Abstract
Neural activity modulates the maturation of synapses and their organization into functional circuits by regulating activity-dependent signaling pathways. Phosphorylation of cyclic AMP/Ca2+-responsive element-binding protein (CREB) is widely accepted as a stimulus-inducible event driven by calcium influx into depolarized neurons. In turn, phosphorylated CREB (pCREB) activates the transcription of brain-derived neurotrophic factor (BDNF), which is needed for synaptic transmission and long-term potentiation. We examined how these molecular events are influenced by sensorineural hearing loss and long-term reactivation via cochlear implants. Sensorineural hearing loss reduced the expression of pCREB and BDNF. In contrast, deafened animals subject to long-term, unilateral intracochlear electrical stimulation exhibited an increased expression of pCREB and BDNF in the contralateral auditory cortical neurons, relative to ipsilateral ones. These changes induced by cochlear implants are further accompanied by the activation of the mitogen-activated protein kinase (MAPK) signaling pathway, which has been implicated in long-lasting forms of synaptic plasticity. Because CREB and BDNF are critical modulators of synaptic plasticity, our data describe for the first time possible molecular candidate genes, which are altered in the auditory cortex, following cochlear implantation. These findings provide insights into adaptive, molecular mechanisms recruited by the brain upon functional electrical stimulation by neural prosthetic devices.
Keywords: activity-dependent gene, BDNF, electrical stimulation, MAPK, sensorineural hearing loss, synaptic plasticity
Introduction
Hearing impairment is one of the most frequently encountered disabilities in our society (http://www.who.int/mediacentre/factsheets/fs300/en/index.html). Although certain forms of deafness are acquired congenitally due to inherited genetic defects, significant hearing loss also becomes more substantial in our population due to aging and more frequent exposure to noise and ototoxic drugs. Over the past 2 decades, there have been significant improvements in the development of hearing aids and cochlear implants to provide deaf patients with important auditory cues for both speech comprehension and their aural environments. The main principle of cochlear implants involves functional electrical stimulation of the primary auditory neurons to restore activity to a sensory-deprived auditory system. This rehabilitative strategy has successfully restored hearing to many but not all patients with severe to profound hearing loss. In neonatally deafened cats, cochlear implants improve the temporal resolution of neurons in the central nucleus of the inferior colliculus (Vollmer et al. 2005), whereas in congenetically deaf cats, cochlear implants restore synaptic structures in the cochlear nucleus (Ryugo et al. 2005) and produce field potentials of larger amplitude in the auditory cortex (AC) (Klinke et al. 1999), suggesting morphological and electrophysiological plasticity changes induced by cochlear implants in the central auditory pathways. However, the molecular mechanisms which drive these changes remain unclear. The increased metabolic activity in the AC of deaf humans after cochlear implantation suggests that neural activity might be a crucial link (Ito et al. 1990, 1993; Herzog et al. 1991; Naito et al. 1995; Syka 2002).
Substantial evidence now shows that modulation of synaptic connection and function is mediated by neural activity (Bliss and Collingridge 1993; Malenka and Nicoll 1999; Lu 2003), which alters the expression of activity-dependent genes or the characteristics of their proteins, for example, their phosphorylation status (Fig. 1). Among these genes, the nuclear transcription factors, c-Fos and cyclic AMP/Ca2+-response element-binding protein (CREB), together with the neurotrophin, brain-derived neurotrophic factor (BDNF) are well-characterized members (West et al. 2002). The immediate early gene, c-Fos, is widely used as a marker to map neuronal activity in multiple brain regions. In particular, visual deprivation has been shown to reduce c-Fos transcription in the visual cortex (Majdan and Shatz 2006), and gene micro arrays also revealed a decrease of c-Fos expression in inferior collicular neurons after deafness (Holt et al. 2005). In response to neural activity or extracellular cues, CREB becomes activated after phosphorylation by several kinases such as the mitogen-activated protein kinase (MAPK), also known as extracellular signal–regulated kinase. This phosphorylation event enables CREB, in association with other transcriptional coactivators, to bind to promoters of specific genes to trigger gene expression (Mayr and Montminy 2001). BDNF is a target gene of CREB-mediated transcription where the promoter element in exon 3—or exon 4 under new nomenclature (Aid et al. 2007)—contains functional CRE (cyclic AMP-response element) regulatory nucleotide sequences (Shieh et al. 1998; Tao et al. 1998). Secreted BDNF binds to the surface-bound neurotrophin receptor, tropomyosin-related kinase B (TrkB), to trigger MAPK-associated signaling pathways leading to increased synthesis of synapse-related genes, such as the activity-regulated cytoskeletal protein, Arg 3.1 (Yin et al. 2002; Ying et al. 2002). Because phosphorylated CREB (pCREB) and BDNF have been consistently shown to produce long-lasting forms of synaptic plasticity (Lonze and Ginty 2002; Lu 2003), we sought to determine if and how these candidate genes and their associated signaling pathways in the AC are affected by intracochlear stimulation. We addressed this question in laboratory rats that have been ototoxically deafened by aminoglycoside antibiotics to achieve profound hearing loss (Tan and Shepherd 2006). These antibiotics destroy hair and supporting cells of the organ of Corti resulting in reduced auditory inputs to the central auditory system. Subsequently, deafened rats receive a unilateral cochlear implant, followed by either an acute or chronic stimulation paradigm.
Figure 1.
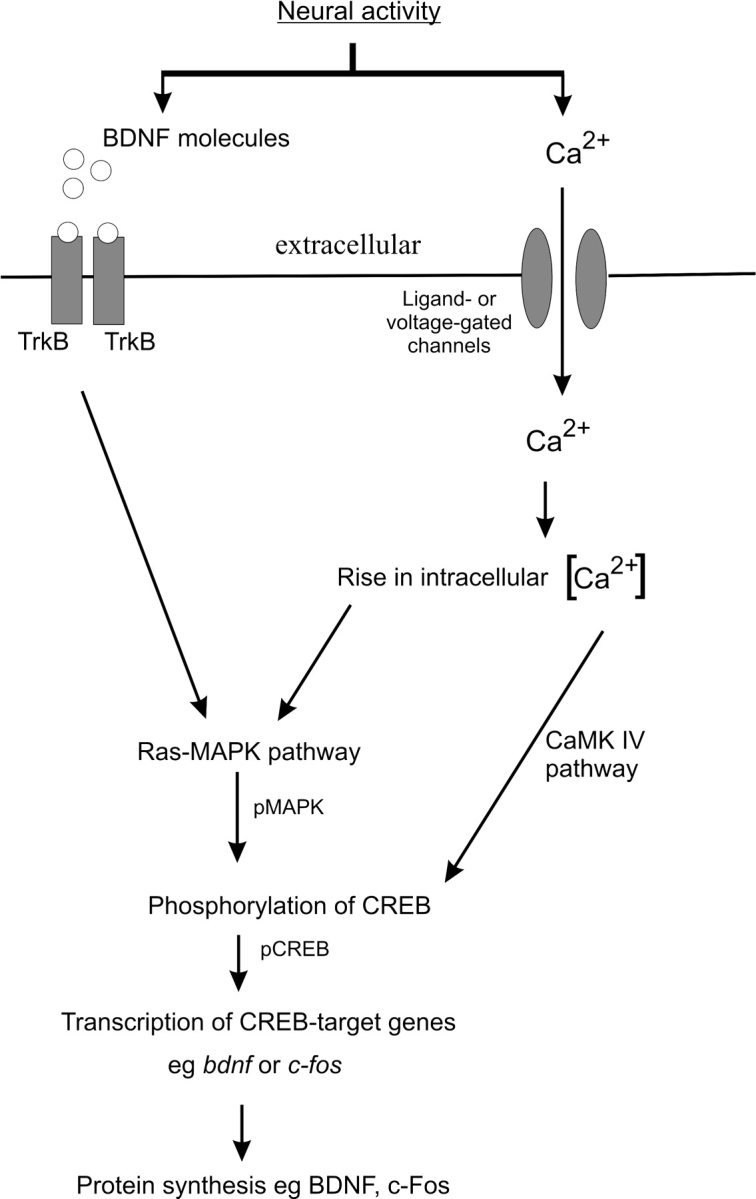
Activity-dependent gene expression in neurons. In response to membrane depolarization and neurotransmitters, an influx of calcium occurs through ligand- and voltage-gated ion channels, resulting in a rise of intracellular calcium levels. This activates both Ras-MAPK and calcium/calmodulin-dependent protein kinase IV (CaMK IV) pathways leading to the phosphorylation of CREB (pCREB). Within the nucleus, pCREB activates the transcription of their target genes, including bdnf and c-fos. BDNF protein is synthesized from their transcripts and released extracellularly. By binding to TrkB receptors, BDNF can augment the Ras-MAPK pathway, causing further phosphorylation of CREB.
Here, we show that reduced peripheral activity in the cochlea significantly decreased the phosphorylation of CREB and MAPK and BDNF expression in the auditory cortical neurons of profoundly deaf rats, in comparison to normal hearing rats. In contrast, reinstating electrical activity unilaterally to the auditory nerve by cochlear implants over a period of 7 weeks dramatically elevated the expression of pCREB, phosphorylated MAPK (pMAPK), and BDNF in contralateral auditory cortical neurons, relative to their ipsilateral counterparts. Associated with these changes, a greater proportion of these contralateral neurons also showed an increased expression of voltage-gated sodium channels when compared with the ipsilateral neurons. To test the hypothesis that neural activation contributes to some of these molecular changes, we also performed acute intracochlear electrical stimulation in deafened rats. Using the same interhemispheric comparison approach, this acute study revealed parallel upregulation of pCREB and MAPK, but not voltage-gated sodium channels, in contralateral auditory cortical neurons. Although BDNF expression was elevated in contralateral auditory cortical neurons, this change was not statistically significant. By identifying distinct molecular regulators of cortical plasticity, these findings provide insights into long-term, plasticity changes within the cerebral cortex in response to functional electrical stimulation which restores afferent activity to central pathways affected by sensory deprivation.
Materials and Methods
A Model of Sensorineural Hearing Loss Using Aminoglycoside Antibiotics
All animal procedures employed in this study were approved by the Royal Victorian Eye and Ear Hospital's Animal Research and Ethics Committee and conformed to guidelines of the National Health and Medical Research Council of Australia. Gentamicin sulphate (420 mg/kg body weight; Sigma, St Louis, MO) and frusemide (200 mg/kg body weight; Troy Laboratories, Smithfield, NSW, Australia) were injected subcutaneously in the skin folds on the lateral abdominal side and the dorsal neck area, respectively, as described (Tan and Shepherd 2006; Hurley et al. 2007). For younger animals (between 2 and 3 weeks old), we used a lower dosage of gentamicin sulphate (350 mg/kg body weight). After deafening, the animals were kept in the colony for a period between 9 and 16 weeks before sacrifice. To evaluate hearing function, we used both the Preyer's reflex in response to a clap startle, followed by click-evoked auditory brain stem response (ABR) measurement. Details of this procedure have been described in an earlier study (Tan and Shepherd 2006). Rats are considered normal hearing if they register a threshold reading of less than 43 dB peak equivalent sound pressure level, whereas profoundly deafened rats display a permanent threshold shift of >50 dB. Together, 6 adult (2–3 months) and 3 younger (2–3 weeks) animals were deafened to analyze the effects of sensorineural hearing loss on activity-dependent gene expression in the AC.
Cochlear Implant Surgery
For cochlear implant surgery, rats weighing above 250 g were used because of reduced morbidity following surgery. Approximately 1 week after deafening, animals were anesthetized with a single intraperitoneal injection of ketamine (75 mg/kg body weight; Parnell Laboratories, Alexandria, NSW, Australia) and xylazil (10 mg/kg body weight; Troy Laboratories) and ABR measurements were performed to confirm profound hearing loss. The animals’ temperature was maintained at 37 °C by using a heating pad. Anesthesia was maintained during surgery using a mixture of isoflurane and oxygen. Surgical details have been described previously (Lu et al. 2005). Briefly, a local anesthetic (lignocaine, 4 mg subcutaneously; Troy Laboratories) was applied on the right, postauricular site before incision. We exposed the bony bulla and a hole was drilled on the dorsal surface to expose the round window niche and the stapedial artery. This artery was carefully cauterized and a cochleostomy performed. The cochlear implant assembly consists of 2 stimulating, platinum electrodes on a silicon carrier, connected to lead wires from an implantable stimulator (Millard and Shepherd 2007). Complete details of our electrode assembly have been described (Shepherd and Xu 2002). To prevent movement of the electrode array, we sealed the cochleostomy with muscle, fixed the implant to the bulla using bone cement (Durelon, ESPE Dental AG, Germany), and further fixed the lead wires to the skull using polyethylene mesh. The stimulator was placed just beneath the skin between the shoulder blades. It generates charge-balanced biphasic current pulses when the animal was placed within a chamber that has a pulsed magnetic field (Millard and Shepherd 2007).
Immediately after surgery, electrically evoked ABRs (eABR) were recorded differentially by using needle electrodes (vertex positive, neck negative, and thorax ground). Biphasic current pulses (0.5 mA; variable pulse width from 20 to 100 μs per phase; 10-μs interphase gap) generated by the computer were delivered to the electrode array and the response recorded using signal averaging techniques (Shepherd et al. 2005). Threshold was defined as the smallest current level required producing a peak-trough response amplitude of >0.25 μV for wave III of the eABR (Millard and Shepherd 2007). At 3 weeks after deafening, implanted rats were stimulated daily for a continuous duration of 3 h over a period of 7 weeks during the mornings. Charge-balanced biphasic current pulses were generated in the stimulator and their charge per phase was set at 3 dB above the individual eABR threshold. Stimulus rate was 200 pulses per second. To eliminate any effects due to surgery, we also surgically implanted nonstimulating “dummy” electrodes in 2 control animals and sacrificed them after 7 weeks. Together, 4 animals were successfully stimulated chronically with cochlear implants.
In the acute study, we adopted similar deafening and surgical procedures but omitted the implantable stimulator. The electrode array was connected to an external current source stimulator, and stimulation parameters used were identical to those for the chronic study. In total, 4 animals were acutely stimulated for 3 h with cochlear implants, whereas sham surgery was performed on 3 additional animals using nonstimulating control electrodes.
Immunohistochemistry
Animals were sacrificed with an overdose of sodium pentobarbitone and perfused intracardially with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. Brains were removed and further fixed for 2 h in the same fixative, before immersing them overnight in 30% sucrose in PBS. Anterior to the AC, near the Bregma, we performed a single coronal cut to generate a flat surface and the brain was embedded with the exposed side facing down in optimum cutting temperature compound (Sakura, Tokyo, Japan). During the cryosectioning, we sectioned from an anterior to posterior direction until the dentate gyrus of the hippocampus could be seen. Sections were then collected at 10 μm and every fifth section was marked and subsequently stained with dilute acidified thionin to identify the starting point where the CA3 region of the hippocampus could be seen lying directly opposite the perirhinal sulcus (Paxinos and Watson 1998; Rutkowski et al. 2003). The AC extends 1.02 mm anterior and 2.14 mm posterior from this point (Paxinos and Watson 1998). By using this landmark feature and the contours of the hippocampus, we could select comparable brain sections from normal hearing and deaf animals in our immunohistochemistry experiments. Dorsoventrally, the AC could also be distinguished by its proximity to the perirhinal sulcus. It lies above this sulcus and has been divided into 2 zones: the temporal cortex 3 (Te3) just above the sulcus, merging into the temporal cortex 1 (Te1), approximately 1 mm above the sulcus (Rutkowski et al. 2003). All analyses were performed in the Te1 area. The labeling of the laminar organization of the cortex was approximate because we could not reliably distinguish layers II and III and layer V begins midway along the cortical depth (Games and Winer 1988).
For immunohistochemistry using the avidin and biotinylated horseradish peroxidise complex(ABC) method, brain sections were first immersed for 1 hour in 0.3 % hydrogen peroxide (diluted from a 30% w/v stock, Merck, Kilsyth, Australia) to remove endogenous peroxidise activity. Sections were then rinsed in PBS and permeabilized with 0.1% Triton-X, blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA) in 0.1% Triton-X for 1 hour. Primary antibodies were diluted in blocking solution and added to the sections for overnight incubation at 4 °C. On the following day, sections were washed 3 X in PBS and biotinylated secondary antibody (1:200) from the rabbit Vectorstain ABC kit (Vector laboratories) was added. This incubation step lasted 2 hours before 3 X PBS washes. Avidin and biotinylated horseradish peroxidase was added following manufacturer's protocol, and after a PBS rinse, diaminobenzidine substrate was applied (Vector laboratories). To intensify the staining, we added nickel solution as provided by the manufacturer and stain development was monitored under the microscope. The staining reaction was stopped by immersing slides from both normal hearing and deaf animals in distilled water at the same time. Because the stain development takes place within 10 minutes, an artifact may arise if we add the substrate first to sections from normal hearing animals before adding them to those from deaf animals. Therefore, in initial studies, we also changed the sequence around and found that this did not affect the results of the experiment. Because speed is essential in the development of the chromogenic signal, we did not analyze more than 6 slides in any one experiment.
For fluorescent-based immunohistochemistry, the hydrogen peroxide step was omitted. The following fluorescein-conjugated secondary antibodies from Molecular Probes (Eugene, Oregon) were used: highly cross-adsorbed Alexa Fluor 488 goat anti-mouse IgG (A-11029) and highly cross-adsorbed Alexa Fluor 594 goat anti-rabbit IgG (A-11037). At the end of the incubation, sections were rinsed 3 × in PBS before mounting in Vectorshield (Vector laboratories) containing the nuclear stain 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI). All sections were viewed with a Zeiss Axioplan 2 microscope with Zeiss Axio Cam camera.
The following primary antibodies were used: antibody against c-Fos (1:50, PC05L, Calbiochem, San Diego, CA), antibody against BDNF targeting the N-terminus of mature BDNF (1:50, sc546, Santa Cruz), antibody against CREB phosphorylated at Ser-133 (1:50, 06-519, Upstate, Lake Placid, NY), antibody against MAPK phosphorylated at threonine and tyrosine residues (1:50, AB3826, Chemicon, Temecula, CA), antibody against all isoforms of voltage-gated sodium channels (1:75, SP19, Alomone, Jerusalem, Israel), and antibody against glial fibrillary acidic protein (GFAP, 1:50, MAB3402, Chemicon), antibody against microtubule-associated protein 2 (MAP2, 1:100, M4403, Sigma). All affinity-purified antibodies were raised in rabbits, except the last 2 antibodies, which were raised in mice. To control for the specificity of the antibodies against c-Fos, BDNF, and voltage-gated sodium channel, blocking peptide recommended by the manufacturer was used. The specificity of the antibodies against pCREB and MAPK was determined by the nuclear localization of the staining with DAPI, as well as Western blotting experiments, to determine if the molecular weights of the immunopositive bands corresponded with published or manufacturer's data.
Densitometric measurements were performed on a region of 0.5995 mm2 of the AC from brain sections processed with the ABC method using the Alpha Imager software (Alpha Innotech, San Leandro, CA). For interhemispheric comparison in the acute and chronic studies, we marked a horizontal line 1 mm above the perirhinal sulcus on the slides to provide a reference point in which the same regions from both ipsilateral and contralateral cortices could be analyzed. To start the densitometric measurements, we first delineated a spot of interest in each immunolabeled neuron. To eliminate the possibility of background staining in the periphery of the spot, we activated the background correction program in the software. This software determines the average of the 10 lowest pixel values and assigns this average value as background. Then, it sums up all the pixel values in the spot after subtracting this background value. By normalizing to the area of the spot, a final integrated density value (IDV) was obtained for each spot. These values were then sorted in descending order. If n (even number) spots have been measured, the IDV of the (n/2)th spot represents the median. We segregated the neurons into 2 populations using the median as a threshold of staining: those with IDVs above the median and those below the median. This approach therefore provides an indication of the proportion of neurons with immunolabeling exceeding a defined threshold value. For each antibody, the median value was determined from each control (normal hearing) section and the number of neurons/spots that exhibited an IDV above this median was counted for both control and deaf animals. For simplicity of reference, neurons expressing an IDV above this median were described as having strong immunoreactivity. This number was divided by the area of the measured section to obtain the density of stained neurons. For unilaterally implanted animals, the comparison was between the left (contralateral) and right (ipsilateral) AC and the median IDV was calculated from the ipsilateral AC. By counting the number of neurons displaying above median value and the area of section counted, the density of stained neurons was similarly obtained for both halves of the cortex. Statistical analyses between normal hearing and deaf animals and left and right auditory cortices of implanted animals were determined using 2-tailed Student's t-test (SigmaStat). Graphs indicate means ± standard error of the mean (SEM). Statistical significance (P < 0.05) is indicated in asterisks.
Western Blotting
Animals were euthanized with an overdose of carbon dioxide, decapitated, and the brains removed, after making an incision mark to indicate the location of Bregma. To delineate the area of the AC, vertical incision marks were made 3 and 6 mm from the Bregma to identify the vertical boundaries. Between these boundaries, horizontal incision marks 1 and 3 mm above the prominent blood vessel running along the perirhinal fissure were made to define the AC. These procedures were adapted from Doron et al. (2002). This cortical tissue was carefully peeled off, judiciously avoiding the underlying hippocampal tissues and snap frozen in liquid nitrogen. From each sample, we used the protein extraction kit from Pierce Technologies (78833, Rockford, IL) to separate the proteins into cytoplasmic and nuclear fractions. Extracted proteins were further enriched using PAGEprep (26800, Pierce Technologies) and concentrations were determined with a Bradford reagent (B6916, Sigma). Equivalent amounts of proteins from auditory cortices of normal hearing and deafened animals were loaded into the gel, specifically 4 μg of nuclear proteins and 10 μg of cytoplasmic proteins. A 12% Bis-Tris gel (3450117, BioRad, Hercules, CA) was used to separate the proteins using a reducing XT-MOPS buffer system (1610793, BioRad) and a running condition of 200 V, ∼1h. At the end of the run, separated proteins were transferred to polyvinylidene difluoride membranes (1620238, BioRad) using a running condition of 30 V, 1 h 20 min and a 1× Tris/glycine buffer (1610771, BioRad) with 20% methanol. Membranes were subsequently blocked for 90 minutes in 5% milk powder (1706404, BioRad) before incubating overnight at 4 °C with primary antibodies. Antibodies against pCREB, pMAPK, c-Fos, and BDNF were used at 1:250 dilution. As a loading control for cytoplasmic and nuclear proteins, we used a rabbit antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:20,000, ab9485, Abcam, Cambridge, UK) and a goat antibody against histone (1:40, sc8616, Santa Cruz), respectively. On the following day, blots were washed 3× for 20 min in a 0.1% Tween 20/PBS before incubating for 1 h with horseradish peroxidase–conjugated secondary antibodies: goat anti-rabbit IgG at a 1:10 000 dilution (1706515, BioRad) or donkey anti-goat IgG at 1:7500 dilution (sc2056, Santa Cruz). The blots were next washed 4× for 30 min in 0.1% Tween 20/PBS and substrate development was performed using ECL Plus Western blotting detection reagents (RPN2132, Amersham Biosciences, Buckinghamshire, UK). Chemiluminescence was detected using ImageQuant 400 (Amersham Biosciences) and conventional Kodak negatives. As described previously, we used the Alpha Imager for our densitometry measurements, normalizing the intensity of the activity-dependent gene to their corresponding housekeeping gene. The background was subtracted during the densitometry measurements. Statistical analyses between normal hearing and deafened groups were determined using the 2-tailed Student's t-test (SigmaStat). Graphs indicate means ± SEM. Statistical significance (P < 0.05) in deafened samples, relative to the control cohort, is indicated in asterisks.
Results
Sensorineural Hearing Loss Causes Reduction of c-Fos Expression in the AC
To validate an alteration of neural activity in the central auditory pathway by aminoglycoside antibiotics, we first used immunohistochemistry to compare any regulation of c-Fos in the AC of deafened animals against those with normal hearing. We observed a reduction in the density of c-Fos expressing neurons in AC of deafened animals (Fig. 2A–C, 10% ± 5%, P < 0.001). An absence of colocalization with an antibody against GFAP, a glial marker, suggested a predominant neuronal expression of c-Fos (Fig. 8G). The c-Fos antibody is specific because preincubation of the antibody with its antigenic peptide completely abolished any signal in cortical neurons (Fig. 2A, inset) and Western blot analysis of nuclear protein fraction of AC revealed a molecular fragment of ∼55 kDa (Fig. 2D). More pertinently, we also found a reduced intensity in the c-Fos immunoreactive band in AC of deafened rats (Fig. 2D), confirming our observation with immunohistochemistry. By normalizing the intensity of this band to a histone immunoreactive band (a loading control), we obtained a significant reduction of c-Fos expression in deafened AC (Fig. 2E, 55% ± 4%, P < 0.05). From these initial findings, we reasoned that aminoglycoside-induced destruction of the organ of Corti diminishes neural activity in the AC, as measured using c-Fos immunochemistry.
Figure 2.
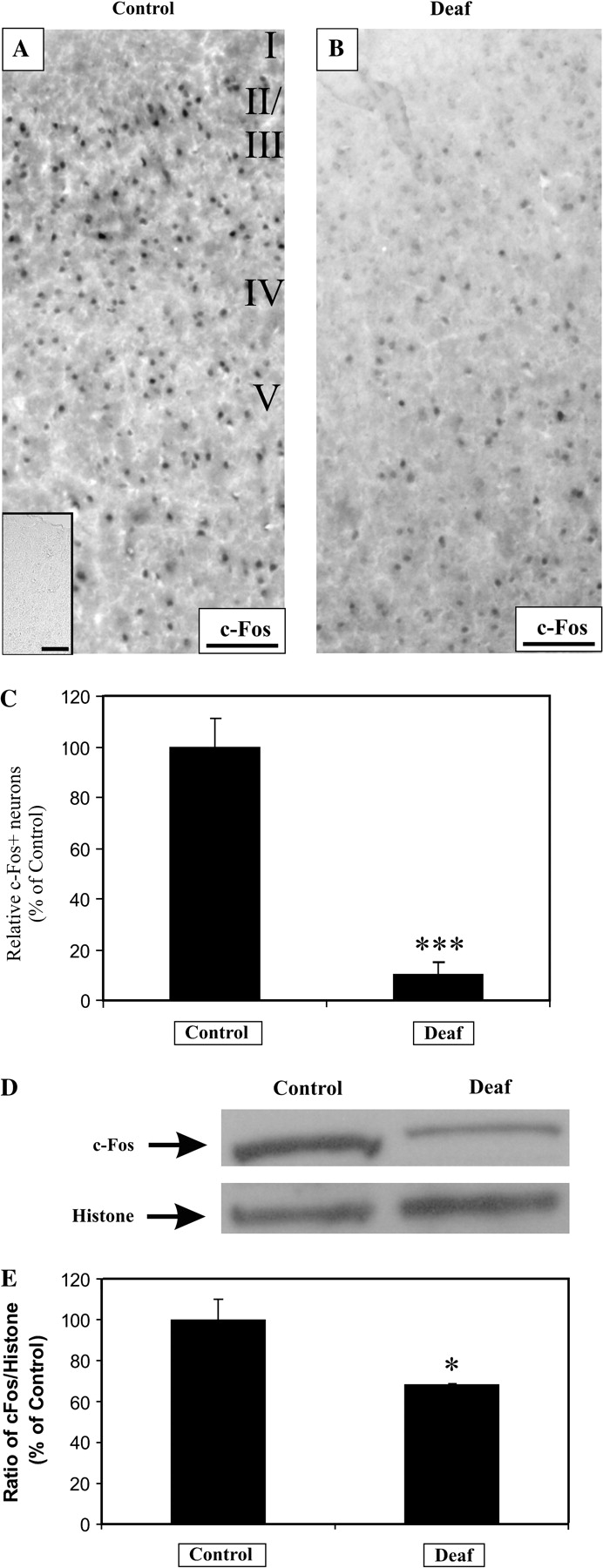
Sensorineural hearing loss reduces c-Fos expression in cortical neurons of the AC. Brain sections of the AC from normal hearing animals (A) and profoundly deaf animals (B) were examined for c-Fos immunoreactivity. After a deafness duration ranging from 9 to 16 weeks, c-Fos expression was prominently decreased in 4 animals, in comparison to normal, hearing controls (A, B). Immunoreactivity against c-Fos was completely abolished after preincubation with excess of its antigenic peptide (A, inset). Densitometric measurement revealed a significant 90% decline in the proportion of c-Fos-positive neurons, relative to controls (C, P < 0.001). From the auditory cortices of 4 normal hearing controls and 4 deafened animals, a representative Western blot showed a decrease in c-Fos expression, whereas the housekeeping nuclear protein, histone, serves as a loading control (D). The intensity of the c-Fos band was normalized to the histone band, and the mean ratio was expressed as a relative percentage of normal hearing controls. There was a 45% reduction in this ratio between controls and deafened animals (E, P < 0.05). Scale bar = 100 μm.
Figure 8.
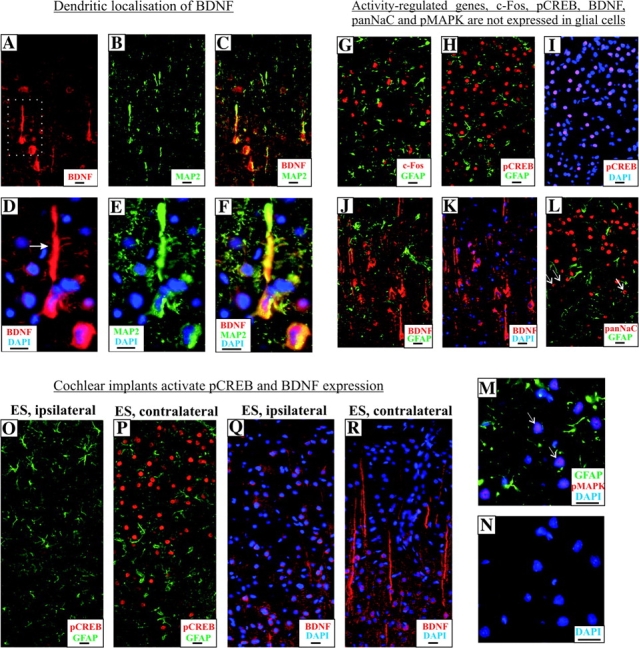
Expression of activity-regulated genes in auditory cortical neurons and their inducibility by cochlear implants. Cortical neurons from the AC of normal hearing rats expressed BDNF in their soma and processes (A). Expression in the processes colocalized with a dendrite marker protein MAP2, indicating a predominant postsynaptic localization of BDNF (B, C). Higher magnification of a BDNF+ pyramidal neuron (from A, dotted box) revealed extensive expression in the apical dendrites (arrow, D), which localizes with MAP2 (E, F). The activity-dependent gene, c-Fos, is expressed exclusively in cortical neurons and not glial cells of the AC, as shown by the absence of c-Fos colocalization with GFAP (G). Similarly, pCREB expression was found in neurons and not glial cells (H) and restricted to nuclei of these neurons, as shown with colocalization staining with DAPI (I). Glial cells in the AC did not express BDNF, as shown by the lack of overlapping immunofluorescence between BDNF and GFAP (J). The use of DAPI staining indicated a cytoplasmic localization of BDNF (K). An antibody against all isoforms of voltage-gated sodium channels (panNaC) detected expression only in the soma bodies (L, arrows), unlike BDNF expression found in both soma and fibers of cortical neurons (K). Lack of colocalization with GFAP immunofluorescence excluded the expression of panNaC in glial cells (L). Neurons in the AC expressed pMAPK only in their nuclei, as shown with DAPI staining (N) and no colocalization was found between pMAPK and GFAP immunostaining (M). Unilateral chronic electrical stimulation with cochlear implants upregulated expression of pCREB in neurons of the contralateral AC (P), relative to those in the ipsilateral cortex (O), whereas GFAP expression in neurons of both cortices did not vary considerably (O, P). In parallel and linked pCREB expression, unilateral chronic intracochlear electrical stimulation also elevated BDNF expression in neurons of the contralateral AC (R), when compared with those of the ipsilateral cortex (Q). Particularly, a more intense BDNF+ immunolabeling was found in the dendrites (R). Scale bar = 20 μm.
Sensorineural Hearing Loss Induces Parallel Downregulation of pCREB and BDNF in the AC
Because neuronal c-Fos expression is highly regulated by depolarization and intracellular calcium signaling, we hypothesized that the activity-dependent phosphorylation of CREB would be similarly altered. We used immunohistochemistry to examine expression of pCREB in AC of normal hearing and deafened rats. We found a significant reduction in the density of pCREB-positive neurons in deafened animals (Fig. 3A–C, 21 ± 8%, P < 0.01) relative to normal hearing controls. The immunoreactivity of pCREB is restricted to nuclei of neurons because no colocalization was observed between GFAP and pCREB antibodies in dual immunofluorescent experiments and the apparent colocalization of pCREB and DAPI signals (Fig. 8H,I). We have previously underlined the specificity of pCREB antibody by demonstrating a nuclear localization using immunohistochemistry and by immunoblots which identified an expected molecular fragment of ∼43 kDa (Tan and Shepherd 2006). Western immunoblots also confirmed a reduction of pCREB in AC of deafened animals (Fig. 3D,E, 67 ± 14%, P < 0.05) against those of normal hearing animals after normalizing to histone.
Figure 3.
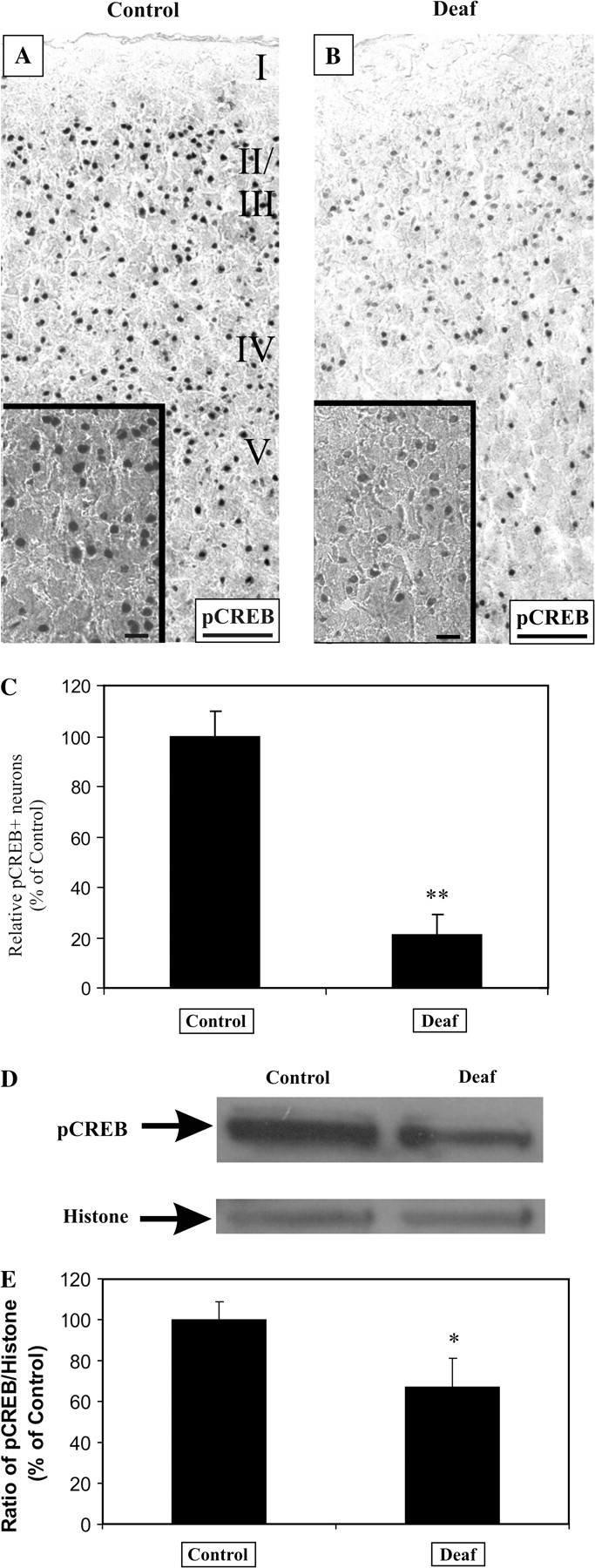
Sensorineural hearing loss downregulates the phosphorylation of CREB in cortical neurons of the AC. In normal hearing animals, pCREB is strongly expressed in nuclei of auditory cortical neurons (A). After 9–16 weeks of profound deafness, there was a noticeable decline in the intensity and density of pCREB+ neurons in 4 animals (B), when compared with normal hearing controls. The density of neurons displaying strong pCREB immunoreactivity was reduced by 79% in profoundly deaf animals relative to normal hearing controls (C, P < 0.01). Validation of this decline using Western blotting in 4 normal hearing and 4 deafened animals showed that pCREB+ band was reduced in nuclear proteins from deafened auditory cortices, whereas the housekeeping nuclear protein, histone, acts as a loading control (D). By normalizing the intensity of the pCREB band to the histone band, pCREB expression level was found to drop by 33% (E, P < 0.05). Scale bar = 100 μm (A, B) and 20 μm (A, B; insets).
Because pCREB can act as a transcription factor triggering the expression of BDNF, we asked if BDNF expression in AC of deafened rats is similarly affected. Because BDNF has been implicated in long-term potentiation and synaptic plasticity in both cortical and hippocampal neurons (Lu 2003), we consider it important as a first step to establish a neuronal expression of BDNF in AC. Dual immunofluorescence did not show any colocalization between BDNF and GFAP, indicating that BDNF expression could not be detected in glial cells (Fig. 8J,K). Next, using an antibody against MAP2, a dendritic marker, we could establish extensive colocalization between BDNF- and MAP2-associated fluorescence in apical and basal dendrites of pyramidal neurons, confirming that BDNF expression is neuron specific and somatodendritic in nature (Fig. 8A–F). A nonfluorescent, enzymatic-based immunohistochemistry also revealed an intense dendrite-like staining in AC of normal hearing animals, which was dramatically reduced in AC of deafened rats (Fig. 4A,B). BDNF immunolabeling was further abolished by preincubation with its antigenic peptide, establishing the specificity of the antibody used (Fig. 4A, inset). Densitometric measurements of the intensity of these dendrites revealed a significant downregulation in deafened AC (Fig. 4C, 30 ± 13%, P = 0.01) against those of normal hearing controls.
Figure 4.
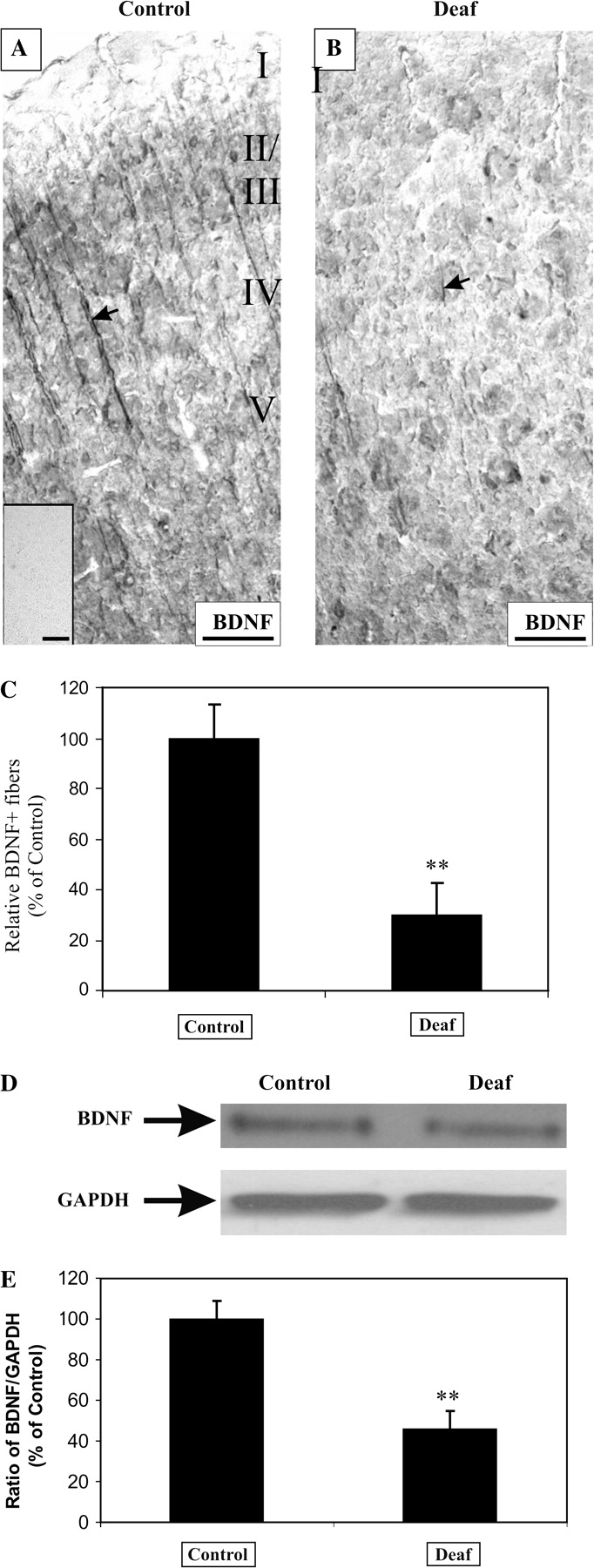
Sensorineural hearing loss reduces BDNF expression in cortical neurons of the AC. In normal hearing animals, BDNF was found to be strongly expressed in neuronal processes of the AC (A, arrow). Immunoreactivity against BDNF was completely abolished after preincubation with excess of its antigenic peptide (A, inset). After 9–16 weeks of deafness, the intensity of BDNF immunoreactivity was downregulated in 4 animals (B, arrow), in comparison to normal hearing controls. An assessment of the intensity of BDNF staining showed a 70% decrease in deafened animals, relative to normal hearing controls (C, P = 0.01). In Western blot experiments, we monitored the expression of the mature BDNF fragment (∼13 kDa) in the auditory cortices of 4 normal hearing controls and 4 deafened animals. There was a decrease in the BDNF-immunopositive band, whereas the housekeeping gene, GAPDH, remained relatively constant (D). The intensity of the BDNF band was normalized to the GAPDH band, and the mean ratio expressed as a relative percentage of control values. There was a 54% reduction in this ratio between controls and deafened animals (E, P < 0.05). Scale bar = 100 μm.
We have previously shown on Western blots that the antibody against BDNF used in this study identifies a larger proneurotrophin form (∼34 kDa) and a smaller mature fragment of ∼13 kDa (Tan and Shepherd 2006). Because mature BDNF is responsible for most of the plasticity-related effects, we monitored the expression levels of mature BDNF in AC of deafened and normal hearing animals, relative to a housekeeping gene, GAPDH. Western immunoblots confirmed a decline in mature BDNF expression in deafened AC (Fig. 4D), and after normalizing to GAPDH we obtained a significant reduction of BDNF (Fig. 4E, 46 ± 9%, P < 0.05) in deafened AC, relative to normal hearing controls. Together, both independent techniques—immunohistochemistry and Western immunoblots—confirmed a reduction of pCREB and BDNF in AC of deafened rats.
Intracochlear Electrical Stimulation Increases pCREB Levels in Contralateral AC
The downregulation of pCREB and BDNF in AC of deafened rats suggests a reduced neural activation in AC resulting from diminishing activity inputs from the peripheral cochlea. To show that these molecular events are related to activity-dependent fluctuations in the auditory system, we inserted cochlear implants unilaterally into deafened rats and performed chronic electrical stimulation over a period of 7 weeks. These cochlear implants stimulate the surviving primary auditory neurons or spiral ganglion neurons, effectively increasing afferent neural activity into the central auditory system. To ensure that cochlear implants remained in the cochleae during the course of stimulation, we monitored ABRs evoked by an electrical impulse transmitted to the implant electrodes. One animal was removed from the study because no ABR was recorded and X-ray radiograph of the skull confirmed a disconnected wire in the implant. Four animals were successfully stimulated and their AC was used for immunohistochemical analysis. Due to the crossover of auditory tracts in the midbrain, a unilateral electrical stimulation in the periphery would predominantly activate the contralateral AC (Kandel et al. 2000). We therefore performed pCREB enzymatic-based immunohistochemistry and compared both ipsilateral and contralateral AC from electrically stimulated animals.
After chronic electrical stimulation, pCREB was dramatically elevated in cortical neurons of the contralateral AC instead of the ipsilateral AC (Fig. 5A–D) but no comparable differences in pCREB expression could be noticed in the ipsilateral and contralateral hippocampus (data not shown). The differential staining pattern observed for both ipsilateral and contralateral AC could also be confirmed using an alternative immunofluorescence approach coupling pCREB and GFAP antibodies (Fig. 8). As shown in Figure 8O,P, pCREB immunofluorescence was drastically reduced, in contrast to GFAP which remained unchanged, suggesting a specific molecular upregulation of pCREB in the contralateral AC as a consequence of unilateral electrical stimulation. We next performed densitometric measurements to evaluate the extent of pCREB elevation. We observed that the density of pCREB-immunopositve neurons in the contralateral AC was almost 2-fold higher (Fig. 5E, 184% ± 11%, P < 0.05), relative to the ipsilateral.
Figure 5.
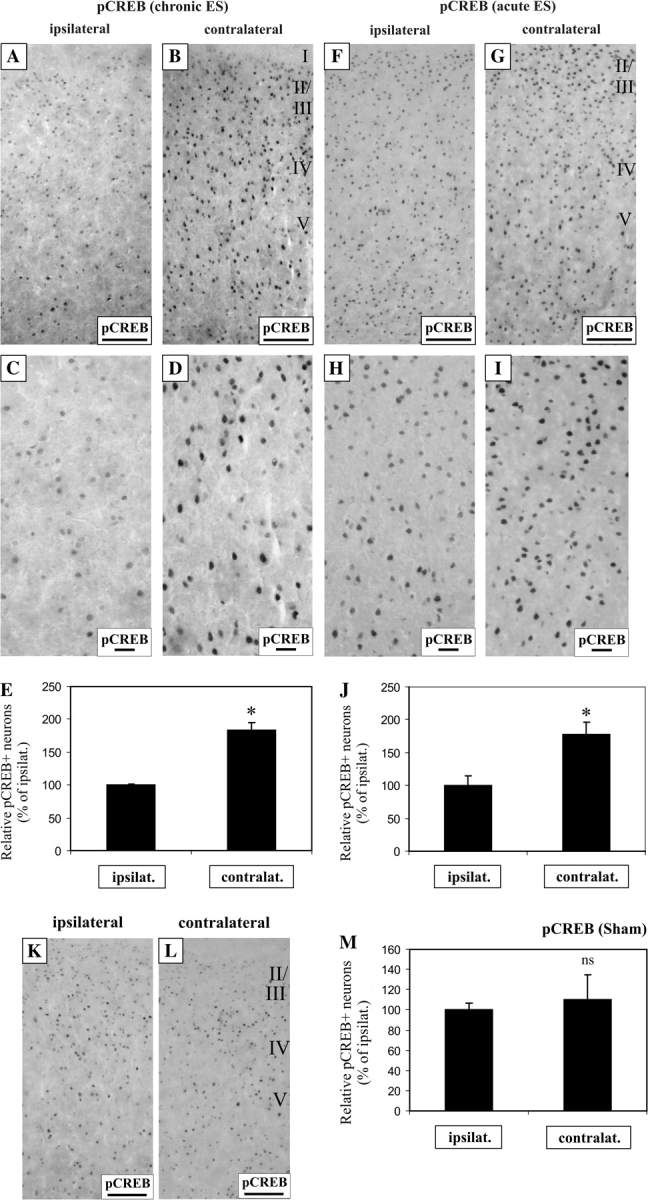
Unilateral electrical stimulation with cochlear implants activates CREB phosphorylation. Chronic intracochlear electrical stimulation increased CREB phosphorylation in the contralateral AC, relative to the ipsilateral (A, B), shown also in higher magnification (C, D). The proportion of neurons expressing above median pCREB immunostaining was elevated by 1.8-fold in the contralateral cortical neurons, relative to the ipsilateral side (E, P < 0.05). Acute intracochlear electrical stimulation also increased CREB phosphorylation in the contralateral AC, relative to the ipsilateral (F, G), shown also in higher magnification (H, I), by 1.8-fold (J, P < 0.05). In contrast, sham surgery using nonstimulating electrodes did not result in comparable differences in pCREB immunostaining between both hemispheres of the AC (K, L), and densitometric measurements did not reveal a statistically significant difference (M, P = 0.7). Scale bar = 100 μm (A, B, F, G). Scale bar = 20 μm (C, D, H, I).
To establish unambiguously a direct role of electrical stimulation in activating CREB, we performed acute 3-h electrical stimulation on deafened animals to determine if a unilateral boost of afferent activity upregulates CREB phosphorylation in the contralateral AC. Immunolabeling of pCREB was increased in the contralateral AC, compared with the ipsilateral cortex (Fig. 5F–I). Similar to chronic electrical stimulation, the density of pCREB-immunopositive neurons in the contralateral AC rose by about 2-fold (Fig. 5J, 178% ± 19%, P < 0.05), when compared with the ipsilateral cortex. If electrical activity increases the phosphorylation of CREB in these cortical neurons, we would deduce that the expression of the immediate early gene, c-Fos, would likewise be elevated. Indeed, immunolabeling of hemispheric halves of AC from acutely stimulated animals demonstrated a 1.4-fold increase in c-Fos expression in contralateral neurons than ipsilateral (Fig. 1, Supplementary Material online, P < 0.05). To further underscore that this alteration in CREB phosphorylation is specific to electrical stimulation and not attributed to surgical procedures, 3 deafened animals were implanted with a nonstimulating electrode and no comparable alterations in pCREB expression could be seen in both contralateral and ipsilateral AC after 3 h (Fig. 5K,L). Densitometric assessment of this labeling did not reveal statistically significant differences (Fig. 5M). Even from 2 animals implanted with a nonstimulating electrode for 7 weeks, no distinguishable differences in pCREB immunolabeling could be observed in both AC hemispheres (data now shown).
BDNF Levels in Contralateral AC Is Significantly Increased by Chronic but Not 3-h Acute Intracochlear Electrical Stimulation
Having established that cochlear implants drive CREB phosphorylation in the AC, we speculated that BDNF expression would be similarly modulated. After chronic electrical stimulation, we observed a marked upregulation of BDNF in the dendrites of the contralateral AC (Fig. 6A–D). We performed densitometric measurements on dendrites in both ipsilateral and contralateral AC. Dendrites in the contralateral AC (Fig. 6E, 181 ± 19%, P < 0.05) exhibited a nearly doubled expression of BDNF, relative to those in the ipsilateral AC. Using a fluorescence-based immunohistochemical approach, we also observed a detectable increase in dendritic expression of BDNF in the contralateral AC when compared with the ipsilateral cortex (Fig. 8Q,R). However, electrical stimulation alone is not entirely responsible for this alteration because an acute 3-h electrical stimulation only weakly elevated the expression of BDNF protein in the dendrites (Fig. 6F–I) of the contralateral AC, when compared with the ipsilateral cortex. Although this stimulation duration increased dendritic BDNF levels (Fig. 6J, 145 ± 18% in contralateral cortex), this change was not statistically significant (Fig. 6J, P = 0.051), indicating that other mechanisms account for the greater activation of BDNF expression in the chronic stimulation paradigm.
Figure 6.
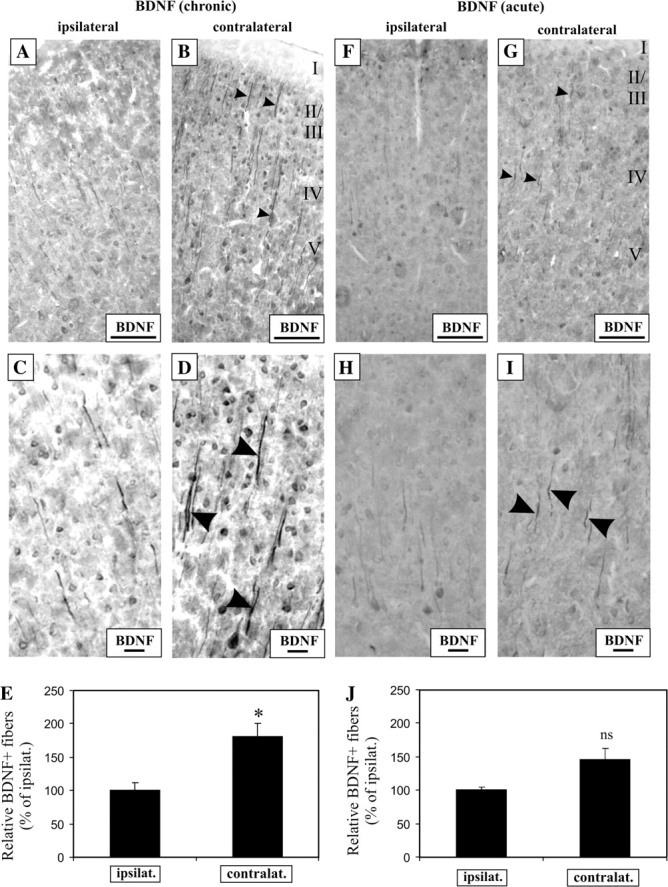
Chronic, but not acute 3-h electrical stimulation with cochlear implants induces robust BDNF expression in contralateral cortical neurons of the deaf AC. After 7 weeks of electrical stimulation, there was an increase in the expression of BDNF protein in the contralateral AC (A, B), and at higher magnification the increased BDNF expression was visibly localized to soma bodies and processes (arrowheads) of the contralateral cortical neurons versus the ipsilateral ones (C, D). BDNF immunoreactivity was increased by 1.8-fold in the processes from the contralateral cortical neurons, when compared with the ipsilateral ones (E, P < 0.05). In contrast, acute electrical stimulation for 3 h resulted only in a mild increase in BDNF immunostaining in the neuronal processes (arrowheads) in the contralateral AC, when compared with the ipsilateral (F–I). Statistical analysis of the BDNF signal in these processes between both hemispheres revealed nonsignificant differences (J, P = 0.051). Scale bar = 100 μm (A, B, F, G). Scale bar = 20 μm (C, D, H, I).
Cochlear Implants and Deafness Differentially Regulate MAPK Phosphorylation in AC
To test the hypothesis that the MAPK signaling cascade might be engaged in the regulation of CREB activity and BDNF expression, we examined pMAPK immunohistochemistry on AC sections, both ipsilateral and contralateral to the chronically stimulated cochlea (Fig. 7). On the contralateral AC, we observed an elevated pMAPK expression prominently in the superficial layers II–IV, relative to the ipsilateral AC (Fig. 7A,B). Densitometric measurements showed a significant increase in the density of pMAPK-immunopositive neurons in the contralateral AC (Fig. 7C, 162 ± 20%, P < 0.005) above the ipsilateral control. Triple fluorescence showed that pMAPK expression was restricted to nuclei of neurons, but not glial cells (Fig. 8M,N). To determine if electrical stimulation could drive the phosphorylation of MAPK in AC, we compared both hemispheres of pMAPK expression in acutely stimulated rats. After 3 h of stimulation, there was a noticeable difference in pMAPK immunolabeling between both hemispheres, with significantly more labeling observed on the contralateral AC (Fig. 7D,E; insets, higher magnification exemplifying pMAPK increase) and the intensity of staining was higher in a significant proportion of neurons (Fig. 7F, 160 ± 17%, P < 0.05).
Figure 7.
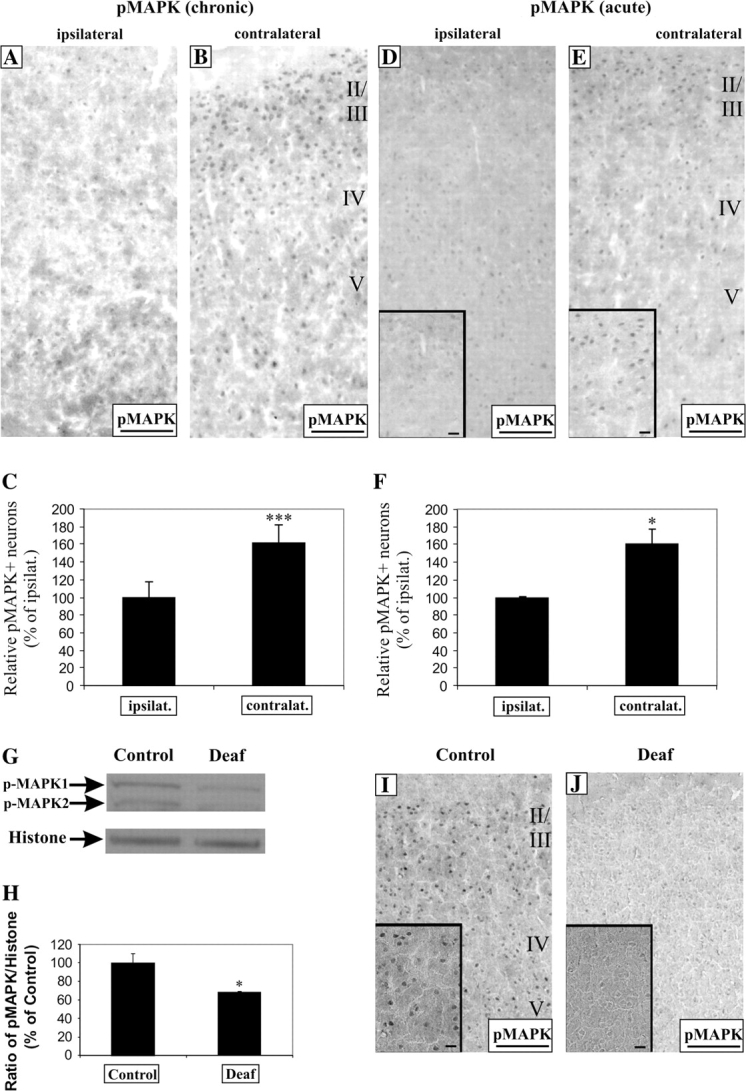
Unilateral electrical stimulation with cochlear implants activates MAPK phosphorylation in cortical neurons of the contralateral AC. Both chronic and acute intracochlear electrical stimulation increased MAPK phosphorylation in the contralateral AC, relative to the ipsilateral (chronic A, B; acute D, E). Higher magnification exemplifying this upregulation is shown as insets (D, E). Densitometric measurements showed a significant increase in the density of pMAPK+ neurons by 1.6-fold in the contralateral cortex versus the ipsilateral in both chronic (C, P < 0.005) and acute (F, P < 0.05) studies. The specificity of the antibody was tested by Western analysis of nuclear protein fractions from the AC of normal hearing animals which showed 2 bands, ∼42 and 44 kDa (G). Both bands diminished in expression in the AC of 4 profoundly deaf animals (9–16 weeks of deafness) with the nuclear housekeeping protein, histone, as a loading control (G). By normalizing the intensity of the upper pMAPK+ band to histone, there was a relative one-third decline in the expression of pMAPK in the deafened AC (H, P < 0.05). This decrease in pMAPK expression could also be confirmed by immunohistochemistry from normal hearing and deaf animals (I, J) and presented at higher magnification in insets. Scale bar = 100 μm (A, B, D, E, I, J) and 20 μm (D, E, I, J, insets).
To obtain further evidence of a parallel association between pMAPK, pCREB, and BDNF, we performed Western analysis of AC from normal hearing and deafened animals. Western blot data identify 2 bands, ∼42 and 44 kDa, corresponding to the dually phosphorylated forms of MAPK in nuclear protein fractions and as expected, their intensities were lower in AC from deafened animals (Fig. 7G). Comparing the ratio of pMAPK to histone, a loading control, revealed a significant reduction of pMAPK in deafened AC compared with normal hearing (Fig. 7H, 68 ± 1%, P < 0.05). This reduction could also be confirmed on sections of AC, where pMAPK labeling was prominently reduced after a period of deafness (Fig. 7I,J) and also shown at higher magnification portraying this downregulation (Fig. 7I,J; insets). These results indicate that sensorineural hearing loss and cochlear implants—2 different paradigms driving neural activity in the AC—can differentially regulate MAPK signaling and this pathway is linked to fluctuations of CREB activity and BDNF expression after chronic stimulation.
Intracochlear Chronic Stimulation and Deafness Affect Voltage-Gated Sodium Ion Channel Expression in AC
The increased phosphorylation of CREB and MAPK and upregulation of BDNF in neurons of AC of chronically stimulated rats would suggest enhanced synaptic activity. In particular, intracochlear chronic electrical stimulation in congenitally deaf cats have been shown in AC to produce field potentials of higher amplitudes (Klinke et al. 1999), but it is not known if cochlear implants alter ion channel expression in the deaf brain. To test this putative link, we examined the expression of voltage-gated sodium channels due to their importance in action potential generation. Because of the diversity and complexity of sodium channel subtypes, we adopted a more general approach by using an antibody that recognized all isoforms of voltage-gated sodium channel (panNaC). After unilateral chronic stimulation, panNaC expression was markedly increased in all layers of cortical neurons in the contralateral AC, relative to the ipsilateral AC (Fig. 9A–D). Unlike BDNF immunoreactivity in the dendrites, panNaC expression was restricted to the neuronal soma (Fig. 9C,D, arrows). Densitometric measurements revealed a statistically significant elevation of panNaC immunoreactivity in the contralateral AC (Fig. 9E, 185 ± 7%, P < 0.01), in comparison to the ipsilateral AC. In contrast, acute electrical stimulation in deafened rats did not result in comparable changes of panNaC expression between both AC (Fig. 9F–I), and densitometry analysis did not reveal any statistically significant differences (Fig. 9J, P > 0.05).
Figure 9.
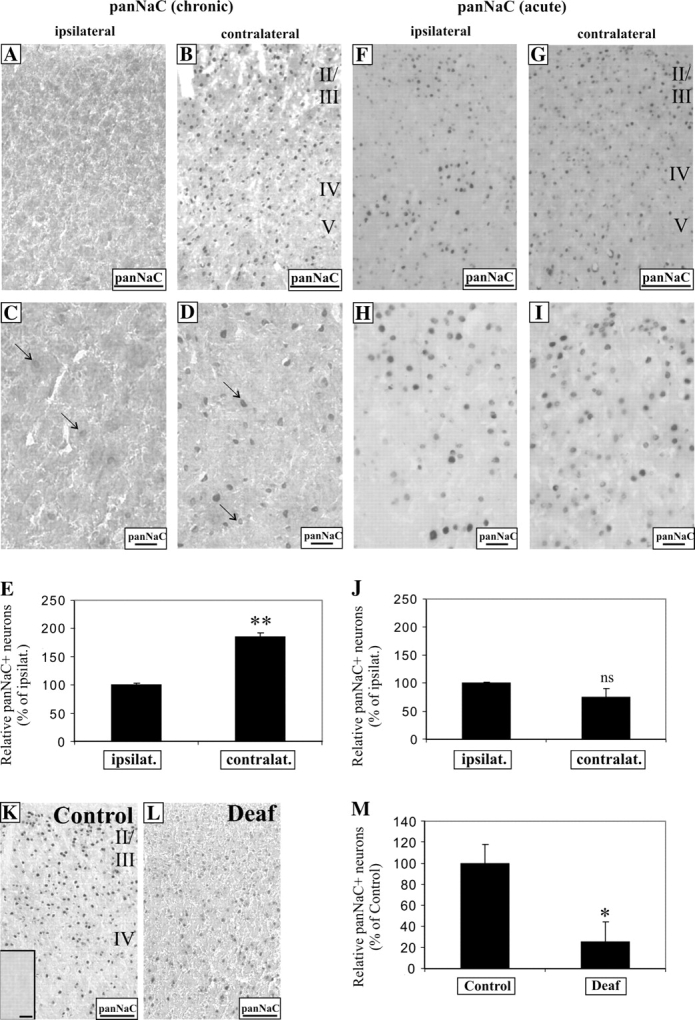
Only chronic unilateral electrical stimulation with cochlear implants activates voltage-gated sodium channel expression in cortical neurons of the contralateral AC. After chronic electrical stimulation, cortical neurons in the contralateral AC were found to upregulate voltage-gated sodium channel expression (panNaC) expression, in comparison to ipsilateral neurons (A, B). At higher magnification, only weak panNaC immunostaining was found in the ipsilateral neurons (C, arrows), whereas a stronger panNaC expression was identified in the soma of stimulated, contralateral neurons (D, arrows). Densitometric measurements showed a significant increase in the density of panNaC+ neurons by 1.8-fold in the contralateral half versus the ipsilateral (E, P < 0.01). In contrast, after an acute 3-h electrical stimulation, no observable differences in panNaC immunolabeling could be seen in neurons from both hemispheres (F, G); in higher magnification (H, I). Statistical analysis between both hemispheres revealed nonsignificant differences (J, P = 0.16). Sensorineural hearing loss led to a decline of panNaC expression in neurons of the AC between normal hearing and deaf animals (K, L), and densitometric measurement showed a significant 80% decline in the proportion of panNaC-positive neurons, relative to controls (M, P < 0.05). Prior incubation of the antibody with its antigenic peptide abolished the signal in cortical neurons of normal hearing animals (K, inset). Scale bar = 100 μm (A, B, F, G, K, L). Scale bar = 20 μm (C, D, H, I).
Because our observations suggest that sensorineural hearing loss and cochlear implants have opposing effects on neural activity inputs in the auditory system, we reasoned that prolonged auditory deprivation by deafness would also alter the expression of panNaC in cortical neurons in the AC. To determine how this expression is altered, we compared panNaC expression in AC from normal hearing and deafened animals. In deafened AC, panNaC expression was detectably less (Fig. 9K,L) and there was a significant decline in the density of neurons showing panNaC immunostaining (Fig. 9M, 25 ± 19%, P < 0.05). Prior incubation of the antibody with its antigenic peptide abolished any immunostaining in AC neurons from normal hearing animals (Fig. 9K, inset), underscoring the specificity of the antibody used.
Taken together, these findings indicated that both sensorineural hearing loss and cochlear implants have profound effects on the regulation of candidate genes affecting neuronal function and synaptic activity.
Discussion
The degeneration of the organ of Corti in the cochlea deprives acoustic inputs into the central auditory system leading to sensorineural hearing loss. In deaf humans, cochlear implants can electrically stimulate the surviving primary auditory neurons, thus reinstating spike activity within the auditory nerve, restoring effective auditory communication in many, but not all patients (Cowan et al. 1997; Fryauf-Bertschy et al. 1997; Francis and Niparko 2003). The success of cochlear implants in deaf patients clearly suggests an adaptive, plastic response of the auditory system (Fallon et al. 2007), but the neural mechanisms underlying this response have not been fully elucidated. In congenitally deaf cats stimulated with cochlear implants, activity in AC not only produced field potentials of higher amplitudes but also was expanded in area, suggesting an enhanced synaptic efficacy (Klinke et al. 1999). What cellular and molecular mechanisms underlie this improved synaptic function induced by cochlear implants?
Using the ototoxically deafened rat as an experimental model, we systematically showed that sensorineural hearing loss perturbs the activity-dependent CREB pathway in the AC by depressing the phosphorylation of CREB and expression of BDNF. Linked to these changes is a downregulation of MAPK signaling cascade in cortical neurons. More significantly, we found that unilateral, chronic electrical stimulation using cochlear implants results in higher numbers of cortical neurons showing intense phosphorylation of CREB and MAPK and somatodendritic expression of BDNF in contralateral AC, when compared with the ipsilateral. Whenever these changes occur, we also observed an increased expression of voltage-gated sodium ion channels in the contralateral cortical neurons. Whereas the regulation of selected candidate genes such as CREB and MAPK can be effected by acute stimulation, a long-lasting mechanism appears to modulate the expression of voltage-gated sodium channels and BDNF. Together, our findings provide a molecular perspective to neural mechanisms recruited by cochlear implants in the central auditory system and complement previous morphological and electrophysiological studies (Klinke et al. 1999; Ryugo et al. 2005).
Sensorineural Hearing Loss and Activity-Dependent Genes
A first, critical step towards elucidating activity-dependent signaling pathways triggered by cochlear implants is to establish the effects of activity deprivation on these pathways within the AC. We have taken advantage of the well-known anatomy of the rat AC and its morphological juxtaposition to the hippocampus to assist us in identifying the AC (Kelly and Sally 1988; Paxinos and Watson 1988; Sally and Kelly 1988; Doron et al. 2002; Rutkowski et al. 2003). Destroying hair cells in the organ of Corti using noise trauma or aminoglycoside antibiotics (Tan and Shepherd 2006) reduces the afferent drive via primary auditory neurons to the central auditory pathway (Tan et al. 2007). Therefore, we would anticipate a reduction in the expression of activity-dependent genes, c-Fos and BDNF, and phosphorylation of CREB; events triggered by calcium influxes through synaptic glutamate receptors and L-type voltage-gated calcium channels (West et al. 2002). Our immunolabeling of cortical neurons in the AC and Western blot semiquantification experiments confirm this hypothesis (Figs 2–4). Moreover, these observations are in close agreement with the decline of c-Fos and BDNF transcription or CREB phosphorylation seen in the visual and piriform cortices after peripheral sensory deprivation (Castren et al. 1992; Kim et al. 2006; Majdan and Shatz 2006), or the downregulation of distinct BDNF transcripts in AC 6 days after traumatic noise exposure (Tan et al. 2007). It should be stressed that the extent of decline for these genes in this study was reportedly less when using Western analysis, in comparison to densitometric measurements of immunolabeling of cortical neurons. This is a technical consideration that can be attributed to the signal amplification associated with biotin–avidin amplification in our immunohistochemical experiment or the lowered degree of sensitivity associated with Western blotting. Nonetheless, both independent methods—immunohistochemistry and Western blotting—verify a reduction of c-Fos, BDNF protein expression, and CREB phosphorylation.
Cochlear Implants and Molecular Plasticity in Cortical Neurons
A prominent feature of neural activation is the increase of intracellular calcium concentration mediated primarily by glutamate activation of N-methyl-D-aspartate (NMDA) receptors and depolarization-induced opening of voltage-gated calcium channels (West et al. 2002). Calcium-dependent kinases such as MAPK and calcium/calmodulin-dependent protein kinase IV phosphorylate CREB (Wu et al. 2001), which then induce the transcription of bdnf and c-fos genes (Shieh et al. 1998; Tao et al. 1998; Mayr and Montminy 2001). Therefore, to investigate if CREB phosphorylation in cortical neurons could be induced by cochlear implants, we focus on unilateral intracochlear stimulation in order to directly compare both ipsilateral and contralateral AC within the same animal. This approach would obviate nonspecific molecular changes associated with differences in fixation procedures.
In both acute and chronic electrical stimulation, our findings reveal, for the first time, increased phosphorylation of CREB in cortical neurons activated by cochlear implants (Figs 5A–J and 8O,P), thus identifying a key molecular marker of plasticity induced by functional electrical stimulation. Increased CREB phosphorylation would have great implications in switching on downstream target genes that are required by neurons to modify their synaptic structure and function in response to the reinstatement of afferent activity.
Unlike CREB phosphorylation, the expression of BDNF protein is dependent on the duration of stimulation, with more robust upregulation seen in somatodendritic compartments of cortical neurons after chronic stimulation (Figs 6 and 8Q,R). Whereas BDNF mRNA transcripts are rapidly inducted in the brain typically 3 h after a seizure-like stimulation (Metsis et al. 1993; Timmusk et al. 1993), synthesis of the protein follows a slower time course and it has been reported that BDNF protein rose significantly at least 6–24 h after a stimulus trigger (Elmer et al. 1998; Chavko et al. 2002; Soya et al. 2007). Elevated BDNF expression in cortical neurons after chronic stimulation via cochlear implants could enhance synaptic transmission in these neurons because BDNF is known to dramatically increase neuronal firing rate and the frequency and amplitude of excitatory postsynaptic currents (Levine et al. 1995). This effect can be attributed to increased phosphorylation of NMDA receptors, which strengthens receptor binding, channel conductance, and synaptic transmission (Suen et al. 1997). Although it cannot be established in this study if BDNF acts presynaptically, we cannot exclude the possibility of such a mechanism because at presynaptic sites, BDNF has been shown to modulate synaptic transmission (Gottschalk et al. 1998) by facilitating the mobilization and docking of synaptic vesicles at active zones (Pozzo-Miller et al. 1999), emphasizing an important mechanism in which BDNF can enhance synaptic transmission and postsynaptic responses in the rat visual cortex and hippocampus (Kang and Schuman 1995; Akaneya et al. 1997).
Thus, it emerges as a tentative possibility that the increase in cortical activity evoked by cochlear implants—as shown in congenitally deaf cats (Klinke et al. 1999)—can be explained by a collective group of molecules acting to increase synaptic activity. On the other hand, an interesting feature demonstrated in this study is the significant upregulation of voltage-gated sodium channels in contralateral cortical neurons, relative to ipsilateral neurons (Fig. 9A–E) only after chronic unilateral stimulation via a cochlear implant. Whereas the physiological significance of this phenomenon cannot be established in the present study, an increase in intrinsic excitability has been shown to be correlated with enhanced voltage-gated sodium currents (Aizenman et al. 2003) or a decrease in action potential threshold (Xu et al. 2005)—features which are related to a regulated synthesis of sodium channels (Aizenman et al. 2003). Moreover, the upregulated expression of voltage-gated sodium channels is consistent with previous findings demonstrating that these channels are linked to BDNF secretion (Balkowiec and Katz 2002) and that the sodium channel Nav1.9 mediates BDNF-evoked depolarizations (Blum et al. 2002).
Due to the reciprocal relationship between BDNF and pCREB, we reason that a presumptive increase in BDNF secretion in stimulated cortical neurons would effectively activate Ras/MAPK signaling downstream of surface-bound TrkB receptors (Finkbeiner et al. 1997; West et al. 2002; Segal 2003). In the present study, we have identified an increased expression of pMAPK in cortical neurons contralateral to stimulation from a cochlear implant (Fig. 7A–F). In recent years, Ras/MAPK signaling has been shown to be important for long-lasting forms of synaptic plasticity by stimulating protein synthesis, which is required at least in memory consolidation and the late phase of long-term potentiation (McGaugh 2000; Kandel 2001; Kelleher et al. 2004). The biological relevance of MAPK signaling in auditory plasticity has been demonstrated in zebra finches, where presentation of novel songs activates MAPK phosphorylation in the auditory forebrain (Cheng and Clayton 2004). If restoration of activity inputs by cochlear implants augments MAPK signaling, it can be argued that sensorineural hearing loss would have the reverse effect. In line with this reasoning, we found that pMAPK expression in nuclear proteins was significantly reduced in AC of profoundly deaf animals when compared with normal hearing controls (Fig. 7G–J).
Taken together, this is the first report demonstrating that cochlear implants activate Ca2+-dependent signaling events in activated neurons of the AC, which involve BDNF synthesis, CREB, and MAPK phosphorylation. These regulatory events may underlie the neural mechanisms necessary to initiate synaptic modification in structure and function to allow the deaf brain to accommodate adaptive changes in response to renewed afferent activity. One of these includes an upregulation of voltage-gated sodium channels that are required for the generation of action potentials—the ultimate, driving force of neurons.
Supplementary Material
Supplementary material can be found at: http//www.cercor.oxfordjournals.org/.
Funding
The National Institute on Deafness and Other Communication Disorders (NO1-DC-3-1005) to R.K.S; The Medical Research and Technology in Victoria, Australia, the Marion and E H Flack Trust, and The Garnett Passe and Rodney Williams Memorial Foundation to J.T.; the Freiwillige Akademische Gesellschaft (Switzerland) to S.W.
Supplementary Material
Acknowledgments
We gratefully thank Helen Feng, Elisa Borg, Rodney Millard, Anne Coco, and Lauren Donley for assistance. Conflict of Interest: None declared.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. doi: 10.1016/s0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Nav1.9. Nature. 2002;419:687–693. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavko M, Nadi NS, Keyser DO. Activation of BDNF mRNA and protein after seizures in hyperbaric oxygen: implications for sensitization to seizures in re-exposures. Neurochem Res. 2002;27:1649–1653. doi: 10.1023/a:1021687011281. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Clayton DF. Activation and habituation of extracellular signal-regulated kinase phosphorylation in zebra finch auditory forebrain during song presentation. J Neurosci. 2004;24:7503–7513. doi: 10.1523/JNEUROSCI.1405-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RS, DelDot J, Barker EJ, Sarant JZ, Pegg P, Dettman S, Galvin KL, Rance G, Hollow R, Dowell RC, et al. Speech perception results for children with implants with different levels of preoperative residual hearing. Am J Otol. 1997;18:S125–S126. [PubMed] [Google Scholar]
- Doron NN, Ledoux JE, Semple MN. Redefining the tonotopic core of rat auditory cortex: physiological evidence for a posterior field. J Comp Neurol. 2002;453:345–360. doi: 10.1002/cne.10412. [DOI] [PubMed] [Google Scholar]
- Elmer E, Kokaia Z, Kokaia M, Carnahan J, Nawa H, Lindvall O. Dynamic changes of brain-derived neurotrophic factor protein levels in the rat forebrain after single and recurring kindling-induced seizures. Neuroscience. 1998;83:351–362. doi: 10.1016/s0306-4522(97)00387-4. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Irvine DRF, Shepherd RK Forthcoming. Cochlear implants and brain plasticity. Hear Res. doi: 10.1016/j.heares.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Francis HW, Niparko JK. Cochlear implantation update. Pediatr Clin North Am. 2003;50:341–361. doi: 10.1016/s0031-3955(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy H, Tyler RS, Kelsay DM, Gantz BJ, Woodworth GG. Cochlear implant use by prelingually deafened children: the influences of age at implant and length of device use. J Speech Lang Hear Res. 1997;40:183–199. doi: 10.1044/jslhr.4001.183. [DOI] [PubMed] [Google Scholar]
- Games KD, Winer JA. Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear Res. 1988;34:1–26. doi: 10.1016/0378-5955(88)90047-0. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog H, Lamprecht A, Kuhn A, Roden W, Vosteen KH, Finendegen LE. Cortical activation in profoundly deaf patients during cochlear implant stimulation demonstrated by H2(15)O PET. J Comput Assist Tomogr. 1991;15:369–375. doi: 10.1097/00004728-199105000-00005. [DOI] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- Hurley PA, Crook JM, Shepherd RK. Schwann cells revert to non-myelinating phenotypes in the deafened rat cochlea. Eur J Neurosci. 2007;26:1813–1821. doi: 10.1111/j.1460-9568.2007.05811.x. [DOI] [PubMed] [Google Scholar]
- Ito J, Iwasaki Y, Sakakibara J, Yonekura Y. Positron emission tomography of auditory sensation in deaf patients and patients with cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:797–801. doi: 10.1177/000348949310201011. [DOI] [PubMed] [Google Scholar]
- Ito J, Sakakibara J, Honjo I, Iwasaki Y, Yonekura Y. Positron emission tomographic study of auditory sensation in a patient with a cochlear implant. Arch Otolaryngol Head Neck Surg. 1990;116:1437–1439. doi: 10.1001/archotol.1990.01870120083015. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of neural science. New York: McGraw-Hill.; 2000. p 1414. [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Sally SL. Organization of auditory cortex in the albino rat: binaural response properties. J Neurophysiol. 1988;59:1756–1769. doi: 10.1152/jn.1988.59.6.1756. [DOI] [PubMed] [Google Scholar]
- Kim HH, Puche AC, Margolis FL. Odorant deprivation reversibly modulates transsynaptic changes in the NR2B-mediated CREB pathway in mouse piriform cortex. J Neurosci. 2006;26:9548–9559. doi: 10.1523/JNEUROSCI.1727-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke R, Kral A, Heid S, Tillein J, Hartmann R. Recruitment of the auditory cortex in congenitally deaf cat by long-term cochlear electrostimulation. Science. 1999;285:1729–1733. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Xu J, Shepherd RK. Cochlear implantation in rats: a new surgical approach. Hear Res. 2005;205:115–122. doi: 10.1016/j.heares.2005.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci. 2006;9:650–659. doi: 10.1038/nn1674. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci USA. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard RE, Shepherd RK Forthcoming. A fully implantable stimulator for use in small laboratory animals. J Neurosci Methods. 2007;166:168–177. doi: 10.1016/j.jneumeth.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Okazawa H, Honjo I, Hirano S, Takahashi H, Shiomi Y, Hoji W, Kawano M, Ishizu K, Yonekura Y. Cortical activation with sound stimulation in cochlear implant users demonstrated by positron emission tomography. Brain Res Cogn Brain Res. 1995;2:207–214. doi: 10.1016/0926-6410(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski RG, Miasnikov AA, Weinberger NM. Characterization of multiple physiological fields within the anatomical core of rat auditory cortex. Hear Res. 2003;181:116–130. doi: 10.1016/s0378-5955(03)00182-5. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Kretzmer EA, Niparko JK. Restoration of auditory nerve synapses in cats by cochlear implants. Science. 2005;310:1490–1492. doi: 10.1126/science.1119419. [DOI] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: sound frequency. J Neurophysiol. 1988;59:1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–310. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172:92–98. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358:961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82:601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Tan J, Ruttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Kopschall I, Rohbock K, et al. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–726. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Tan J, Shepherd RK. Aminoglycoside-induced degeneration of adult spiral ganglion neurons involves differential modulation of tyrosine kinase B and p75 neurotrophin receptor signaling. Am J Pathol. 2006;169:528–543. doi: 10.2353/ajpath.2006.060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Vollmer M, Leake PA, Beitel RE, Rebscher SJ, Snyder RL. Degradation of temporal resolution in the auditory midbrain after prolonged deafness is reversed by electrical stimulation of the cochlea. J Neurophysiol. 2005;93:3339–3355. doi: 10.1152/jn.00900.2004. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


