Abstract
Objective
To determine if levels of soluble intercellular adhesion molecule-1 (sICAM-1), a marker of alveolar epithelial and endothelial injury, differ in patients with hydrostatic pulmonary edema and acute lung injury (ALI) and are associated with clinical outcomes in patients with ALI.
Design, setting, and participants
Measurement of sICAM-1 levels in (1) plasma and edema fluid from 67 patients with either hydrostatic pulmonary edema or ALI enrolled in an observational, prospective single center study, and (2) in plasma from 778 patients with ALI enrolled in a large multi-center randomized controlled trial of ventilator strategy.
Results
In the single-center study, levels of sICAM-1 were significantly higher in both edema fluid and plasma (median 938 and 545 ng/ml, respectively) from ALI patients compared to hydrostatic edema patients (median 384 and 177 ng/ml, P < 0.03 for both comparisons). In the multi-center study, higher plasma sICAM-1 levels were associated with poor clinical outcomes in both unadjusted and multivariable models. Subjects with ALI whose plasma sICAM-1 levels increased over the first 3 days of the study had a higher risk of death, after adjusting for other important predictors of outcome (odds ratio 1.48; 95% CI 1.03–2.12, P = 0.03).
Conclusions
Both plasma and edema fluid levels of sICAM-1 are higher in patients with ALI than in patients with hydrostatic pulmonary edema. Higher plasma sICAM-1 levels and increasing sICAM-1 levels over time are associated with poor clinical outcomes in ALI. Measurement of sICAM-1 levels may be useful for identifying patients at highest risk of poor outcomes from ALI.
Keywords: Acute respiratory distress syndrome, Acute lung injury, Intracellular adhesion molecule-1, Pulmonary edema
Introduction
Previous studies on biomarkers in the plasma [1–3], edema fluid,[4] and urine[5] of patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) have demonstrated that specific markers may provide insight into both the pathogenesis and prognosis of this frequently fatal syndrome. For instance, biomarker studies that demonstrated elevation of markers of endothelial activation and dysregulated coagulation [6] led to recognition of this mechanism of injury as a key component of ALI [7, 8], and subsequently to the development of novel therapeutic approaches focused on pulmonary vascular microthrombi [9, 10]. Similarly, ancillary studies of plasma biomarkers in patients enrolled in the NHLBI ARDS Network’s trial of low tidal volume ventilation have provided important insights into the mechanism of benefit of this effective supportive therapy [1–3].
Soluble intercellular adhesion molecule-1 (sICAM-1) is an adhesion molecule expressed on both alveolar epithelial cells and vascular endothelium [11, 12] and is thought to play an important role in neutrophil recruitment and trafficking into the lung [13]. Experimental animal studies have demonstrated increased sICAM-1 expression in the setting of acute lung injury [14, 15], and elevated plasma levels of sICAM-1 were associated with poor outcomes in a small cohort of adults with acute lung injury [16] and in pediatric lung injury [17]. Since injury to the alveolar epithelium and vascular endothelium is of central importance in the pathogenesis of ALI and ARDS [7], we hypothesized that release of sICAM-1 into the airspaces would be an important prognostic indicator in adult patients with ALI/ARDS. To test this hypothesis, we first determined whether sICAM-1 could be detected in the airspaces of patients with ALI in a small, single-center study, with the plan to validate and further explore the prognostic and pathogenic significance of sICAM-1 in a larger cohort if the single-center results were promising [3, 4, 18]. Because high levels of sICAM-1 were measured in both the plasma and pulmonary edema fluid of these subjects and were associated with poor clinical outcomes, we then determined the prognostic value of plasma sICAM-1 in a large multi-center study of patients with ALI/ARDS. We also tested the prognostic value of changes in sICAM-1 levels over time (in both edema fluid and plasma in the single-center study, and plasma alone in the multi-center trial). Some of the findings of this study have been previously reported in abstract form [19–21].
Materials and methods
Single-center study
The study was approved by the Committee on Human Research at the University of California, San Francisco. Sixteen patients with hydrostatic pulmonary edema and 51 patients with ALI/ARDS were selected, based on availability of adequate sample volume, from a database of over 300 patients who were previously enrolled in a prospective study to collect pulmonary edema fluid and plasma from patients with acute pulmonary edema. Hydrostatic pulmonary edema [22] and ALI/ARDS [23] were defined based on previously published definitions. At the time of entry into the prospective database, all patients had pulmonary edema fluid and plasma collected within the first 4 h after endotracheal intubation. Pulmonary edema fluid was aspirated with a 14Fr suction catheter as previously described [23]. A subset of patients with ALI/ARDS (n = 31) had serial samples of pulmonary fluid obtained over the subsequent 8 h. All samples were immediately centrifuged at 3,000×g for 10 min, and supernatants were aliquoted and frozen until assayed for sICAM-1.
Multi-center study
Clinical data and biological samples for the multi-center study were obtained from patients enrolled in a randomized controlled trial of lower tidal volume, plateau-pressure limited ventilation conducted by the National Heart, Lung, and Blood Institute’s ARDS Network [24]. This trial enrolled 861 patients to test the hypothesis that ventilation with a lower tidal volume, plateau pressure-limited strategy would reduce mortality in patients with ALI [24]. The details and results of the clinical trial have been published earlier [24]. Briefly, patients were randomized to ventilation with a tidal volume of either 6 or 12 ml/kg predicted body weight; once the benefit of the lower tidal volume strategy had been demonstrated, an additional 41 patients were assigned to the lower tidal volume to complete a factorialized trial of lisofylline versus placebo [25] and these patients are also included in the current analysis. All patients were followed until death, 180 days or until discharge to home with unassisted breathing. The trial demonstrated a significant reduction in 180-day mortality in the lower tidal volume treatment group (31 vs. 40%, P = 0.007). In a factorial design, ketoconazole [26] and lisofylline [25] were also administered to subsets of patients (n = 117 and n = 116, respectively) as investigational therapies for ALI, neither of which affected mortality. Inclusion and exclusion criteria are described in the original publication, and the institutional review boards of all involved hospitals approved the trial [24]. Plasma samples were obtained from all the patients at enrollment prior to randomization, day 1 and day 3 of the clinical trial. Plasma samples were centrifuged at 3,000×g, and the supernatants were aliquoted and frozen for later use.
Measurement of sICAM-1 in plasma and pulmonary edema fluid
sICAM-1 levels were measured in duplicate in plasma and pulmonary edema fluid samples by commercially available ELISA (Biosource International, Camarillo CA) against a standard curve of recombinant human sICAM-1 provided by the manufacturer. The lower limit of detection was 1.6 ng/ml; the intra-assay coefficient of variation was 9.5%, while the inter-assay coefficient of variation was 7.8%. Diluent was provided by the manufacturer.
Statistical analysis
Statistical analysis was performed with STATA/SE 9.2 (College Station, TX) and SPSS 13.0 for Macintosh (Chicago, IL). For comparison of clinical and demographic characteristics and clinical outcomes between the two groups, Student’s T-tests were used to compare normally distributed data, and the Mann Whitney U test was used for non-normally distributed data; Chi-square or Fisher’s Exact Test was used for categorical variables. For multiple group comparisons, the Kruskal–Wallis test was used. In the single center pilot study, ventilator-free days were dichotomized at 5 based on the results of exploratory analyses. To analyze serial levels of sICAM-1 in the pulmonary edema fluid in patients with ALI/ARDS, patients in the single-center study were categorized into two groups: those with an increase in sICAM-1 in the edema fluid during the first 8 h after enrollment and those with no change or a fall in sICAM-1 in the edema fluid during the first 8 h after enrollment.
In the multi-center study, multivariable linear and logistic regression models were used to control for potential confounding variables. Because sICAM-1 levels were not normally distributed, we applied natural log-transformation to the plasma sICAM-1 levels in order to fit linear models. We first tested the impact of sICAM-1 on mortality in an unadjusted logistic regression, and then created a multivariable logistic model to control for baseline clinical features that may have affected outcomes, including age, sepsis or trauma as etiology of lung injury, PaO2/FiO2, and severity of illness, as reflected by APACHE III score [27]. These covariates were selected on the basis of the bivariate analysis results and prior clinical research about predictors of outcomes in ALI [28]. We performed similar analyses using linear regression models for the outcomes of ventilator-free days and organ failure-free days. Logistic models were checked using the Hosmer–Lemeshow test and the linktest [29]; linear models were evaluated using residual-based diagnostics. The Wilcoxon signed-rank test was used to evaluate changes in sICAM-1 levels between Day 0 and Day 3, and analysis of covariance was used to test the impact of lower tidal volume ventilation on the change in sICAM-1 levels from Day 0 to Day 3.
Results
Single-center study
Clinical Characteristics of Subjects
Sixteen patients with hydrostatic pulmonary edema and 51 patients with ALI/ARDS were enrolled in the single-center study. Patient characteristics are summarized in Table 1. Causes of hydrostatic pulmonary edema included acute myocardial infarction/ischemia (n = 9), congestive heart failure (n = 4) and acute volume overload (n = 3). Causes of ALI/ARDS included pneumonia (n = 16), nonpulmonary sepsis (n = 12), aspiration of gastric contents (n = 6), reperfusion injury after lung transplantation (n = 6), multiple transfusions (n = 4), drug overdose (n = 3) and other causes (n = 4). Compared with the hydrostatic edema group, significantly more patients with ALI/ARDS were female and smokers. Severity of illness was higher in patients with ALI/ARDS, and clinical outcomes were worse in this group, including significantly fewer days alive and free of mechanical ventilation (ventilator-free days) (Table 1).
Table 1.
Clinical characteristics of patients enrolled in the single center study (n = 67) and the multicenter study (n = 778)
| Patient characteristic | Hydrostatic pulmonary edema n = 16 |
Single-center ALI/ARDS n = 51 |
Multi-center ALI/ARDS n = 778 |
|---|---|---|---|
| Male gender | 88%* | 51% | 59% |
| Caucasian | 69% | 60% | 73% |
| Current smoker | 21% * | 36% | N/A |
| Age (years) | 59 ± 21 | 48 ± 17 | 51 ± 17 |
| A – a O2 difference | 467 ± 175 | 466 ± 146 | N/A |
| PaO2/FiO2 | N/A | N/A | 132 ± 62 |
| Lung injury score | 2.8 ± 0.6 | 2.7 ± 0.7 | 2.7 ± 0.6 |
| Tidal volume, cc/kg PBW | N/A | N/A | 10.7 ± 2.6** |
| Plateau pressure, cm H20 | N/A | N/A | 30 ± 7.7** |
| SAPSII Score | 40 ± 6* | 49 ± 15 | N/A |
| APACHE III score | N/A | N/A | 83 ± 28 |
| Alveolar fluid clearance (%/h) | 9.4 (3.2, 26.1) | 3.0 (0, 8.3) | N/A |
| Ventilator free days | 22 (2, 26) * | 0 (0, 23) | 10 (0, 22) |
| Hospital survival | 75% | 49% | 65% |
| Plasma sICAM-1, ng/ml | 177 (137, 703)* | 545 (232, 1330) | 604 (328, 1022) |
| Edema fluid sICAM-1, ng/ml | 384 (217, 801)* | 938 (675, 1743) | N/A |
SAPSII Simplified Acute Physiology Score II, APACHE III acute physiology and chronic health evaluation III, LIS lung injury score [46]
P-value for comparison with single center ALI patients <0.05
Pre-randomization data
Data as percent, mean ± SD or median (interquartile range), as appropriate
sICAM-1 levels and etiology of pulmonary edema
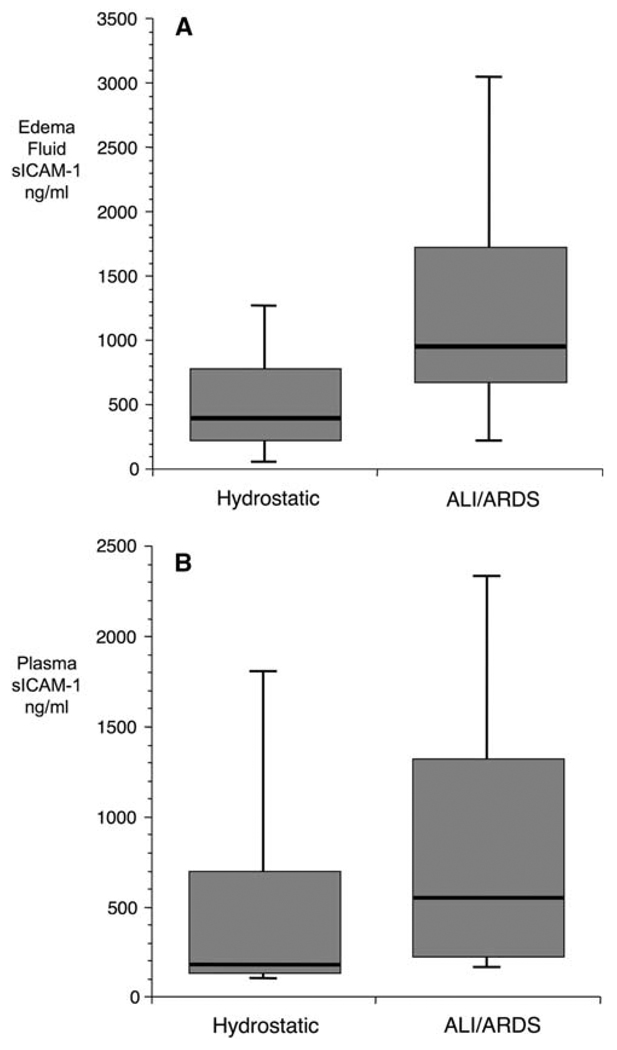
Baseline levels of sICAM-1 were significantly higher in both the pulmonary edema fluid (Fig. 1a) and plasma (Fig. 1b) from patients with ALI/ARDS compared to patients with hydrostatic pulmonary edema. In both the patient groups, baseline edema fluid levels were higher than simultaneous plasma levels, although this difference did not reach statistical significance in the hydrostatic group (data not shown).
Fig. 1.
Boxplot summary of edema fluid (a) and plasma (b) levels in patients with hydrostatic pulmonary edema (n = 16) and ALI/ARDS (n = 51) from the single-center study. Edema fluid sICAM-1 levels were significantly higher in subjects with ALI (median 938 ng/ml, IQR 675–1743) compared to subjects with hydrostatic pulmonary edema (median 384 ng/ml, IQR 217–801) (a; P value for comparison 0.004). Plasma sICAM-1 levels demonstrated a similar pattern [b; median in ALI/ARDS (545 ng/ml, IQR 232–1330) vs. median in hydrostatic (177 ng/ml, IQR 137–703; P = 0.021)]
sICAM-1 Levels and clinical characteristics
Among patients with ALI/ARDS, several clinical characteristics were associated with higher levels of sICAM-1 (Table 2). Smokers had higher levels of sICAM-1 in the plasma [median 1191 (IQR 465–1480) ng/ml] and edema fluid [1,361(920–2,716) ng/ml] compared to non-smokers [plasma 354(194–974) ng/ml, P = 0.03; edema fluid 886 (390–1845) ng/ml, P = 0.04]. Patients with liver failure, as defined by serum bilirubin ≥4 g/dl, had higher levels of sICAM-1 in the plasma [1,191 (629–2229) ng/ml] compared to patients without liver failure [387 (233–1345) ng/ml, P = 0.023]. Gender, race-ethnicity, renal failure, and presence or absence of sepsis were not significantly associated with sICAM-1 levels.
Table 2.
Clinical characteristics significantly associated with plasma sICAM-1 Levels in patients with ALI, single center and multi-center studies
| Dichotomous variables | |||
|---|---|---|---|
| Study | Characteristic | Plasma sICAM, ng/ml (median [IQR]) | P value |
| Single centera | Active smoker | 1,191 (465, 1,480) | 0.04 |
| Not active smoker | 354 (390, 1,845) | ||
| Liver failure (serum bilirubin >4 g/dL) | 1,191 (629, 2,229) | 0.02 | |
| No liver failure | 387 (233, 1,345) | ||
| Multi-centerb | Sepsis | 724 (436, 1,341) | <0.0001 |
| No sepsis | 555 (310, 956) | ||
| Trauma | 346 (237, 605) | <0.0001 | |
| No trauma | 639 (351, 1,044) | ||
| AIDS | 596 (321, 993) | 0.002 | |
| No history of AIDS | 842 (522, 1,512) | ||
| Hepatic encephalopathy | 1,526 (996, 1,586) | 0.02 | |
| No hepatic encephalopathy | 603 (325, 1021) | ||
| Cirrhosis | 1,330 (965, 1,968) | <0.0001 | |
| No cirrhosis | 595 (322, 986) | ||
| Caucasian | 562 (310, 959) | 0.0008 | |
| Non-Caucasian | 713 (414, 1,192) | ||
| Continuous variables | |||
|---|---|---|---|
| Study | Characteristic | Spearman rank coefficient, with sICAM-1 |
P value |
| Multi-center | APACHE III | 0.16 | <0.0001 |
| Age | −0.11 | 0.001 | |
| PaO2:FiO2 | −0.07 | 0.03 | |
| Creatinine | 0.14 | 0.0002 | |
| Plateau pressure | 0.15 | 0.0003 | |
| Respiratory compliance | −0.11 | 0.005 | |
APACHE III Acute physiology and chronic health evaluation III, AIDS acquired immune deficiency syndrome
Not associated with sICAM-1 levels: gender, race-ethnicity, sepsis
Not associated with sICAM-1 levels: gender
sICAM-1 Levels and clinical outcomes of patients with ALI/ARDS
There was a trend towards higher edema fluid and plasma levels of sICAM-1 in patients who eventually died, but these differences did not reach statistical significance (Table 3). Patients with fewer days alive and free of mechanical ventilation (<5 ventilator free days) had significantly higher levels of both plasma and edema fluid sICAM-1 (Table 3).
Table 3.
Clinical outcomes and sICAM-1 levels among patients with ALI/ARDS in the single center study (n = 51)
| Outcome | Value | Plasma sICAM-1 (ng/ml) | P | Edema fluid sICAM-1 (ng/ml) | P |
|---|---|---|---|---|---|
| Ventilator-free days | <5 days | 745 (336, 1,345) | 0.036 | 1327 (880, 2,501) | 0.037 |
| ≥5 days | 295 (175, 999) | 820 (286, 1,394) | |||
| Hospital survival | Died | 737 (316, 1,344) | 0.14 | 1221 (845, 1,959) | 0.14 |
| Lived | 338 (182, 1,033) | 847 (353, 1,534) |
Data as median (interquartile range)
In a subset of patients with ALI/ARDS (n = 31), serial samples of pulmonary edema fluid and plasma were obtained over the 8 h after collection of the first sample. Hospital survival was threefold higher in patients with stable or decreasing levels of sICAM-1 in the edema fluid compared to patients with increasing levels (63% survival vs. 20% survival, P = 0.029). In contrast, changes in plasma ICAM-1 had no association with survival (P = 1.0).
Multi-center study
Baseline clinical characteristics of subjects
Baseline plasma samples were available for study from 778 of the patients enrolled in the clinical trial. Clinical characteristics of these patients are summarized in Table 1 and are similar to clinical characteristics of the 124 patients who were not included in the study because plasma was not available (data not shown). The patients were equally distributed between the two treatment groups (6 and 12 ml/kg tidal volume). Several clinical characteristics were significantly associated with higher baseline sICAM-1 levels in this cohort (Table 2), including sepsis as a risk factor for ALI, higher APACHE III score, lower PaO2/FiO2 ratio, non-Caucasian race-ethnicity, history of AIDS, history of hepatic encephalopathy or cirrhosis, higher baseline creatinine, higher plateau pressure, and lower compliance. Trauma as a risk factor for ALI and increasing age were both associated with lower sICAM-1 levels.
sICAM-1 levels and clinical outcomes
In analyses, adjusting solely for ventilator strategy, higher baseline plasma sICAM-1 levels were associated with poor clinical outcomes. Specifically, in a logistic regression model controlling for ventilator strategy, increasing baseline plasma sICAM-1 was associated with an increased odds of death [odds ratio (OR) 1.22 per one-log increase in sICAM-1 (95% CI 1.02–1.46); P = 0.03] (Table 4). Likewise, higher baseline plasma sICAM-1 levels were strongly associated with fewer ventilator-free days and fewer organ failure-free days in analyses controlling for ventilator strategy alone (Table 4). In multivariable models controlling for other important clinical covariates including sepsis, trauma, APACHE III score, PaO2:FiO2 ratio, and age, the association between sICAM-1 and each outcome remained strong, although the confidence interval was slightly wider and did not exclude unity in the case of mortality (Table 4). There was no evidence of a significant interaction between ventilator strategy and baseline plasma sICAM-1 level. As a sensitivity analysis, we evaluated the effect of treatment with ketoconazole or lisofylline on the multivariable model for mortality and found that they had no impact on the results.
Table 4.
Baseline plasma sICAM-1 levels and clinical outcomes in a multicenter study of 778 patients with ALI/ARDS
| Measurement | Risk of death |
Ventilator-free days |
Organ failure-free days |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) |
P value |
Mean difference (days) |
P value |
Mean difference (days) |
P value |
|
| Baseline plasma ICAM-1, per one-log increase, adjusted for ventilator strategy |
1.22 (1.02, 1.46) | 0.03 | −1.87 (−2.76, −0.97) | <0.001 | −2.32 (−3.24, −1.40) | <0.001 |
| Baseline plasma ICAM-1, per one-log increase, multivariable modela |
1.22 (0.99, 1.49) | 0.06 | −1.57 (−2.45, −0.69) | 0.001 | −1.67 (−2.56, −0.78) | <0.001 |
Controlling for ventilator strategy, APACHE III score, PaO2/FiO2 ratio, sepsis or trauma as risk factors for ALI, and age
Changes in plasma sICAM-1 levels over time
Paired plasma samples from Days 0 and 3 were available for 686 subjects. At baseline, the median plasma sICAM-1 level was 604 ng/ml (IQR 327–1022), similar to plasma levels in the ALI/ARDS patients in the single-center study. On Day 3 after randomization, the median plasma sICAM-1 level was slightly higher, 653 ng/ml (IQR 390–1153; P < 0.0001 for comparison with day 0). Subjects whose plasma sICAM-1 level increased over these 3 days had a trend towards an increased risk of death compared with those whose plasma level decreased over time (mortality 36.0 vs. 29.5%, P = 0.07; Table 5). After adjusting for other important predictors of outcome (age, APACHE III score, P:F ratio, presence of sepsis or trauma, and low tidal volume ventilation), the association between increasing sICAM-1 and death was statistically significant [odds ratio 1.48 (95% CI 1.03–2.12); Table 5]. The change in sICAM-1 levels over time was not affected by ventilator strategy.
Table 5.
Change over time in plasma sICAM-1 levels and mortality in ALI
| Predictor | Odds ratio for death at 180 days (95% CI)a |
P value |
|---|---|---|
| Increase in plasma sICAM-1 over first 3 days, adjusted for ventilator strategy |
1.33 (0.96, 1.84) | 0.09 |
| Increase in plasma sICAM-1 over first 3 days, multivariable modelb |
1.48 (1.03, 2.12) | 0.03 |
Referent group: subjects whose sICAM-1 decreased over first 3 days
Controlling for ventilator strategy, APACHE III score, PaO2/FiO2 ratio, sepsis or trauma as risk factors for ALI, and age
Discussion
This study demonstrates that elevations in sICAM-1, a marker of lung epithelial and endothelial injury, were associated with poor clinical outcomes in two separate cohorts of patients with ALI. In addition, both edema fluid and plasma sICAM-1 levels were higher in subjects with ALI than in a control group of subjects with acute hydrostatic pulmonary edema. These results suggest that sICAM-1 may be a useful biomarker of lung injury in patients with ALI/ARDS. sICAM-1 has been associated with adverse clinical outcomes in patients at risk for ALI/ARDS from trauma or other critical illness [30, 31], pediatric patients with ALI/ARDS [17] and other critical illness [32], and in patients with acute lung injury due to primary graft dysfunction (reperfusion edema) after lung transplantation [33]. Higher levels of plasma sICAM-1 were also associated with adverse clinical outcomes in a small study of adults with ALI/ARDS [16]. However, the current study is the first to show that sICAM-1 is independently associated with adverse clinical outcomes in a large cohort of adults with ALI.
While baseline plasma sICAM-1 levels had prognostic value, increasing plasma sICAM-1 levels over the first 3 days of ALI were also associated with poor outcomes in the larger multi-center cohort. In the single-center study, increasing edema fluid sICAM-1 levels over the first 8 h were associated with poor outcomes, while changes in plasma sICAM-1 over that same time period were not. There may be several explanations for the difference in the prognostic value of the plasma sICAM-1 levels between the single-center and multi-center cohorts. First, the difference in time elapsed between measurements in the two cohorts (8 h in single-center study vs. 3 days in multi-center study) may be responsible, particularly if plasma sICAM-1 levels change more slowly than edema fluid levels. Second, the single-center study was likely underpowered to detect an association between change in plasma sICAM-1 levels over time and clinical outcomes, given its small sample size. Finally, the differences in the underlying patient populations of the two cohorts may be responsible for these differing results.
In addition to its potential prognostic value, measurement of sICAM-1 in patients with acute lung injury may provide insight into the pathogenesis of this complex syndrome. ICAM-1 is a counter receptor for the β2 integrins CD11a/CD18 and CD11b/CD18 and plays an important role in leukocyte migration and localization. ICAM-1 is widely expressed in human tissues, including the vascular endothelium and inflammatory cells, and can be induced by pro-inflammatory stimuli [11]. In the lung, ICAM-1 is highly constitutively expressed by the alveolar epithelium [34] where it is localized predominantly to the apical surface of alveolar epithelial type I cells under baseline conditions [12, 35]. In cell culture, exposure of lung epithelial cells to pro-inflammatory and infectious stimuli leads to increased ICAM-1 expression [36–39]. Similarly, in experimental models, pneumonia and acute lung injury up-regulate expression of ICAM-1 by alveolar epithelial type II cells [34, 40] and lead to shedding of a soluble form of ICAM-1 (sICAM-1) into the airspaces [14, 15]. Although the biologic function of sICAM-1 in the alveolar compartment remains unclear, sICAM-1 has been reported to bind alveolar macrophages and enhance inflammatory cytokine production by alveolar macrophages in response to injurious stimuli [41]. Thus, elevated sICAM-1 levels may be not only a marker of poor prognosis but also an important mediator involved in the pathogenesis of lung epithelial injury. Alternatively, endothelial sources of sICAM-1 may be an important contributor to elevated plasma and/or edema fluid levels, as pulmonary vascular endothelium has also been shown to up-regulate ICAM-1 expression in response to inflammatory stimuli [42].
The specific origin of the sICAM-1 measured in plasma in these study subjects cannot be definitively identified. In the single-center study, the finding that pulmonary edema fluid levels of sICAM-1 were consistently higher than simultaneous plasma levels suggests that the alveolar epithelium is the primary source of plasma sICAM-1. In contrast, the lack of either an interaction between plasma sICAM-1 levels and ventilator strategy or an impact of ventilator strategy on the change in plasma sICAM-1 levels over time in the multi-center study argues against the alveolar epithelium as the sole source of sICAM-1 levels in these subjects. Since both alveolar epithelial and endothelial injury are hallmarks of ALI/ARDS [7], both sources probably contribute to elevated plasma levels in these subjects. Of note, the association between elevated sICAM-1 levels and liver disease observed in both the single-center and multi-center cohorts has been previously demonstrated in patients with diverse types of chronic liver disease. Elevated serum ICAM-1 levels have been previously observed in patients with chronic liver diseases including Hepatitis C [43], non-alcoholic steatohepatitis [44], and hepatocellular carcinoma [45], though the mechanism of these associations remains unclear.
This study has some limitations. First, because plasma was not available on all 902 subjects enrolled in the original multi-center trial, sICAM-1 levels were measured on a subset of the larger study sample. Plasma availability was the sole factor determining inclusion in this study, and important clinical characteristics of the included patients were similar to those of the patients for whom plasma was unavailable, suggesting that the 778 included patients are representative of the larger study population. However, we cannot definitively exclude selection bias. Second, the source of sICAM-1 levels in these patients cannot be definitively identified; however, a biological marker that reflects injury to more than one source may still be valuable in ALI because the pathogenesis of this syndrome involves injury to both the endothelium and epithelium. Finally, participants in randomized controlled trials of novel clinical therapies may not be similar to the broader population of patients with ALI/ARDS. However, the similarity of the results obtained in the single-center study, which was by nature less selective than a randomized controlled trial, suggests that these findings are generalizable to other populations of ALI patients, as do the results of prior analyses of sICAM-1 levels in pediatric ALI [17] and ALI after lung transplantation [33].
In conclusion, elevations in both plasma and pulmonary edema fluid levels of sICAM-1 are associated with poor outcomes in patients with ALI. Measurement of plasma sICAM-1, particularly in combination with other biologic markers, may be useful for identifying patients at highest risk of poor clinical outcomes. In addition, serial measurements of plasma sICAM-1 levels may have value in assessing prognosis and clinical response to new therapies.
Acknowledgments
Supported by NIH/NCRR KL2RR024130 to Dr. Calfee, NIH HL 081332 to Dr. Ware, NIH HL 51856 and P50 HL74005 to Dr. Matthay, NIH HL 04201 to Dr. Eisner and contracts NO-1 HR 46054, 46055, 46056, 46057, 46058, 46060, 46061, 46062, 46063, 46064 with the National Heart Lung and Blood Institute. Dr. Calfee is also supported by the Flight Attendant Medical Research Insitute.
National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network
Network Participants: Cleveland Clinic Foundation, Herbert P. Wiedemann, M.D.,* Alejandro C. Arroliga, M.D., Charles J. Fisher, Jr., M.D., John J Komara, Jr., B.A., R.R.T., Patricia Periz-Trepichio, B S., R.R.T.; Denver Health Medical Center, Polly E. Parsons, M.D., Denver VA Medical Center, Carolyn Welsh, M.D.; Duke University Medical Center, William J. Fulkerson, Jr., M.D.,* Neil MacIntyre, M.D., Lee Mallatratt, R.N., Mark Sebastian, M.D., John Davies, R.R.T., Elizabeth Van Dyne, R.N., Joseph Govert, M.D.; Johns Hopkins Bayview Medical Center, Jonathan Sevransky, M.D., Stacey Murray, R.R.T.; Johns Hopkins Hospital, Roy G. Brower, M.D., David Thompson, M.S., R.N., Henry E. Fessler, M.D.; LDS Hospital, Alan H. Morris, M.D.,* Terry Clemmer, M.D., Robin Davis, R.R.T., James Orme, Jr., M.D., Lindell Weaver, M.D., Colin Grissom, M.D., Frank Thomas, M.D., Martin Gleich, M.D. (posthumous); McKay-Dee Hospital, Charles Lawton, M.D., Janice D’Hulst, R.R.T.; MetroHealth Medical Center of Cleveland, Joel R. Peerless, M.D., Carolyn Smith, R.N.; San Francisco General Hospital Medical Center, Richard Kallet, M.S., R.R.T., John M. Luce, M.D.; Thomas Jefferson University Hospital, Jonathan Gottlieb, M.D., Pauline Park, M.D., Aimee Girod, R.N., B⋅S.N., Lisa Yannarell, R.N., B⋅S.N.; University of California, San Francisco, Michael A. Matthay, M.D.,* Mark D. Eisner, M.D., M.P⋅H., Brian Daniel, R.C⋅P., R.R.T.; University of Colorado Health Sciences Center, Edward Abraham, M.D.,* Fran Piedalue, R.R.T., Rebecca Jagusch, R.N., Paul Miller, M.D., Robert McIntyre, M.D., Kelley E. Greene, M.D.; University of Maryland, Henry J. Silverman, M.D.,* Carl Shanholtz, M.D., Wanda Corral, B⋅S.N., R.N., University of Michigan, Galen B. Toews, M.D.,* Deborah Arnoldi, M.H⋅S.A., Robert H. Bartlett, M.D., Ron Dechert, R.R.T., Charles Watts, M.D.; University of Pennsylvania, Paul N. Lanken, M.D.,* Harry Anderson, III, M.D., Barbara Finkel, M.S⋅N., R.N., C. William Hanson, III, M.D.; University of Utah Hospital, Richard Barton, M.D., Mary Mone, R.N.; University of Washington/Harborview Medical Center, Leonard D. Hudson, M.D.,* Greg Carter, R.R.T., Claudette Lee Cooper, R.N., Annemieke Hiemstra, R.N., Ronald V. Maier, M.D., Kenneth P. Steinberg, M.D.; Utah Valley Regional Medical Center, Tracy Hill, M.D., Phil Thaut, R.R.T.; Vanderbilt University, Arthur P. Wheeler, M.D.,* Gordon Bernard, M.D.,* Brian Christman, M.D., Susan Bozeman, R.N., Linda Collins, Teresa Swope, R.N., Lorraine B. Ware, M.D.
Clinical Coordinating Center: Massachusetts General Hospital, Harvard Medical School, David A. Schoenfeld, Ph.D.,* B. Taylor Thompson, M.D., Marek Ancukiewicz, Ph.D., Douglas Hayden, M.A., Francine Molay, M.S⋅W., Nancy Ringwood, B⋅S.N., R.N., Gail Wenzlow, M.S⋅W., M.P⋅H., Ali S. Kazeroonin, B⋅S.
NHLBI Staff: Dorothy B. Gail, Ph.D., Andrea Harabin, Ph.D.,* Pamela Lew, Myron Waclawiw, Ph.D.
*Steering Committee: Gordon R. Bernard, M.D., Chair; Principal Investigator from each center as indicated by an asterisk.
Data and Safety Monitoring Board: Roger G. Spragg, M.D., Chair, James Boyett, Ph.D., Jason Kelley, M.D., Kenneth Leeper, M.D., Marion Gray Secundy, Ph.D., Arthur Slutsky, M.D.
Protocol Review Committee: Joe G. N. Garcia, M.D., Chair, Scott S. Emerson, M.D., Ph.D., Susan K. Pingleton, M.D., Michael D. Shasby, M.D., William J. Sibbald, M.D.
Footnotes
The members of the NHLBI ARDS Clinical Trials Network are listed in the Appendix.
Contributor Information
Carolyn S. Calfee, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of California, San Francisco, 505 Parnassus Avenue, San Francisco, CA 94143-0111, USA, carolyn.calfee@ucsf.edu, Tel.: +1-415-4761079, Fax: +1-415-502-2126
Mark D. Eisner, Divisions of Pulmonary and Critical Care Medicine and Occupational and Environmental Medicine, Department of Medicine, University of California, San Francisco, San Francisco, CA, USA
Polly E. Parsons, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Fletcher Allen Health Care, University of Vermont, Burlington, VT, USA
B. Taylor Thompson, Pulmonary/Critical Care Unit, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Edward R. Conner, Jr, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of California, San Francisco, 505 Parnassus Avenue, San Francisco, CA 94143-0111, USA.
Michael A. Matthay, Cardiovascular Research Institute, Departments of Medicine and Anesthesia, University of California, San Francisco, CA, USA
Lorraine B. Ware, Division of Allergy, Pulmonary and Critical Care Medicine, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA
References
- 1.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. (discussion 230–232) [DOI] [PubMed] [Google Scholar]
- 3.Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB, Conner ER, Matthay MA. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med. 2001;29:2325–2331. doi: 10.1097/00003246-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 5.McClintock D, Starcher B, Eisner MD, Thompson BT, Hayden DL, Church GD, Matthay MA. Higher urine desmosine levels are associated with mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L566–L571. doi: 10.1152/ajplung.00457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin DB, Wiener-Kronish JP, Murray JF, Green DR, Turner J, Luce JM, Montgomery AB, Marks JD, Matthay MA. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 8.Sapru A, Wiemels JL, Witte JS, Ware LB, Matthay MA. Acute lung injury and the coagulation pathway: potential role of gene polymorphisms in the protein C and fibrinolytic pathways. Intensive Care Med. 2006;32(9):1293–1303. doi: 10.1007/s00134-006-0223-5. [DOI] [PubMed] [Google Scholar]
- 9.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 10.Calfee CS, Matthay MA. Non-ventilatory management of acute lung injury and the acute respiratory distress syndrome. Chest. 2007;131:913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 12.Kang BH, Crapo JD, Wegner CD, Letts LG, Chang LY. Intercellular adhesion molecule-1 expression on the alveolar epithelium and its modification by hyperoxia. Am J Respir Cell Mol Biol. 1993;9:350–355. doi: 10.1165/ajrcmb/9.4.350. [DOI] [PubMed] [Google Scholar]
- 13.Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome—how neutrophils migrate into the lung. Crit Care. 2004;8:453–461. doi: 10.1186/cc2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendez MP, Morris SB, Wilcoxen S, Greeson E, Moore B, Paine R., III Shedding of soluble ICAM-1 into the alveolar space in murine models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;290:L962–L970. doi: 10.1152/ajplung.00352.2005. [DOI] [PubMed] [Google Scholar]
- 15.Beck-Schimmer B, Schimmer RC, Warner RL, Schmal H, Nordblom G, Flory CM, Lesch ME, Friedl HP, Schrier DJ, Ward PA. Expression of lung vascular and airway ICAM-1 after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 1997;17:344–352. doi: 10.1165/ajrcmb.17.3.2861. [DOI] [PubMed] [Google Scholar]
- 16.Agouridakis P, Kyriakou D, Alexandrakis MG, Prekates A, Perisinakis K, Karkavitsas N, Bouros D. The predictive role of serum and bronchoalveolar lavage cytokines and adhesion molecules for acute respiratory distress syndrome development and outcome. Respir Res. 2002;3:25. doi: 10.1186/rr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flori HR, Ware LB, Glidden D, Matthay MA. Early elevation of plasma soluble intercellular adhesion molecule-1 in pediatric acute lung injury identifies patients at increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med. 2003;4:315–321. doi: 10.1097/01.PCC.0000074583.27727.8E. [DOI] [PubMed] [Google Scholar]
- 18.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 19.Conner ER, Ware LB, Modin G, Matthay MA. Elevated pulmonary edema fluid concentrations of soluble intercellular adhesion molecule-1 in patients with acute lung injury: biological and clinical significance. Chest. 1999;116 doi: 10.1378/chest.116.suppl_1.83s. 83S–84S. [DOI] [PubMed] [Google Scholar]
- 20.Ware LB, Conner ER, Modin G, Matthay MA. Clinical significance of endothelial and epithelial markers in pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med. 1998;157:A549. [Google Scholar]
- 21.Ware LB, Conner ER, Modin G, Matthay MA. An early rise in sICAM-1 levels in pulmonary edema fluid predicts mortality in patients with acute lung injury. Am J Respir Crit Care Med. 1999;159:A375. [Google Scholar]
- 22.Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol. 1999;87:1301–1312. doi: 10.1152/jappl.1999.87.4.1301. [DOI] [PubMed] [Google Scholar]
- 23.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 24.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 25.The Acute Respiratory Distress Syndrome Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 26.The Acute Respiratory Distress Syndrome Network. Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2000;283:1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 27.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 28.Eisner MD, Thompson T, Hudson LD, Luce JM, Hayden D, Schoenfeld D, Matthay MA. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 29.Vittinghoff E, Glidden D, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival and repeated measures models. New York, NY: Springer Science Business + Media; 2005. [Google Scholar]
- 30.Kayal S, Jais JP, Aguini N, Chaudiere J, Labrousse J. Elevated circulating E-selectin, intercellular adhesion molecule 1, and von Willebrand factor in patients with severe infection. Am J Respir Crit Care Med. 1998;157:776–784. doi: 10.1164/ajrccm.157.3.9705034. [DOI] [PubMed] [Google Scholar]
- 31.Boldt J, Wollbruck M, Kuhn D, Linke LC, Hempelmann G. Do plasma levels of circulating soluble adhesion molecules differ between surviving and nonsurviving critically ill patients? Chest. 1995;107:787–792. doi: 10.1378/chest.107.3.787. [DOI] [PubMed] [Google Scholar]
- 32.Briassoulis G, Papassotiriou I, Mavrikiou M, Lazaropoulou C, Margeli A. Longitudinal course and clinical significance of TGF-beta1, sL-and sE-Selectins and sICAM-1 levels during severe acute stress in children. Clin Biochem. 2007;40:299–304. doi: 10.1016/j.clinbiochem.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Covarrubias M, Ware LB, Kawut SM, De Andrade J, Milstone A, Weinacker A, Orens J, Lama V, Wille K, Bellamy S, Shah C, Demissie E, Christie JD. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7:2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 34.Burns AR, Takei F, Doerschuk CM. Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol. 1994;153:3189–3198. [PubMed] [Google Scholar]
- 35.Piedboeuf B, Frenette J, Petrov P, Welty SE, Kazzaz JA, Horowitz S. In vivo expression of intercellular adhesion molecule 1 in type II pneumocytes during hyperoxia. Am J Respir Cell Mol Biol. 1996;15:71–77. doi: 10.1165/ajrcmb.15.1.8679224. [DOI] [PubMed] [Google Scholar]
- 36.Barton WW, Wilcoxen S, Christensen PJ, Paine R. Disparate cytokine regulation of ICAM-1 in rat alveolar epithelial cells and pulmonary endothelial cells in vitro. Am J Physiol. 1995;269:L127–L135. doi: 10.1152/ajplung.1995.269.1.L127. [DOI] [PubMed] [Google Scholar]
- 37.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996;153:1850–1856. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 38.Madjdpour C, Oertli B, Ziegler U, Bonvini JM, Pasch T, Beck-Schimmer B. Lipopolysaccharide induces functional ICAM-1 expression in rat alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2000;278:L572–L579. doi: 10.1152/ajplung.2000.278.3.L572. [DOI] [PubMed] [Google Scholar]
- 39.Yu ML, Limper AH. Pneumocystis carinii induces ICAM-1 expression in lung epithelial cells through a TNF-alpha-mediated mechanism. Am J Physiol. 1997;273:L1103–L1111. doi: 10.1152/ajplung.1997.273.6.L1103. [DOI] [PubMed] [Google Scholar]
- 40.Kang BH, Manderschied BD, Huang YC, Crapo JD, Chang LY. Contrasting response of lung parenchymal cells to instilled TNF alpha and IFN gamma: the inducibility of specific cell ICAM-1 in vivo. Am J Respir Cell Mol Biol. 1996;15:540–550. doi: 10.1165/ajrcmb.15.4.8879188. [DOI] [PubMed] [Google Scholar]
- 41.Schmal H, Czermak BJ, Lentsch AB, Bless NM, Beck-Schimmer B, Friedl HP, Ward PA. Soluble ICAM-1 activates lung macrophages and enhances lung injury. J Immunol. 1998;161:3685–3693. [PubMed] [Google Scholar]
- 42.Grau GE, Mili N, Lou JN, Morel DR, Ricou B, Lucas R, Suter PM. Phenotypic and functional analysis of pulmonary microvascular endothelial cells from patients with acute respiratory distress syndrome. Lab Invest. 1996;74:761–770. [PubMed] [Google Scholar]
- 43.Pata C, Yazar A, Altintas E, Polat G, Aydin O, Tiftik N, Konca K. Serum levels of intercellular adhesion molecule-1 and nitric oxide in patients with chronic hepatitis related to hepatitis C virus: connection fibrosis. Hepatogastroenterology. 2003;50:794–797. [PubMed] [Google Scholar]
- 44.Ito S, Yukawa T, Uetake S, Yamauchi M. Serum intercellular adhesion molecule-1 in patients with nonalcoholic steatohepatitis: comparison with alcoholic hepatitis. Alcohol Clin Exp Res. 2007;31:S83–S87. doi: 10.1111/j.1530-0277.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Mei MH, Zeng SE, Shi QF, Liu YM, Qin LL. Expressions of ICAM-1 and its mRNA in sera and tissues of patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:120–125. doi: 10.3748/wjg.v7.i1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]