requirements.
Choi and Ballermann (1995) reported that 2ng/ml TGF-β1 can induce apoptosis and capillary formation of cultured wild-type glomerular capillary endothelial cells; capillary morphogenesis can be inhibited by neutralizing antibody to TGF-β1 (Figure 6, K) or by transfection of the endothelial cells with a mutant TGF-β receptor type II gene, which blocks apoptosis and capillary formation. Moreover, Pollman et al.(1999) showed that 1ng/ml of TGF-β can induce significant apoptosis in bovine aortic endothelial cells (BAEC) cultured in serum free media.
Diffuse intimal thickening and atherogenesis
The hormetic morphogen theory of curvature suggests that general lessening of the inhibitory signaling in the vasculature (Figure 3A, red lines) would lead to increased field cell proliferation on the concave side of a concave structure, leading to diffuse intimal thickening (DIT), and that focal loss of inhibitory signaling could lead to focal intimal thickening resembling atherosclerotic lesions. It seems likely that atheromas may also form due to release of hormetic morphogens by platelets depositing on the intima or by proliferating cells inside the lesions.
Morphostats
Walshe et al.(2009) suggested that constitutive TGF-β expression may serve important maintenance functions in the post-natal vasculature. Thus, TGF-β may to some extent serve as a morphostat, a term coined by Potter (2001) for agents that exert morphogen-like effects and maintain fidelity of form in adult tissues. Potter proposed that morphostatic fields induced by morphostats provide morphostasis, the maintenance of adult tissue micro-architecture; he suggested that derailed morphostasis, particularly at the interface between different morphostatic fields, is associated with metaplasia and cancer (Potter 2007). The Potter morphostat theory seems to provide valuable insights into morphogenesis. In line with the hormetic morphogen theory, it supports the idea that aberrant morphogen signaling is associated with the risk of cancer. However, it fails to consider both the morphogenetic effects of hormetic morphogens and the importance of their gradients in curvature control during morphogenesis, curvature maintenance, and tissue remodeling and repair.
Cancer
In 2008, I proposed that below a threshold of mitochondrial basic ATP synthesis, bimodal morphogen signals fail to adequately modulate mitochondrial ATP synthesis, which leads to loss of morphogen gradient control of normal tissue curvature that causes formation of abnormal and neoplastic tissue morphology (Fosslien 2008). This concept is based upon several reported observations. For example, germline mutations of genes that encode specific mitochondrial proteins promote formation of benign and malignant tumors. In response to mitochondrial dysfunction, cells shift their metabolism from deriving ATP via mitochondrial oxidative phosphorylation to glycolysis together with lactate accumulation. Otto Warburg, who first observed this effect, considered oxygen metabolism a sine qua non for cell differentiation and development of higher life forms and viewed the cause of cancer as a lack of mitochondrial ATP. I suggest that the Warburg effect causes cancer by derailing hormetic morphogen modulation of mitochondrial ATP generation, which disrupts the flow of genetic information that controls normal curvature formation and morphogenesis. Accordingly, development of benign and malignant tumors and invasiveness of cancer cells may be understood as a loss of morphogen gradient control of tissue curvature. As an example, during development of cancer from follicular tissue, follicle sizes decrease until in undifferentiated cancer tissue no curvature is visible. Thus, the increased cell proliferation of cancer tissues may represent a futile attempt to restore normal cell differentiation and normal morphogenesis when proper mitochondrial function in response to morphogens is missing: The more hormetic morphogen-induced mitochondrial ATP modulation is deranged, the more rapid the cell proliferation and the less differentiated the tumor will appear because feedback of cell differentiation as part of normal morphogenesis is never generated.
It is quite conceivable that in a tumor nodule, oxygen may be a convex morphogen: Along gradients of declining oxygen concentration from the nodule outside towards the nodule center mitochondrial respiration, ATP synthesis and cell proliferation would be decreasing, resulting in curvature of the tissue away from the high concentration end of the oxygen gradients.
Inflammation and morphogen signaling
COX-1, the constitutively expressed isoform of cyclooxygenase, regulates in vitro endothelial cell tubulogenesis (Tsujii et al. 1998). By comparison, COX-2 is a multifunctional isoform that in vivo is commonly expressed at sites of inflammation. Chronic inflammation is involved in the development of atherosclerosis (Stastny et al. 1986a; 1986b). Moreover, chronic inflammation is a cancer risk factor, and COX-2 is over-expressed in many types of human neoplasia, and COX-2 expression is involved in cancer-induced angiogenesis (Fosslien 2000a, 2000b, 2001b).
Transgenic overexpression of COX-2 reduces TGF-beta type II receptor (RII) expression leading to resistance to inhibitory signaling by TGF-beta (Hahm et al. 2002). This mechanism may explain why many cancer cells are resistant to inhibition by TGF-β. In vitro, TGF-β can induce cyclooxygenase-2 (COX-2) and COX-1, however, the effect is cell specific (Luo et al. 1998). For example, TGF-β induces COX-2 transcription of cultured pulmonary artery SMCs (Bradbury et al. 2002). Increased expression of COX-1 or COX-2 affects the synthesis of prostaglandin (PG). McAnulty et al.(1997) showed that in vitro, PGE2 can participate in the inhibitory signaling pathway of TGF-β: 40 pg/ml of TGF-β3 or more inhibited lung fibroblast proliferation. The inhibition could be completely suppressed by the non-selective cyclooxygenase inhibitor indomethacin (by comparison, 5 pg/ml (0.2 pM) of TGF-β induced maximal stimulation of fibroblast growth). PGE2 can induce expression of uncoupling protein (UCP), which reduces ATP synthesis by diverting energy from the mitochondrial inner membrane potential (Δψm) to generation of heat (Fosslien 2008). Volcik et al.(2003) found that UCP2 mutations can cause neural tube defects, which corroborates other findings that UCP function can affect morphogenesis.
TGF-β signaling via COX-2 expression can also affect the synthesis of PGJ2 and its derivative, 15-deoxy-Δ12,14-J2 (15d-PGJ2). These prostaglandins can exhibit biphasic, hormetic effects on cell proliferation: PGJ2 and 15d-PGJ2 can up- and down-regulate the activity of NADH-ubiquinone reductase (Complex I) of the mitochondrial respiratory chain and induce apoptosis in a concentration-dependent manner (Emi and Maeyama 2004), i.e. they can behave like a hormetic morphogen. At very high concentrations, PGJ2 induces mitochondrial formation of radical oxygen species (ROS), lowers mitochondrial Δψm, and can cause death of cultured cancer cells even without caspase activation (Pignatelli et al. 2005).
These findings suggest that chronic inflammation with sustained elevation of COX-2 expression may disrupt hormetic morphogen signaling and thereby cause aberrant morphogenesis; this mechanism may help explain why chronic inflammation is a risk factor for atherogenesis and cancer.
Other theories
Several theories of morphogenesis assume the presence and action of two opposing agents: an activator and an inhibitor. As examples, Turing (1990) proposed a ‘reaction-diffusion’ mechanism of pattern formation involving at least two ‘morphogens’. Schiffmann (1989) noted that cyclic AMP (cAMP) is the morphogenetic field activator-morphogen and ATP is the inhibitor; it is assumed that the activator and the inhibitor have different diffusion constants. Cummings (2001, 2006) developed mathematical models of curvature formation for sheets of epithelial cells induced by two morphogens, each signaling via its own cell surface receptors. He presented an ‘interacting signaling pathway’ mathematical model of morphogenesis involving two free interacting pairs of extracellular ligands signaling via coupled signaling pathways; activation of any one of the signaling pathways would suppress the other.
Lewis Wolpert (1969, 1971,1981, 1989, 1994) introduced the concept of positional information and suggested that it can explain gradient-induced pattern formation; His ‘French Flag’ model proposes a polar coordinate system where a diffusible morphogen provides static positional information that determines cell fate based upon the coordinates within the gradient field; a cell at a particular coordinate location could interpret the positional information according to its genetic program and developmental history. He also noted that mechanisms for providing positional information are similar in Drosophila and vertebrates.
Jaeger and Reinitz (2006) commenting on the French Flag model of morphogenesis proposed that positional information is not static, but a dynamic process involving feed-back. Kerszberg and Wolpert (2007) proposed that cell-cell interactions play important roles for cell positional interpretation.
By comparison, the hormetic morphogen theory of curvature is based upon in vitro findings that a single morphogen can induce concentration-dependent opposite, biphasic, hormetic effects and that, at very high concentration, such morphogens can induce cell disintegration and thus enable the formation of tubular lumens and tissue cavities. It assumes that in vivo tissue gradients of such a morphogen can control curvature and cell differentiation along the gradients and thereby control the morphogenesis of tubular and other curved structure. The theory considers changes in field cell hormesis during developmental cell differentiation to be an important feedback mechanism during hormetic morphogen curvature formation.
The hormetic morphogen theory of curvature raises several questions relating to morphogenesis. For example, during atherogenesis, SMCs from the media migrate into the intima where they proliferate and contribute to the formation of atheromas. Could this migration and proliferation be caused by a shift in hormetic responses to hormetic morphogen gradients? Do synthetic SMCs exhibit altered hormetic responses compared to contractile SMCs? Is cell differentiation during morphogenesis a major feed-back mechanism during hormetic morphogen curvature formation? Is cancer a disease due to loss of curvature control? In order to answer these and similar questions it will be necessary to investigate in vitro hormetic responses to hormetic morphogens by different cell types of the vasculature and differing oxygen concentrations, and, in the case of cancer, to compare hormetic responses of cancer cells with those of corresponding cancer-free cells.
Information processing
Benková et al. (2003) noted that during development of a variety of organs morphogen gradients may be employed in the form of modules; in plants for example, auxin gradients are involved in the formation of many types of plant organs. One could also compare information processing during morphogenesis to a genetic program that utilizes hormetic morphogen gradient-induced fields like subroutines of a main, overall genetically-directed whole body morphogenetic developmental program. Based upon in vitro results (Figure 1) the hormetic morphogen theory of curvature assumes that within a cell-specific response range, field cells can perform a log conversion and signal inversion of the hormetic morphogen signal, and that in addition, the signal conversion includes a signal offset feature, which would explain the morphogen response curve showing inhibition of growth below the morphogen equidyne concentration. Such signal conversion modules are common in electronic circuitry, and the theory assumes that field cells harbor biological circuits that can perform similar information-decoding functions. One solution has been offered by Foster et al. (1952) who performed a theoretical analysis of the kinetics of two-point attachments of auxin to its receptor and concluded that the enzyme kinetics of such attachments were in complete agreement with experimental results and could fully explain the biphasic effects of auxin on plant growth.
Reinhardt et al. (2000) investigated plant patterning and proposed an auxin-independent apical-basal pattern and a second, auxin-dependent radial pattern, which may control phyllotaxis, the positioning of leaves on the stem of plants. Although the investigators did not relate the radial pattern directly to curvature, there is nevertheless a similarity of thoughts between their suggestion of two separate patterns in plant morphogenesis, a longitudinal and a radial, and my 2002 hypothesis of bimodal morphogen-induced control of curvature of tubular structures and a second regulator of longitudinal growth, a hypothesis that was developed independently, and, as noted above, was based upon laboratory findings of bimodal effects of TGF-betas on human cells.
The basic theoretical models of curvature formation presented in this paper assume that mural field cells have the same hormetic response to the hormetic morphogen. However, in vivo, curved structures may have layers of different cell types that may exhibit different hormetic responses. Furthermore, it seems likely that in vivo there is an interaction between cell differentiation, cell type hormesis, and tissue curvature. Thus, along a gradient of a hormetic morphogen, as cells in the morphogen field differentiate according to differing morphogen gradient concentrations, curvature responses may change for the different cell populations that emerge. As example, a multilayered, curved mural structure such as a blood vessels wall contains morphologically different cell types, typically separated by elastic laminas, located at interfaces between the major cell types. The hormetic morphogen theory of curvature proposes that in vivo mural cells may exhibit cell-type specific hormetic responses, i.e. they may exhibit different hormetic response curves (compare Figure 1). The different hormetic responses would ensures smooth transitions of curvatures between the different layers of multilayered curved structures, i.e. an inner layer would exhibit more curvature than the next outer layer. This concept assumes that cell differentiation and cellular hormetic responses are interlinked.
It has been suggested that the morphogenesis of complex structures in distantly related biological systems may involve similar mechanisms (Wolpert 1989; Meinhardt 1996), but a role for hormesis in mammalian morphogenesis seems to have evaded major attention. This may possibly be due to ‘discipline-specific isolation’ (Calabrese 2003), even though hormesis is a basic phenomenon found not only in mammals but also across species barriers. The ideas expressed in the theory of curvature that emerge from a combination of the principles of hormesis and the observations of biphasic responses to morphogens suggest that concepts of the hormetic morphogen theory of curvature may apply across species barriers as well.
CONCLUSION
In vitro, morphogens such as TGF-β can induce biphasic growth responses by SMCs and fibroblasts, stimulating growth at low concentrations and inhibiting growth at high concentrations. Similarly, the plant auxin indolacetic acid (IAA) can induce biphasic growth responses by coleoptile segments in vitro. These dose responses are characteristic of hormesis. Therefore, I propose that TGF-β and other morphogens known to induce biphasic morphogenetic responses may be referred to as hormetic morphogens. In vitro, TGF-β can modulate mammalian mitochondrial ATP synthesis, and IAA can modulate the synthesis of ATP by plant mitochondria. In both cases, the induced ATP modulations are linearly related to the log of the TGF-β and IAA concentrations.
The hormetic morphogen theory of curvature proposes that in vivo, tissue gradients of exponentially declining concentration of a hormetic morphogen such as TGF-β in mammals and auxin in plants can modulate mitochondrial ATP generation by cells in the gradient field (field cells) in a hormetic, concentration-dependent manner, inhibiting and stimulating ATP generation at high and low morphogen concentrations respectively. The morphogen gradient-induced amplitude modulation of field cell ATP establishes linear gradients of field cell ATP generation along the morphogen gradients, which modulates field cell growth and induces linear growth gradients along the hormetic gradients.
Tissue gradients of hormetic morphogens can thus induce concave tissue curvature as seen from the high concentration end of the morphogen gradients. Radial growth gradients can induce tubulogenesis where the morphogen concentration, the slope of the gradients and the hormetic response of field cells determine the radius of curvature and thereby the tubular inside diameter. At very high concentrations, hormetic morphogens such as TGF-β and retinoic acid induce cell disintegration, necrosis, or apoptosis, which enables tubular and cystic lumen formation and tissue cavity formation.
The hormetic morphogen theory views developmental morphogenesis and post-natal adaptations of tissue architecture as a dynamic process: Abnormal hormetic morphogen signaling causes aberrant morphogenesis that is associated with human developmental and post-natal pathologies such as malformations, vascular disease, and cancer. The theory may help explain the role of hormetic morphogens in curvature formation during vasculogenesis and angiogenesis and elucidate the mechanism involved in vascular abnormalities associated with too much or too little TGF-β signaling. The biphasic, hormetic effects induced by TGF-β in vitro, applied to curvature control by theoretical tissue gradients, suggest that it is the correct balance of hormetic morphogen gradient-induced, inhibitory and stimulatory signaling that is a sine qua non for proper in vivo morphogenesis. The central role of mitochondrial energetics in hormetic morphogen signaling may explain why primary and secondary mitochondrial dysfunction is a significant cause of abnormal morphogenesis in teratogenesis, vascular diseases, carcinogenesis, and other human maladies. Furthermore, involvement of COX-2 and prostanoids in hormetic morphogen signaling links chronic inflammation to atherogenesis and to carcinogenesis.
Hormesis is a basic phenomenon that occurs in distantly related organisms. It therefore seems likely that the hormetic morphogen theory of curvature may apply across species barriers as well and that the morphogenesis of many curved structures in biology may be based on similar, hormetically regulated morphogenetic mechanisms.
ACKNOWLEDGEMENTS
I thank Edward Calabrese, Ph.D. for his interest in my 2002 paper and my ideas on biphasic morphogen-induced curvature in biology, for contacting me in 2008, and for providing literature and references on hormesis. I also appreciate our valuable discussion about hormesis and hormetic morphogens at the 8th Annual Meeting of the International Dose Response Society, where some of this material was presented.
REFERENCES
- Agah R, Prasad KS, Linnemann R, Firpo MT, Quertermous T, Dichek DA. Cardiovascular over-expression of transforming growth factor-beta(1) causes abnormal yolk sac vasculogenesis and early embryonic death. Circ Res. 2000;86(10):1024–1030. doi: 10.1161/01.res.86.10.1024. [DOI] [PubMed] [Google Scholar]
- Ahringer J. Embryonic tissue differentiation in Caenorhabditis elegans requires dif-1, a gene homologous to mitochondrial solute carriers. EMBO J. 1995;14(10):2307–2316. doi: 10.1002/j.1460-2075.1995.tb07225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewiz M, Sauer M, Teichman T, Seifertová D, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115(5):591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Bialek K, Meudt WJ, Cohen JD. Indole-3-acetic Acid (IAA) and IAA conjugates applied to bean stem sections: IAA content and the growth response. Plant Physiol. 1983;73(1):130–134. doi: 10.1104/pp.73.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. Studies on the growth hormone of plants: IV. On the mechanism of the action Proc Natl Acad Sci U S A. 1933;9(7):717–719. doi: 10.1073/pnas.19.7.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury DA, Newton R, Zhu YM, Stocks J, Corbett L, Holland ED, Pang LH, Knox AJ. Effect of bradykinin, TGF-beta1, IL-1beta, and hypoxia on COX-2 expression in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283(4):L717–L725. doi: 10.1152/ajplung.00070.2002. [DOI] [PubMed] [Google Scholar]
- Braun AC, Wood HN. Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci U S A. 1976;73(2):496–500. doi: 10.1073/pnas.73.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookman JJ, Jermyn KA, Kay RR. Nature and distribution of the morphogen DIF in the Dictyostelium slug. Development. 1987;100(1):119–124. doi: 10.1242/dev.100.1.119. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis as a biological hypothesis. Environ Health Perspect. 1998;106(Suppl 1):357–362. doi: 10.1289/ehp.98106s1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Agonist concentration gradients as a generalizable regulatory implementation strategy. Crit Rev Toxicol. 2001;31(4–5):471–473. doi: 10.1080/20014091111758. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Editorial. Nonlinearity in Biology, Toxicology, and Medicine. 2003;1:1. doi: 10.1080/15401420390844438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Cancer biology and hormesis: human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol. 2005;35(6):463–582. doi: 10.1080/10408440591034502. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. U-shaped dose response in behavioral pharmacology: historical foundations. Crit Rev Toxicol. 2008a;38(7):591–598. doi: 10.1080/10408440802026307. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008b;66(5):594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Blain RB. Hormesis and plant biology. Environ Pollut. 2009;157(1):42–48. doi: 10.1016/j.envpol.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, McDevitt TC. Homogeneous and organized differentiation within embryoid bodies induced by micros-phere-mediated delivery of small molecules. Biomaterials. 2009;30(13):2507–2515. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RL, Jonker L, Goumans MJ, Larsson J, Bouwman P, Karlsson S, Dijke PT, Arthur HM, Mummery CL. Defective paracrine signalling by TGFbeta in yolk sac vasculature of endoglin mutant mice: a paradigm for hereditary haemorrhagic telangiectasia. Development. 2004;131(24):6237–6247. doi: 10.1242/dev.01529. [DOI] [PubMed] [Google Scholar]
- Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, Ten Dijke P, Mummery CL. Compensatory signaling induced in the yolk sac vasculature by deletion of TGFbeta receptors in mice. J Cell Sci. 2007;120(Pt 24):4269–4277. doi: 10.1242/jcs.013169. [DOI] [PubMed] [Google Scholar]
- Cedergreen N, Streibig JC, Kudsk P, Mathiassen SK, Duke SO. The occurrence of hormesis in plants and algae. Dose Response. 2006;5(2):150–162. doi: 10.2203/dose-response.06-008.Cedergreen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child CM. A Dynamic Conception of the Organic Individual. Proc Natl Acad Sci U S A. 1915;1(3):164–172. doi: 10.1073/pnas.1.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi ME, Ballermann BJ. Inhibition of capillary morphogenesis and associated apoptosis by dominant negative mutant transforming growth factor-beta receptors. J Biol Chem. 1995;270(36):21144–21150. doi: 10.1074/jbc.270.36.21144. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Ting SA, Widelitz RB, Lee YS. Mechanism of skin morphogenesis. II Retinoic acid modulates axis orientation and phenotypes of skin appendages Development. 1992;115(3):839–852. doi: 10.1242/dev.115.3.839. [DOI] [PubMed] [Google Scholar]
- Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT. TGF-beta1, -beta2, and -beta3 in vitro: biphasic effects on Tenon’s fibroblast contraction, proliferation, and migration. Invest Ophthalmol Vis Sci. 2000;41(3):756–763. [PubMed] [Google Scholar]
- Cummings FW. The interaction of surface geometry with morphogens. J Theor Biol. 2001;212(3):303–313. doi: 10.1006/jtbi.2001.2377. [DOI] [PubMed] [Google Scholar]
- Cummings FW. On the origin of pattern and form in early Metazoans. Int J Dev Biol. 2006;50(2–3):193–208. doi: 10.1387/ijdb.052058fc. [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121(6):1845–1654. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- Edlund S, Lee SY, Grimsby S, Zhang S, Aspenström P, Heldin CH, Landström M. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2005;25(4):1475–1488. doi: 10.1128/MCB.25.4.1475-1488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi M, Maeyama K. The biphasic effects of cyclopentenone prostaglandins, prostaglandin J2 and 15-deoxy-Δ12,14 -prostaglandin J2 on proliferation and apoptosis in rat basophilic leukemia (RBL-2H3) cells. Biochem Pharmacol. 2004;67(7):1259–1267. doi: 10.1016/j.bcp.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Fierlbeck W, Liu A, Coyle R, Ballermann BJ. Endothelial cell apoptosis during glomerular capillary lumen formation in vivo. J Am Soc Nephrol. 2003;14(5):1349–1354. doi: 10.1097/01.asn.0000061779.70530.06. [DOI] [PubMed] [Google Scholar]
- Fosslien E, Qiu L, Yamashiroya H. Uterine leiomyoma cells: growth responses to epidermal growth factor and transforming growth factor beta. Ann Clin Lab Sci. 1997;27:318–319. [Google Scholar]
- Fosslien E. Adverse effects of nonsteroidal anti-inflammatory drugs on the gastrointestinal system. Ann Clin Lab Sci. 1998;28(2):67–81. [PubMed] [Google Scholar]
- Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000a;30(1):3–21. [PubMed] [Google Scholar]
- Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000b;37(5):431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- Fosslien E. Mitochondrial medicine—molecular pathology of defective oxidative phosphorylation. Ann Clin Lab Sci. 2001a;31(1):25–67. [PubMed] [Google Scholar]
- Fosslien E. Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci. 2001b;31(4):325–348. [PubMed] [Google Scholar]
- Fosslien E. Establishment, maintenance, and remodeling of curvature in biology. Med Hypotheses. 2002;59(3):233–238. doi: 10.1016/s0306-9877(02)00206-2. [DOI] [PubMed] [Google Scholar]
- Fosslien E. Cardiovascular complications of non-steroidal anti-inflammatory drugs. Ann Clin Lab Sci. 2005;35(4):347–385. [PubMed] [Google Scholar]
- Fosslien E. Cancer morphogenesis: role of mitochondrial failure. Ann Clin Lab Sci. 2008;38(4):307–329. [PubMed] [Google Scholar]
- Foster RJ, McRae DH, Bonner J. Auxin-induced growth inhibition a natural consequence of two-point attachment. Proc Natl Acad Sci U S A. 1952;38(12):1014–1022. doi: 10.1073/pnas.38.12.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick AB, Flanders KC, Danielpour D, Yuspa SH, Sporn MB. Retinoic acid induces transforming growth factor-beta 2 in cultured keratinocytes and mouse epidermis. Cell Regul. 1989;1(1):87–97. doi: 10.1091/mbc.1.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm KB, Lim HY, Sohn S, Kwon HJ, Lee KM, Lee JS, Surh YJ, Kim YB, Joo HJ, Kim WS, Cho SW. In vitro evidence of the role of COX-2 in attenuating gastric inflammation and promoting gastric carcinogenesis. J Environ Pathol Toxicol Oncol. 2002;21(2):165–176. [PubMed] [Google Scholar]
- Hannan RL, Kourembanas S, Flanders KC, Rogelj SJ, Roberts AB, Faller DV, Klagsbrun M. Endothelial cells synthesize basic fibroblast growth factor and transforming growth factor beta. Growth Factors. 1988;1(1):7–17. doi: 10.3109/08977198809000242. [DOI] [PubMed] [Google Scholar]
- Hernandez RE, Putzke AP, Myers JP, Margaretha L, Moens CB. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134(1):177–187. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AR, Margaryan NV, Seftor EA, Hendrix MJ. Deciphering the signaling events that promote melanoma tumor cell vasculogenic mimicry and their link to embryonic vasculogenesis: role of the Eph receptors. Dev Dyn. 2007;236(12):3283–3296. doi: 10.1002/dvdy.21190. [DOI] [PubMed] [Google Scholar]
- Illmensee K, Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976;73(2):549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh F, Itoh S, Carvalho RL, Adachi T, Ema M, Goumans MJ, Larsson J, Karlsson S, Takahashi S, Mummery CL, Dijke PT, Kato M. Poor vessel formation in embryos from knock-in mice expressing ALK5 with L45 loop mutation defective in Smad activation. Lab Invest. 2009;89(7):800–810. doi: 10.1038/labinvest.2009.37. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Reinitz J. On the dynamic nature of positional information. Bioessays. 2006;28(11):1102–1111. doi: 10.1002/bies.20494. [DOI] [PubMed] [Google Scholar]
- Jones CM, Armes N, Smith JC. Signalling by TGF-beta family members: short-range effects of Xnr-2 and BMP-4 contrast with the long-range effects of activin. Curr Biol. 1996;6(11):1468–1475. doi: 10.1016/s0960-9822(96)00751-8. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11(4):415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Kay RR, Large S, Traynor D, Nayler O. A localized differentiation-inducing-factor sink in the front of the Dictyostelium slug. Proc Natl Acad Sci U S A. 1993;90(2):487–491. doi: 10.1073/pnas.90.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefford NP, Bonner J. Steady state growth of avena coleoptile sections in high auxin concentrations. Plant Physiol. 1961;36(3):323–325. doi: 10.1104/pp.36.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerszberg M, Wolpert L. Mechanisms for positional signalling by morphogen transport: a theoretical study. J Theor Biol. 1998;191(1):103–114. doi: 10.1006/jtbi.1997.0575. [DOI] [PubMed] [Google Scholar]
- Kerszberg M, Wolpert L. Specifying positional information in the embryo: looking beyond morphogens. Cell. 2007;130(2):205–209. doi: 10.1016/j.cell.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Krause MM, Brand MD, Krauss S, Meisel C, Vergin H, Burmester GR, Buttgereit F. Nonsteroidal antiinflammatory drugs and a selective cyclooxygenase 2 inhibitor uncouple mitochondria in intact cells. Arthritis Rheum. 2003;48(5):1438–1444. doi: 10.1002/art.10969. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, Hendrix MJ. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A. 2006;103(10):3752–3757. doi: 10.1073/pnas.0506977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AK, Gupta D, Levy S, Wallace DC, McKeon RJ, Buck CR. TGF-beta1 induction of the adenine nucleotide translocator 1 in astrocytes occurs through Smads and Sp1 transcription factors. BMC Neurosci. 2004;5:1–14. doi: 10.1186/1471-2202-5-1. Available at http://biomedcentral.com/1471-2202/5/1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233(4):1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- Leite S, Martins NM, Dorta DJ, Curti C, Uyemura SA, dos Santos AC. Mitochondrial uncoupling by the sulindac metabolite, sulindac sulfide. Basic Clin Pharmacol Toxicol. 2006;99(4):294–299. doi: 10.1111/j.1742-7843.2006.pto_490.x. [DOI] [PubMed] [Google Scholar]
- Li R, Hodny Z, Luciakova K, Barath P, Nelson BD. Sp1 activates and inhibits transcription from separate elements in the proximal promoter of the human adenine nucleotide translocase 2 (ANT2) gene. J Biol Chem. 1996;271(31):18925–18930. doi: 10.1074/jbc.271.31.18925. [DOI] [PubMed] [Google Scholar]
- Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transportand the carnitine cycle. Am J Med Genet C Semin Med Genet 15. 2006;142C(2):77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Lang JA, Miller MW. Transforming growth factor beta1 regulates the expression of cyclooxygenase in cultured cortical astrocytes and neurons. J Neurochem. 1998;71(2):526–534. doi: 10.1046/j.1471-4159.1998.71020526.x. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7(1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty RJ, Hernández-Rodriguez NA, Mutsaers SE, Coker RK, Laurent GJ. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-beta isoforms on fibroblast cell cultures. Biochem J. 1997;321(Pt 3):639–643. doi: 10.1042/bj3210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell N, Zorn AM, Crease DJ, Gurdon JB. Activin has direct long-range signalling activity and can form a concentration gradient by diffusion. Curr Biol. 1997;7(9):671–681. doi: 10.1016/s0960-9822(06)00294-6. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mille F, Thibert C. Morphogens and cell survival during development. J Neurobiol. 2005;64(4):357–366. doi: 10.1002/neu.20167. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Models of biological pattern formation: common mechanism in plant and animal development. Int J Dev Biol. 1996;40(1):123–134. [PubMed] [Google Scholar]
- Millerd A, Bonner J, Axelrod B, Bandurski R. Oxidative and phosphorylative activity of plant mitochondria. Proc Natl Acad Sci U S A. 1951;37(12):855–862. doi: 10.1073/pnas.37.12.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro MA, Palomino H. Induction of cleft palate in mice by inhibitors of prostaglandin synthesis. J Craniofac Genet Dev Biol. 1990;10(1):83–94. [PubMed] [Google Scholar]
- Moss NS, Benditt EP. Human atherosclerotic plaque cells and leiomyoma cells. Comparison of in vitro growth characteristics Am J Pathol. 1975;78(2):175–190. [PMC free article] [PubMed] [Google Scholar]
- Nulton-Persson AC, Szweda LI, Sadek HA. Inhibition of cardiac mitochondrial respiration by salicylic acid and acetylsalicylate. J Cardiovasc Pharmacol. 2004;44(5):591–595. doi: 10.1097/00005344-200411000-00012. [DOI] [PubMed] [Google Scholar]
- Oey NA, Ijlst L, van Roermund CW, Wijburg FA, Wanders RJ. dif-1 and colt, both implicated in early embryonic development, encode carnitine acylcarnitine translocase. Mol Genet Metab. 2005;85(2):121–124. doi: 10.1016/j.ymgme.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Orvis GD, Jamin SP, Kwan KM, Mishina Y, Kaartinen VM, Huang S, Roberts AB, Umans L, Huylebroeck D, Zwijsen A, Wang D, Martin JF, Behringer RR. Functional redundancy of TGF-beta family type I receptors and receptor-Smads in mediating anti-Müllerian hormone-induced Müllerian duct regression in the mouse. Biol Reprod. 2008;78(6):994–1001. doi: 10.1095/biolreprod.107.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179(1):297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- Palmieri F. Diseases caused by defects of mitochondrial carriers: a review. Biochim Biophys Acta. 2008;1777(7–8):564–578. doi: 10.1016/j.bbabio.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Sánchez-Rodríguez J, Santos A, Perez-Castillo A. 15-deoxy-Δ12,14 -prostaglandin J2 induces programmed cell death of breast cancer cells by a pleiotropic mechanism. Carcinogenesis. 2005;26(1):81–92. doi: 10.1093/carcin/bgh308. [DOI] [PubMed] [Google Scholar]
- Pollman MJ, Naumovski L, Gibbons GH. Vascular cell apoptosis: cell type-specific modulation by transforming growth factor-beta1 in endothelial cells versus smooth muscle cells. Circulation. 1999;99(15):2019–2026. doi: 10.1161/01.cir.99.15.2019. [DOI] [PubMed] [Google Scholar]
- Potter JD. Morphostats: a missing concept in cancer biology. Cancer Epidemiol Biomarkers Prev. 2001;10(3):161–170. [PubMed] [Google Scholar]
- Potter JD. Morphogens, morphostats, microarchitecture and malignancy. Nat Rev Cancer. 2007;7(6):464–474. doi: 10.1038/nrc2146. [DOI] [PubMed] [Google Scholar]
- Qiu L. Regulatory role of hormones and growth factors in the pathophysiology of human uterine leiomyoma. Ph.D. Thesis. Graduate College of the UIC Health Sciences Center, University of Illinois at Chicago; 1995. [Google Scholar]
- Rasmusson AG, Soole KL, Elthon TE. Alternative NAD(P)H dehydrogenases of plant mitochondria. Annu Rev Plant Biol. 2004;55:23–39. doi: 10.1146/annurev.arplant.55.031903.141720. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12(4):507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann Y. The second messenger system as the morphogenetic field. Biochem Biophys Res Commun 29. 1989;165(3):1267–1271. doi: 10.1016/0006-291x(89)92739-3. [DOI] [PubMed] [Google Scholar]
- Segura I, Serrano A, De Buitrago GG, González MA, Abad JL, Clavería C, Gómez L, Bernad A, Martínez-A C, Riese HH. Inhibition of programmed cell death impairs in vitro vascular-like structure formation and reduces in vivo angiogenesis. FASEB J. 2002;16(8):833–841. doi: 10.1096/fj.01-0819com. [DOI] [PubMed] [Google Scholar]
- Shaulsky G, Loomis WF. Mitochondrial DNA replication but no nuclear DNA replication during development of Dictyostelium. Proc Natl Acad Sci U S A. 1995;92(12):5660–5663. doi: 10.1073/pnas.92.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroot B. The physiology and biochemistry of retinoic acid. Nouv Rev Fr Hematol. 1990;32(1):26–29. [PubMed] [Google Scholar]
- Stastny J, Fosslien E, Robertson AL., Jr Human aortic intima protein composition during initial stages of atherogenesis. Atherosclerosis. 1986a;60(2):131–139. doi: 10.1016/0021-9150(86)90005-5. (Erratum in: Atherosclerosis 1986 62(3):277) [DOI] [PubMed] [Google Scholar]
- Stastny J, Robertson AL, Jr, Fosslien E. Basic proteins in the human aortic intima: nonequilibrium two-dimensional electrophoretic analysis of tissue extracts. Exp Mol Pathol. 1986b;45(3):279–286. doi: 10.1016/0014-4800(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Taya Y, O’Kane S, Ferguson MW. Pathogenesis of cleft palate in TGF-beta3 knockout mice. Development. 1999;126(17):3869–3879. doi: 10.1242/dev.126.17.3869. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93(5):705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. 1953. Bull Math Biol. 1990;52(1–2):153–197. doi: 10.1007/BF02459572. [DOI] [PubMed] [Google Scholar]
- Volcik KA, Shaw GM, Zhu H, Lammer EJ, Finnell RH. Risk factors for neural tube defects: associations between uncoupling protein 2 polymorphisms and spina bifida. Birth Defects Res A Clin Mol Teratol. 2003;67(3):158–161. doi: 10.1002/bdra.10019. [DOI] [PubMed] [Google Scholar]
- Walshe TE, Saint-Geniez M, Maharaj AS, Sekiyama E, Maldonado AE, D’Amore PA. TGF-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS ONE. 2009;2009;4(4):e5149. doi: 10.1371/journal.pone.0005149. doi:10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- Wilcox JN, Derynck R. Developmental expression of transforming growth factors alpha and beta in mouse fetus. Mol Cell Biol. 1988;8(8):3415–3422. doi: 10.1128/mcb.8.8.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and pattern formation. Curr Top Dev Biol. 1971;6(6):183–224. doi: 10.1016/s0070-2153(08)60641-9. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and pattern formation. Philos Trans R Soc Lond B Biol Sci. 1981;295(1078):441–450. doi: 10.1098/rstb.1981.0152. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information revisited. Development. 1989;107(Suppl):3–12. doi: 10.1242/dev.107.Supplement.3. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and pattern formation in development. Dev Genet. 1994;15(6):485–490. doi: 10.1002/dvg.1020150607. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. TGF-β superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16(3):329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Zaid A, Hodny Z, Li R, Nelson BD. Sp1 acts as a repressor of the human adenine nucleotide translocase-2 (ANT2) promoter. Eur J Biochem. 2001;268(21):5497–5503. doi: 10.1046/j.1432-1033.2001.02453.x. [DOI] [PubMed] [Google Scholar]
- Zaid A, Li R, Luciakova K, Barath P, Nery S, Nelson BD. On the role of the general transcription factor Sp1 in the activation and repression of diverse mammalian oxidative phosphorylation genes. J Bioenerg Biomembr. 1999;31(2):129–135. doi: 10.1023/a:1005499727732. [DOI] [PubMed] [Google Scholar]