Abstract
Background
Hyperglycemia is associated with worse outcome in acute stroke patients.
Methods
We conducted a prospective, randomized, multicenter, 3 arm trial [tight control (target 70 – 110 mg/dL), loose control (target 70 – 200 mg/dL), and control usual care (70 – 300 mg/dL)] to assess the feasibility and safety of two insulin infusion protocol targets in acute ischemic stroke patients. The planned sample was 72 subjects.
Results
A total of 74 subjects were enrolled. Seventy two (97%) had data available for the primary analyses and 73 (99%) had three month clinical outcome data. Median age was 67 years, median NIHSS score was 8, median glucose was 163 mg/dL and median time to randomization was 10. 7 hours. Fifty-nine percent of patients were diabetic, 35% received thrombolysis, and 14% of subjects died within 3 months. The loose control and usual care groups had median glucose concentrations of 151 mg/dL. The tight control group had a median glucose concentration of 111 mg/dL. The loose control group spent 90% of the first 24 hours in target and the tight group 44% of time in target. There was only 1 symptomatic hypoglycemic patient in the loose control group (4%) and 0 in the tight control group. The overall rates of hypoglycemia (<55 mg/dL) were 4% in control, 4% in loose and 30% in tight. Exploratory efficacy analysis was conducted.
Conclusions
Insulin infusion for acute ischemic stroke patients is feasible and safe using the insulin infusion protocol in the GRASP trial. Exploratory efficacy analysis supports further comparative study.
Keywords: Stroke, glucose, clinical trial
The association between hyperglycemia and poor outcome following acute ischemic stroke has been of interest for nearly three decades 1. The lack of evidence to guide clinical care in the setting of hyperglycemia has been communicated in the 2003 American Heart Association/American Stroke Association (AHA/ASA) guidelines which suggest that treatment of hyperglycemia may be necessary if levels exceed 300 mg/dL(2). AHA/ASA guidelines from 2007 suggest treating at lower glucose levels based on expert opinion 3. Both of these statements acknowledge the lack of adequate evidence and call for additional clinical research to inform best practice for hyperglycemic acute stroke patients.
Preclinical data in animal models of focal ischemia and reperfusion have long demonstrated the association between hyperglycemia and poor outcome(4,5). Even mild to moderate elevations in glucose portend greater injury. Insulin treatment that controls hyperglycemia can reduce infarct volume and improve performance on functional outcome measures(6,7). Imaging studies in humans demonstrate greater infarct volumes, worse functional outcome(8) and reduced salvageable tissue(9) in hyperglycemic patients.
Data from the critical care literature have supported aggressive insulin infusion therapy to improve clinical outcome in critically ill patients(10). More recently the NICE-SUGAR study suggested that application of a tight control strategy to a broadly selected ICU population resulted in greater mortality in the tight control group (11). These contradictory results have resulted in clinical equipoise for the critical care community and even more uncertainty in other populations. We designed and conducted the Glucose Regulation in Acute Stroke Patients (GRASP) Trial to understand the therapeutic potential for tight versus more conventional glucose control in hyperglycemic acute ischemic stroke patients. The primary objective of the GRASP trial was to assess the safety and feasibility of glucose/insulin/potassium (GIK) therapy, at two different target glucose concentrations in acute ischemic stroke patients.
Methods
The GRASP trial was a multicenter, prospective, randomized, unblinded (with blinded outcomes) study of acute hyperglycemic stroke patient treated with GIK within 24 hours of stroke symptom onset. The trial was designed to assess the safety and feasibility of glucose concentration control using GIK in acute ischemic stroke patients. The International Clinical Trials Registry number is NCT00282867.
Study Population
Patients were enrolled at the University of Virginia (UVA) and Medical College of Georgia (MCG). Eligible patients were older than17, with ischemic stroke onset within 24 hours, with glucose >110 mg/dL, and able to be treated within 2 hours. Potential subjects were excluded for renal dysfunction (creatinine 2. 5 mg/dL or greater), confounding illness, experimental therapy for the enrollment stroke, pregnancy, life threatening condition limiting follow up, missing stratification information, or for standard care indication for insulin infusion. The protocol was approved at both sites by the local Institutional Review Board and signed informed consent was obtained from the patient or legal representative prior to enrollment in all cases.
Randomization and Blinding
Randomization was stratified by glucose concentration and predicted probability of outcome as determined by a previously developed and validated model ( (14,15). Randomization was 1:1:1 and used randomly permuted blocks of randomly chosen block sizes of three or six. The treating physician was unblinded to the assigned treatment but was excluded from assessing three month clinical outcomes. Outcome assessments were blinded.
Treatment Protocol
Patients randomized to the control group were treated as per the standard at that Institution. Treatment of hyperglycemia, excluding IV insulin, was at the discretion of the treating physician but patients were required to get insulin if their blood glucose was > 300 mg/dL as per the AHA/ASA guidelines at that time(2). The target glucose concentration range was prespecified as 70 mg/dL to 300 mg/dL.
All patients who were assigned an insulin infusion received Novolin brand insulin in normal saline (1u/1 ml) as a continuous infusion. Dosing was guided by the eProtocol-insulin electronic support system (16). The eProtocol-insulin provided an individual recommendation for each dose adjustment for each patient based on a validated algorithm. Glucose concentrations were checked by point of care (Accu-chek®) testing of capillary blood every one to four hours (as recommended by eProtocol-insulin) unless the subject was hypoglycemic (<55 mg/dL), in which case the hypoglycemia protocol of 15 minute checks was initiated. A glucose and potassium infusion including one liter of D5NS with 20 mEq of potassium was delivered at a constant rate of 100 mLs per hour during periods of insulin infusion but not if insulin infusion was stopped. In addition, meal insulin (subcutaneous Humalog 1u/15 g carbohydrate) was provided with each meal in the insulin infusion groups. Meals were specified (grams of carbohydrate and protein) by nutrition teams and those who were cleared to eat were fed orally. Tube feeds were allowed per standard care in those NPO. The target glucose concentration for the loose control group was prespecified as 70mg/dL to 200 mg/dL and for tight control was 70 mg/dL to 110 mg/dL. All patients received investigational treatment for five days or until discharge, whichever came first.
Standardized stroke care was provided across sites as recommended by the ASA/AHA stroke guidelines.(2,3) Additionally fluid status and electrolyte status were closely monitored in the insulin infusion patients.
Outcomes
The primary outcomes were safety, as determined by hypoglycemia (blood glucose <55 mg/dL), symptomatic hypoglycemia, and feasibility as determined by in-target success at 24 hours. Symptomatic hypoglycemia was determined using a standardized symptom questionnaire of patient symptoms if the glucose fell below 55 mg/dL (17). Patients unable to communicate were assessed for physiologic signs and if present were assigned symptomatic status. Patients were evaluated for safety throughout the treatment period but were evaluated for feasibility at 24 hours. For purposes of calculating sample size, feasibility was defined as having two of three glucose concentrations closest to 24 hours within the target range. Further analyses assessed the glucose profiles by treatment group over the first 24 hours, and the proportion of time on treatment that the patient’s glucose concentrations were in the target range.
Additional feasibility information included patient enrollment success, duration of treatment, and rate of acceptance of eProtocol-insulin recommendations.
Blinded follow up evaluations for clinical outcomes occurred at 6 weeks (phone) and 3 months (in person). Two pre-specified subgroup studies were conducted and included a continuous glucose monitoring study and a Matrix MetalloProteinase study, both of which will be described elsewhere.
Safety
An independent safety monitor (BMK) reviewed and adjudicated all adverse events in a blinded fashion for relationship to investigational treatment. An NIH-NINDS appointed Data Safety and Monitoring Board (DSMB) consisting of two neurologists, an endocrinologist and a statistician, oversaw the conduct and safety of the trial.
Statistical Analysis
Using the method of Blackwelder (18), we determined that a sample of 24 observations per group would provide 90% power for declaring the treatments not equivalent with a 16% hypoglycemic rate in the treated group assuming a 1% rate in the control group. Type I error was 2.5% to allow for both loose and tight control rates to be compared to control. We allowed for a 10% withdrawal rate.
Feasibility for the loose and tight control groups was defined as having at least 19 of the 24 patients brought into target within 24 hours. An exploratory time in target analysis for the first 24 hours was also conducted.
Additionally, an exploratory efficacy analysis was completed exploring the unadjusted and adjusted mRankin. A post hoc analysis examining the subgroup of patients that resembled the Treatment of Hyperglycemic In Stroke Trial (THIS) patients (19) was conducted and included only those patients with NIHSS score of 3–22 and enrollment glucose of 150 mg/dL or greater. A responder analysis (20,21) that defines favorable outcome based on severity of baseline NIHSS score was conducted as a post hoc analysis as well. Adjusted analyses included adjustment for predicted probability of outcome (20), glucose concentration and tPA treatment.
Results
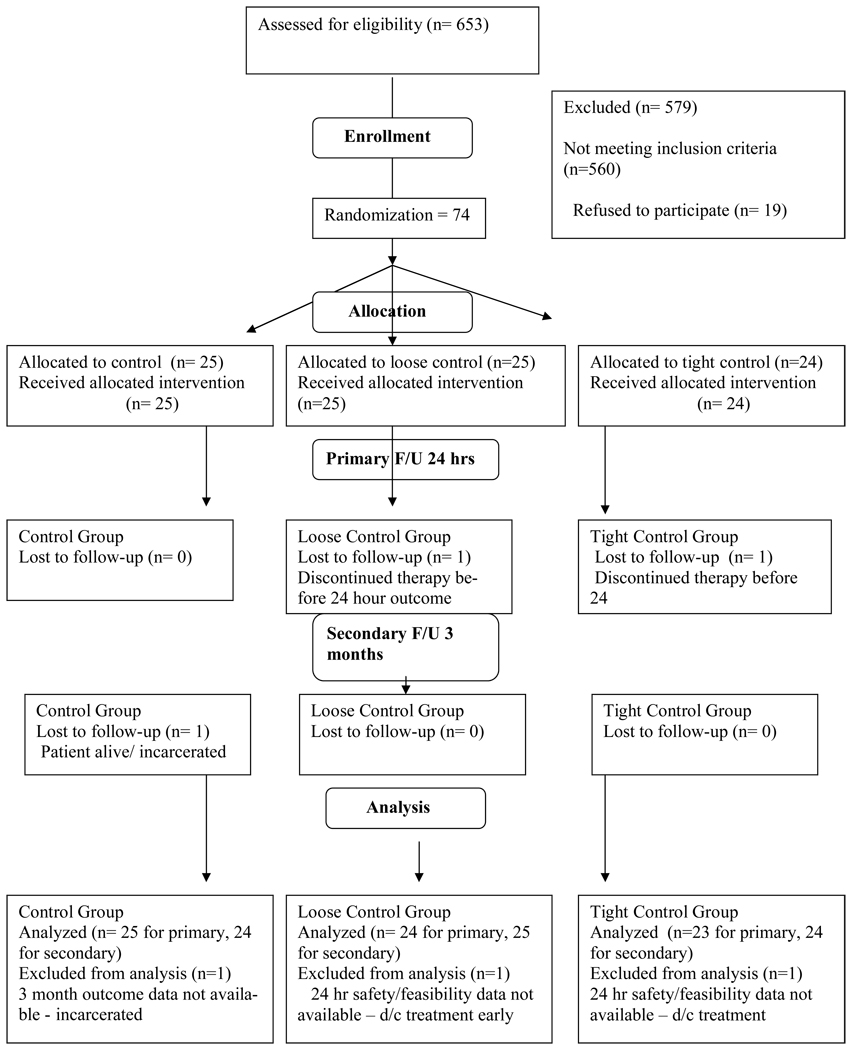
Study enrollment occurred between May 20, 2006 and November 26, 2007. A total of 653 patients were screened and 74 subjects were enrolled. Three reasons for ineligibility (glucose level, not stroke, time) accounted for 85% of the 579 exclusions (Figure 1). Twenty five patients were randomized to control treatment, 25 to loose control therapy and 24 to tight control therapy. One subject from loose control and one from tight control were discharged prior to the primary safety and feasibility outcome assessments. Both of those patients provided three month efficacy outcome data. One subject in usual care was lost to follow up (incarcerated) and no three month data was available. Enrollment occurred slightly ahead of schedule as demonstrated in the online Figure. All subjects received their assigned treatment and were analyzed in the treatment group to which they were assigned.
Figure 1.
The diagram demonstrates the CONSORT style patient enrollment flow diagram
A total of 38 patients were enrolled at UVA and 36 at MCG. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Baseline characteristics of all enrolled patients (n = 74)
| Characteristic | Usual care (N=25) |
Loose control (N=25) |
Tight control (N=24) |
|---|---|---|---|
| Median age, years (IQR) | 66 (53–73) | 71 (62–80) | 68 (58–74) |
| Men, N (%) | 15 (60%) | 13 (52%) | 13 (54%) |
| Median NIHSS (IQR) | 8 (4–16) | 8 (5–16) | 8 (6–10) |
| Diabetes mellitus*, N (%) | 19 (76%) | 13 (52%) | 12 (50%) |
| Treatment with IV rt-PA, N (%) | 10 (40%) | 7 (28%) | 9 (38%) |
| Lacunar stroke subtype N (%) | 3 (12%) | 9 (36%) | 7 (29%) |
| Median blood glucose, mg/dL (IQR) | 143 (133–185) | 168 (133–221) | 167 (142–229) |
| African American N (%) | 7 (28%) | 10 (40%) | 7 (29%) |
| Median hours to randomization (IQR) | 10.4 (6.4–16.8) | 10.6 (7.0–15.2) | 12.3 (7.1–15.2) |
NIHSS – NIH Stroke Scale; IQR – interquartile range,
Diabetes mellitus defined as known history of diabetes prior to enrollment in trial
Primary Outcome Results
The tight control group had a 30% rate of at least one hypoglycemic event while the usual care group and the loose control group each had a rate of 4% (p = 0.05 for tight versus control, Fisher’s exact test). The single subject in the trial that had symptomatic hypoglycemia was in the loose control group.
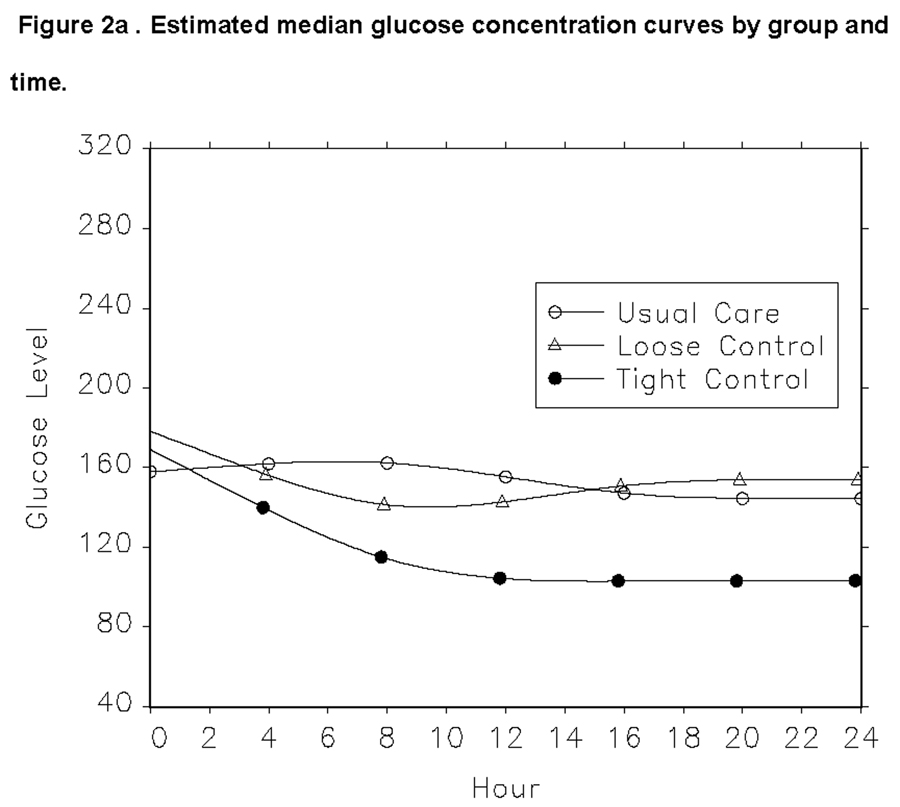
The other primary outcome was feasibility of glucose control. The median glucose concentrations for the entire treatment period were 151, 151 and 111 mg/dL, for usual care, loose control and tight control groups respectively. Figure 2 demonstrates how glucose concentrations changed over the first 24 hour period. Overall, there was a 97% adherence to the eProtocol-insulin recommendations for the two insulin infusion groups. Additionally, our trial demonstrated that in the usual care group 88% received subcutaneous insulin as standard care treatment.
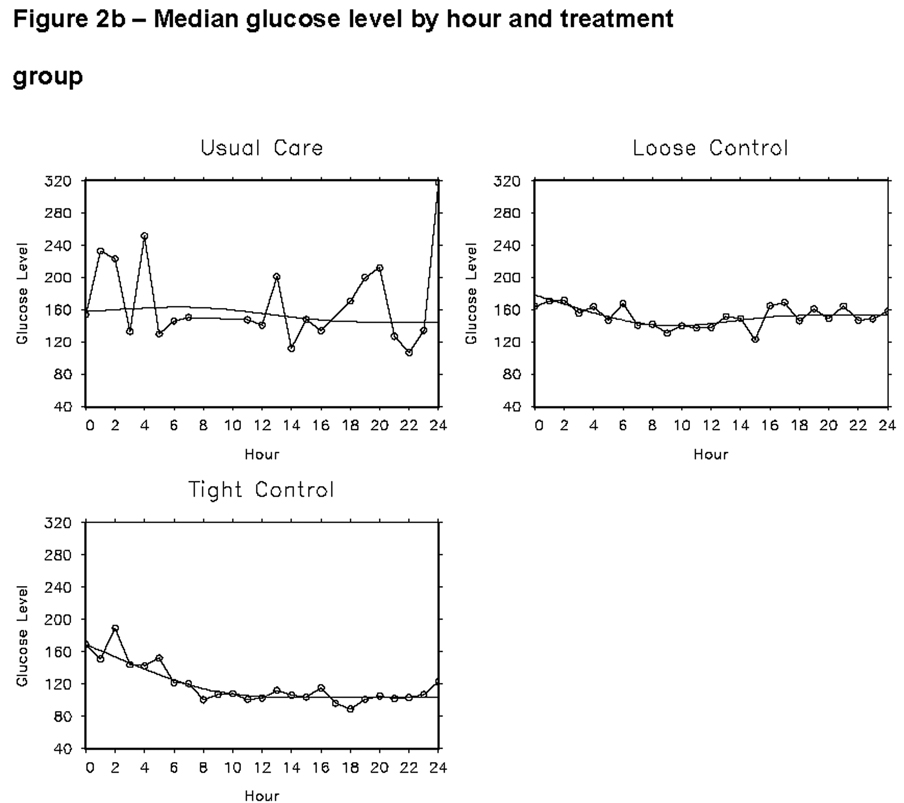
Figure 2.
Estimated median glucose concentrations for each of the three treatment groups over the first 24 hours are shown in Figure 2a. The curves were fit using quantile regression and 4-knot restricted cubic splines. The data points for figure 2a are shown in 2b.
Of the 23 patients assessed for feasibility in the tight control group, 44% (95% CI: 23%, 66%) met the pre-specified feasibility criterion of being in target at 24 hours. Significantly greater feasibility (p< 0. 001 Fisher’s exact test) was observed in the loose control group, where 92% (95% CI 73%, 99%) of the 24 patients were in target at 24 hours.
A secondary analysis was conducted to assess the percentage of time in the first 24 hours that patients’ glucose levels were in the treatment-specific target range. In the loose control group, the median time in range was 90%; for the tight control group, the median time in range was 44%. In a post hoc analysis of the 70–130 mg/dL range used in the THIS trial(19) the median time in target was 64% for the tight control group.
Additional feasibility data included that 58% of GRASP patients were enrolled within 12 hours of symptom onset and 98% were started on therapy within 2 hours of established eligibility. The median time on the protocol was 75 hours and standard care patient discharge was the primary reason for early discontinuation.
Safety
Table 2 lists the frequencies of death and serious adverse events in the three groups. A total of 10 patients were dead at 3 months. The loose control group had a significantly greater proportion of deaths (25%) than the usual care group (4%), p = 0. 05, Fisher’s exact test. There was no significant difference in the rate of death observed in the tight control group (13%) and the usual care group (p = 0. 26, Fisher’s exact test). One of the deaths in the tight control group occurred after the 3 month follow up evaluation was complete but prior to the close of the 3 month window. The most commonly reported serious adverse events (SAE)s included neuroworsening, stroke extension or new stroke, congestive heart failure (CHF), pneumonia/pneumonitis, brain edema and cellulitis. Of these, 1 was definitely related (CHF) and 4 were probably related (1 CHF, 3 cellulitis (in same patient) to treatment. No safety issues occurred related to treatment with tPA in combination with the intervention.
Table 2.
Serious Adverse Events (SAEs)
| Treatment Group | ||||
|---|---|---|---|---|
| Serious Adverse Event | Usual Care (N=25) |
Loose Control (N=25) |
Tight Control (N=24) |
Total (N=74) |
| None | 19 (76%) | 13 (52%) | 16 (67%) | 48 |
| Non-fatal | 5 (20%) | 6 (24%) | 5 (21%) | 16 |
| Fatal | 1 (4%) | 6 (24%) | 3 (13%) | 10 |
chi-squared p= 0.29
Exploratory efficacy
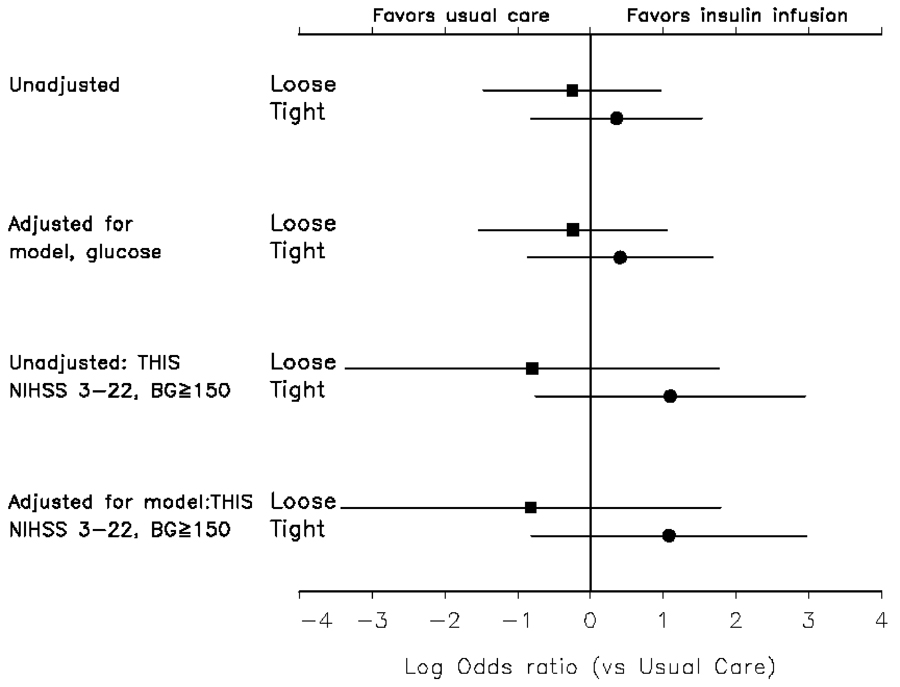
This pilot trial of safety and feasibility was not designed or powered to assess efficacy. None of the comparisons between treatment groups were statistically significant, and the efficacy analysis was purely exploratory. The unadjusted outcomes are shown in Table 3. Adjustment for predicted probability of outcome and glucose level did not substantially change the estimate of treatment effect (Figure 3). The analysis using the subgroup of patients with NIHSS score of 3–22 and baseline glucose of 150 mg/dL (to resemble the THIS trial) or greater demonstrated a larger odds of favorable outcome in the tight control group but not in the loose control group. Additionally, a responder analysis (20,21) of this same subgroup demonstrated an 18% favorable outcome in the control group and a 40% favorable outcome in the tight control group.
Table 3.
Functional Clinical Outcomes at 3 Months in GRASP Trial
| Outcome | Usual Care (N=24) |
Loose Control (n=25) |
Tight Control (N=24) |
|---|---|---|---|
| Modified Rankin Scale 0–1, N (%) | 8 (33%) | 7 (25%) | 10 (42%) |
| Barthel Index 95–100, N (%) | 12 (50%) | 15 (40%) | 13 (54%) |
| NIHSS ≤1, N* (%) | 7/22 (32%) | 5 /21 (24%) | 6/23 (26%) |
| SIS score (± SD) (mean of 8 domains) | 71.7 (20.4) | 66.2 (25.5) | 68.7 (19.1) |
| Death, N (%) | 1 (4%) | 6 (24%) | 3 (13%) |
NIHSS – NIH Stroke Scale; SIS – Stroke Impact Scale. None of these comparisons are statistically different.
Two patients in the usual care group, 4 in the loose control group and 3 in the tight control group were evaluated only by phone at 3 months.
Figure 3.
Unadjusted and adjusted odds of favorable outcome by mRankin (0,1) at 3 months in the loose control group compared to usual care and the tight control group compared to usual care. The null line (0) reflects the usual care group. THIS is the Treatment of Hyperglycemia in Ischemic Stroke Trial and the adjusted analysis for THIS included the subgroup that resembles the THIS Trial patients.
Adjustment for tPA did not change any results.
Discussion
These data demonstrate the safety and feasibility of glucose concentration control in an acute ischemic stroke population using insulin infusion. Only a single symptomatic hypoglycemia event occurred in the insulin infusion groups and none occurred in the tight control group though there was a 30% asymptomatic event rate. The loose control group had similar glucose concentrations and the same rate of overall hypoglycemic events as the control group. These data suggest no benefit to a loose control approach using insulin infusion therapy as subcutaneous insulin therapy is safer, less resource intense and produced nearly identical glucose concentration control results. Though a typical tight control patient spent only 44% of the first 24 hours in target, this includes the initiation time. Additionally, when the post hoc analysis of the 70–130 mg/dL target was assessed, a typical subject spent 64% of the first 24 hours in target, an acceptable rate for a tight control protocol.
In this trial, patients were enrolled successfully, randomized rapidly, received their appropriate treatment, there was excellent adherence to the eProtocol-insulin recommendations and glucose concentration levels were appropriately discriminated between the control group and the tight control group.
Though any comments on efficacy are not warranted based on these data, this trial has informed future trials on the control group rates of favorable outcome as well as the population most likely to benefit from the treatment. The post hoc analysis of the population of patients constructed to resemble the THIS trial (19) suggested that a more narrow population of hyperglycemic diabetics with moderate to severe strokes may benefit most from this intervention
Another finding that informs future trial design is demonstrated by the success of our stratification by predicted probability of outcome. This predicted probability included 6 major risk predictors which could not have been individually stratified or balanced by randomization in a small middle phase trial. Stratifying by this predicted probability allowed us to balance risk across treatment groups despite a small sample.
These data can now be added to the data from the other glucose control trials (19, 22–24) which have provided valuable information to guide future trials. The THIS trial, consistent with our data, suggest potential benefit of aggressive glucose regulation in a hyperglycemic diabetic population with moderate to severe strokes (19). The GIST- UK trial22, Kriesel trial23 and Walters 24 trial all support excluding the non diabetics from such a trial as they tend to self correct and enter the target range without intervention.
Our study has limitations. It was powered to assess safety and feasibility, not efficacy, and the encouraging results should be interpreted with extreme caution. Our study design allowed the control patients to be on usual care stroke wards whereas most of the insulin infusion patients were in ICUs or neurological step down units. This differential care may have impacted the outcomes of patients, the rates of reported SAEs and may have confounded our results. In addition, as eProtocol-insulin was used in the insulin infusion groups and not in the control group, the frequency of glucose checks was increased in these two groups relative to the control and again may have confounded results. If this confounding effect were present, we would have expected benefit in both insulin infusion treatment groups. The lack of suggested benefit in the loose control group but a hint of suggested benefit in the tight group, argues against this possibility. Finally, we recognize that this patient population was acquired from only two sites so a larger sampling will be necessary to assess the generalizability of these results.
Overall, this middle phase trial has provided information to inform future trials on safety, feasibility, target range (“dose”), duration of treatment, time to treatment, the community accepted usual care, safety with tPA, expected lost to follow up rates and the population most likely to respond to treatment. These data are critically important to support the design of future trials which are supported by current AHA/ASA guidelines. An appropriately powered phase III trial of glucose concentration control in acute ischemic stroke patients is warranted.
Supplementary Material
Acknowledgment
Funded by NIH-NINDS R01 NS050192 and supported by the UVA GCRC
Footnotes
The authors report no conflicts of interest to report related to this work.
This work was presented, in part, at the 6th World Stroke Congress in Vienna, Austria on 9/25/2008 and was also presented, in part, at the International Stroke Conference in San Diego, California on February 18, 2009.
References
- 1.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 2.Adams HP, Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, Grubb RL, Higashida R, Kidwell C, Kwiatkowski TG, Marler JR, Hademenos GJ. Stroke Council of the American Stroke Association. Guidelines for the early management of patients with ischemic stroke. Stroke. 2003;34:1056–1083. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Frulan A, Grubb RL, Higashida RT, Jauch JC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks FM. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 4.Voll CL, Auer RN. The effect of postischemic blood glucose levels on ischemic brain damage in the rat. Ann Neurol. 1988;24:638–646. doi: 10.1002/ana.410240508. [DOI] [PubMed] [Google Scholar]
- 5.Aur R. Insulin, blood glucose levels and ischemic brain damage. Neurology. 1998;51:S39–S43. doi: 10.1212/wnl.51.3_suppl_3.s39. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton MG, Tranmer BI, Auer RN. Insulin reduction of cerebral infarction due to transient focal ischemia. J Neurosurg. 1995;82:262–268. doi: 10.3171/jns.1995.82.2.0262. [DOI] [PubMed] [Google Scholar]
- 7.Izumi Y, Pinard E, Roussel S, Seylaz J. Insulin protects brain tissue against focal ischemic in rats. Neuroscience Letters. 1992;144:121–123. doi: 10.1016/0304-3940(92)90730-u. [DOI] [PubMed] [Google Scholar]
- 8.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 9.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 10.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 11.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 12.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, Zivin J. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. 1999;30:2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 13.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 14.Johnston KC, Connors AF, Wagner DP, Haley EC. Predicting Outcome in Ischemic Stroke: External Validation of Predictive Risk Models. Stroke. 2003;34:200–202. doi: 10.1161/01.str.0000047102.61863.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston KC, Wagner DP, Wang X, Newman GC, Thijs V, Sen S. Warach S for the GAIN, Citicoline and ASAP Investigators. Validation of an Acute Ischemic Stroke Model: Does DWI lesion volume offer a clinically significant improvement in prediction of outcome? Stroke. 2007;38:1820–1825. doi: 10.1161/STROKEAHA.106.479154. [DOI] [PubMed] [Google Scholar]
- 16.Morris AH, Orme J, Jr, Truwit JD, Steingrub J, Grissom C, Lee KH, Li GL, Thompson BT, Brower R, Tidswell M, Bernard GR, Sorenson D, Sward K, Zheng H, Schoenfeld D, Warner H. A replicable method for blood glucose control in critically ill patients. Crit Care Med. 2008;36:1787–1795. doi: 10.1097/CCM.0b013e3181743a5a. [DOI] [PubMed] [Google Scholar]
- 17.Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes. 1993;42:1791–1798. doi: 10.2337/diab.42.12.1791. [DOI] [PubMed] [Google Scholar]
- 18.Blackwelder W. “Proving the null hypothesis" in clinical trials. Controlled Clinical Trials. 1982;3(4):345–353. doi: 10.1016/0197-2456(82)90024-1. [DOI] [PubMed] [Google Scholar]
- 19.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39:384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP, Jr, Leclerc JR, Bluhmki E, Clarke W, Hansen MD, Hacke W. Measuring outcomes as a function of baseline severity of ischemic stroke. Cerebrovasc Dis. 2004;18:124–129. doi: 10.1159/000079260. [DOI] [PubMed] [Google Scholar]
- 21.Saver JL, Yafeh B. Confirmation of tPA treatment effect by baseline severity- Confirmation of tPA treatment effect by baseline severity adjusted end point reanalysis of the NINDS-tPA stroke trials. Stroke. 2007;38:414–416. doi: 10.1161/01.STR.0000254580.39297.3c. [DOI] [PubMed] [Google Scholar]
- 22.Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 23.Kreisel SH, Berschin UM, Hammes HP, Leweling H, Bertsch T, Hennerici MG, Schwarz S. Pragmatic management of hyperglycaemia in acute ischaemic stroke: safety and feasibility of intensive intravenous insulin treatment. Cerebrovasc Dis. 2009;27:167–175. doi: 10.1159/000185608. [DOI] [PubMed] [Google Scholar]
- 24.Walters MR, Weir CJ, Lees KR. A randomised, controlled pilot study to investigate the potential benefit of intervention with insulin in hyperglycaemic acute ischaemic stroke patients. Cerebrovascular Diseases. 2006;22:116–122. doi: 10.1159/000093239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.