Summary
Background
Vertebrate oocytes are arrested in metaphase II of meiosis prior to fertilization by cytostatic factor (CSF). CSF enforces a cell cycle arrest by inhibiting the anaphase promoting complex (APC), an E3 ubiquitin ligase that targets Cyclin B for degradation. Although Cyclin B synthesis is ongoing during CSF arrest, constant Cyclin B levels are maintained. To achieve this, oocytes allow continuous slow Cyclin B degradation, without eliminating the bulk of Cyclin B, which would induce release from CSF arrest. However, the mechanism that controls this continuous degradation is not understood.
Results
We report here the molecular details of a negative feedback loop wherein Cyclin B promotes its own destruction through Cdc2/Cyclin B-mediated phosphorylation and inhibition of the APC inhibitor, Emi2. Emi2 bound to the core APC and this binding was disrupted by Cdc2/Cyclin B, without affecting Emi2 protein stability. Cdc2 mediated phosphorylation of Emi2 was antagonized by PP2A, which could bind to Emi2 and promote Emi2-APC interactions.
Conclusions
Constant Cyclin B levels are maintained during a CSF arrest through the regulation of Emi2 activity. A balance between Cdc2 and PP2A controls Emi2 phosphorylation, which in turn controls the ability of Emi2 to bind to and inhibit the APC. This balance allows proper maintenance of Cyclin B levels and Cdc2 kinase activity during CSF arrest.
Introduction
Vertebrate oocytes remain arrested for prolonged periods in metaphase II of meiosis through the action of cytostatic factor (CSF), an activity defined based on the ability of injected cytoplasm from unfertilized Xenopus eggs to block blastomere cleavage [1]. Fertilization triggers release from this arrest, promoting entry into the early embryonic cell cycles. The nature of CSF has not been fully defined, but Mos, the apical kinase in a MAPK cascade, is critical to the establishment of the CSF arrest [2–6]. Cdk2/Cyclin E has also been implicated as a CSF component [7].
A defining feature of the CSF arrest is maintenance of high Cdc2/Cyclin B activity. CSF inhibits the APC, an E3 ubiquitin ligase that promotes destruction of Cyclin B and other proteins whose degradation is important for M phase exit. Cdc20 or Cdh1 promotes APC activation, depending upon the precise cell cycle stage [8–10]. CSF arrest establishment has been reported to rely on factors that enforce APC inhibition by the spindle assembly checkpoint (e.g. Mad2) [11, 12]. However, Mad2 is not necessary for maintaining the CSF arrest, suggesting that CSF might rely upon factors other than spindle checkpoint components to maintain APC inhibition [13].
Previously, it was reported that a protein known as Emi1 could bind Cdc20 to inhibit the APC [14]. Immunodepletion of Emi1 from CSF-arrested Xenopus egg extracts promoted release from CSF arrest. Moreover, recombinant Emi1 promoted a CSF-like arrest in egg extracts. Despite its ability to inhibit the APC, the importance of Emi1 in the CSF arrest was called into question by observations suggesting that Emi1 was present at insufficient levels in CSF-arrested eggs/extracts to be responsible for the arrest [15]. Moreover, Emi1 stabilized both A and B-type Cyclins, while the endogenous arrest promoted stabilization of only Cyclin B [16]. Recently, an Emi1-related protein, Emi2, has been implicated in the CSF arrest. This APC inhibitor, which also contains a putative Cdc20 binding domain, can promote a CSF-like arrest and is sufficiently abundant in CSF extracts to potentially contribute to the arrest. Moreover, its depletion resulted in spontaneous APC activation and release from the CSF arrest [17, 18]. Importantly, the increase in free intracellular calcium that accompanies fertilization and triggers release from the CSF arrest, leads to Emi2 degradation, alleviating APC inhibition and promoting CSF release [19, 20]. Thus, it seems likely that Emi2, inadvertently depleted by antibodies prepared against Emi1, is the more important mediator of the CSF arrest.
Calcium-induced Emi2 degradation is triggered by the CaMKII-mediated phosphorylation of Emi2 at Thr 195 (of Xenopus Emi2) [20, 21], providing a docking site for the Polo-like kinase, Plx1, whose phosphorylation of Emi2 creates a phosphodegron recognized by the β-TrCP E3 ubiquitin ligase. Together, CaMKII and Plx1 promote Emi2 degradation.
Although Cyclin B degradation occurs primarily at exit from CSF arrest (and with each subsequent M phase exit), there is evidence to suggest that Cyclin B degradation occurs at continuous low levels during a CSF arrest to counterbalance ongoing Cyclin B synthesis [22, 23]. Without this, Cyclin B levels would rise unabated throughout the arrest, resulting in a gradual, rather than a precipitous, exit from the CSF arrest upon fertilization (due to the presence of a large bolus of Cyclin B to degrade). This ongoing Cyclin B degradation appears to be triggered by Cdc2/Cyclin B kinase activity itself, such that Cyclin B synthesis leads to higher Cdc2 kinase activity and results in compensatory Cyclin B degradation [24]. Although this regulatory loop has been well-documented, the molecular mechanisms linking Cdc2/Cyclin B kinase and APC activation during a CSF arrest have not been reported. We show here that Emi2 is phosphorylated and inhibited by Cdc2/Cyclin B at sites distinct from the CaMKII/Plx1 sites, though Cdc2 phosphorylation does not promote Emi2 degradation. Rather, Emi2 can bind and inhibit the full APC (rather than Cdc20 alone) and Emi2-APC interactions are inhibited by Cdc2 phosphorylation of Emi2. Thus, Cdc2 mediated Emi2 phosphorylation relieves APC inhibition, allowing Cyclin B degradation. In addition, Cdc2’s effects on Emi2 are antagonized by PP2A-mediated Emi2 dephosphorylation, which facilitates the Emi2-APC interaction. These findings establish an auto-regulatory loop comprised of Cdc2/Cyclin B, PP2A, Emi2, and the APC that controls Cyclin B levels to properly maintain the CSF arrest and allow for rapid M phase exit upon fertilization.
Results
Cdc2/Cyclin B targets Emi2 to regulate Cyclin B
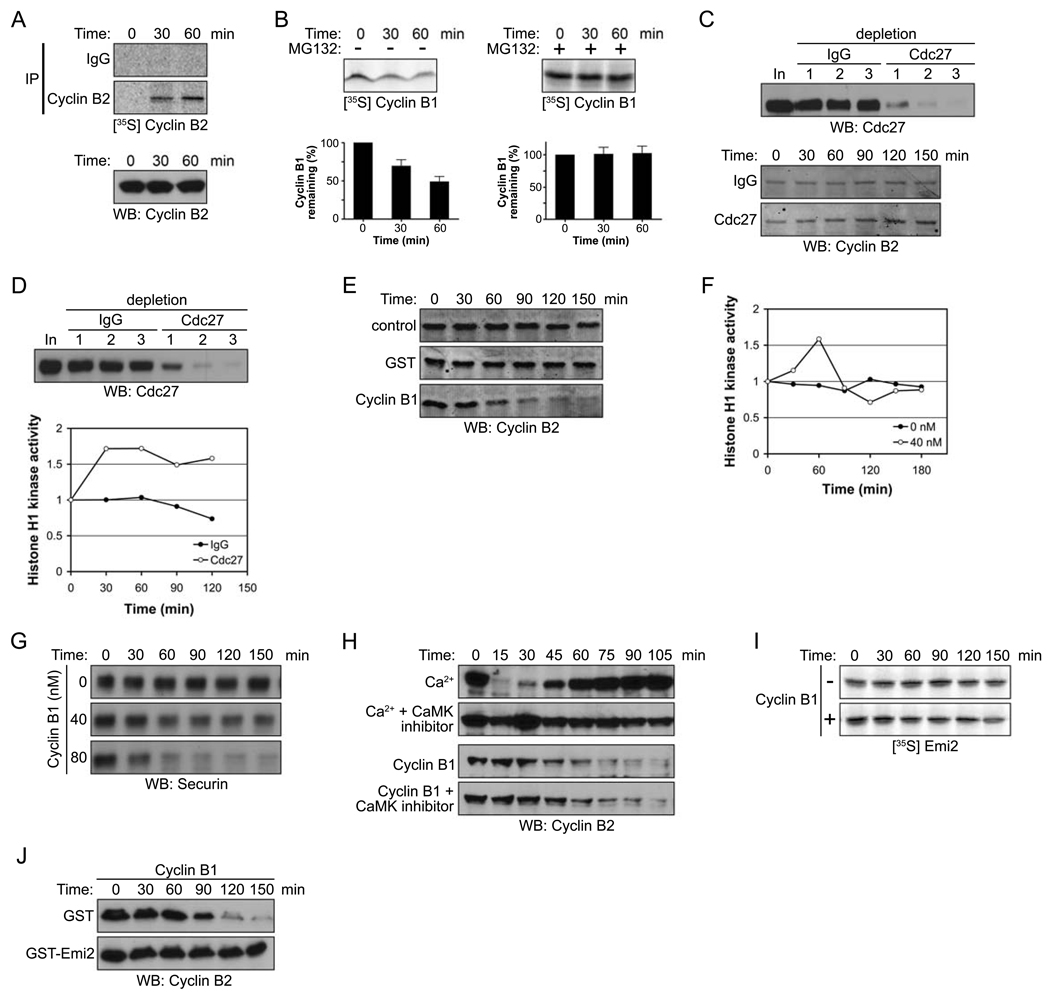
As reported by Kishimoto and colleagues [24], Cyclin B synthesis continues during a CSF arrest, a finding we have repeated with similar results (Fig. 1A, top). Continued protein synthesis would be expected to result in a steady increase in Cyclin B levels during a CSF arrest, which is not observed. Rather, we observe constant levels of Cyclin B throughout CSF arrest (Fig. 1A, bottom). Thus, to maintain constant Cdc2/Cyclin B activity during the arrest, a continual low level of Cyclin B degradation is required, as demonstrated by the degradation of radiolabeled Cyclin B in a CSF-arrested extract (Fig. 1B, left). This degradation is prevented by the proteosome inhibitor, MG132 (Fig 1B, right). Additionally, immunodepletion of the APC using anti-Cdc27 antibodies resulted in a gradual increase in Cyclin B, accompanied by increased Cyclin B/Cdc2 kinase activity (Fig. 1C and D). We note that Histone H1 kinase activity eventually plateaued, even in the absence of the APC. This raises the interesting possibility that Cdc2/Cyclin B1 kinase activity might not only promote Cyclin B degradation, but might also limit its translation. Confirming previously reported results, we also found that addition of excess Cyclin B to CSF extracts to activate latent Cdc2 enhanced APC-mediated Cyclin B degradation, consistent with a role for Cdc2/Cyclin B activity in triggering Cyclin B destruction (Fig. 1E). Moreover, Cdc2/Cyclin B kinase activity increased only transiently upon addition of recombinant Cyclin B, consistent with the induction of compensatory degradation (Fig. 1F). As shown in Figure 1G, Cyclin B addition also induced the degradation of another APC substrate (Securin) in a dose dependent manner.
Figure 1. Emi2 antagonizes Cdc2/Cyclin B-induced degradation of Cyclin B.
(A) (Top): CSF-arrested Xenopus egg extracts were incubated in the presence of 35S-labeled methionine and cysteine. At the indicated times, endogenous Cyclin B2 protein was immunoprecipitated and newly synthesized Cyclin B2 was detected by autoradiography. (Bottom): Total Cyclin B2 levels were measured in a CSF extract at the indicated times by immunoblotting.
(B) (Top): 35S-labeled Cyclin B1 was added into CSF extracts in the absence (−, left) or the presence (+, right) of MG132 (100 µM). Aliquots were withdrawn at the indicated times and the amount of Cyclin B1 was detected by autoradiography. (Bottom): The radiolabeled Cyclin B1 remaining at each time point was quantified. Error bars represent standard deviation of four measurements.
(C) (Top): The APC was depleted from CSF extracts with three sequential incubations with IgG or Cdc27 antibodies. Samples from each extract were resolved by SDS-PAGE and immunoblotted with anti-Cdc27 antibodies. (Bottom): Samples were taken from control or APC (Cdc27) depleted extracts at the indicated times after incubation with energy regenerating mix at room temperature. The amount of Cyclin B2 was examined by immunoblotting (10 µg total protein was loaded per lane to clearly visualize the changes in endogenous Cyclin B levels).
(D) (Top) The APC was depleted from CSF extracts as described in (C). (Bottom): After 30 min incubation at room temperature, the Cdc2/Cyclin B kinase activity in control or APC (Cdc27) depleted extracts was measured at the indicated times using Histone H1 kinase assays. The phosphorylation of Histone H1 was quantified, normalized and plotted.
(E) Buffer (control), GST, or 40 nM Cyclin B1 was added to CSF extracts. At the indicated times, samples were withdrawn and endogenous Cyclin B2 levels were measured by immunoblotting.
(F) At the indicated times, Cdc2/Cyclin B kinase activity in the CSF extracts was measured in the presence or absence of recombinant Cyclin B1 (40 nM) using Histone H1 as an exogenous substrate. The phosphorylation of Histone H1 was quantitated, normalized, and plotted.
(G) Buffer (control) or Cyclin B1 (40 nM or 80 nM) was added to CSF extracts. At the indicated times, samples were withdrawn and Securin levels were measured by immunoblotting.
(H) Ca2+ or recombinant Cyclin B1 was added into CSF extracts in the presence or absence of the CaMKII peptide inhibitor (281–309, 400 µM). Samples were taken at the indicated times and the amount of endogenous Cyclin B2 was measured by immunoblotting.
(I) Radiolabeled Emi2 was added into CSF extracts supplemented with 40 nM recombinant Cyclin B1. The levels of Emi2 in the extract were examined at the indicated times by autoradiography.
(J) Cyclin B1 was added into CSF extracts in the presence of GST or GST-Emi2. At the indicated times, samples were taken and the amount of endogenous Cyclin B2 was determined by immunoblotting.
With the discovery that Emi2 protein plays a key role in maintaining CSF arrest, we hypothesized that Cdc2/Cyclin B might control its own degradation by modulating Emi2 function. CaMKII-mediated phosphorylation of Emi2 at fertilization promotes Emi2 degradation, relieving APC inhibition. However, Cdc2/Cyclin B-induced Cyclin B degradation was not inhibited by CaMKII inhibitors at concentrations that prevented Ca2+-induced Cyclin B degradation (Fig. 1H, [25]). Moreover, addition of 40 nM Cyclin B to extracts did not promote Emi2 destruction (Fig. 1I); similar results were obtained with Cyclin B added up to 80 nM, although some Emi2 degradation could be observed at later time points, consistent with previous reports that very high Cdc2 kinase activity induces Emi2 degradation [21]. Therefore, we have restricted our use of recombinant Cyclin B1 to levels that do not induce Emi2 degradation. Interestingly, adding excess Emi2 to CSF extracts blocked acceleration of Cyclin B degradation by exogenous Cyclin B protein (Fig. 1J). In that excess Emi2 could circumvent the ability of Cdc2/Cyclin B to increase Cyclin B degradation, these data suggested that Cdc2/Cyclin B might control Cyclin B levels by antagonizing Emi2.
Cdc2/Cyclin B disrupts the association of Emi2 and APC/Cdc20
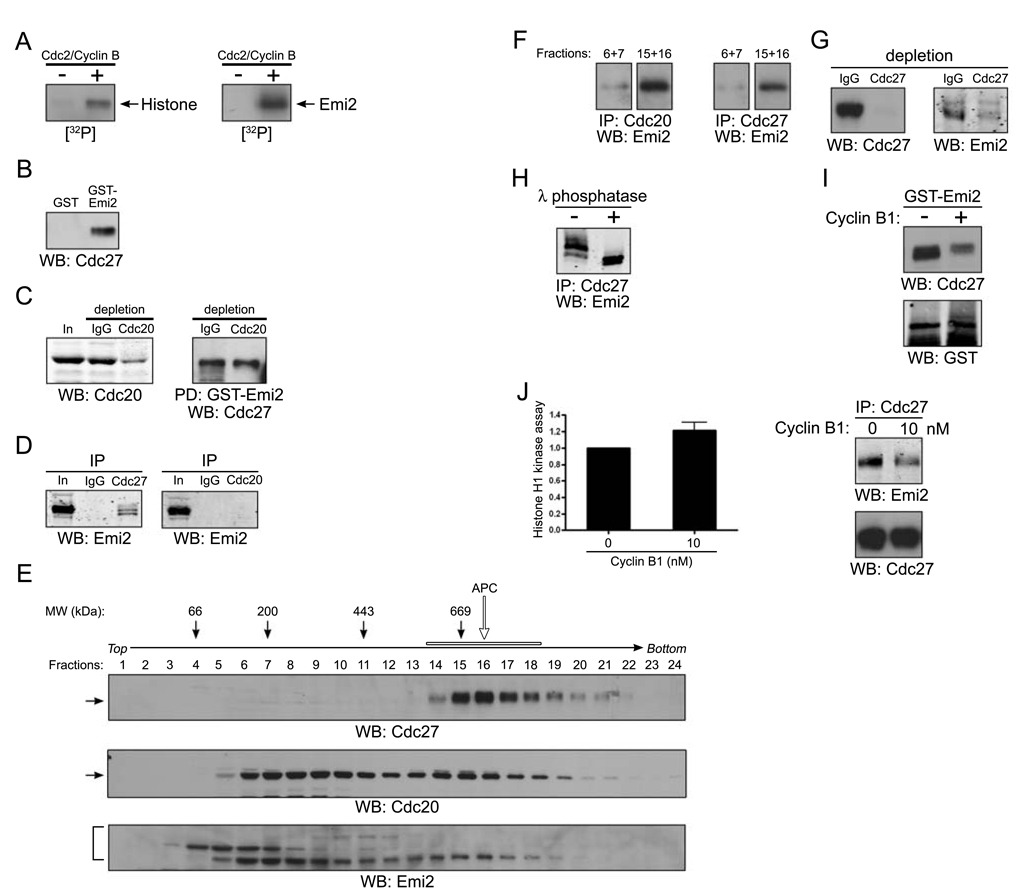
Consistent with the hypothesis that Cdc2/Cyclin B could modulate Emi2, we found that Cdc2 could phosphorylate Emi2 in vitro (Fig. 2A). To determine if there were consequences of this phosphorylation, we analyzed Emi2-APC interactions. The Emi2 relative, Emi1, interacts with the APC activator Cdc20 to disrupt APC-Cdc20 interactions [26]. In examining Emi2-APC interactions, we found that Emi2 could co-precipitate with the APC core component, Cdc27 (Fig. 2B). To see if Cdc20 was required for this interaction, we generated a Cdc20-depleted extract and found that Emi2 could still co-precipitate with Cdc27, even in an egg extract immunodepleted of ~80% of Cdc20 (Fig. 2C). Consistent with this, Emi2 was detected in Cdc27 immunoprecipitates, but was absent from Cdc20 immunoprecipitates (Fig. 2D). We next separated CSF extracts on 5–30% sucrose gradients and identified APC-containing fractions by immunoblotting for Cdc27 and APC2 (fractions 14–18; Fig 2E and data not shown). That the Cdc27 co-precipitation reflected a true interaction of Emi2 with the APC is suggested by the fact that a portion of Emi2 co-sedimented with the intact APC (Emi2 antibody recognizes three bands, as discussed in Fig. 2H). Although high levels of GST-Emi2 associated with and precipitated small amounts of Cdc20 from extracts even after APC immunodepletion (data not shown), we could not detect independent interaction between Emi2 and Cdc20 when Cdc20 was immunoprecipitated from gradient fractions (6–7) containing both Emi2 and Cdc20, but lacking other APC components (Fig. 2F, left). In contrast, Emi2 was detected in anti-Cdc20 immunoprecipitates formed from gradient fractions containing the full APC (15–16), although there was considerably less Emi2 present (Fig. 2F, left). Emi2 also co-immunoprecipitated with Cdc27 in the fractions containing the full APC (15–16; Fig 2F, right). Since Cdc20 did not bind Emi2 in the monomeric Cdc20 fractions (6–7) or in CSF extract (2D, left), Emi2-Cdc20 interactions in the higher molecular weight fractions may be mediated by the core APC (which binds both Emi2 and Cdc20). These data suggest that Emi2 associates more strongly with the full APC than with Cdc20 alone and that Cdc20, Emi2 and the core APC co-sediment and co-precipitate, indicating that Emi2 is not likely to inhibit the APC by preventing APC-Cdc20 interactions. Moreover, the majority of Emi2 was co-depleted from CSF extracts when Cdc27 was immunodepleted (Fig 2G). These data suggest that the bulk of Emi2 in extracts is APC-associated. Emi2 antibody recognizes more than one band on SDS-PAGE; phosphatase treatment of Emi2 immunoprecipitates indicates that some of this complexity arises from phosphorylated Emi2 species (Fig. 2H).
Figure 2. Cdc2/Cyclin B disrupts the association of Emi2 and APC/Cdc20.
(A) Histone H1 (left) or in vitro translated Emi2 (right) was mixed with [γ-32P] ATP in the presence or absence of recombinant Cdc2/Cyclin B kinase at room temperature for 10 min. The reactions were separated by SDS-PAGE and the phosphorylation of Histone H1 or Emi2 was monitored by autoradiography.
(B) GST or GST-Emi2 proteins bound to glutathione Sepharose beads were incubated in CSF extracts. After incubation, the protein beads were pelleted, washed, and immunoblotted for the core APC component, Cdc27.
(C) (Left): CSF extracts were depleted of Cdc20 by two consecutive incubations with Cdc20 antibody. The mock (IgG) and Cdc20 depleted extracts were resolved by SDS-PAGE and immunoblotted with anti-Cdc20 antibodies. (Right): GST-Emi2 linked to glutathione Sepharose was incubated in control or Cdc20 depleted extracts, retrieved, washed, and immunoblotted for Cdc27.
(D) Control IgG, Cdc27 (left) or Cdc20 (right) antibodies were immobilized on Protein A Sepharose beads and incubated in CSF extracts. The beads were retrieved, washed and immunoblotted for Emi2.
(E) CSF extracts was fractionated on a 5–30% sucrose gradient. Fractions were collected, analyzed by SDS-PAGE, and immunoblotted for Cdc27, Cdc20 and Emi2. The open arrow and bar indicate the APC complex.
(F) Cdc20 (left) or Cdc27 (right) antibodies were immobilized on Protein A Sepharose beads and incubated in sucrose gradient fractions 6 and 7 (pooled) or 15 and 16 (pooled). After incubation, the beads were retrieved by centrifugation, washed, and immunoblotted for Emi2.
(G) Cdc27 antibody was used to deplete CSF extract of the APC. IgG or Cdc27 depleted extracts were immunoblotted for Cdc27 (left) or Emi2 (right).
(H) Emi2 antibodies immobilized on Protein A Sepharose beads were incubated in CSF extracts. After incubation, the beads were retrieved, washed and incubated in the presence (+) or the absence (−) of λ phosphatase before being resolved by SDS-PAGE and immunoblotting for Emi2.
(I) GST-Emi2 linked to glutathione Sepharose was incubated in CSF extracts in the presence or absence of 80 nM recombinant Cyclin B1 proteins. After incubation, the protein beads were retrieved by centrifugation, washed, and immunoblotted for Cdc27 or GST.
(J) (Left): Cdc2/Cyclin B kinase activity was measured in CSF extracts in the presence or absence of exogenous recombinant Cyclin B1 (10 nM) using Histone H1 as an exogenous substrate. The phosphorylation of Histone H1 was quantitated and plotted. Error bars represent standard deviation of three measurements. (Right): Cdc27 antibodies were immobilized on Protein A Sepharose beads and incubated in CSF extracts in the presence or absence of Cyclin B1 (10 nM). The beads were retrieved, washed and immunoblotted for Emi2 or Cdc27.
To determine how Cdc2 might affect Emi2-APC interactions, we treated CSF extracts with excess Cyclin B and added GST-Emi2. As shown in Fig. 2I, elevating Cyclin B levels interfered with the ability of GST-Emi2 to precipitate the APC (detected by Cdc27 immunoblotting). Moreover, excess Cyclin B prevented precipitation of Emi2 with the APC from the high molecular weight gradient fractions (fractions 15–16; data not shown). At lower levels of Cyclin B addition (10 nM), resulting in slight elevations in Cdc2 kinase activity (Fig. 2J, left), we also observed loss of Emi2 from Cdc27 immunoprecipitates, suggesting that even small increases in Cyclin B promote some Emi2 dissociation from the APC (Fig 2J, right). These observations suggested that Cdc2/Cyclin B might dissociate the Emi2-APC complex, allowing compensatory Cyclin B degradation when Cyclin B levels rise during a CSF arrest.
Cdc2/Cyclin B disrupts interactions between the C-terminal region of Emi2 and the APC
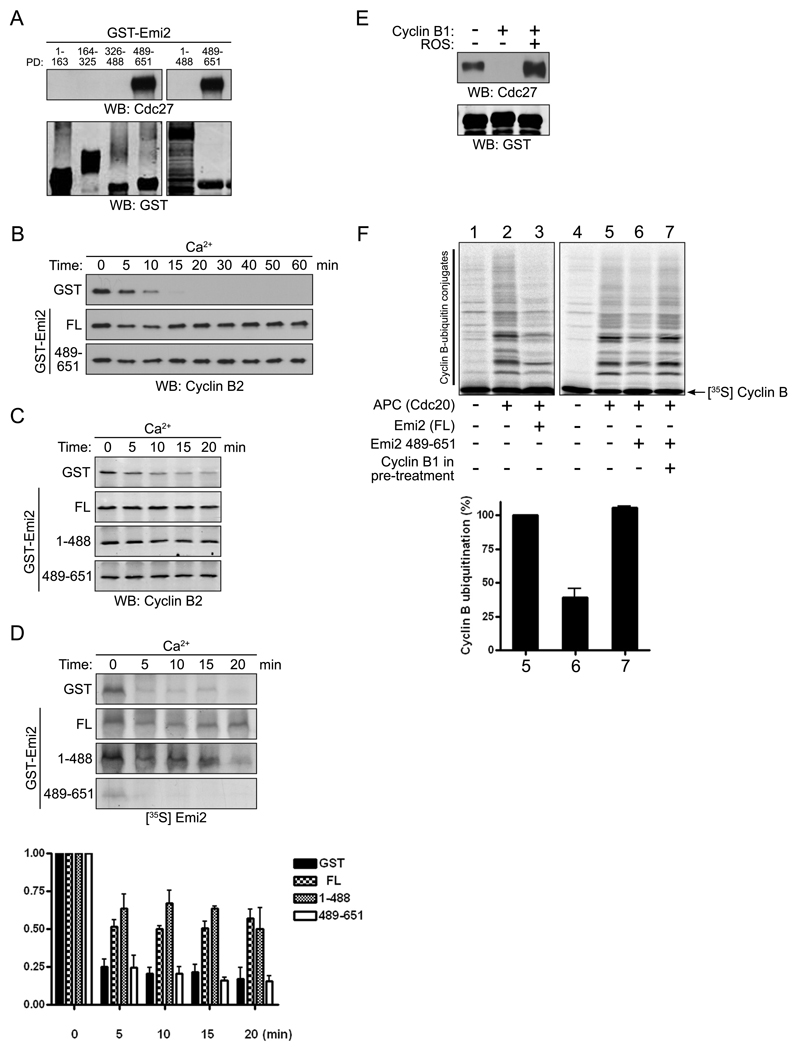
As it has been reported that a C-terminal fragment of Emi2 containing the zinc binding region (ZBR, aa 583–624) could rescue the release from CSF arrest caused by Emi2 depletion [17], we suspected that APC binding might reside within this region of Emi2. To confirm this, we assayed the ability of GST-fused protein fragments spanning the length of Emi2 to precipitate the APC core component, Cdc27. Only the Emi2 fragment containing amino acids 489 to 651 could precipitate Emi2 (Fig. 3A). Moreover, Emi2 protein lacking only this fragment (aa 1–488) was unable to bind the APC (Fig. 3A). In addition, the 489–651 Emi2 fragment was sufficient to prevent Cyclin B degradation and Ca+2-mediated release from the CSF arrest (Fig. 3B). Surprisingly, the N-terminal fragment of Emi2 (aa 1–488) that could not bind the APC also inhibited Ca2+-induced Cyclin B degradation (Fig. 3C). However, because the N-terminus contains sites of CaMKII (T195) and Plx1 phosphorylation (S33/38), we hypothesized that expression of the 1–488 fragment might inhibit degradation of endogenous Emi2 by competing for these kinases [19]. As shown in Fig. 3D, this was the case, suggesting that the ability of the 1–488 fragment to inhibit Cyclin B degradation was mediated by endogenous full length Emi2, whose degradation was inhibited by excess 1–488 fragment. To determine if Cdc2/Cyclin B targeted the region of Emi2 contained within the 489–651 fragment to disrupt its interactions with the APC, we repeated binding assays between GST-Emi2 (aa 489–651) and Cdc27 in CSF extracts supplemented with excess Cyclin B. High levels of Cyclin B disrupted interactions between the C-terminus of Emi2 (aa 489–651) and the APC (Fig. 3E). Moreover, Cdc2 kinase activity was required for this dissociation because the Cdk inhibitor roscovitine restored the interaction between Emi2 and APC even in the presence of high Cyclin B levels (Fig. 3E). Similarly, depleting Cdc2 from extracts using p13-Sepharose restored Emi2-APC interactions (data not shown). To demonstrate that Cdc2/Cyclin B-induced modification of the Emi2 C-terminus could alter its APC-inhibitory properties, we showed that the Emi2 C-terminus could inhibit the APC in vitro as well as full length Emi2 (Fig 3F, lanes 3 and 6). We then immobilized the GST-Emi2 C-terminus on glutathione Sepharose, dipped this protein into CSF extract or extract treated with excess Cyclin B protein and retrieved the Emi2 protein by centrifugation. The “dipped” Emi2 was then eluted from the Sepharose with free glutathione and added to an in vitro APC assay. As shown in Fig. 3F (lanes 4–7, see also Fig. 4I), while the GST-Emi2 C-terminus dipped into control extract could retard APC-mediated ubiquitination of Cyclin B protein in vitro, the same protein exposed to Cyclin B-treated CSF extracts lost its APC-inhibitory activity. Collectively, these data suggest that the Emi2 C-terminus is a downstream target of Cdc2/Cyclin B in regulating the APC and raise the possibility that phosphorylation of the Emi2 C-terminus by Cdc2 might interfere with its APC binding and inhibition.
Figure 3. Cdc2/Cyclin B disrupts interactions between the C-terminal region of Emi2 and the APC.
(A) GST-Emi2 protein fragments bound to glutathione Sepharose were incubated in CSF extracts, retrieved, washed, and immunoblotted for Cdc27 or GST.
(B) GST or GST-Emi2 proteins (full-length (FL) or C-terminal (aa 489–651)) were added into CSF extracts supplemented with Ca2+. Aliquots removed at the indicated times were analyzed by SDS-PAGE and immunoblotted for Cyclin B2.
(C) Emi2 full-length (FL), N-terminal (aa 1–488), or C-terminal (aa 489–651) GST proteins were added into CSF extracts supplemented with Ca2+. Aliquots were removed at the indicated times and immunoblotted for Cyclin B2.
(D) (Top): 35S-labeled Emi2 was added to CSF extracts supplemented with GST or the indicated GST-Emi2 proteins. After Ca2+ addition, aliquots were removed at the indicated times and the amount of 35S-labeled Emi2 was examined by autoradiography. (Bottom): The amount of 35S-labeled Emi2 at each time point was quantified, normalized, and plotted. Error bars represent standard deviation of three replicates.
(E) GST-Emi2 (aa 489–651) (4 µM) was incubated in CSF extracts in the presence or absence of 80 nM recombinant Cyclin B1 with or without the Cdc2 kinase inhibitor, roscovitine (ROS, 100 µM). GST-Emi2 was retrieved from the extracts by centrifugation, washed, and immunoblotted for Cdc27 or GST.
(F) (Top): GST-Emi2 FL (lanes 1–3) or GST-Emi2 (aa 489–651, lanes 4–7) immobilized on glutathione Sepharose was incubated in CSF extracts in the presence or absence of excess recombinant Cyclin B1. The protein beads were retrieved and washed thoroughly and the proteins were then eluted using glutathione. Eluted proteins were then added to an in vitro APC assay and conversion of radiolabeled Cyclin B1 to ubiquitinated forms was monitored by SDS-PAGE and autoradiography. (Bottom): The amount of ubiquitin conjugated Cyclin B was quantified, normalized and plotted (lanes 5–7). Error bars represent standard deviation of four experiments.
Figure 4. Phosphorylation of Emi2 by Cdc2/Cyclin B at T545 and T551 disrupts APC binding and inhibition.
(A) GST-Emi2 protein (aa 489–651) was incubated in CSF extracts in the absence (top) or presence (bottom) of recombinant Cyclin B1. After incubation, the protein was retrieved on glutathione Sepharose, resolved by SDS-PAGE, and the relevant bands were excised from the gel, trypsin digested, and analyzed by LC/MS mass spectrometry. The mass spectra of tryptic peptides were examined and the phosphorylated peptide (bottom: residues 545–556, [M2+H]2+ at m/z 649.6, retention time at 7.0 min) shows a 80 Da mass increase compared to the non-phosphorylated form (top: [M2+H]2+ at m/z 609.6, retention time at 7.8 min). Asterisks indicate tryptic peptides derived from GST protein.
(B) The same experiment was repeated as in (A) with GST-Emi2 (aa 489–651) T545/551A protein in the presence of Cyclin B1. (Top): LC/MS extracted ion chromatograms (EIC) at m/z 1157.6 shows that unphosphorylated mutant peptide elutes at 6.8 min. (Middle): EIC at m/z 1237.6 shows that presumed monophosphorylated mutant peptide is not detected. (Bottom): EIC at m/z 1317.6 shows that presumed diphosphorylated mutant peptide is not detected.
(C) GST, GST-Emi2 (aa 489–651) WT or GST-Emi2 (aa 489–651) T546/551A proteins were incubated in CSF extract supplemented with 80 nM recombinant Cyclin B1 protein in the presence of [γ-32P] ATP. Phosphorylation of the indicated proteins was examined by autoradiography at 0 and 10 min.
(D) (Top): Cdc2 was depleted from CSF extracts by three consecutive incubations with recombinant His-p13 coupled to CnBr-activated Sepharose. Control and Cdc2-depleted extracts were immunoblotted with anti-Cdc2 antibody. (Bottom): GST-Emi2 (aa 489–651) WT protein was incubated in the depleted extracts supplemented with 80 nM recombinant Cyclin B1 in the presence of [γ-32P] ATP. The phosphorylation of GST-Emi2 (aa 489–651) WT protein was examined by autoradiography at 0 and 10 min.
(E) GST, GST-Emi2 (aa 489–651) WT or GST-Emi2 (aa 489–651) T546/551A proteins were incubated with [γ-32P] ATP in the presence or absence of recombinant Cdc2/Cyclin B kinase for 10 min. The reactions were separated by SDS-PAGE and the phosphorylation of the indicated proteins was monitored by autoradiography.
(F) GST-Emi2 protein (aa 489–651; WT, T545/551A, T545A, or T551A) linked to glutathione Sepharose was incubated in CSF extracts in the presence or absence of exogenous Cyclin B1. After incubation, the protein beads were retrieved by centrifugation, washed, and immunoblotted for Cdc27 or GST.
(G) Recombinant Cyclin B1 was added into CSF extracts also supplemented with either GST, GST-Emi2 (aa 489–651) WT, or GST-Emi2 (aa 489–651) T545/551A proteins. At the indicated times, samples were withdrawn and immunoblotted for endogenous Cyclin B2.
(H) MBP, MBP-Emi2 (aa 489–651) WT, or MBP-Emi2 (aa 489–651) T545/551A proteins were added into CSF extracts. Aliquots were removed at the indicated times and the Cdc2/Cyclin B kinase activity was measured by Histone H1 kinase assays. The phosphorylation of Histone H1 was measured, normalized, and plotted.
(I) (Top): An in vitro APC assay was performed as described in Figure 3F using WT or T545/551A mutant Emi2 proteins (aa 489–651). (Bottom): The amount of ubiquitin conjugated Cyclin B was quantified, normalized, and plotted (lanes 2–6). Error bars represent standard deviation of four experiments.
Phosphorylation of Emi2 by Cdc2 at T545 and T551 disrupts APC binding and inhibition
In order to identify the sites of Cdc2-mediated Emi2 phosphorylation, we incubated GST-Emi2 C-terminus in CSF extract in the presence or absence of excess Cyclin B and, following in-gel trypsin digestion, subjected the Emi2 proteins to LC/MS analysis. Cdc2 phosphorylation sites were found within a 12-mer peptide containing T545 and T551 (Fig. 4A). In the absence of Cyclin B, no phosphorylation on the same peptide was detected by LC/MS analysis at the expected LC retention time (data not shown). To determine if these sites were important for the ability of Cdc2/Cyclin B to disrupt Emi2-APC interactions, we mutated T545 and T551 to the nonphosphorylatable Ala, either singly or in combination. We performed LC/MS again using GST-Emi2 (aa 489–651) T545/551A proteins. Neither mono nor diphosphorylated peptide (residues 545–556) was detected in extracted ion chromatographic analysis (Fig. 4B). Moreover, mutation of either T545 or T551 to Ala somewhat diminished phosphorylation of Emi2 in CSF extracts supplemented with excess Cyclin B, but mutation of these sites together largely abrogated phosphorylation (data now shown and Fig. 4C). Depletion of Cdc2 from CSF extracts prior to addition of excess Cyclin B1 greatly diminished Emi2 (aa 489–651) phosphorylation (Fig. 4D). Finally, phosphorylation of Emi2 by Cdc2/Cyclin B in vitro was abrogated by mutation of T545 and T551 to Ala, consistent with the fact that these are the primary sites of Cdc2 phosphorylation on Emi2 (Fig 4E). Since Emi2 phosphorylation by Cdc2/Cyclin B caused Emi2 to dissociate from the APC, we predicted that mutation of these sites would abrogate this dissociation. Mutation of either T545 or T551 to Ala partially blocked the dissociation of Emi2 from the APC when excess Cyclin B was added to CSF extracts (Fig. 4F). However, dissociation of Emi2 from the APC was completely inhibited by Cyclin B addition when both T545 and T551 were mutated, suggesting that phosphorylation of both sites contributes to disruption of Emi2-APC interactions (Fig. 4F). In addition, the T545/551A mutant Emi2 C-terminus was a more potent inhibitor of Cdc2/Cyclin B-induced endogenous Cyclin B degradation than the WT C-terminal Emi2 in CSF extracts (Fig. 4G). Addition of the T545/551A mutant Emi2 protein to CSF extracts also promoted increased endogenous Cdc2 kinase activity, as measured by Histone H1 phosphorylation, while addition of similar amounts of WT Emi2 did not (Fig 4H). Finally, we repeated the experiment demonstrating that Emi2 “dipped” into Cyclin B supplemented CSF extracts lost APC-inhibitory activity (as in Fig. 3F) using the T545/551A mutant. As shown in Fig. 4I, incubating WT Emi2 in a Cyclin B-treated extract caused loss of APC inhibition and ubiquitination of Cyclin B, while mutation of T545/551A prevented Emi2 inhibition and precluded APC ubiquitination of Cyclin B. Taken together, these data argue strongly that Cdc2 sows the seeds for Cyclin B destruction, at least in part, by phosphorylating the APC inhibitor Emi2 at T545 and T551.
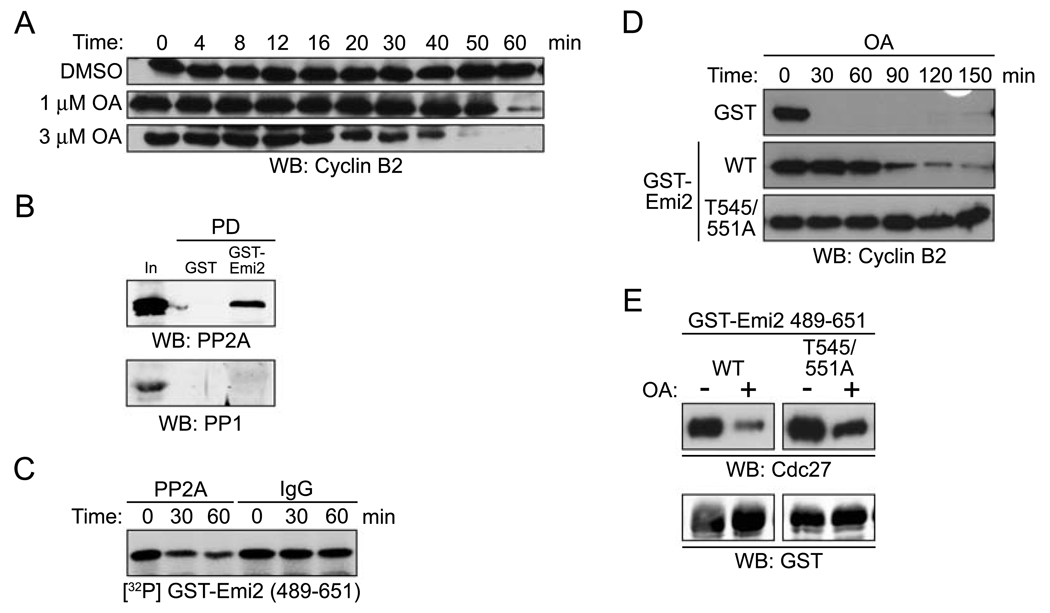
An okadaic acid-sensitive phosphatase keeps Emi2 active to maintain CSF arrest
Although excess Cyclin B impeded Emi2 binding to the APC, we were surprised to find that isolated Emi2-APC complexes could not be fully dissociated by addition of purified Cdc2/Cyclin B complexes (see partial dissociation, Fig. 5A). While this might reflect a low stoichiometry of phosphorylation in vitro, we speculated that it might also indicate the presence of an Emi2-bound phosphatase acting in opposition to Cdc2. In this regard, it is interesting that addition of the phosphatase inhibitor okadaic acid (OA) to the Emi2-APC complex also resulted in partial dissociation of the complex (Fig. 5A). This suggested that both an OA-sensitive phosphatase and an Emi2-directed kinase were present in the Emi2-APC precipitate. Interestingly, when we added both OA and Cdc2/Cyclin B to the Emi2-APC complexes, we achieved nearly full dissociation of the complex (Fig. 5A). Thus, inhibiting an OA-sensitive phosphatase, while enhancing Cdc2 mediated phosphorylation, was sufficient to dissociate Emi2 from the APC. OA did not affect Cdc2 kinase activity directly, as shown using recombinant Cdc2/Cyclin B1 and Histone H1 as an exogenous substrate (Fig. 5B).
Figure 5. An okadaic acid-sensitive phosphatase keeps Emi2 active to maintain CSF arrest.
(A) GST-Emi2 (FL) protein beads were incubated in CSF extracts to bind Cdc27. The beads were retrieved, washed, and then incubated in fresh buffer containing MgCl2 and ATP in the presence of DMSO (−), okadaic acid (OA), recombinant Cdc2/Cyclin B1 or in combination as indicated. After incubation, the protein beads were pelleted, washed, and immunoblotted for Cdc27 and GST.
(B) Histone H1 protein was incubated with [γ-32P] ATP and recombinant Cdc2/Cyclin B kinase in the presence or absence of or OA for 10 min. The reactions were separated by SDS-PAGE and the phosphorylation of Histone H1 was monitored by autoradiography.
(C) DMSO or 5 µM OA was added to CSF extracts. Samples were taken at the indicated times and the endogenous Cyclin B2 levels were examined by immunoblotting.
(D) CSF extracts were incubated with or without OA and the Cdc2 kinase activity of the extracts was examined using Histone H1 as a substrate. The reactions were separated by SDS-PAGE and the phosphorylation of Histone H1 was monitored by autoradiography.
(E) GST-Emi2 (FL) protein linked to glutathione Sepharose was incubated in CSF extracts supplemented with either DMSO or OA. After incubation, the protein beads were retrieved, thoroughly washed, and transferred into fresh CSF extracts supplemented with OA. Samples were taken at the indicated times to monitor Cyclin B2 levels by immunoblotting.
(F) 35S-labeled Cyclin B1 and Emi2 proteins were synthesized in vitro and added to CSF extracts. After 15 min incubation, Ca2+, DMSO or OA (or both Ca2+ and OA) were added. Aliquots were removed at the indicated times and the degradation or gel mobility shifting of both Cyclin B1 and Emi2 were examined by autoradiography using a phosphorimager.
OA alone (in the absence of calcium addition) can induce release from a CSF arrest [27] (Fig. 5C). We have also examined Cdc2 kinase activity in CSF extracts in the presence or absence of OA to exclude the possibility that OA induces CSF release and Cyclin B degradation solely through enhancing the Cdc2 kinase activity. As shown in Fig. 5D, OA did not measurably affect the Histone H1-directed kinase activity in CSF extracts. Note that Histone H1 phosphorylation was measured after 10 min incubation with OA, before the CSF extract could enter interphase (which would have resulted in decreased Cdc2 activity).
Given the effects of OA on dissociation of the Emi2-APC complex, we speculated that both an OA-sensitive phosphatase and Cdc2/Cyclin B regulated the phosphorylation status of Emi2. Furthermore, this OA-sensitive phosphatase might be required to dephosphorylate Emi2 to maintain inhibition of the APC and CSF arrest. To evaluate whether Emi2 was the relevant target in OA-induced CSF release, we immobilized GST-Emi2 on glutathione Sepharose beads and dipped the beads into either DMSO or OA-treated CSF extracts, with the idea that Emi2 would become phosphorylated by endogenous Cdc2/Cyclin B during the incubation if an Emi2-directed phosphatase was inhibited by OA. The protein beads were then retrieved by centrifugation, washed, and added into fresh CSF extract treated with OA to induce release. As shown in Fig 5E, the Emi2 that had been pre-incubated in the presence of OA was a less potent inhibitor of Cyclin B degradation than control Emi2 protein. These data suggest that an OA-sensitive phosphatase promotes activation of Emi2 as an APC inhibitor. Moreover, OA addition to CSF extracts resulted in an electrophoretic mobility shift in Emi2, appearing just prior to release from the CSF arrest (Fig. 5F; note the subsequent down-shift at 60 min in the OA-treated sample, probably reflecting dephosphorylation of Emi2 when the extracts entered interphase). Furthermore, the addition of OA neither induced degradation of Emi2, nor interfered with the calcium-induced degradation (Fig. 5F). These observations indicate that enhancing Emi2 phosphorylation by Cdc2/Cyclin B or inhibiting an Emi2-directed phosphatase can induce dissociation of Emi2 from the APC, allowing APC activation and exit from CSF arrest.
Emi2 associates with PP2A, opposing Cdc2-mediated Emi2 phosphorylation
OA can inhibit both PP1 and PP2A, albeit at different concentrations. To determine which phosphatase was being inhibited by OA to allow Emi2 phosphorylation and CSF release, we performed an OA titration. As shown in Fig. 6A, release from the CSF arrest occurred at low concentrations (1 µM) of OA, a concentration that inhibits PP2A, but not PP1, in the Xenopus egg extract [28] (Fig 6A). We also found that PP2A, but not PP1, could be retrieved from CSF extracts in association with GST-Emi2 (Fig. 6B). Moreover, PP2A immunoprecipitated from CSF extracts could catalyze the in vitro dephosphorylation of Cdc2-phosphorylated Emi2 (Fig. 6C). These data suggest that PP2A is an Emi2-directed phosphatase and that inhibition of PP2A is important for OA-induced CSF release.
Figure 6. Emi2 associates with PP2A acting in opposition of Cdc2/Cyclin B kinase activity.
(A) DMSO, 1 or 3 µM OA was added to CSF extracts. Aliquots were removed at the indicated times and immunoblotted for endogenous Cyclin B2.
(B) GST or GST-Emi2 (FL) protein beads were incubated in CSF extracts. After incubation, the protein beads were retrieved, washed, and immunoblotted for PP2A (top) and PP1 (bottom).
(C) IgG or PP2A antibody was immobilized on Protein A Sepharose beads and incubated in CSF extracts. The beads were retrieved, washed and mixed with GST-Emi2 (aa 489–651) WT protein that was pre-phosphorylated with recombinant Cdc2/Cyclin B kinase in the presence of [γ-32P] ATP. The phosphorylation of GST-Emi2 (aa 489–651) WT was examined by autoradiography after 0, 30 and 60 min.
(D) OA was added into CSF extracts supplemented with either GST, GST-Emi2 WT, or GST-Emi2 T545/551A proteins. At the indicated times, samples were withdrawn and immunoblotted for endogenous Cyclin B2.
(E) GST-Emi2 protein beads (aa 489–651; WT or T545/551A) were incubated in CSF extracts in the presence of DMSO (−) or OA. After incubation, the protein beads were retrieved, washed, and immunoblotted for Cdc27 and GST.
Although OA synergized with Cdc2/Cyclin B to induce release of Emi2 from the APC, it remained possible that PP2A did not act on the Cdc2 phosphorylation sites of Emi2. To address this, we examined the ability of OA to promote release from the CSF arrest in the presence of excess WT or T545/551A Emi2 protein. As shown in Fig. 6D, WT Emi2 slowed OA-induced release from a CSF arrest, while the mutant Emi2 was considerably more potent at delaying the CSF release. Moreover, when we phosphorylated Emi2 in vitro with Cdc2/Cyclin B, and added it to OA-treated CSF extracts, dephosphorylation of Emi2 was inhibited (data not shown). These data demonstrate that PP2A antagonizes the effects of Cdc2 on Emi2. However, the effects of OA in inducing release might not have been solely due to inhibition of T545 and T551 dephosphorylation because the double mutant was only partially resistant to OA-induced dissociation of the Emi2-APC complex (Fig. 6E). Thus, although PP2A opposes Cdc2 in regulating Emi2, these data suggest that another kinase, in addition to Cdc2, can also regulate Emi2-APC interactions.
Discussion
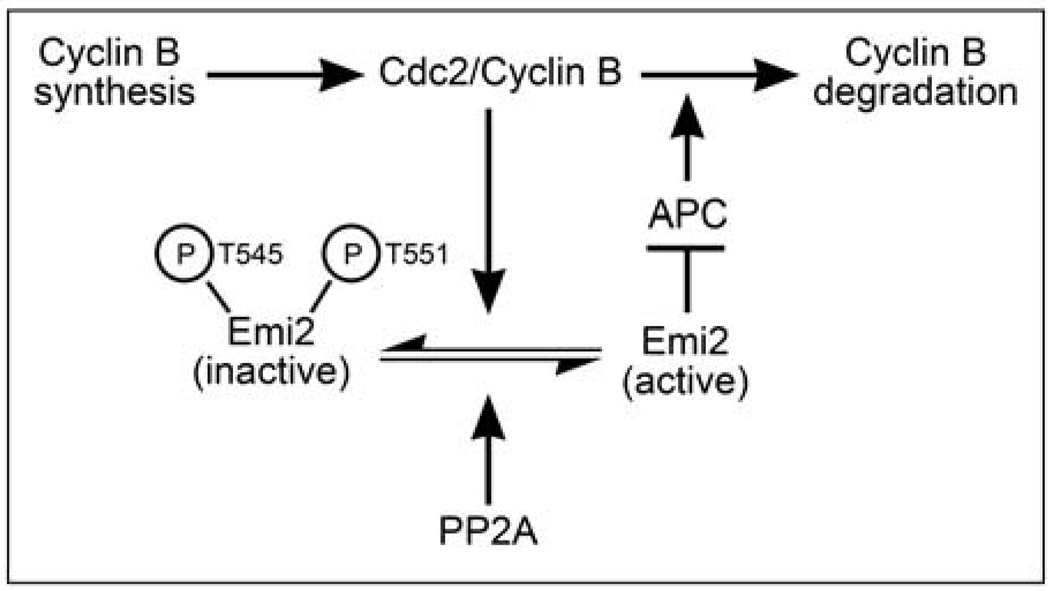
M phase exit requires APC-mediated degradation of B-type Cyclins [29]. To transition sharply from M to S phase, cells must degrade Cyclins rapidly and efficiently. Therefore, unrestrained Cyclin B accumulation during a CSF arrest could pose a problem: if the degradative machinery were overwhelmed by high Cyclin B levels, M phase exit following fertilization might be gradual rather than abrupt [30]. Interestingly, our preliminary results also indicate that excess Cyclin B may predispose CSF-arrested eggs, which are normally quite refractory to apoptosis, to undergo premature cell death (Perry, Wu, and Kornbluth, unpublished). Therefore, eggs that might have difficulties properly executing the first post-fertilization divisions could be eliminated. To minimize these problems, eggs have developed mechanisms to maintain relatively constant Cyclin B levels during the meiotic arrest. As Cyclin B levels rise, Cdc2-mediated phosphorylation of Emi2 suppresses its APC-inhibitory activity, allowing the APC to degrade excess Cyclin B. However, when Cyclin B levels drop, dephosphorylation of Emi2 by bound PP2A predominates, restoring Emi2-mediated APC inhibition. Thus, ambient Cyclin B levels during a CSF arrest are controlled by an equilibrium between Cyclin B synthesis and degradation, influenced directly by Emi2 (Fig. 7).
Figure 7. Cyclin B levels are regulated by Cdc2 and PP2A modulation Emi2 during a CSF arrest.
Cyclin B proteins are continually synthesized and degraded during a CSF arrest. When Cyclin B levels become elevated due to ongoing synthesis, the resulting increase in Cdc2 kinase activity tilts the balance between Cdc2/Cyclin B and PP2A, allowing for the phosphorylation of Emi2. This promotes the dissociation of Emi2 from the APC, thus activating a proportion of APC, and allowing for some Cyclin B degradation. Degradation of Cyclin B lowers Cdc2 kinase activity, allowing PP2A to predominate, thus shifting the balance towards dephosphorylated Emi2, which can inhibit the APC to block further Cyclin B degradation.
Cdc2 modulates APC inhibition by Emi2 during a CSF arrest
Although it was known that Cyclin B could auto-regulate to maintain constant levels during a CSF arrest, the mechanism was not clear [24]. That Emi2 is a relevant target of Cdc2/Cyclin B was demonstrated in several ways. First, Emi2 pre-incubated in CSF extracts treated with excess Cyclin B lost the ability to inhibit the APC in vitro. Importantly, this effect of pre-incubation was abrogated when Cdc2 phosphorylation sites were mutated. Moreover, mutant Emi2 was a more effective inhibitor of Cyclin B degradation and CSF exit than WT Emi2, arguing that Cdc2 targets Emi2 to modulate Cyclin B degradation during the CSF arrest. Although APC depletion promoted endogenous Cyclin B accumulation, there was also evidence that Cyclin B controls its own translation, since Cdc2/Cyclin B kinase activity plateaued, even in extracts unable to degrade Cyclin B.
In exploring the basis for Emi2 modulation by Cdc2, we found that excess Cyclin B diminished Emi2-APC interactions. This effect was abrogated by mutation of Thr 545 and Thr 551. Additionally, the effects of Cdc2 phosphorylation were unlikely to be exerted through overall conformational changes in the protein because Cdc2 disrupted association of the isolated Emi2 C-terminus with the APC as readily as it dissociated full-length Emi2-APC complexes. Mutation of the Cdc2 phosphorylation sites did not prevent calcium addition induced Emi2 degradation, suggesting that Cdc2 and CaMKII regulation of Emi2 are unlinked. Indeed, Cdc2 phosphorylation did not alter Emi2 stability.
PP2A antagonizes Cdc2 in regulation of Emi2
Although excess Cyclin B dissociated Emi2 from the APC in CSF extracts, purified Cdc2/Cyclin B was a weak trigger of dissociation when the isolated in vitro Emi2-APC complex was assayed. While this may reflect a low stoichiometry of phosphorylation in vitro, it also suggested that there might be a phosphatase antagonizing Cdc2 within the in vitro complex. Consistent with this, addition of OA in concert with Cdc2/Cyclin B efficiently dissociated the Emi2-APC complex. Although OA alone can promote a release from CSF arrest [21, 27], we do not believe that the sites of Cdc2 phosphorylation are the only important PP2A targets on Emi2. Additional phosphorylated peptides were detected when Emi2 was analyzed by LC/MS mass spectrometry after incubation in OA-treated CSF extracts (Wang, Wu, and Kornbluth, unpublished observations). Interestingly, OA alone could induce a partial release of Emi2 from the co-precipitated APC in vitro. Thus, there must have been an Emi2-directed kinase also present in the precipitate.
Although we have seen that Emi2 can bind Cdc2 (data not shown), it is unlikely that this is the only relevant Emi2-directed kinase in the precipitate as we have noted partial dissociation of Emi2-APC complexes by OA even when mutant Emi2 lacking Cdc2 phosphorylation sites was used to retrieve the APC from extracts. It is an interesting possibility that this additional Emi2-bound kinase(s) might be CaMKII or Plx1. Although these kinases promote Emi2 degradation in the full CSF extract (or intact egg), in the purified in vitro reaction, absent E3 ligases or proteosomes, it may be that the consequence of such phosphorylation is dissociation, rather than degradation. These (or other) kinases might promote degradation of Emi2 in a two-step manner, triggering dissociation of the Emi2-APC complex prior to degradation. This possibility merits further investigation, as does the possibility that PP2A antagonizes Emi2 degradation. Data presented here have shown that Emi2 can be regulated directly by phosphorylation as well as by degradation. It will be of interest to determine how these pathways are integrated and how Emi2-directed kinase and phosphatase activities are coordinated to control M phase exit.
Experimental procedures
Constructs and protein purification
Wild type Emi2 was isolated from a Xenopus oocyte cDNA library and subcloned into the XbaI and SacI sites of pGEX-KG using the following primers: 5’-GGCTCTAGACATGG CAAATCTCTTAGAGAA-3’ and 5’-GCCGAGCTCTCAAA GTCTCTTCAAATTCC-3’. Emi2 fragments were PCR amplified and subcloned into pGEX-KG. Emi2 mutants (T545/551A; T545A; T551A) were prepared using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). GST fusion proteins expressed in bacteria were purified on glutathione Sepharose beads (Amersham) and eluted using with 10 mM glutathione. Protein was dialyzed in XB-CSF buffer (100 mM KCl, 50 mM sucrose, 10 mM Hepes, 5 mM EGTA, 2 mM MgCl2, and 100 µM CaCl2 and 1mM DTT, pH 7.8).
Emi2 (aa 489–651) WT or T545/551A proteins were cloned into the XbaI and SalI sites of the pMAL-c2X vector. MBP fusion proteins expressed in bacteria were purified on maltose Sepharose beads (New England Biolabs) and eluted with 10 mM maltose. All proteins were dialyzed in XB-CSF buffer.
His-p13 protein expressed in bacteria was purified on Ni-NTA Agarose beads (Qiagen) and eluted with imidazole (200 mM). The eluted proteins were dialyzed in PBS buffer before being cross-linked to CnBr-activated Sepharose beads (Amersham).
Emi2 was cloned into the NotI site of the pSP64T vector and protein was in vitro translated using the TNT Quick Coupled Transcription/Translation System (Promega). FLAG-Emi2 was then purified on mouse anti-FLAG M2 Agarose beads (Sigma). Radiolabeled proteins, including Xenopus Emi2 and Xenopus Cyclin B1 [31], were generated using the same TNT Quick Coupled Transcription/Translation System in the presence of 35S-labeled methionine and cysteine (MP Biomedicals).
Throughout this work, the use of recombinant Cyclin B refers to human His-Cyclin B1 lacking the first 13 amino acids (Δ13), which is more easily produced and purified than full-length Cyclin B1. This protein was made as described previously in baculovirus-infected Sf9 cells[32].
Preparation of egg extracts
Mature eggs were obtained as described[33]. CSF extracts and interphase extracts were prepared according the methods of Murray[34] and Smythe and Newport, respectively[35]. All extracts were prepared at 4°C and all incubations were done at room temperature and supplemented with energy regenerating mix. Where indicated, 0.6 mM Ca2+ was added to extracts to induce release from CSF arrest. Sucrose gradient separation of extract components was performed as described previously[36].
Immunoblot analysis, immunoprecipitation and depletion
The antibodies used for immunoblotting were: rabbit anti-Emi2 [18], mouse anti-Cyclin B2 [37], mouse anti-Cdc27 (Transduction), mouse anti-Cdc20 (Abcam), mouse anti-PP2A (Upstate), and rabbit anti-PP1 (Upstate), rabbit anti-Cdc2 [32], and rabbit anti-Securin (a gift from H. Zou, University of Texas Southwestern Medical Center, Dallas, TX).
For Cdc20 immunoprecipitations, 5 µg of mouse anti-Cdc20 (Abcam) or mouse IgG was coupled to Protein A Sepharose (Sigma) and incubated in 200 µl of CSF extract for 2 hours at 4°C. The beads were washed with PBS (supplemented with 300 mM NaCl and 0.1% Triton), eluted with SDS-PAGE sample buffer and resolved by SDS-PAGE for immunoblotting. For Cdc27 immunoprecipitations, 4 µg of mouse anti-Cdc27 (Santa Cruz) or mouse IgG was used as described above.
20 µg of Cdc27 antibody (Santa Cruz) was used to deplete CSF extracts of the APC. Anti-Cdc27 antibodies or control IgG were coupled to Protein A Sepharose, split into three aliquots and incubated sequentially in CSF extract for 30 min at 4°C.
For Cdc20 depletion, 25 µg of rabbit anti-Cdc20 [38] or control IgG were coupled to Protein A dynabeads (Dynal Biotech). The beads were then incubated with CSF extracts and two consecutive 20 min depletions at room temperature were performed.
To affinity deplete CSF extracts of Cdc2, recombinant His-p13 coupled to CnBr-activated Sepharose was incubated in CSF extracts and three consecutive 30 min depletions at 4°C were performed.
Kinase assays and phosphatase assays
To generate in vitro phosphorylated Emi2, recombinant Cdc2/Cyclin B (Calbiochem) and Emi2 proteins, including in vitro translated FLAG-Emi2 (FL) or recombinant GST-Emi2 (aa 489–651) WT or T545/551A, were incubated in kinase buffer (10 mM Tris-HCl pH 7.5, 10 mM MgCl2, 1 mM DTT, 100 µM ATP) with 2 µCi [γ-32P] ATP at room temperature for the indicated times.
Histone H1 kinase assays were performed by adding 2 µl of extract to 28 µl of HH1 Kinase reaction mix (10 mM Hepes pH 7.2, 5 mM MgCl2, 50 mM NaCl, 100 µM ATP, 4.2 mM DTT, 5 µg Histone H1 and 2 µCi [γ-32P] ATP) for 10 min at room temperature. The reactions were resolved by SDS-PAGE and the bands corresponding to Histone H1 were quantified with a phosphorimager (Molecular Dynamics).
Emi2 immunoprecipitated from CSF extracts with 2 µg of Emi2 antibody [18] coupled to Protein A Sepharose was treated with λ phosphatase (New England Biolabs) according to the manufacturer’s instructions.
PP2A phosphatase assays were performed using PP2A immunoprecipitated from CSF extracts. Control IgG or mouse anti-PP2A antibody (Upstate) was coupled to Protein A Sepharose beads and incubated in CSF extracts for 2 hours at 4°C. After incubation, the beads were retrieved, washed, and mixed with PP2A phosphatase buffer (Upstate) and GST-Emi2 (aa 489–651) WT protein that was pre-phosphorylated with recombinant Cdc2/Cyclin B kinase in the presence of [γ-32P] ATP. The reactions were stopped at the indicated times by the addition of SDS-PAGE sample buffer and the phosphorylation of Emi2 was examined by autoradiography.
Mass spectrometry
GST-Emi2 protein (aa 489–651) WT or T545/551A linked to glutathione Sepharose was incubated in CSF extracts with or without recombinant Cyclin B1. The beads were retrieved, thoroughly washed with PBS (supplemented with 300 mM NaCl and 0.1% Triton), resolved by SDS-PAGE, and stained with Coomassie blue stain. The relevant bands were excised from the gel and subject to alkylation and trypsin digestion prior to peptide mass fingerprinting analysis. LC/MS mass spectra of protein tryptic peptides were obtained by HPLC electrospray ionization mass spectrometry on an Agilent LC/MSD Trap mass spectrometer[39].
In vitro APC assay
The APC assay was performed as described previously[40] with minor modifications. GST-Emi2 (36 µM), Cdc20, and immunoprecipitated APC were mixed, incubated at room temperature for 1 hour, and then 35S-labeled human Cyclin B was added in the presence of recombinant E1, E2, and ATP.
Acknowledgements
We are grateful to Dr. James Edwin Hall (School of Pharmacy, The University of North Carolina at Chapel Hill, Chapel Hill, NC) for providing Mass Spectrometry resources. This work was supported by RO1 GM67225 to SK and RO1 GM64348 to MA.
References
- 1.Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 2.Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- 3.Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt RR, Ferrell JE., Jr The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science. 1999;286:1362–1365. doi: 10.1126/science.286.5443.1362. [DOI] [PubMed] [Google Scholar]
- 5.Gross SD, Schwab MS, Lewellyn AL, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by the protein kinase p90Rsk. Science. 1999;286:1365–1367. doi: 10.1126/science.286.5443.1365. [DOI] [PubMed] [Google Scholar]
- 6.Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk) Curr Biol. 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 7.Tunquist BJ, Schwab MS, Chen LG, Maller JL. The spindle checkpoint kinase bub1 and cyclin e/cdk2 both contribute to the establishment of meiotic metaphase arrest by cytostatic factor. Curr Biol. 2002;12:1027–1033. doi: 10.1016/s0960-9822(02)00894-1. [DOI] [PubMed] [Google Scholar]
- 8.Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 9.Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- 10.Kramer ER, Gieffers C, Holzl G, Hengstschlager M, Peters JM. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr Biol. 1998;8:1207–1210. doi: 10.1016/s0960-9822(07)00510-6. [DOI] [PubMed] [Google Scholar]
- 11.Tunquist BJ, Eyers PA, Chen LG, Lewellyn AL, Maller JL. Spindle checkpoint proteins Mad1 and Mad2 are required for cytostatic factor-mediated metaphase arrest. J Cell Biol. 2003;163:1231–1242. doi: 10.1083/jcb.200306153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwab MS, Roberts BT, Gross SD, Tunquist BJ, Taieb FE, Lewellyn AL, Maller JL. Bub1 is activated by the protein kinase p90(Rsk) during Xenopus oocyte maturation. Curr Biol. 2001;11:141–150. doi: 10.1016/s0960-9822(01)00045-8. [DOI] [PubMed] [Google Scholar]
- 13.Tsurumi C, Hoffmann S, Geley S, Graeser R, Polanski Z. The spindle assembly checkpoint is not essential for CSF arrest of mouse oocytes. J Cell Biol. 2004;167:1037–1050. doi: 10.1083/jcb.200405165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reimann JD, Jackson PK. Emi1 is required for cytostatic factor arrest in vertebrate eggs. Nature. 2002;416:850–854. doi: 10.1038/416850a. [DOI] [PubMed] [Google Scholar]
- 15.Ohsumi K, Koyanagi A, Yamamoto TM, Gotoh T, Kishimoto T. Emi1-mediated M-phase arrest in Xenopus eggs is distinct from cytostatic factor arrest. Proc Natl Acad Sci U S A. 2004;101:12531–12536. doi: 10.1073/pnas.0405300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, Mayer TU. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 2005;19:502–513. doi: 10.1101/gad.320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR, 3rd, Jackson PK. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc Natl Acad Sci U S A. 2005;102:4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Maller JL. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- 21.Hansen DV, Tung JJ, Jackson PK. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc Natl Acad Sci U S A. 2006;103:608–613. doi: 10.1073/pnas.0509549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubiak JZ, Weber M, de Pennart H, Winston NJ, Maro B. The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. Embo J. 1993;12:3773–3778. doi: 10.1002/j.1460-2075.1993.tb06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thibier C, De Smedt V, Poulhe R, Huchon D, Jessus C, Ozon R. In vivo regulation of cytostatic activity in Xenopus metaphase II-arrested oocytes. Dev Biol. 1997;185:55–66. doi: 10.1006/dbio.1997.8543. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto TM, Iwabuchi M, Ohsumi K, Kishimoto T. APC/C-Cdc20-mediated degradation of cyclin B participates in CSF arrest in unfertilized Xenopus eggs. Dev Biol. 2005;279:345–355. doi: 10.1016/j.ydbio.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, Kornbluth S. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 2005;123:89–103. doi: 10.1016/j.cell.2005.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 27.Lorca T, Fesquet D, Zindy F, Le Bouffant F, Cerruti M, Brechot C, Devauchelle G, Doree M. An okadaic acid-sensitive phosphatase negatively controls the cyclin degradation pathway in amphibian eggs. Mol Cell Biol. 1991;11:1171–1175. doi: 10.1128/mcb.11.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis SS, Walsh S, Weiser DC, Yoshida M, Shenolikar S, Kornbluth S. PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. Embo J. 2003;22:5734–5745. doi: 10.1093/emboj/cdg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasch R, Cross FR. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature. 2002;418:556–562. doi: 10.1038/nature00856. [DOI] [PubMed] [Google Scholar]
- 30.Sha W, Moore J, Chen K, Lassaletta AD, Yi CS, Tyson JJ, Sible JC. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc Natl Acad Sci U S A. 2003;100:975–980. doi: 10.1073/pnas.0235349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Bardes ES, Moore JD, Brennan J, Powers MA, Kornbluth S. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 1998;12:2131–2143. doi: 10.1101/gad.12.14.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh S, Margolis SS, Kornbluth S. Phosphorylation of the cyclin b1 cytoplasmic retention sequence by mitogen-activated protein kinase and Plx. Mol Cancer Res. 2003;1:280–289. [PubMed] [Google Scholar]
- 33.Ohsumi K, Sawada W, Kishimoto T. Meiosis-specific cell cycle regulation in maturing Xenopus oocytes. J Cell Sci. 1994;107(Pt 11):3005–3013. doi: 10.1242/jcs.107.11.3005. [DOI] [PubMed] [Google Scholar]
- 34.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 35.Smythe C, Newport JW. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 1991;35:449–468. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- 36.Casaletto JB, Nutt LK, Wu Q, Moore JD, Etkin LD, Jackson PK, Hunt T, Kornbluth S. Inhibition of the anaphase-promoting complex by the Xnf7 ubiquitin ligase. J Cell Biol. 2005;169:61–71. doi: 10.1083/jcb.200411056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochegger H, Klotzbucher A, Kirk J, Howell M, le Guellec K, Fletcher K, Duncan T, Sohail M, Hunt T. New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development. 2001;128:3795–3807. doi: 10.1242/dev.128.19.3795. [DOI] [PubMed] [Google Scholar]
- 38.Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- 39.Garcia BA, Shabanowitz J, Hunt DF. Analysis of protein phosphorylation by mass spectrometry. Methods. 2005;35:256–264. doi: 10.1016/j.ymeth.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Tang Z, Yu H. Functional analysis of the spindle-checkpoint proteins using an in vitro ubiquitination assay. Methods Mol Biol. 2004;281:227–242. doi: 10.1385/1-59259-811-0:227. [DOI] [PubMed] [Google Scholar]