Abstract
We recently developed a procedure to study fear incubation in which rats given 100 tone-shock pairings over 10 days show low fear 2 days after conditioned fear training and high fear after 30 or 60 days. Here, we studied the role of the stress-related peptides, neuropeptide Y (NPY) and corticotropin-releasing factor (CRF), in fear incubation. We gave rats either 10 or 100 30-sec tone-0.5-sec footshock pairings over 1 day (short training) or 10 days (long training) and then assessed tone-cue-induced conditioned suppression of lever responding 2 days after short training or 2 days and 1 month after long training. Prior to testing, we injected NPY (5-10 μg, i.c.v.), the NPY Y1 receptor antagonist BIBO3304 (20-40 μg, i.c.v.), the NPY Y2 receptor antagonist BIIE0246 (2.5-5 mg/kg, s.c.), the non-selective CRF receptor antagonist D-Phe CRF(12-41) (10 μg, i.c.v.), or the CRF1 receptor antagonist MTIP (0-20 mg/kg, s.c.). Conditioned suppression after long training was higher after 1 month than after 2 days (fear incubation); conditioned suppression was robustly expressed 2 days after short training (non-incubated fear). Both incubated and non-incubated fear responses were attenuated by NPY. In contrast, D-Phe CRF(12-41), MTIP, BIBO3304, or BIIE0246 had no effect on conditioned fear at the different time points. Results confirm previous work on the potent effect of exogenous NPY administration on conditioned fear, but the negative results with BIBO3304 and BIIE0246 question whether endogenous NPY contributes to incubated (or non-incubated) fear. Results also suggest that CRF receptors are not involved in cue-induced fear in the conditioned suppression procedure.
Keywords: D-Phe CRF(12-41), fear conditioning, anxiety, stress, incubation, MTIP, neuropeptide Y, PTSD
Introduction
In fear conditioning studies, an initially neutral environment (context) or discrete cue (e.g., tone or spoken word) is paired with a noxious stimulus (e.g., electric shock). Under certain conditions, responses to fear cues increase over time in the absence of further stress exposure, a phenomenon termed ‘fear incubation’ (McAllister et al., 1967). Fear incubation has been demonstrated in humans (Diven, 1937; Golin, 1961) and laboratory animals (Balogh et al., 2002; Houston et al., 1999; McMichael, 1966). However, most fear incubation studies involve intervals of 24 h or less (McAllister et al., 1967), and beyond this interval, fear responses typically remain stable over many weeks (Gale et al., 2004; Gleitman et al., 1967; Hendersen, 1978). Despite years of research, the neuronal mechanisms of fear incubation are unknown.
Recently, we developed a fear incubation procedure in which conditioned fear increases over time (Pickens et al., 2009). We trained food-restricted rats to lever-press for food in daily 90-min sessions. We then gave each rat one-hundred 30-s tones co-terminating with a 0.5-s mild footshock over 10 days (10 pairings/day). Rats trained using this procedure showed low fear responses to the discrete tone cue 2 days after conditioned fear training, moderate fear after 15 days, and high fear after 31 or 61 days. We also showed that rats given 1 day of fear conditioning (10 tone-shock pairings) show high fear to the tone cue 2 days later; the fear induced by short training does not incubate over time (Pickens et al., 2009). Here, we studied the roles of neuropeptide Y (NPY) and corticotropin-releasing factor (CRF), neuropeptides involved in anxiety and stress responses (Heilig et al., 1994), in fear incubation.
Results of many studies demonstrate that ventricular or localized brain injections of NPY and systemic or central injections of CRF receptor antagonists reduce unconditioned anxiety and stress (Heilig, 2004; Kask et al., 2002; Zorrilla et al., 2004). The effects of NPY and CRF receptor antagonists on conditioned fear responses were also examined in several studies. Although both NPY agonism and CRF antagonism have been reported to suppress expression of conditioned fear, important differences are also noted. Ventricular or basolateral amygdala (BLA) injections of NPY decrease fear-potentiated startle; additionally, ventricular NPY injections decrease fear-induced tachycardia and amygdala NPY injections decrease discrete cue conditioned freezing (Broqua et al., 1995; Fendt et al., 2009; Gutman et al., 2008; Tovote et al., 2004). Similarly, systemic injections of the CRF1 receptor antagonists antalarmin or CP-154,126 or ventricular injections of the non-selective CRF receptor antagonist α-helical CRF(9-41) decrease the expression of contextual fear conditioned freezing (Deak et al., 1999; Hikichi et al., 2000; Kalin et al., 1990). Contextual fear-potentiated startle expression is also decreased by oral delivery of the CRF1 receptor antagonist GSK876008 (Walker et al., 2009) and by genetic deletion of CRF1 and CRF2 receptors (Risbrough et al., 2009).
However, the effects of CRF receptor blockade on discrete-cue-induced fear-potentiated startle are mixed. While systemic injections of CP-154,126 or ventricular or caudal pontine reticular nucleus injections of α-helical CRF(9-41) decreased the expression of discrete-cue-induced fear-potentiated startle (Fendt et al., 1997; Schulz et al., 1996; Swerdlow et al., 1989), systemic injections of the CRF1 receptor antagonist NBI-30775, oral administration of GSK876008, or ventricular injections of α-helical CRF(9-41) were ineffective (de Jongh et al., 2003; Risbrough et al., 2009; Walker et al., 2009). CRF1 and CRF2 receptor knockout mice also show intact discrete-cue-induced fear-potentiated startle (Risbrough et al., 2009). Together, while CRF receptors play an important role in the expression of contextual fear conditioning, their role in conditioned fear induced by discrete cues has not been clearly established.
Here, we examined the effects of ventricular injections of NPY and the non-selective CRF receptor antagonist D-Phe CRF(12-41) (Menzaghi et al., 1994), and of systemic injections of the selective CRF1 receptor antagonist MTIP (Gehlert et al., 2007), on the expression of incubated fear 1 month after long (100 tone-shock pairings; 10 pairings/day) discrete-cue fear conditioning training. We found that NPY, but not the CRF receptor antagonists, decreased the expression of incubated fear. Subsequently, we studied the specificity of these effects (or lack thereof) to incubated fear, by examining NPY and D-Phe CRF(12-41) effects on non-incubated fear 2 days after short (1 day, 10 tone-shock pairings) training, and NPY effects on weak pre-incubated fear 2 days after long (10 days) training. Finally, in an additional experiment, we examined the role of endogenous NPY in fear incubation. For this purpose, we attempted (1) to increase fear 2 days after long training by decreasing NPY transmission with ventricular injections of the NPY Y1 receptor antagonist BIBO3304 (Wieland et al., 1998), and (2) to decrease fear 1 month after long training by increasing NPY transmission with systemic injections of the NPY Y2 receptor antagonist BIIE0246 (Doods et al., 1999). The Y2 receptor is a putative NPY presynaptic autoreceptor that inhibits NPY release, while Y1 is an NPY postsynaptic receptor (Larhammar et al., 2004; Wahlestedt et al., 1986).
Experimental procedures
Male Long-Evans rats (total n=229, Charles River, Raleigh, NC, 250-390 g) were individually housed in a colony room under a reverse 12-h:12-h light-dark cycle with lights off at 9 am. We excluded 13 rats due to equipment failure, misplaced cannula, lost headcap, or illness. The rats were deprived to 85% of their free-feeding body weight at the beginning of the experiment and kept at that weight throughout the experiment, with free access to water. All procedures followed the guidelines outlined in the “Principles of Laboratory Animal Care” (NIH publication no. 85-23) and were approved by the local Animal Care and Use Committee. Experiments were conducted in 12 self-administration chambers (Med Associates, St Albans, VT). Each chamber had two levers 9 cm above the floor, but only one lever (“active,” retractable lever) activated the pellet dispenser, delivering 45-mg food pellets (# F00021, 5.5% fat, 60% carbohydrate, 4.5% fiber; Bioserv, Frenchtown, NJ). The chambers’ grid floors were connected to electric shock generators.
Drugs
All drugs were prepared fresh before testing. NPY (Bachem, Torrance, CA, catalog number: H-6375), D-Phe CRF(12-41) ([D-Phe12, Nle21,38, Cα MeLeu37] h/rCRF12-41) (Bachem, Torrance, CA, catalog number: H-3266) and BIBO3304 ((R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoroacetate) (Tocris Bioscience, Ellisville, MO, catalog number: 2412) were each dissolved in saline. MTIP (3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine) (Lilly Research Laboratories, Indianapolis, IN) was dissolved in 10% Tween-80. BIIE0246 ((S)-N(2)-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl] cylopentyl] acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamid) (Tocris Bioscience, Ellisville, MO, catalog number: 1700) was dissolved in 30% polyethylene glycol (Sigma-Aldrich, St. Louis, MO). The injection volume for systemic injections was 1 ml/kg. The injection volume for ventricular (i.c.v.) delivery was 1 μl. D-Phe CRF(12-41) (0 and 10 μg, i.c.v.) and BIBO3304 (0, 20 and 40 μg, i.c.v.) were injected 10 min before testing. MTIP (0, 10 and 20 mg/kg, s.c.) and NPY (0, 5 and 10 μg, i.c.v.) were injected 30 min before testing. BIIE0246 (0, 2.5 and 5 mg/kg, i.p.) was injected 40 min before testing. Doses were chosen based on previous studies in rats showing effects on fear conditioning, ethanol self-administration and stress-induced reinstatement, stress-induced defecation, and blockade of the effects of Y2 agonism on food seeking (Gehlert et al., 2007; Ghitza et al., 2007; Gutman et al., 2008; Kask et al., 2000; Liu et al., 2002; Scott et al., 2005; Valdez et al., 2002).
Intracranial surgery and intracranial injections
The rats were anaesthetized with a mixture of sodium pentobarbital and chloral hydrate (60 and 25 mg/kg, respectively, i.p.). They were then implanted with guide cannulae (23-gauge; Plastics One, Roanoke, VA) 2 mm above the right lateral ventricle: antero-posterior: -0.9 mm, medio-lateral: +1.4 mm and dorso-ventral: -2.0 mm (Paxinos et al., 2005) using a stereotaxic instrument (Kopf, Tujunga, CA). The analgesic buprenorphine (0.1 mg/kg, s.c.) was given after surgery and the rats were allowed to recover for at least 5 days. The rats to be tested 2 days after training were implanted with guide cannulae before training. With the exception of 2 rats that were implanted with guide cannulae prior to training, the rats to be tested 1 month after training were implanted with cannulae 13-19 days after the last training day. Cannulae placements were verified by a positive dipsogenic response to angiotensin II (50 ng in 2 μl; Sigma, St. Louis, MO). Placements were considered accurate if a rat started drinking within 2 min of the injection and sustained drinking for 3-4 min (Sakai et al., 1995). Ventricular injections of D-Phe CRF(12-41) (0 or 10 μg in 1 μl of physiological saline), NPY (0, 5 or 10 μg in 1 μl of physiological saline) and BIBO3304 (0, 20 or 40 μg in 1 μl of physiological saline) were made with Harvard infusion pumps, using 10-ml Hamilton syringes that were connected to 30-gauge injectors (Plastics One) via polyethylene-50 tubing. Injections lasted 1 min and injectors were left in place for an additional minute before being replaced with cannula blockers. Rats were placed in the operant chambers 10 min (D-Phe CRF(12-41) and BIBO3304) or 30 min (NPY) after the completion of the injection and the test program was then started immediately. One rat with a blocked cannula in Exp. 2 was tested without the vehicle injection and included in the vehicle group.
Procedures
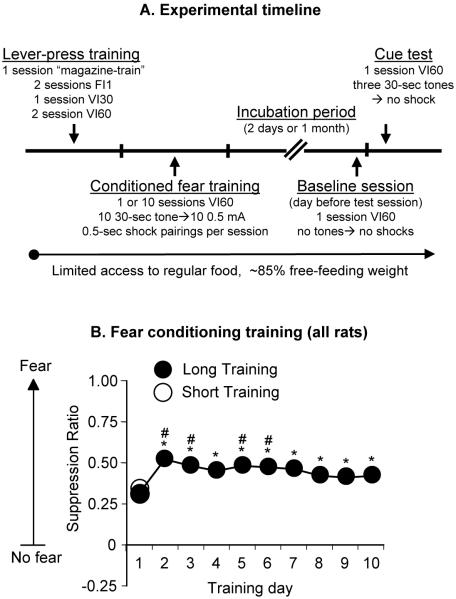
We used a fear incubation protocol consisting of 6 phases (Fig. 1A): magazine training (1 session), lever-press acquisition (5 sessions), fear conditioning (1 or 10 sessions), incubation period (2 days or 1 month), baseline session (1 session), and test for cue-induced fear conditioning (1 session). Rats were trained during the dark cycle. Sessions began with extension of the active lever and illumination of a red houselight. Rats were weighed and fed after the daily sessions.
Figure 1. Experimental timeline and fear conditioning training.
Fear incubation procedure: (A) Timeline of the fear incubation protocol. (B) Acquisition of fear conditioning: suppression ratios across 1 (n=44) or 10 (n=172) sessions of tone presentations for Exp. 1-3. * Different from Session 1, p<0.05; # Different from Session 10, p<0.05.
Food self-administration training
During the first session, the rats were given 60-min magazine training (pellet delivery every 125 s). The following day, 2 sessions of fixed-interval-1 (FI-1) reinforcement schedule (lever-presses could earn a pellet each sec) were run 2-4 h apart. These sessions ended when rats received 50 pellets (up to 1 h). A third session of up to 3 h was run immediately after the second session for rats that did not earn 50 pellets in the second session. All rats except 2 achieved 50 pellets in this session. These 2 rats were given an additional session the following day after their usual VI-30 session and they both earned 50 pellets within this session. The rats were then given one 90-min session in which pellets were earned under a variable-interval-30 (VI-30) reinforcement schedule (pellet availability for lever-presses ranging from 1 to 59 sec), and 2 daily 90-min sessions on a VI-60 schedule (pellet availability ranging from 1 to 119 sec). Rats were maintained on the VI-60 schedule for the rest of the experiment.
Fear conditioning
Fear conditioning training occurred over 1 or 10 90-min sessions during which the rats were given ten 30-sec tones (2900 Hz, 20 dB above background), ranging from 3 to 14 min apart and co-terminating with an electric shock (0.5-sec, 0.5-mA, scrambled, shock intensity adjusted for inter-chamber variability) while earning pellets on a VI-60 schedule. Conditioned inhibition of lever-pressing for food pellets was our measure of fear (Estes et al., 1941; Hunt et al., 1951; Miczek, 1973). Lever-presses were recorded during the 30-sec prior to tone presentation (Precue) and during the 30-sec tone presentation (Cue), and were converted into a suppression ratio: Suppression ratio = ((Precue-Cue)/(Precue+Cue)). The suppression ratio normalizes lever-pressing during the tone for baseline Precue responding (Annau et al., 1961; Armony et al., 1997). A value of 1 indicates total conditioned suppression of lever-pressing during tone presentation (high fear). A value of 0 reflects no lever-press suppression during tone presentation (no fear). In each experiment, the rats assigned to the different treatments were matched for their suppression ratios during training.
Conditioned fear testing
During the incubation periods, the food-restricted rats were weighed and handled 5-7 times per week. On the last day of each incubation period, lever-pressing was re-stabilized in a 90-min baseline session with no tones or shocks. The following day, conditioned fear to the tone was tested by presenting four 30-sec tones, without shock, over 35 min. The first tone occurred after 6.5 min and subsequent tones occurred after inter-trial intervals of 4, 7, and 11 min. The suppression ratios across the first 3 extinction trials were used as our measure of conditioned fear in order to assess the strength of the incubated fear response over repeated trials and to avoid a potential ceiling effect of a high fear response on the first trial. No effects were seen during the fourth trial in any of the experiments in this paper or in our previous report (Pickens et al., 2009), thus this trial was not included in our analyses. During testing, the rats ate all pellets earned regardless of drug treatment.
Experiment 1: Effect of NPY on conditioned fear
We studied the effect of NPY on the expression of conditioned fear 2 days or 30-32 days (1 month) after long (10 days of 10 tone-shock pairings/day) training. We also studied the effect of NPY on the expression of conditioned fear 2 days after short (1 day of 10 tone-shock pairings) training. All of the rats given 1 day training and half of the rats given 10 day training were tested for responses to the fear tone 2 days after training. The other half of the rats given 10 day training was tested for responses to the tone 1 month after training. In the long-training condition, the experimental design included the between-subjects factors of Incubation Period (2 days or 1 month) and NPY dose (0, 5 or 10 μg) (n=11-16 per dose per incubation period). In the short-training condition, the experimental design included the between-subjects factor of NPY dose (0, 5 or 10 μg, n=8 per dose).
Experiment 2: Effect of NPY Y1 and Y2 receptor antagonists on conditioned fear
We studied the effect of the selective Y1 receptor antagonist BIBO3304 and the selective Y2 receptor antagonist BIIE0246 on the expression of fear conditioning 2 and 32 days (1 month) after long (10 days) training, respectively. The experimental design included the between-subjects factor of BIBO3304 dose (0, 20, or 40 μg, i.c.v., n=7-8 rats per dose) or BIIE0246 dose (0, 2.5 or 5 mg/kg, s.c., n=7-8 rats per dose).
Experiment 3: Effect of CRF receptor antagonists on conditioned fear
We studied the effect of the selective CRF1 receptor antagonist MTIP and the non-selective CRF antagonist D-Phe CRF(12-41) on the expression of fear conditioning 32-36 days (termed 1 month in the rest of the text and figures) after long (10 days) training. We also studied the effect of D-Phe CRF(12-41) on the expression of fear 2 days after short (1 day) training. In the long-training condition, the experimental design included the between-subjects factor of MTIP dose (0, 10, or 20 mg/kg, s.c., n=7-8 rats per dose) or D-Phe CRF(12-41) dose (0 or 10 μg, i.c.v., n=12-13 per dose). In the short-training condition, the experimental design included the between-subjects factor of D-Phe CRF(12-41) dose (0 or 10 μg, i.c.v., n=10 per dose).
Statistical analyses
Data were analyzed by Statistica 5.1 software (Tulsa, OK). The main dependent measure was suppression ratio that is defined as: ((Precue-Cue)/(Precue+Cue)). The factors used in the statistical analyses are described in the Results section and significant effects (p<0.05) in the different ANOVAs were followed by post-hoc Fischer PLSD tests.
Results
Conditioned fear training (Exp. 1-2)
In the long training condition (10 days), conditioned suppression increased towards the beginning of fear training and decreased as training continued (Fig. 1B). The maximum fear seen during days 2, 3, 5 and 6 of training was significantly more than that on the first and last day of training (all p values<0.05). In the short training condition (1 day), the conditioned suppression value was similar to that observed in the long training condition on training day 1 and day 10 (Fig. 1B).
Experiment 1: Effect of NPY on fear conditioning
Long training (10 days)
Ventricular injections of NPY decreased incubated fear 1 month after training (Fig. 2A). The statistical analysis included the between-subjects factors of Incubation Period (2 days or 1 month) and NPY Dose (0, 5 or 10 μg), and the within-subjects factor of Cue Trial (the 3 tone presentations). This analysis revealed significant main effects of Incubation Period (F(1,74)=13.1, p<0.01) and Cue Trial (F(2,148)=64.1, p<0.01), and a significant interaction of Incubation Period x Cue Trial (F(2,148)=6.6, p<0.01). No other main effects or interactions were significant (p values >0.05).
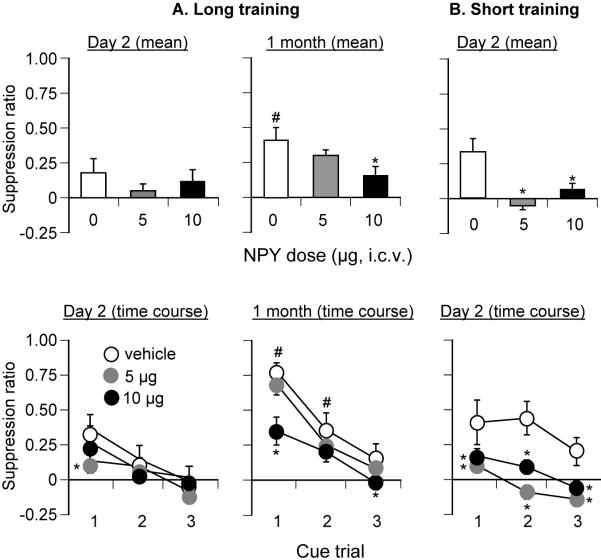
Figure 2. Effect of ventricular injections of NPY on conditioned fear: long (10 d) and short (1 d) training.
NPY injections reduced conditioned fear. (A) Long training (10 days): Mean (±sem) test suppression ratios after ventricular injections of vehicle (saline) or NPY (5 and 10 μg) in rats given 100 tone-shock pairings over 10 days and tested for fear conditioning 2 days or 1 month after training (n=80; 11-16 per dose). (B) Short training (10 days): Mean (±sem) test suppression ratios after ventricular injections of vehicle or NPY in rats given 10 tone-shock pairings over 1 day and tested for fear conditioning 2 days after training (n=24, n=8 per dose). Top panels represent mean suppression ratios across the 3 cue trials. Bottom panels represent trial-by-trial suppression ratios. * Different from vehicle within each incubation interval, p<0.05; # Different from the corresponding vehicle in day 2, p<0.05.
An ANOVA limited to the rats tested at 1 month revealed significant effects of NPY Dose (F(2,44)=4.1, p<0.05) and Cue Trial (F(2,88)=69.4, p<0.01) and a significant interaction of NPY Dose X Cue Trial (F(4,88)=2.5, p<0.05). An ANOVA limited to the rats at the 2 day test revealed a significant effect of Cue Trial (F(2,60)=12.3, p<0.01), but no significant effect of NPY Dose or an interaction between the two factors (p values >0.1). Post-hoc group differences in suppression ratios are indicated in Fig. 2A. NPY injections had no effect on the 30-sec precue lever presses or on lever presses during the test session (p>0.1, Table 1).
Table 1.
Food-reinforced responding during the fear conditioning tests. Data are the mean±sem responses per minutes during (1) the 30 sec prior to exposure to the tone cues, (2) the first 5 min of the session before the first cue presentation that occurred 6.5 min into the session, and (3) the entire 35 min session
| Exp. 1: NPY | |||
| Long training: 1 month test | Vehicle | 5 μg | 10 μg |
| 30 sec pre-cue | 29.8±4.2 | 28.3±3.8 | 33.3±4.3 |
| First 5 minutes | 23.9±2.9 | 24.4±2.3 | 29.1±3.9 |
| 35 min test session | 29.3±4.2 | 27.6±3.4 | 33.8±3.4 |
| Exp. 1: NPY | |||
| Long training: Day 2 test | Vehicle | 5 μg | 10 μg |
| 30 sec pre-cue | 28.3±3.5 | 29.2±5.0 | 33.0±5.4 |
| First 5 minutes | 25.9±3.9 | 24.8±2.7 | 24.6±2.6 |
| 35 min test session | 31.1±3.6 | 31.5±2.6 | 31.5±5.0 |
| Exp. 1: NPY: | |||
| Short training: Day 2 test | Vehicle | 5 μg | 10 μg |
| 30 sec pre-cue | 20.1±3.7 | 24.8±2.4 | 34.7±4.4* |
| First 5 minutes | 17.2±2.4 | 22.6±2.7 | 27.9±4.3 |
| 35 min test session | 20.1±3.1 | 27.8±2.2 | 35.5±4.2* |
| Exp. 2: BIBO3304 | |||
| Long training: Day 2 test | Vehicle | 20 μg | 40 μg |
| 30 sec pre-cue | 34.1±5.7 | 35.6±5.9 | 35.8±6.1 |
| First 5 minutes | 23.6±5.5 | 28.2±5.0 | 28.1±4.7 |
| 35 min test session | 30.9±5.7 | 33.7±6.0 | 35.5±6.0 |
| Exp. 2: BIIE0246 | |||
| Long training: 1 month test | Vehicle | 2.5 mg/kg | 5 mg/kg |
| 30 sec pre-cue | 33.3±6.0 | 28.1±7.2 | 27.8±7.2 |
| First 5 minutes | 31.3±6.1 | 34.8±7.9 | 27.3±5.7 |
| 35 min test session | 28.6±5.3 | 28.9±7.6 | 28.6±7.0 |
| Exp. 3: MTIP | |||
| Long training: 1 month test | Vehicle | 10 mg/kg | 20 mg/kg |
| 30 sec pre-cue | 21.0±1.5 | 27.4±5.0 | 25.0±3.3 |
| First 5 minutes | 23.7±2.6 | 33.9±6.5 | 25.6±4.5 |
| 35 min test session | 20.0±2.5 | 26.7±4.6 | 22.6±3.3 |
| Exp. 3: D-Phe CRF(12-41) | Vehicle | 10 μg | |
| Long training: 1 month test | 23.8±2.7 | 27.6±3.8 | |
| 30 sec pre-cue | 25.9±3.3 | 31.2±4.9 | |
| First 5 minutes | 24.5±2.9 | 27.5±3.6 | |
| 35 min test session | |||
| Exp. 3: D-Phe CRF(12-41) | |||
| Short training: Day 2 test | Vehicle | 10 μg | |
| 30 sec pre-cue | 22.5±4.6 | 20.5±2.1 | |
| First 5 minutes | 18.5±4.0 | 17.2±1.9 | |
| 35 min test session | 20.6±4.8 | 20.5±2.2 | |
different from vehicle, p<0.05 following a significant ANOVA.
Short training (1 day)
Ventricular NPY injections decreased non-incubated fear that is expressed 2 days after training (Fig. 2B). The statistical analysis included the between-subjects factor of NPY Dose (0, 5 or 10 μg), and the within-subjects factor of Cue Trial. This analysis revealed significant main effects of NPY Dose (F(2,21)=11.6, p<0.01) and Cue Trial (F(2,42)=9.8, p<0.01), but no significant interaction between the two factors (p>0.1). Post-hoc group differences in suppression ratios are indicated in Fig. 2B. Unlike the long training condition, NPY injections increased the 30-sec precue lever presses and total lever presses during the test session (p values<0.05; Table 1).
Experiment 2: Effect of Y1 and Y2 receptor antagonism on conditioned fear
NPY Y1 receptor antagonism (day 2 test)
Ventricular injections of BIBO3304 had no effect on the expression of conditioned fear 2 days after long training (10 days). The mean±sem suppression ratio values of the test session were: Vehicle: 0.29±0.08; 20 μg BIBO3304: 0.23±0.07; and 40 μg BIBO3304: 0.16±0.05 (n=22; 7-8 per dose). The statistical analysis included the between-subjects factor of BIBO3304 Dose, and the within-subjects factor of Cue Trial. The statistical analysis demonstrated a significant effect of Cue Trial (F(2,38)=10.6, p<0.01), but no significant effect of BIBO3304 Dose or an interaction between the two factors (p values >0.1). BIBO3304 injections had no effect on precue or total session lever presses (p>0.1, Table 1).
NPY Y2 receptor antagonism (1 month test)
Systemic injections of BIIE0246 had no effect on the expression of conditioned fear 1 month after long training. The mean±sem suppression ratio values of the test session were: Vehicle: 0.58±0.09; 2.5 mg/kg BIIE0246: 0.58±0.11; and 5 mg/kg BIIE0246: 0.53±0.11 (n=23; 7-8 per dose). The statistical analysis included the between-subjects factor of BIIE0246 Dose and the within-subjects factor of Cue Trial. This analysis demonstrated a significant effect of Cue Trial (F(2,20)=43.0, p<0.01), but no significant effect of BIIE0246 Dose or an interaction between the two factors (p values >0.1). BIIE0246 injections had no effect on precue or total session lever presses (p>0.1, Table 1).
Experiment 3: Effect of CRF receptor antagonists on conditioned fear
Long training (10 days)
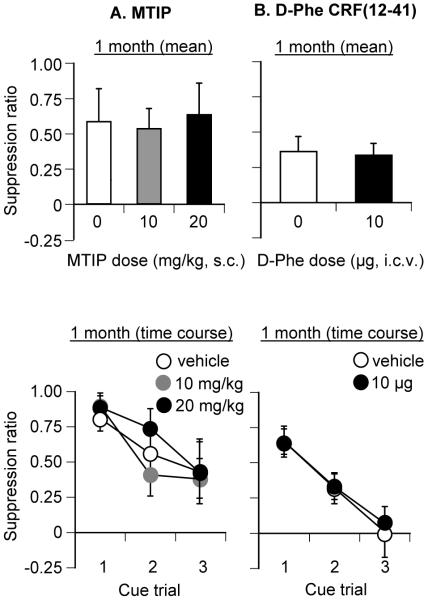
Systemic injections of MTIP or ventricular injections of D-Phe CRF(12-41) had no effect on the expression of conditioned fear 1 month after training (Fig. 3A,B). The statistical analyses for each test included the between-subjects factor of MTIP Dose (0, 10 or 20 mg/kg) or D-Phe CRF(12-41) Dose (0 or 10 μg), and the within-subjects factor of Cue Trial. The analysis for MTIP revealed a significant effect of Cue Trial (F(2,38)=24.9, p<0.01), but no significant effect of MTIP Dose or an interaction between the two factors (p values>0.1). The analysis for D-Phe CRF(12-41) revealed a significant effect of Cue Trial (F(2,46)=20.1, p<0.01), but no significant effect of D-Phe CRF(12-41) Dose or an interaction between the two factors (p values >0.1). MTIP or D-Phe CRF(12-41) injections had no effect on precue or total session lever presses (p>0.1, Table 1).
Figure 3. Effect of MTIP and D-Phe CRF (12-41) on conditioned fear: long (10 d) training.
CRF receptor blockade had no effect on conditioned fear: (A) MTIP: Mean (±sem) test suppression ratios after systemic injections of vehicle (10% Tween 80) and MTIP (10 and 20 mg/kg, s.c.) in rats tested one month after long training (10 days) (n=22; 7-8 per dose). (B) D-Phe CRF(12-41): Mean (±sem) test suppression ratios after ventricular injections of vehicle (saline) and D-Phe CRF(12-41) (10 μg) in rats tested one month after long training (10 days) (n=25, n=12-13 per dose). Top panels represent mean suppression ratios across the 3 cue trials. Bottom panels represent trial-by-trial suppression ratios. Abbreviations: D-Phe: D-Phe CRF(12-41).
Short training (1 day)
Ventricular injections of D-Phe CRF(12-41) had no effect on conditioned fear 2 days after training. The mean±sem suppression ratio values of the test session were: Vehicle: 0.49±0.09, and 10 μg D-Phe CRF(12-41): 0.57±0.09 (n=10 per group). The statistical analysis demonstrated a significant effect of Cue Trial (F(2,36)=12.7, p<0.01), but no significant effect of D-Phe CRF(12-41) Dose or an interaction between the two factors (p values >0.1). Injections of D-Phe CRF(12-41) had no effect on precue or total session lever presses (p>0.1, Table 1).
Discussion
We found that the fear response, as measured by conditioned suppression of lever pressing during a discrete tone cue previously paired with shock, was higher 1 month after 10 sessions of fear training than after 2 days. We interpret these findings, which replicate those from our recent study (Pickens et al., 2009), to suggest that conditioned fear following an extended training period incubates over time. We also found that ventricular NPY injections decreased the expression of the incubated fear response one month after training. In contrast, ventricular injections of the non-selective CRF receptor antagonist D-Phe CRF(12-41) or systemic injections of the selective CRF1 receptor antagonist MTIP were ineffective. We also assessed whether NPY and D-Phe CRF(12-41) injections differentially affect incubated fear (one month after long training) and non-incubated fear (high fear 2 days after short, 1 day training). We found no evidence that this is the case: as with incubated fear, NPY but not D-Phe CRF(12-41) injections decreased non-incubated fear. Additionally, we found that ventricular injections of the Y1 receptor antagonist BIBO3304 or systemic injections of the Y2 receptor antagonist BIIE0246 had no effect on conditioned suppression 2 days or 1 month following long training, respectively. Thus, under our experimental conditions an endogenous role of NPY transmission in fear incubation has not been established. Our results extend previous work on the profound effect of exogenous NPY on conditioned fear responses in several procedures, but do not support the notion that NPY transmission differentially modulates incubated versus non-incubated fear. Somewhat surprisingly, we did not find evidence that activation of CRF receptors plays a role in conditioned fear, as assessed in the conditioned suppression procedure.
Methodological considerations
Two main methodological issues should be considered in interpreting our data. The first is that ventricular NPY injections cause hunger and increase feeding (Clark et al., 1984; Leibowitz, 1995; Levine et al., 1984). Thus, effects of NPY on fear measured via food-reinforced or food-associated responding could be secondary to its effect on appetite. This possibility is unlikely, because while NPY (10 but not 5 μg) increased precue lever-pressing in rats tested 2 days after short training (Table 1), this was not the case in rats tested either 2 days or 1 month after long training. Also, conditioned suppression was decreased two days after short training at a dose that did not increase baseline lever pressing (5 μg). Additionally, the conditioned suppression score is based on a ratio of lever-pressing during the tone with lever-pressing in the period before the tone, and consequently is relatively insensitive to changes in baseline (precue) lever-pressing (Millenson et al., 1974). Finally, the effects of NPY to decrease conditioned fear in the present study are consistent with an extensive body of evidence indicating that central administration of NPY decreases conditioned, as well as unconditioned, fear (Heilig, 2004). This effect of NPY was observed in conditioned fear tasks with no appetitive component (Broqua et al., 1995; Gutman et al., 2008; Tovote et al., 2004) or in a task with a feeding component (conflict test) where the unpunished feeding response was unaffected by central amygdala NPY injections (Heilig et al., 1993).
A second potential methodological issue in Exp. 2 is the use of a single dose of D-Phe CRF(12-41). However, the dose we used (10 μg) is either similar or higher than the D-Phe CRF(12-41) doses that (1) decrease footshock-induced reinstatement of cocaine or alcohol seeking (Erb et al., 1998; Le et al., 2000; Liu et al., 2002), (2) decrease defensive burying in cocaine withdrawn rats (Basso et al., 1999), and (3) increase time in open arms of an elevated plus maze after social defeat stress (Menzaghi et al., 1994). Additionally, D-Phe CRF(12-41) is a more potent CRF receptor antagonist than alpha-helical CRF(9-41) (Menzaghi et al., 1994), and the dose of D-Phe CRF(12-41) we used was higher than the alpha-helical CRF(9-41) dose (5 μg) that reduced cued fear-potentiated startle (Swerdlow et al., 1989). Finally, we found that doses of MTIP similar to or higher than those that reversed alcohol withdrawal-induced anxiety, dependence-induced alcohol drinking, and stress-induced reinstatement of alcohol seeking (Gehlert et al., 2007) had no effect on the expression of incubated fear. Together, it is unlikely that the negative findings with the CRF receptor antagonists are due to the doses used in our study.
Role of NPY in conditioned fear
We found that NPY injections decreased fear expression and that this effect was independent of the duration of training and the incubation period. Thus, NPY injections decreased both incubated fear (1 month after long training), and non-incubated high fear (2 days after short training). Our findings are in agreement with those from previous studies on the effect of ventricular NPY injections on signaled punished responding (Heilig et al., 1992) in the Geller-Seifter operant conflict test (Geller et al., 1960) and on conditioned fear responses. Broqua et al. (1995) and Gutman et al. (2008) reported that NPY decreases fear-potentiated startle and Tovote et al. (2004) reported that NPY decreases fear-induced tachycardia.
In Exp. 2 we used Y1 and Y2 receptor antagonists to study the role of endogenous NPY in fear incubation. We used this approach because blockade of post-synaptic Y1 receptors (resulting in decreased NPY transmission) increases unconditioned anxiety- and stress-like responses (Kask et al., 2000; Kask et al., 1996), while blockade of presynaptic Y2 receptors (resulting in increased NPY transmission) has the opposite effect (Bacchi et al., 2006). To this end, we found no evidence that the observation that exogenous NPY decreased both incubated and non-incubated fear reflects a potential role of endogenous NPY, which is activated by fear cues. Thus, the Y1 receptor antagonist BIBO3304 did not increase fear on day 2 and the Y2 receptor antagonist BIIE0246 did not decrease fear after 1 month in our fear incubation procedure.
Together, while interpretation of negative pharmacological data is not straightforward, our data do not support the notion that endogenous NPY plays a role in conditioned fear. Our negative results with the Y1 receptor antagonist are in agreement with those from previous studies on fear-potentiated startle, conditioned freezing and fear-induced tachycardia (Fendt et al., 2009; Gutman et al., 2008; Tovote et al., 2004). These observations may reflect the general principle that NPY and other neuropeptides serve as “alarm systems”, which are endogenously activated only under extreme stress conditions, and typically are not engaged under more mild stressful conditions (Hokfelt et al., 1984). Finally, a question for future research is which NPY receptors mediate the effect of exogenous NPY on fear incubation. We did not pursue this question for two main reasons. The first reason is that we could not establish that endogenous NPY plays a role in fear incubation. The second reason is that our data indicate that, under our experimental conditions, the effect of exogenous NPY is not selective to fear incubation, which is the focus of our research.
Role of CRF in conditioned fear
We found that neither the CRF1 receptor antagonist MTIP nor the non-selective CRF receptor antagonist D-Phe CRF(12-41) had an effect on incubated fear 1 month after long training. Additionally, D-Phe CRF(12-41) had no effect on non-incubated fear 2 days after short training. These results are in agreement with those from previous studies demonstrating that CRF receptor blockade had no effect on fear potentiated startle after 20 pairings of short discrete cues (3.7-30 sec) with a mild intensity shock (0.4-0.6 mA) (de Jongh et al., 2003; Risbrough et al., 2009; Walker et al., 2009). In contrast, in experiments in which investigators paired short discrete cues (3-3.2 sec) with 120 mild footshocks (0.4 mA) or 20 stronger shocks (1.2 mA), they reported that CRF receptor blockade decreased cued fear-potentiated startle (Schulz et al., 1996; Swerdlow et al., 1989). These results are consistent with those of Griebel (2002) who reported that CRF receptor blockade appears to affect measures of unconditioned anxiety only in strongly stressed, but not in minimally stressed, laboratory rodents, as well as results from the operant conflict model in which alpha-helical CRF(9-41) had no effect under relatively low stress conditions (Britton et al., 1986). In our studies, however, CRF receptor antagonism was ineffective regardless of the number of previous tone-shock pairings (10 in the short training or 100 in the long training).
The lack of an effect of CRF receptor blockade in our procedure may be due to the short duration of our fear cue. Walker et al. (2008; 2009) found that blockade of CRF1 receptors with GSK876008 decreased fear potentiated startle induced by an 8-minute cue but not by a 3.7-second cue. These authors interpreted their data to suggest that CRF has a role in what they refer to as sustained fear (or anxiety), but not in phasic (or acute) fear. Walker et al. (2009) suggested that cues of 1 min or less are short duration cues. Their negative data with a CRF1 receptor antagonist for 3.7-sec fear cues, and our negative data with both D-Phe CRF(12-41) and MTIP with 30-sec fear cues, suggest that the acute fear state induced by short duration cues is CRF independent.
Finally, a role for CRF receptors in fear induced by long duration cues would be in line with previous results demonstrating that CRF receptor antagonism decreases the expression of contextual fear conditioning (Deak et al., 1999; Hikichi et al., 2000; Kalin et al., 1990; Skorzewska et al., 2008; Walker et al., 2009). Additionally, the effect of ventricular or bed nucleus of the stria terminalis injections of alpha-helical CRF(9-41) (a non-selective CRF receptor antagonist) on shock-induced reinstatement of fear-potentiated startle (Waddell et al., 2008) is in agreement with a putative role of CRF in contextual fear, since shock-induced reinstatement of conditioned fear depends on associations between the context and shock (Bouton et al., 1979).
Concluding remarks
Using the conditioned suppression procedure (Estes et al., 1941), we found that injections of NPY, but not CRF receptor antagonists, reduced the expression of both incubated and non-incubated fear. These results extend previous reports on the role of NPY in the expression of fear conditioning using fear-potentiated startle (Broqua et al., 1995; Gutman et al., 2008) and fear-induced tachycardia (Tovote et al., 2004), and are in agreement with results from studies showing that CRF receptor antagonism has no effect on fear potentiated-startle induced by exposure to short duration discrete cues (de Jongh et al., 2003; Risbrough et al., 2009; Walker et al., 2009).
In our initial pharmacological study of fear incubation, we found similar effects (or lack of effects) of pharmacological manipulations on incubated versus non-incubated fear, suggesting that the two fear phenomena are mechanistically similar. Additionally, under our experimental conditions, the magnitude of long-training incubated fear and short-training non-incubated fear is similar. These observations raise the possibility that what controls fear incubation is not a sensitization-like mechanism that leads to enhanced fear 1 month after long training, but a tolerance-like mechanism that develops during the 10 training days and causes inhibition of fear in the days immediately after training.
Finally, to the degree that our fear incubation model is relevant to the human condition of delayed-onset PTSD, which occurs in almost 40% of military PTSD cases and 15% of civilian cases (Andrews et al., 2007), our data suggest that pharmacological manipulations that increase central NPY transmission should be considered in PTSD treatment.
Acknowledgments
Research was supported by the Intramural Research Programs of the NIDA and NIAAA, NIH. We thank Evan Goldart for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319–1326. doi: 10.1176/appi.ajp.2007.06091491. [DOI] [PubMed] [Google Scholar]
- Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. J Comp Physiol Psychol. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7(2):157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Bacchi F, Mathe AA, Jimenez P, Stasi L, Arban R, Gerrard P, Caberlotto L. Anxiolytic-like effect of the selective neuropeptide Y Y2 receptor antagonist BIIE0246 in the elevated plus-maze. Peptides. 2006;27(12):3202–3207. doi: 10.1016/j.peptides.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Radcliffe RA, Logue SF, Wehner JM. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behav Neurosci. 2002;116(6):947–957. doi: 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145(1):21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5(4):368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Britton KT, Lee G, Vale W, Rivier J, Koob GF. Corticotropin releasing factor (CRF) receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat. Brain Res. 1986;369(12):303–306. doi: 10.1016/0006-8993(86)90539-1. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6(3):215–222. [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115(1):427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- de Jongh R, Groenink L, van der Gugten J, Olivier B. Light-enhanced and fear-potentiated startle: temporal characteristics and effects of alpha-helical corticotropin-releasing hormone. Biol Psychiatry. 2003;54(10):1041–1048. doi: 10.1016/s0006-3223(03)00468-2. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong ML, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140(1):79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Diven K. Certain determinants in the conditioning of anxiety reactions. J Psychol Interdisciplinary and Applied. 1937;3:291–308. [Google Scholar]
- Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F, Engel W, Eberlein W, Rudolf K. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol. 1999;384(23):R3–5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18(14):5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK, Skinner BF. Some quantitative properties of anxiety. J Exp Psychol. 1941;29:390–400. [Google Scholar]
- Fendt M, Burki H, Imobersteg S, Lingenhohl K, McAllister KH, Orain D, Uzunov DP, Chaperon F. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacology (Berl) 2009;206(2):291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. Corticotropin-releasing factor in the caudal pontine reticular nucleus mediates the expression of fear-potentiated startle in the rat. Eur J Neurosci. 1997;9(2):299–305. doi: 10.1111/j.1460-9568.1997.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24(15):3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27(10):2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller I, Seifter J. The effects of meprobamate, barbiturates, d-amphetamine and promazine on experimentally induced conflict in the rat. Psychopharmacologia (Berl) 1960;1(6):482–492. [Google Scholar]
- Ghitza UE, Nair SG, Golden SA, Gray SM, Uejima JL, Bossert JM, Shaham Y. Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J Neurosci. 2007;27(43):11522–11532. doi: 10.1523/JNEUROSCI.5405-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleitman H, Holmes PA. Retention of incompletely learned CER in rats. Psychon Sci. 1967;7(1):19–20. [Google Scholar]
- Golin S. Incubation effect: role of awareness in an immediate versus delayed test of conditioned emotionality. J Abnorm Soc Psychol. 1961;63:534–539. doi: 10.1037/h0043261. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301(1):333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28(48):12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38(4):213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17(2):80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8(4):357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Koob GK, Britton KT. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regul Pept. 1992;41(1):61–69. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- Hendersen RW. Forgetting and conditioned fear inhibition. Learn Motiv. 1978;9:16–30. [Google Scholar]
- Hikichi T, Akiyoshi J, Yamamoto Y, Tsutsumi T, Isogawa K, Nagayama H. Suppression of conditioned fear by administration of CRF receptor antagonist CP-154,526. Pharmacopsychiatry. 2000;33(5):189–193. doi: 10.1055/s-2000-7587. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Goldstein M. Chemical anatomy of the brain. Science. 1984;225(4668):1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6(2):111–119. [PMC free article] [PubMed] [Google Scholar]
- Hunt HF, Brady JV. Some effects of electro-convulsive shock on a conditioned emotional response (“anxiety”) J Comp Physiol Psychol. 1951;44(1):88–98. doi: 10.1037/h0059967. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Takahashi LK. Fear-motivated behavior induced by prior shock experience is mediated by corticotropin-releasing hormone systems. Brain Res. 1990;509(1):80–84. doi: 10.1016/0006-8993(90)90311-x. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J. Inhibition of amphetamine- and apomorphine-induced behavioural effects by neuropeptide Y Y(1) receptor antagonist BIBO 3304. Neuropharmacology. 2000;39(7):1292–1302. doi: 10.1016/s0028-3908(99)00199-9. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26(3):259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kask A, Rago L, Harro J. Anxiogenic-like effect of the neuropeptide Y Y1 receptor antagonist BIBP3226: antagonism with diazepam. Eur J Pharmacol. 1996;317(23):R3–4. doi: 10.1016/s0014-2999(96)00838-2. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38(4):141–151. doi: 10.1016/j.npep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Watchus W, Juzytsch W, Shalev U, Shaham Y. The role of corticotropin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Brain peptides and obesity: pharmacologic treatment. Obes Res. 1995;3(Suppl 4):573S–589S. doi: 10.1002/j.1550-8528.1995.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5(6):1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22(18):7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister DE, McAllister WR. Incubation of fear: an examination of the concept. J Exp Res Personality. 1967;2:180–190. [Google Scholar]
- McMichael JS. Incubation of anxiety and instrumental behavior. J Comp Physiol Psychol. 1966;61(2):208–211. doi: 10.1037/h0023148. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, Koob GF. Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J. Pharmacol. Exp. Ther. 1994;269:564–572. [PubMed] [Google Scholar]
- Miczek KA. Effects of scopolamine, amphetamine and benzodiazepines on conditioned suppression. Pharmacol Biochem Behav. 1973;1(4):401–411. doi: 10.1016/0091-3057(73)90006-3. [DOI] [PubMed] [Google Scholar]
- Millenson JR, Leslie J. The conditioned emotional response (CER) as a baseline for the study of anti-anxiety drugs. Neuropharmacology. 1974;13(1):1–9. doi: 10.1016/0028-3908(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5 edn. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-Lasting Incubation of Conditioned Fear in Rats. Biol Psychiatry. 2009;65(10):881–886. doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34(6):1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai RR, Ma LY, He PF, Fluharty SJ. Intracerebroventricular administration of angiotensin type 1 (AT1) receptor antisense oligonucleotides attenuate thirst in the rat. Regul Pept. 1995;59(2):183–192. doi: 10.1016/0167-0115(95)00111-n. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, 3rd, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A. 1996;93(19):10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3-36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol. 2005;17(7):452–457. doi: 10.1111/j.1365-2826.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- Skorzewska A, Bidzinski A, Hamed A, Lehner M, Turzynska D, Sobolewska A, Maciejak P, Szyndler J, Wislowska-Stanek A, Plaznik A. The influence of CRF and alpha-helical CRF(9-41) on rat fear responses, c-Fos and CRF expression, and concentration of amino acids in brain structures. Horm Behav. 2008;54(5):602–612. doi: 10.1016/j.yhbeh.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9-41) Neuropsychopharmacology. 1989;2(4):285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Tovote P, Meyer M, Beck-Sickinger AG, von Horsten S, Ove Ogren S, Spiess J, Stiedl O. Central NPY receptor-mediated alteration of heart rate dynamics in mice during expression of fear conditioned to an auditory cue. Regul Pept. 2004;120(13):205–214. doi: 10.1016/j.regpep.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26(10):1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Waddell J, Bouton ME, Falls WA. Central CRF receptor antagonist a-helical CRF9-41 blocks reinstatement of extinguished fear: the role of the bed nucleus of the stria terminalis. Behav Neurosci. 2008;122(5):1061–1069. doi: 10.1037/a0013136. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Yanaihara N, Hakanson R. Evidence for different pre-and post-junctional receptors for neuropeptide Y and related peptides. Regul Pept. 1986;13(34):307–318. doi: 10.1016/0167-0115(86)90048-0. [DOI] [PubMed] [Google Scholar]
- Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009;34(6):1533–1542. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213(12):29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol. 1998;125(3):549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13(7):799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]