Abstract
The drug discrimination procedure in animals has been extensively utilized to model the abuse related, subjective effects of drugs in humans, but it has seldom been used to examine abused volatile inhalants like toluene. The present study sought to characterize the temporal aspects of toluene's discriminative stimulus as well assess toluene blood concentrations under identical exposure conditions. B6SJLF1/J mice were trained to discriminate 10 min of exposure to 6000 ppm inhaled toluene vapor from air. Toluene vapor concentration dependently substituted for the training exposure condition with longer exposures to equivalent concentrations producing greater substitution than shorter exposures. Toluene's discriminative stimulus effects dissipated completely by 60 min after the cessation of exposure. Injected liquid toluene dose-dependently substituted for toluene vapor as well as augmenting the discriminative stimulus effects of inhaled toluene. Toluene blood concentrations measured under several exposure conditions which produced full substitution were all nearly identical suggesting that the concentration of toluene in the animals tissues at the time of testing determined discriminative performance. These results indicate that the discriminative stimulus effects of inhaled toluene vapor are likely mediated by CNS effects rather than by it's pronounced peripheral stimulus effects.
Keywords: drug discrimination, toluene, inhalant, mice, blood concentration, time course
Introduction
A broad range of volatile and gaseous compounds are abused by inhalation and abuse of these chemicals is a serious public health problem (Lubman et al. 2008, Neumark et al. 1998). Inhalant abuse is of particular concern since children, either because of the ubiquitous nature of abuseable inhalants or for other less apparent reasons, are the most likely to inhale these compounds (Johnston et al. 2008, Seth et al. 2005, Thiesen et al. 2007). While the list of inhalants is long, consumer products containing toluene, such as paint and lacquer thinners, spot removers, glues, polishes and motor fuels are very frequently cited as being abused (Bowen et al. 1999a, Cairney et al. 2002, Giovacchini 1985).
Our understanding of the abuse-related behavioral and neurochemical effects of many drugs is relatively good. Unfortunately, the same cannot be said for inhalants like toluene. While there are undoubtedly a multitude of causes underlying our poor understanding of inhalants, one of the most salient may be the lack of appropriate behavioral procedures which can be or have been adapted to studying the abuse-related effects of these substances. One behavioral paradigm which has been used extensively to examine the abuse-related subjective effects of many drugs is the drug discrimination procedure (Balster 1991, Colpaert 1999, Stolerman 1992).
The discriminative stimulus effects of drugs in animals are thought to model subjective drug effects in humans and may have particular utility for examining drugs, such as most inhalants, which have toxic properties that preclude their study in humans (Colpaert 1987). In the drug discrimination procedure, subjects are trained to attend to a drug's interoceptive stimulus effects. This is accomplished by reinforcing drug-appropriate operant responding during daily training sessions which are preceded by non-contingent training drug administration. Likewise, the subjects are also trained to attend to the absence of a drug's interoceptive stimulus effects by reinforcing vehicle-appropriate operant responding during daily training sessions which are preceded by non-contingent vehicle administration. After many days of training subjects will accurately choose the reinforced operant based on whether the test session was preceded by non-contingent training drug or vehicle administration. The major utility of the drug discrimination paradigm lies in its ability to compare test drugs to the training drug. Of particular importance is the relative selectivity of the assay. In general, only test compounds which have CNS effects similar to the training drug will elicit training-drug appropriate responding, while test compounds that have no CNS effects or CNS effects dissimilar to the training drug elicit vehicle responding (Colpaert 1999).
To date, the primary application of the drug discrimination paradigm to inhalants has been to conduct cross-tests of inhalants in animals which have been trained to discriminate injected drugs from vehicle (Bowen 2006, Bowen and Balster 1997, Bowen et al. 1999b, Rees et al. 1987a, Rees et al. 1987b, Shelton and Balster 2004). Given that inhalants appear to act upon multiple receptor systems (Bowen et al. 2006), cross-test data is informative but cannot serve as a substitute for experiments in which the inhalants serve as the training stimuli (Stolerman et al. 1999). Few studies have examined inhalants themselves as discrimination training drugs (Knisely et al. 1990, Rees et al. 1987c). Two recent studies, both from our laboratory, showed that the abused inhalants toluene and 1,1,1-trichloroethane could be trained as discriminative stimuli using the inhalational route (Shelton 2007, 2008).
While these experiments demonstrated that inhalant vapors could be trained as discriminative stimuli, fundamental questions regarding the nature of inhalants such as toluene as discriminative stimuli have not been addressed. For instance what is the time course of toluene's discriminative stimulus and does the length of toluene vapor exposure alter that time course. Another question which has been examined with few drugs, toluene not among them, is the relationship between drug blood or brain levels and discriminative stimulus performance (Kimmel et al. 2008, Lamas et al. 1995, Quertemont et al. 2003). Elucidating this relationship may be particularly important for inhalants given that their uptake and elimination kinetics are often very rapid (Gerasimov et al. 2002). Lastly, while our prior studies provided strong initial support for the conclusion that the CNS effects toluene and TCE were responsible for their discriminative stimulus effects, examining the relationship between discriminative stimulus effects and toluene blood concentrations across different exposure conditions might further strengthen this hypothesis. The present study designed was to address these basic questions in mice which had been trained to discriminate 6000 ppm inhaled toluene vapor from air.
Materials and methods
Subjects
Sixteen adult male B6SJLF1/J mice (Jackson Laboratory, Bar Harbor, Maine) trained in two groups of 8 animals served as subjects for the drug discrimination experiments. Sixteen additional B6SJLF1/J mice were used only for blood sampling. B6SJLFI/J mice are a F1 hybrid derived from C57BL6/J female and SJL/J male parents. This strain has previously been used in our laboratory for drug discrimination studies with ethanol, 1,1,1-trichloroethane and toluene (Shelton 2007, 2008, Shelton et al. 2004). The mice were 9-10 weeks old at the start of the experiment. The mice were individually housed on a 12 hr light/dark cycle (lights on 7 AM) and allowed to acclimate to the laboratory for a period of one week prior to the start of training. To promote operant responding the mice were fed 3-5 g of standard rodent chow (Harlan, Teklad, Madison, WI) once daily after the test session. Feeding was adjusted to maintain a healthy, stable weight of between 25-31g for the duration of the study. Feeding was increased if the animals showed any signs of adverse consequences resulting from this mild food restriction. These studies were reviewed and approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Apparatus
Drug discrimination sessions were conducted in standard mouse operant conditioning chambers (Med-associates model ENV-307AW, St. Albans, VT). Each chamber was equipped with two low-force, optically switched levers (Med-associates model ENV-310M) on the front wall approximately 2.5 cm above the chamber floor. Above each lever was a yellow LED stimulus lamp. Equidistant between the levers was a recessed receptacle into which a 0.01 ml liquid dipper cup could be elevated via an electrically operated dipper mechanism. A single 5-watt incandescent houselight was located at the top center of the chamber rear wall. The operant conditioning chambers were individually housed in sound-attenuating and ventilated cubicles. Drug discrimination schedule conditions and data recording were accomplished using a Med-associates interface and Med-PC version 4 control software running on a PC-compatible computer (Med-Associates, St. Albans, VT). The milk solution reinforcer consisted of 25% sugar, 25% nonfat powdered milk and 50% tap water (by volume).
The static vapor chambers and general procedures used to expose the mice to toluene vapor prior to drug discrimination testing have been previously described (Shelton 2007, Shelton et al. 2004). Briefly, each exposure chamber consisted of a 26-liter cylindrical glass bell jar. A foam rubber gasket was fixed to the rim of the bell. A removable clear acrylic lid with an attached 110v fan motor was fitted to the top of the jar. The motor drive shaft passed through a seal bearing in the lid down into the bell jar where it was connected to a plastic fan blade. Directly below the fan blade was a suspended wire mesh platform to which a filter paper disk was attached. Vapors were produced by injecting toluene via a stoppered port in the lid onto the filter paper using gas-tight glass microliter syringes. The fan was then activated which rapidly volatilized the toluene and produced vapor. As the inhalation chambers were sealed and of a fixed volume, the amount of liquid necessary to produce a given vapor concentration could accurately be calculated using the ideal gas law equation simplified for room temperature (Nelson 1971). At the concentrations examined in the present study, normal daily variations in laboratory temperature and atmospheric pressure were predicted to have negligible effects on the parts per million (ppm) of vapor produced by a given volume toluene. Closed-loop recirculation of chamber atmosphere through a single wavelength IR spectrometer indicated that toluene vapor concentration in the chambers reached equilibrium in less than 1 min for all tested concentrations and did not decrease by more than 10% over the course of 20 min, which was the longest exposure period used in the present study [see (Shelton 2007) for more details of this procedure].
Prior to vapor exposure each mouse was weighed and placed into individual 7.5 cm diameter × 8 cm tall, cylindrical stainless steel containers with wire mesh tops (Oneida, Oneida, NY). The stainless steel containers were then inserted into the inhalation chamber and the lid attached. The calculated volume of toluene necessary to produce a given chamber concentration was then injected onto the filter paper and the fan activated. Air exposure sessions were identical to those with toluene except that no toluene was injected onto the filter paper.
Discrimination training
After the animals were acclimated to the laboratory, daily (M-F) 15-min training sessions were initiated. At the onset of each training session both lever lights and the houselight were illuminated for the duration of the session. During lever-press training there were no differential stimuli other than reinforcer presentations with which to associate the active lever. The mice were first reinforced for responding on only one of the two levers on a fixed ratio 1 response (FR1) schedule for several daily sessions. Upon completion of the FR requirement, a 0.01 ml liquid dipper cup was elevated into the dipper receptacle for 3 sec. Responses occurring while the dipper was elevated did not count toward completion of the next ratio requirement. Responding on the inactive lever reset the FR requirement on the correct lever. The animals were then reinforced for responding only on the opposite lever under the FR1 schedule for several daily sessions. Once the animals were reliably responding at FR1 on either lever, the operant session length was decreased to 5 min and behavior was again allowed to stabilize prior to the initiation of toluene discrimination training. During each 5 min toluene or air discrimination training session, one of the two levers was designated as correct. The correct lever was determined by whether the subject received a 10 min exposure to toluene vapor or a 10 min air exposure immediately before the discrimination training session. Completion of the FR requirement on the correct lever resulted in a 3 sec dipper presentation. The lever corresponding to toluene vapor and air exposure remained constant throughout the study for a given animal but was counterbalanced across mice. Training exposures were presented according to a double alternation schedule (i.e. two toluene vapor days followed by two air days). Responses emitted on the incorrect lever were recorded and reset the FR requirement on the correct lever. Over the course of 10-20 sessions, the response requirement was increased to FR12. These training conditions were in effect for the remainder of the study. Animals were determined to have acquired the toluene vapor versus air discrimination when the first FR was completed on the correct lever, prior to the completion of a FR on the incorrect lever, in 8 out of 10 consecutive training sessions. Additionally, the mice were required to emit greater than 80% of responses on the correct lever during all 10 of these sessions.
Substitution Test Procedure
Following acquisition of the toluene vapor and air discrimination, substitution tests were conducted on Tues and Fri, providing that the mice continued to exhibit accurate stimulus control on the Mon, Wed and Thurs training sessions. Test sessions were suspended if an animal did not emit the first FR on the correct lever and produce greater than 80% correct-lever responding during all training sessions since the last test session. If a mouse failed to meet the criteria for testing it received additional daily toluene vapor and air training sessions until the correct first FR, as well as greater than 80% correct-lever responding, were emitted for a minimum of 3 consecutive training sessions before being returned to the Tues and Fri testing, Mon, Wed, Thurs training schedule. Between substitution tests, the double alternation sequence of toluene vapor and air training sessions was continued. Each vapor substitution test was preceded by an exposure to a single concentration of toluene vapor for 10 or 20 min. Following this exposure the animals were promptly removed from the exposure chamber and in the case of the concentration-effect curve studies were immediately placed into the operant conditioning chambers for a 5-min drug discrimination test session. In the time-course delayed testing experiment the animals were removed from the exposure chamber after 10 min of 6000 ppm toluene vapor exposure and left under the fume hood until the appropriate period of time had elapsed for discrimination testing. In tests for i.p. toluene substitution, the injections were given and the animals were then exposed to air or toluene vapor for 10 min prior to the start of the 5 min discrimination test session. On test days, both levers were active and completion of the FR requirement on either lever resulted in dipper presentation. Vapor concentrations were generally administered in an ascending order and each test condition was tested once without regard for the prior days training condition (toluene vapor or air). A minimum of six mice were used for each concentration-effect curve and all substitution data shown in each graph was generated in the same group of animals. Prior to each test curve, control substitution test sessions were conducted with 6000 ppm toluene and air.
Blood toluene level analysis
The mice used for drug discrimination tests were also used for toluene blood level analysis. Since only a limited number of total blood samples could be taken from each mouse they were supplemented by an additional 16 mice used solely for assessment of toluene blood levels. The blood sampling mice were exposed to 10 min of 6000 ppm toluene vapor and air on the same double alternation schedule as the drug discrimination animals but did not undergo drug discrimination training. Blood was collected from each mouse on up to 4 occasions over the course of several months. A minimum of 3 weeks was allowed between each sample collection to allow for healing of the sampling site as well as full recovery of lost blood volume.
Toluene exposure conditions prior to blood sampling were identical to drug discrimination exposure conditions except that rather than being tested in the discrimination procedure each mouse was briefly restrained and approximately 0.1 ml of blood was obtained from the submandibular vascular bundle using a 5 mm lancet (Golde et al. 2005). Blood droplets were captured in a micro collection tube containing EDTA (BD lavender top Microtainer). The tube was briefly agitated and a 20 ul blood sample was then removed and placed into a 20 ml headspace vial to which 960 ul of type 1 ultrapure water and 20 ul of o-xylene internal standard had been previously added. The blood samples were then immediately tested for toluene concentration using a Hewlett Packard model 5890A gas chromatograph (GC) equipped with a flame ionization detector, 2.5 meter 10% TCEP 100/120 Chromosorb PAW column (Restek, Bellefonte, PA) and CTC Combi-Pal headspace autosampler. The GC parameters were: 5 min sample incubation at 90C, headspace sample volume 1.25 ml, 7 min sample run time, injector temp 200C, oven temp isothermal 110C, detector temp 200C, helium carrier gas flow rate 30 ml/min, FID hydrogen flame flow rate 25 ml/min and FID air flow rate 400 ml/min. Data were collected and analyzed by Clarity GC software (Apex data systems, Prague, CZ) using a linear regression analysis with no weighting. A 7 point calibration curve preceded the analysis of blood samples and quality control toluene standards were interspersed with each set of blood samples. Blood toluene concentrations were calculated by the internal standard method. Up to 3 replicates were analyzed from each animal and averaged if sufficient blood was collected. Each blood concentration data point represents a mean (±SEM) toluene blood concentration (ug/ml) generated from at least 3 mice.
Data analysis
Since the behavioral effects of toluene were expected to dissipate rapidly, potentially resulting in switching of responding between levers within the test session, only the choice of the lever upon which the first fixed ratio was completed was used to assess toluene-like discriminative stimulus effects. Response rates during the entire session were used to assess the inhalants effects on operant performance. Group means (± SEM) were calculated for first fixed ratio responding as well as response rate in responses/sec. A criterion of 80% or greater mean toluene vapor-appropriate responding was selected to indicate full substitution for the training concentration of toluene vapor. Mean toluene vapor-lever responding between 20% and 79% was defined as partial substitution. Mean toluene vapor-lever responding of less than 20% was considered evidence of no substitution. When possible EC50 values (and 95% confidence limits) for toluene vapor-lever selection were calculated based on the linear portion of each mean dose-effect curve using a Microsoft Excel spreadsheet based on SAS Pharm/PCS version 4 (Tallarida and Murray 1986). EC50 values for individual concentration effect curves were considered significantly different from each other when their respective 95% confidence limits did not overlap.
Compounds
HPLC grade toluene (99.8% purity) and 99% o-xylene were purchased from Fisher Scientific (Fair Lawn, NJ). Toluene used for i.p. injections was diluted in 20% Intralipid emulsion (Sigma-Aldrich, St. Louis, MO) to produce a 10 ml/kg injection volume for all doses tested.
3. Results
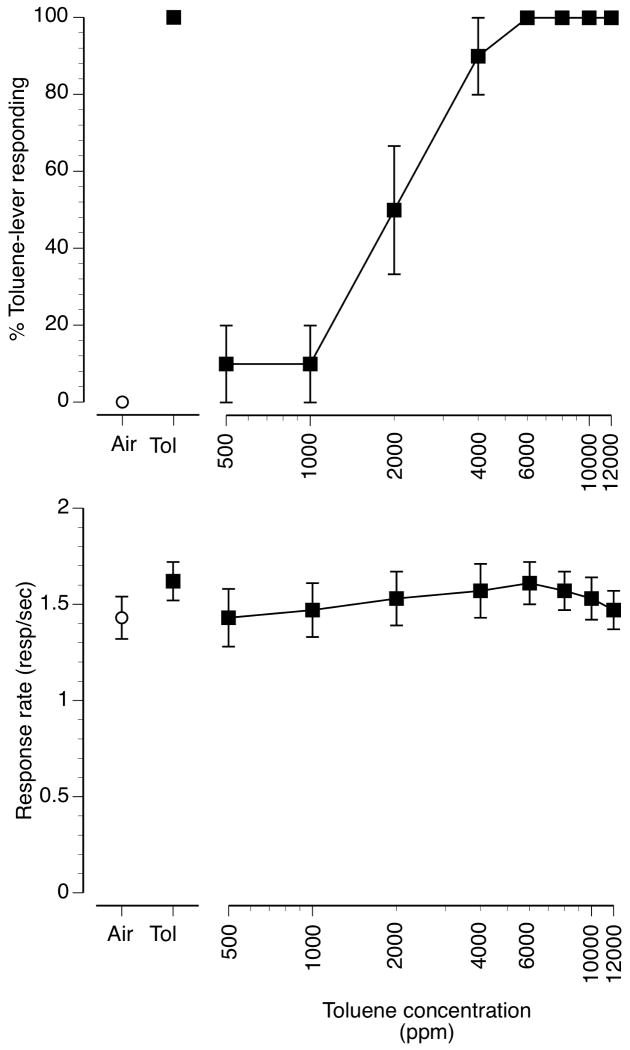
Toluene vapor concentration-dependently substituted for the 6000 ppm training concentration (figure 1, upper panel) with an EC50 value of 2000 ppm (CL: 1551-2578 ppm). The 1000 ppm toluene vapor concentrations produced 10% and the 2000 ppm toluene concentration, 50% toluene vapor-appropriate responding. Concentrations of 4000-12000 ppm all produced full substitution. Control tests following 10 min of exposure to air and 6000 ppm toluene resulted in 0% and 100% toluene-lever selection, respectively. Toluene concentrations up to 12,000 ppm had no significant effect on mean 5 min rates of responding (figure 1, lower panel) despite visible ataxia as well as signs of respiratory tract irritation, notably profuse lacrimation and salivation upon removal from the vapor exposure chamber at this concentration. Higher toluene concentrations were not examined for this reason.
Figure 1.
Concentration-effect curve for inhaled toluene vapor in mice (n=10) trained to discriminate 10 min of exposure to 6000 ppm inhaled toluene from air. Points above Air and Tol represent the results of air (open circle) and 6000 ppm inhaled toluene (filled square) exposure control sessions. Mean (± SEM) percentages toluene-lever responding are shown in the upper panel, Mean (± SEM) response rates in responses/sec are shown in the bottom panel.
Table 1 shows the results of toluene vapor concentration-effect curves following 10 and 20 min of toluene vapor exposure prior to discrimination testing. Also shown are toluene blood concentrations resulting from exposure to the same toluene concentrations and durations. Both 10 and 20 min of exposure to toluene vapor produced concentration-dependent full substitution for the 10 min, 6000 ppm toluene training exposure. The 10 min toluene vapor exposure duration resulted in an EC50 for toluene substitution of 3137 ppm (CL: 2458-4003 ppm). Increasing the toluene vapor exposure duration to 20 min significantly shifted the substitution curve to the left with an EC50 of 1662 ppm (CL: 1222-2262 ppm). None of the concentrations of toluene tested affected rates of operant responding at either exposure duration (data not shown). Toluene vapor exposure concentration- and exposure time-dependently increased toluene blood levels (Table 1). Toluene blood concentration following 10 min and 20 min of exposure to 6000 ppm toluene was 81.1 (±3.9) ug/ml and 127.1 (±11.4) ug/ml, respectively.
Table 1.
Mean (±SEM) toluene lever selection resulting from 10 or 20 min of exposure to increasing concentrations of toluene vapor in mice (n=6) trained to discriminate 10 min of exposure to 6000 ppm toluene vapor from air. Also show are mean (±SEM) blood levels achieved under similar toluene vapor exposure conditions.
| 10 min vapor exposure | 20 min vapor exposure | |||
|---|---|---|---|---|
| Toluene conc. ppm |
% Toluene-lever (±SEM) |
ug/ml blood conc. (±SEM) |
% Toluene-lever (±SEM) |
ug/ml blood conc. (±SEM) |
| Air control | 0 (±0) | - | 0 (±0) | - |
| Toluene control | 100 (±16.7) | - | 100 (±0) | - |
| 500 | 0 (±0) | - | 16.7 (±16.7) | - |
| 1000 | 0 (±0) | 12.9 (±1.1) | 16.7 (±16.7) | 18.9 (±3.5) |
| 2000 | 16.7 (±16.7) | 32.6 (±0.9) | 66.7 (±21.1) | 46.1 (±5.5) |
| 4000 | 66.7 (±21.1) | 66.0 (±5.7) | 100 (±0) | 81.9 (±5.4) |
| 6000 | 100 (±0) | 81.1 (±3.9) | 100 (±0) | 127.1 (±11.4) |
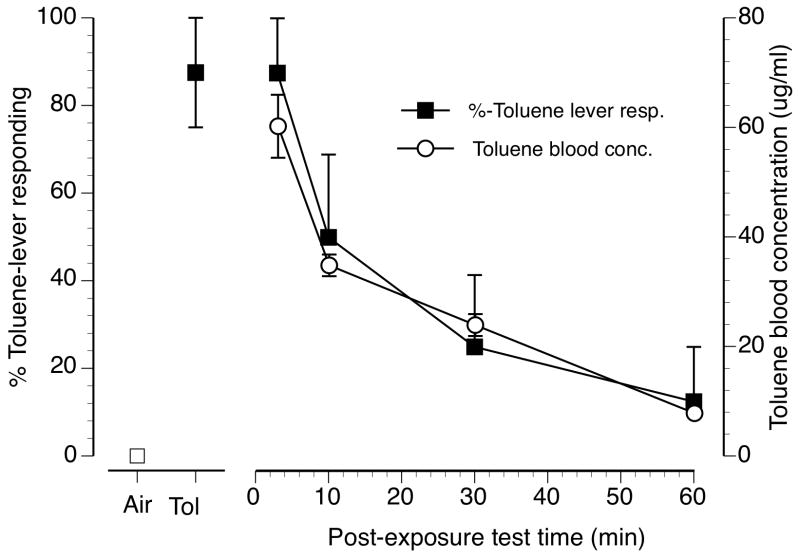
Figure 2 shows the results of post-exposure testing delays on the substitution produced by 10 min of exposure to 6000 ppm toluene vapor (figure 2, filled squares). Also shown are toluene blood levels taken at similar delay intervals (figure 2, open circles). Increasing the delay between the end of the 10 min, 6000 ppm toluene vapor exposure and the beginning of discrimination test session resulted in a time-dependent reduction in toluene-lever selection. A 3-minute delay between the cessation of vapor exposure and the start of discrimination testing still produced full substitution. Increasing the delay to 10 and then 30 minutes produced decreasing levels of partial substitution. A delay of 60 min resulted in no substitution for the training condition. Control tests with air and 6000 ppm toluene vapor in which the delay between the end of the 10 min exposure and the start of testing were as short as practical (generally less than 1 min) resulted in 0% and 88% toluene-lever selection, respectively. Reductions in toluene blood levels over time followed the same temporal pattern as did the discrimination results (open circles). Specifically at the 3 min post-exposure sampling delay, toluene blood concentration was 60.2 (±5.8) ug/ml declining over successive samples to 7.8 (±0.4) ug/ml at the longest sampling delay of 60 min.
Figure 2.
Discriminative stimulus and toluene blood concentration time course following 10 min of exposure to 6000 ppm toluene vapor (n=8). Filled squares represent mean (± SEM) toluene-lever selection. Open circles represent mean (± SEM) toluene blood concentrations in ug/ml. Points above Air and Tol represent toluene lever selection on air (open square) and 6000 ppm inhaled toluene (filled square) exposure control sessions.
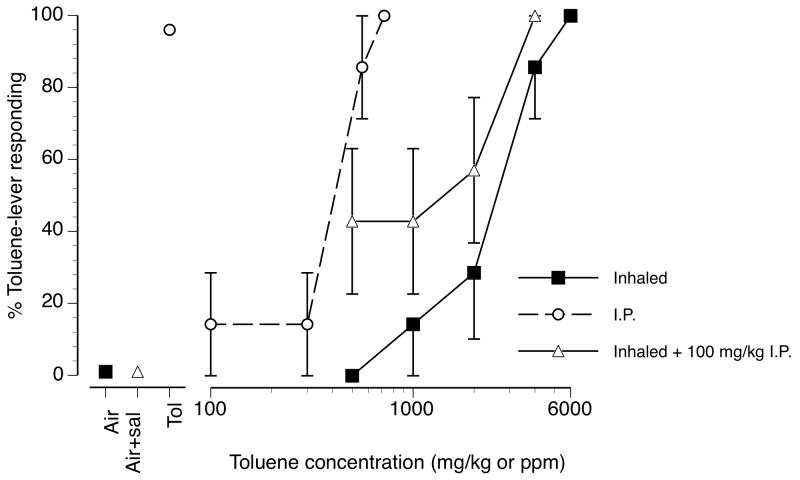
Figure 3 shows the substitution of inhaled toluene vapor alone, i.p. injected liquid toluene alone and the combination of 100 mg/kg i.p. toluene + toluene vapor for the 6000 ppm toluene vapor training condition. Increasing concentrations of toluene vapor alone (filled squares, solid line) concentration-dependently substituted for 6000 ppm toluene vapor with an EC50 of 2298 ppm (CL: 1582-3335 ppm). I.P. injected toluene (open circles, dashed line) also dose-dependently and fully substituted for the 6000 ppm toluene vapor training stimulus with an ED50 of 447 mg/kg (CL: 410-487 mg/kg). Neither inhaled nor i.p. administered toluene affected rates of operant responding (data not shown). Toluene blood concentrations were 7.0 (±0.9), 29.2 (±1.6) and 80.7 (±21.8) ug/ml at doses of 100, 320 and 560 mg/kg i.p. toluene, respectively. The administration of 100 mg/kg i.p. toluene shifted the inhaled toluene concentration-effect curve upward relative to the inhaled toluene alone concentration effect curve (open triangles, solid line).
Figure 3.
Concentration-effect curves for inhaled toluene vapor, i.p. injected toluene and a combination of 100 mg/kg i.p. toluene + inhaled toluene vapor in mice trained to discriminate 10 min of exposure to 6000 ppm inhaled toluene vapor from air (n=7). Filled squares represent mean (± SEM) toluene-lever selection resulting from inhaled toluene exposure. Open circles represent mean (± SEM) toluene-lever selection resulting from i.p. injected toluene. Open triangles represent mean (± SEM) toluene-lever selection resulting from combining 100 mg/kg i.p. injected toluene with inhaled toluene. Points above Air, Air+veh and Tol represent toluene lever selection on air (open square), air + i.p. toluene vehicle and 6000 ppm inhaled toluene (filled square) exposure control sessions, respectively.
4. Discussion
The current results confirm our prior work that abused inhalant vapors can serve as discriminative stimuli in mice (Shelton 2007, 2008) The substitution pattern of toluene vapor for the 6000 ppm training condition was concentration dependent and stable over repeated testing. Full substitution was produced by concentrations of 4000-6000 ppm toluene vapor across three separate toluene concentration-effect curve determinations. As was also the case in our prior discrimination study with toluene vapor, concentrations up to 12,000 ppm had little effect on operant response rates (figure 1, lower panel) while at the same time having strong signs of respiratory tract irritation as well as observable, but very short duration, effects on motor coordination. This lack of response rate suppression is somewhat surprising given that 20 min of exposure to 6000 ppm toluene reduced operant responding by greater than 50% in mice trained to discriminate PCP or diazepam from saline (Bowen et al. 1999b). The shorter length of exposure in the present study does not appear to be responsible since 10 min of exposure to 6000 ppm toluene suppressed operant responding by over 50% in mice trained to discriminate dizocilpine from saline (Shelton 2004). One common element in both of these prior studies was that the discrimination test sessions were 3 min shorter than that used in the present experiment. Given that the mice appeared to recover extremely rapidly from the locomotor incoordinating effects of inhaled toluene, the longer test sessions may have obscured any transient effects on operant responding. It has also been shown that animals can become tolerant to the response rate suppressing effects of the discrimination training drug (Colpaert et al. 1976, Haug 1984, York and Winter 1975), which could also account for the lack of an effect of toluene on operant response rates in the present study. Follow up toluene discrimination experiments will be necessary to determine if either or both of these factors impacted the present results.
Pharmacokinetic studies in rats have shown that the duration of toluene vapor exposure necessary to reach asymptotic blood levels may be as much as 3-4 hours (Kishi et al. 1988). However, our goal was to more fully explore the discriminative stimulus effects of toluene vapor at the relatively short exposure durations and high concentrations that are thought to be encountered by inhalant abusers (Chakroun et al. 2008, Thiesen et al. 2007). Much of the available data on toluene blood concentrations resulting from high concentration exposure comes from post-mortem blood samples from persons suspected of inhalant overdose (Chao et al. 1993). The multiple uncertainties inherent in these case reports make them poorly suited as a comparator for our findings. However, one recent study examined toluene blood concentrations in homeless adolescents in Brazil (Thiesen et al. 2007). Mean toluene blood concentration in the 68 subjects was 15.3 ug/ml with greater than 80 ug/ml toluene detected in two subjects. Since the blood samples were obtained well after cessation of toluene exposure and none of the adolescents appeared visibly intoxicated, the authors suggested that the reported blood levels were likely an underestimate of peak concentrations. These data would suggest that the toluene concentrations generated in the mice in the present study are well within the range of those achieved in toluene abusers.
Since our toluene exposure durations were shorter than those required to produce asymptotic blood concentrations, one can conceptualize inhaled toluene dose as being a function of both exposure concentration and duration. The relationship between the two in mice at short exposure durations and high toluene concentrations is unclear. Blood toluene concentrations have, however, been examined in rats following somewhat longer exposures. In one experiment, 95% of asymptotic blood concentrations were estimated to have been reached after 53 min of toluene exposure (Benignus et al. 1981). This data is in good agreement with a second study that reported the half-time of uptake of inhaled toluene concentrations from 1000-3000 ppm to be approximately 34 min (Rees et al. 1985). In another experiment in pregnant rats, toluene blood levels approximately doubled when the exposure duration was increased from 15 to 30 min (Bowen et al. 2007), suggesting a linear relationship between exposure duration and blood concentration at least up to 30 min of exposure. If in the present study, toluene blood levels were increasing at a linear rate over 20 min, it would be predicted that 10 min of exposure to toluene vapor would result in blood concentrations 50% of those produced by 20 min of exposure. The present data showing that 10 min of 1000-6000 ppm toluene vapor exposure produced blood concentrations which were between 64% and 81% of that generated by 20 min of exposure suggests a non-linear relationship even at these short exposure durations. Our data also suggest that the uptake of inhaled toluene may be more rapid in mice than rats, which would be consistent with their smaller size and more rapid heart and respiratory rate. It might have been possible to conduct discrimination tests and collect toluene blood concentrations at even shorter time points to delineate this relationship further. However, we chose not to do so based on reports indicating that the correlation between toluene concentrations in venous blood, arterial blood and brain deteriorate as exposure duration decreases (Benignus et al. 1998, Bowen et al. 2007, Carlsson 1982).
Regardless of the shape of the function between toluene exposure duration and blood concentrations, our data clearly indicated that 10 min of exposure to toluene vapor does not produce asymptotic blood levels in mice. Therefore, the total dose of toluene administered to the mice could be manipulated by increasing exposure duration while maintaining a fixed exposure concentration. Indeed, increasing the duration of toluene exposure from 10 to 20 minutes significantly shifted the entire toluene concentration-effect curve to the left, such that 20 min of exposure to 4,000 ppm produced the same level of full substitution, as well as virtually identical toluene blood concentrations, as did 10 min of exposure to 6,000 ppm toluene. These data support the hypothesis that toluene's discriminative stimulus is controlled by the concentration of toluene in the tissues of the animal resulting from the training exposure conditions (exposure concentration X exposure time) rather than the absolute toluene concentration to which the animal was exposed. This relationship provides additional evidence that the CNS effects of toluene are likely responsible for it's discriminative stimulus effects (Shelton 2007).
In the present study toluene vapor's discriminative stimulus effects were relatively short lived (figure 2). Imposing a 10 min delay between the end of vapor exposure and the beginning of discrimination testing resulted in only 50% toluene-lever selection. Toluene lever-responding continued to decreased over successively longer delays to 12.5% after a 60 min post-exposure testing delay. Our discrimination time course data is in good agreement with a previously published experiment in which the ability of toluene vapor to substitute for 1.25 g/kg i.p. ethanol in mice was examined (Bowen 2008). In that study, full substitution of 6000 ppm toluene vapor for ethanol was produced when there was no delay prior to testing. A 10 min delay prior to testing resulted in 43% ethanol-lever selection and their longest testing delay of 40 min resulted in no ethanol-lever responding. The correspondence in the toluene discriminative stimulus time-course data between the present study in which the animals were trained to discriminate inhaled toluene and the previous report in which the animals were trained to discriminate injected ethanol suggests a centrally-mediate mechanism of action. Specifically, since there was little or no possibility that the substitution of toluene for ethanol in the study by Bowen was the result of the peripheral stimulus effects of toluene being similar to those of ethanol, it is therefore probable that toluene's discriminative stimulus effects were mediated by CNS effects in both experiments.
Well over 400 time-course studies on the discriminative stimulus effects of drugs have been reported in the literature. It is generally assumed, but seldom explicitly stated, that the deterioration of a drug's discriminative stimulus over time is a function of the drug being metabolized or otherwise eliminated. A few studies have, however, examined both the time course of blood, plasma or brain drug levels and drug-appropriate responding (Hiltunen et al. 1989, Kimmel et al. 2008, Lamas et al. 1995, Quertemont et al. 2003). These experiment have shown that the time course of discriminative stimulus effects and drug levels correlate fairly well but that discriminative stimulus effects under some conditions deteriorate more rapidly than do blood or brain drug concentrations. For example in rhesus monkeys, cocaine's discriminative stimulus effects diminished at a faster rate than did plasma cocaine levels (Lamas et al. 1995). In contrast, clearance of radiolabeled cocaine as measured by PET scan correlated well with the time course of cocaine's discriminative stimulus in a second rhesus monkey experiment (Kimmel et al. 2008). In a third study, brain ethanol concentrations were measured from CSF samples collected by microdialysis from rats which were discriminating ethanol from vehicle (Quertemont et al. 2003). Here also the authors noted that the discriminative stimulus effects of ethanol deteriorated somewhat more quickly than did brain ethanol concentrations. They hypothesized that this uncoupling of brain ethanol levels and discriminative stimulus effects was the result of functional tolerance to the discriminative stimulus effects of ethanol, perhaps due to receptor desensitization. In contrast, our results show that toluene's discriminative stimulus and blood concentration time courses correspond almost perfectly. This finding suggests that functional tolerance does not occur to the discriminative stimulus effects of toluene, although it is certainly possible that the present exposure or testing procedure did not have the necessary sensitivity to detect functional tolerance. Additional testing with longer toluene exposure durations that result in more prolonged elevations in toluene blood concentrations would be necessary to definitively answer this question.
In drug discrimination studies the choice of route of training drug administration may be based on a number of scientific or practical rationales. While administration route can alter the pharmacokinetics of a drug, substitution patterns in animals administered a training drug by different routes are generally consistent (Grant et al. 1991, Sanger 1993, Shelton and Balster 1994), although exceptions have been noted (Baker et al. 2004). We chose to administer toluene via inhalation primarily because that is the means by which it is abused. However, as noted in the Introduction, inhaled toluene has pronounced odor as well as other peripheral stimulus properties at concentrations well below those which produce robust CNS effects (Cometto-Muniz and Cain 1995, Cometto-Muniz et al. 2002) which might also function as discriminative stimuli. Intraperitoneal administration of toluene, which while perhaps not completely eliminating, would be expected to substantially reduce the salience of these olfactory and trigeminal cues when compared with inhalation exposure. In our prior study using toluene vapor as a training stimulus we found that i.p. injection of toluene would substitute for toluene vapor (Shelton 2007). The results from the present study confirm that initial finding as well as extend it by showing that i.p. injection of toluene can enhance the discriminative stimulus effects of inhaled toluene vapor. These finding support the hypothesis that the discriminative stimulus effects of toluene are likely mediated by its CNS rather than peripheral stimulus effects. This hypothesis is further supported by highlighting the almost identical toluene blood levels achieved by the i.p. dosing and inhalation conditions which produced full substitution. Specifically, 560 mg/kg i.p toluene produced a blood concentration of 80.7 (±21.8) ug/ml and 10 min of 6000 ppm toluene vapor exposure resulted in a toluene blood concentration of 81.1 (±3.9) ug/ml.
While the present study implicates CNS mechanisms as underlying the discriminative stimulus of inhaled toluene vapor, it does not directly address the involvement of any individual neurotransmitter system. Previous drug discrimination studies have implicated GABAA and NMDA receptor mechanisms as underlying the discriminative stimulus effects of toluene (Bowen and Balster 1997, Bowen et al. 1999b, Rees et al. 1987a, Rees et al. 1987b, Shelton and Balster 2004) although a recent study has also suggested that toluene may also have amphetamine-like discriminative stimulus effects (Bowen 2006). Toluene has also been shown to effect recombinant GABAA, NMDA, acetylcholine and glyince receptors expressed in Xenopus oocytes (Bale et al. 2002, Bale et al. 2005, Beckstead et al. 2000, Cruz et al. 2000). These in vitro experiments have reported EC50 toluene concentrations as low as 0.17 mM (Cruz et al. 1998) which is in the range of the blood toluene concentrations generated in the present study. Taken together these data support the conclusion that multiple receptor systems were likely to have been involved in transducing toluene's discriminative stimulus but additional studies will be necessary to directly address this question.
In summary, the present data show that inhaled toluene vapor can serve as a discriminative stimulus and that toluene's discriminative stimulus can be controlled by manipulating exposure concentration as well as exposure duration. Interperitoneal injection of toluene produces full substitution as well as enhances the effects of inhaled toluene, suggesting route of administration plays little role in defining inhaled toluene's discriminative stimulus. The high degree of correspondence between the level of toluene's substitution and toluene blood concentrations suggest that toluene's discriminative stimulus is controlled by amount of toluene present in the subject's tissues at the time of testing and that no functional tolerance occurs to toluene's discriminative stimulus effects. Taken as a whole these data support the conclusion that an inhaled toluene discrimination can serve as a valid method for examining the abuse-related subjective effects of toluene vapor and similar discrimination studies may also be possible with other abused inhalants.
References
- Baker LE, Pynnonen D, Poling A. Influence of reinforcer type and route of administration on gamma-hydroxybutyrate discrimination in rats. Psychopharmacology (Berl) 2004;174:220–7. doi: 10.1007/s00213-003-1744-z. [DOI] [PubMed] [Google Scholar]
- Bale AS, Smothers CT, Woodward JJ. Inhibition of neuronal nicotinic acetylcholine receptors by the abused solvent, toluene. Br J Pharmacol. 2002;137:375–83. doi: 10.1038/sj.bjp.0704874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale AS, Tu Y, Carpenter-Hyland EP, Chandler LJ, Woodward JJ. Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons following exposure to the abused inhalant toluene. Neuroscience. 2005;130:197–206. doi: 10.1016/j.neuroscience.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Balster RL. Drug abuse potential evaluation in animals. Br J Addict. 1991;86:1549–58. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–205. [PubMed] [Google Scholar]
- Benignus VA, Boyes WK, Bushnell PJ. A dosimetric analysis of behavioral effects of acute toluene exposure in rats and humans. Toxicol Sci. 1998;43:186–95. doi: 10.1006/toxs.1998.2458. [DOI] [PubMed] [Google Scholar]
- Benignus VA, Muller KE, Barton CN, Bittikofer JA. Toluene levels in blood and brain of rats during and after respiratory exposure. Toxicol Appl Pharmacol. 1981;61:326–34. doi: 10.1016/0041-008x(81)90353-7. [DOI] [PubMed] [Google Scholar]
- Bowen SE. Increases in amphetamine-like discriminative stimulus effects of the abused inhalant toluene in mice. Psychopharmacology (Berl) 2006;186:517–24. doi: 10.1007/s00213-006-0381-8. [DOI] [PubMed] [Google Scholar]
- Bowen SE. Time course of the ethanol-like discriminative stimulus effects of abused inhalants in mice. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. Desflurane, enflurane, isoflurane and ether produce ethanol-like discriminative stimulus effects in mice. Pharmacol Biochem Behav. 1997;57:191–8. doi: 10.1016/s0091-3057(96)00308-5. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol. 2006;28:636–47. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Daniel J, Balster RL. Deaths associated with inhalant abuse in Virginia from 1987 to 1996. Drug Alcohol Depend. 1999a;53:239–45. doi: 10.1016/s0376-8716(98)00139-2. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Hannigan JH, Irtenkauf S. Maternal and fetal blood and organ toluene levels in rats following acute and repeated binge inhalation exposure. Reprod Toxicol. 2007;24:343–52. doi: 10.1016/j.reprotox.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Jones HE, Balster RL. Phencyclidine- and diazepam-like discriminative stimulus effects of inhalants in mice. Exp Clin Psychopharmacol. 1999b;7:28–37. doi: 10.1037//1064-1297.7.1.28. [DOI] [PubMed] [Google Scholar]
- Cairney S, Maruff P, Burns C, Currie B. The neurobehavioural consequences of petrol (gasoline) sniffing. Neurosci Biobehav Rev. 2002;26:81–9. doi: 10.1016/s0149-7634(01)00040-9. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Exposure to toluene: uptake, distribution and elimination in man. Scand J Work Environ Health. 1982;8:43–55. doi: 10.5271/sjweh.2497. [DOI] [PubMed] [Google Scholar]
- Chakroun R, Faidi F, Hedhili A, Charbaji K, Nouaigui H, Laiba MB. Inhalant abuse detection and evaluation in young Tunisians. J Forensic Sci. 2008;53:232–7. doi: 10.1111/j.1556-4029.2007.00623.x. [DOI] [PubMed] [Google Scholar]
- Chao TC, Lo DS, Koh J, Ting TC, Quek LM, Koh TH, Koh-Tan CY, Zubaidah A. Glue sniffing deaths in Singapore--volatile aromatic hydrocarbons in post-mortem blood by headspace gas chromatography. Med Sci Law. 1993;33:253–60. doi: 10.1177/002580249303300312. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in behavioral toxicology. Zentralbl Bakteriol Mikrobiol Hyg [B] 1987;185:48–51. [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–45. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Kuyps JJ, Niemegeers CJ, Janssen PA. Discriminative stimulus properties of fentanyl and morphine: tolerance and dependence. Pharmacol Biochem Behav. 1976;5:401–8. doi: 10.1016/0091-3057(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Cometto-Muniz JE, Cain WS. Relative sensitivity of the ocular trigeminal, nasal trigeminal and olfactory systems to airborne chemicals. Chem Senses. 1995;20:191–8. doi: 10.1093/chemse/20.2.191. [DOI] [PubMed] [Google Scholar]
- Cometto-Muniz JE, Cain WS, Abraham MH, Gola JM. Psychometric functions for the olfactory and trigeminal detectability of butyl acetate and toluene. J Appl Toxicol. 2002;22:25–30. doi: 10.1002/jat.822. [DOI] [PubMed] [Google Scholar]
- Cruz SL, Balster RL, Woodward JJ. Effects of volatile solvents on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;131:1303–8. doi: 10.1038/sj.bjp.0703666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;286:334–40. [PubMed] [Google Scholar]
- Gerasimov MR, Ferrieri RA, Schiffer WK, Logan J, Gatley SJ, Gifford AN, Alexoff DA, Marsteller DA, Shea C, Garza V, Carter P, King P, Ashby CR, Jr, Vitkun S, Dewey SL. Study of brain uptake and biodistribution of [11C]toluene in non-human primates and mice. Life Sci. 2002;70:2811–28. doi: 10.1016/s0024-3205(02)01542-4. [DOI] [PubMed] [Google Scholar]
- Giovacchini RP. Abusing the volatile organic chemicals. Regul Toxicol Pharmacol. 1985;5:18–37. doi: 10.1016/0273-2300(85)90017-0. [DOI] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Grant KA, Knisely JS, Tabakoff B, Barrett JE, Balster RL. Ethanol-like discriminative stimulus effects of non-competitive N-methyl-d-aspartate antagonists. Behav Pharmacol. 1991;2:87–95. [PubMed] [Google Scholar]
- Haug T. Tolerance to the depressant effects of diazepam in the drug discrimination paradigm. Pharmacol Biochem Behav. 1984;21:409–15. doi: 10.1016/s0091-3057(84)80103-3. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU, Hellstrom-Lindahl E, Croon LB, Jones AW. Concentrations of ethanol in rebreathed air of rats: correlation with the discriminative stimulus effects of ethanol. Alcohol. 1989;6:39–43. doi: 10.1016/0741-8329(89)90071-2. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. University of Michigan News and Information Services. Ann Arbor, MI: 2008. Various stimulant drugs show continuing gradual declines among teens in 2008, most illicit drugs hold steady. [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90:453–62. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, Harabuchi I, Ikeda T, Yokota H, Miyake H. Neurobehavioural effects and pharmacokinetics of toluene in rats and their relevance to man. Br J Ind Med. 1988;45:396–408. doi: 10.1136/oem.45.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely JS, Rees DC, Balster RL. Discriminative stimulus properties of toluene in the rat. Neurotoxicol Teratol. 1990;12:129–33. doi: 10.1016/0892-0362(90)90124-u. [DOI] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Hall E, Mello NK. Relationship between the discriminative stimulus effects and plasma concentrations of intramuscular cocaine in rhesus monkeys. Psychopharmacology (Berl) 1995;121:331–8. doi: 10.1007/BF02246072. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Lawrence AJ. Inhalant abuse among adolescents: neurobiological considerations. Br J Pharmacol. 2008;154:316–26. doi: 10.1038/bjp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumark YD, Delva J, Anthony JC. The epidemiology of adolescent inhalant drug involvement. Arch Pediatr Adolesc Med. 1998;152:781–6. doi: 10.1001/archpedi.152.8.781. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Green HL, Grant KA. Brain ethanol concentrations and ethanol discrimination in rats: effects of dose and time. Psychopharmacology (Berl) 2003;168:262–70. doi: 10.1007/s00213-003-1437-7. [DOI] [PubMed] [Google Scholar]
- Rees DC, Knisely JS, Balster RL, Jordan S, Breen TJ. Pentobarbital-like discriminative stimulus properties of halothane, 1,1,1-trichloroethane, isoamyl nitrite, flurothyl and oxazepam in mice. J Pharmacol Exp Ther. 1987a;241:507–15. [PubMed] [Google Scholar]
- Rees DC, Knisely JS, Breen TJ, Balster RL. Toluene, halothane, 1,1,1-trichloroethane and oxazepam produce ethanol-like discriminative stimulus effects in mice. J Pharmacol Exp Ther. 1987b;243:931–7. [PubMed] [Google Scholar]
- Rees DC, Knisely JS, Jordan S, Balster RL. Discriminative stimulus properties of toluene in the mouse. Toxicol Appl Pharmacol. 1987c;88:97–104. doi: 10.1016/0041-008x(87)90273-0. [DOI] [PubMed] [Google Scholar]
- Rees DC, Wood RW, McCormick JP, Cox C. Toxicokinetics of toluene in the rat. Scand J Work Environ Health. 1985;11:301–6. doi: 10.5271/sjweh.2219. [DOI] [PubMed] [Google Scholar]
- Sanger DD. Substitution by NMDA antagonists and other drugs in rats trained to discriminate ethanol. Behav Pharmacol. 1993;4:523–8. [PubMed] [Google Scholar]
- Seth R, Kotwal A, Ganguly KK. Street and working children of Delhi, India, misusing toluene: an ethnographic exploration. Subst Use Misuse. 2005;40:1659–79. doi: 10.1080/10826080500222792. [DOI] [PubMed] [Google Scholar]
- Shelton KL. Substitution profiles of N-methyl-D-aspartate antagonists in ethanol-discriminating inbred mice. Alcohol. 2004;34:165–75. doi: 10.1016/j.alcohol.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Shelton KL. Inhaled toluene vapor as a discriminative stimulus. Behav Pharmacol. 2007;18:219–29. doi: 10.1097/FBP.0b013e328157f460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL. Discriminative stimulus effects of inhaled 1,1,1-trichloroethane in mice: comparison to other hydrocarbon vapors and volatile anesthetics. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1380-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL, Balster RL. Ethanol drug discrimination in rats: substitution with GABA agonists and NMDA antagonists. Behav Pharmacol. 1994;5:441–51. [PubMed] [Google Scholar]
- Shelton KL, Balster RL. Effects of abused inhalants and GABA-positive modulators in dizocilpine discriminating inbred mice. Pharmacol Biochem Behav. 2004;79:219–28. doi: 10.1016/j.pbb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Dukat M, Allan AM. Effect of 5-HT3 receptor over-expression on the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2004;28:1161–71. doi: 10.1097/01.alc.0000138687.27452.e2. [DOI] [PubMed] [Google Scholar]
- Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci. 1992;13:170–6. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mariathasan EA, White JA, Olufsen KS. Drug mixtures and ethanol as compound internal stimuli. Pharmacol Biochem Behav. 1999;64:221–8. doi: 10.1016/s0091-3057(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacological Calculations with Computer Programs. 2. New York: Springer-Verlag; 1986. [Google Scholar]
- Thiesen FV, Noto AR, Barros HM. Laboratory diagnosis of toluene-based inhalants abuse. Clin Toxicol (Phila) 2007;45:557–62. doi: 10.1080/15563650701365891. [DOI] [PubMed] [Google Scholar]
- York JL, Winter JC. Assessment of tolerance to barbital by means of drug discrimination procedures. Psychopharmacologia. 1975;42:283–7. doi: 10.1007/BF00421269. [DOI] [PubMed] [Google Scholar]