Summary
During regional patterning of the anterior neural plate, a medially positioned domain of cells is specified to adopt retinal identity. These eye field cells remain coherent as they undergo morphogenetic events distinct from other prospective forebrain domains. We show that two branches of the Wnt signaling pathway coordinate cell fate determination with cell behavior during eye field formation. Wnt/β-catenin signaling antagonizes eye specification through the activity of Wnt8b and Fz8a. In contrast, Wnt11 and Fz5 promote eye field development, at least in part, through local antagonism of Wnt/β-catenin signaling. Additionally, Wnt11 regulates the behavior of eye field cells, promoting their cohesion. Together, these results allow us to postulate a model in which Wnt11 and Fz5 signaling promotes early eye development through the coordinated antagonism of signals that suppress retinal identity and promotion of coherence of eye field cells.
Introduction
During central nervous system (CNS) development, regional fate determination must be coupled to the morphogenetic processes that shape the various structures of the brain. The highly specialized vertebrate eye is one of the organs in which the integration of fate determination and morphogenesis is most evident. The optic vesicles are formed as evaginations of the forebrain, but prior to this, the group of cells that will give rise to the eyes exists as a single bilateral domain called the eye field. The eye field is readily detectable within the anterior neural plate (ANP) by the overlapping expression of a number of transcription factors known as the eye specification network of genes (reviewed in Chuang and Raymond, 2002).
During gastrulation, the ANP becomes subdivided into domains that will generate telencephalic, eye field, diencephalic, and hypothalamic fates. The signaling pathways responsible for this regional patterning are beginning to be unraveled (reviewed in Wilson and Houart, 2004), and one of the pathways that has received the most attention is the Wnt/β-catenin signaling cascade (Logan and Nusse, 2004). Activation of this signaling cascade is initiated by interaction of Wnt ligands with a receptor complex formed by Frizzled (Fz) and low-density lipoprotein receptor-related proteins (LRP). Downstream of the receptor, a protein complex containing glycogen synthase kinase 3β (GSK3β), axin, and adenomatous polyposis coli (APC) promotes phosphorylation and, consequently, proteasome-mediated degradation of β-catenin. Inactivation of the GSK3β/axin/β-catenin complex upon pathway activation leads to accumulation and nuclear translocation of β-catenin, where it interacts with transcription factors such as the lymphoid enhancer binding factor 1 (LEF1) or the T cell-specific transcription factor (TCF) to modulate transcription. Various other proteins modulate the activity of the pathway, including the cytoplasmic protein Dishevelled that facilitates pathway activation upon ligand/receptor binding.
Wnts can also activate alternative signaling cascades, including one branch that shares components with the planar cell polarity pathway (PCP) described in Drosophila (Veeman et al., 2003). Noncanonical Wnt pathways are GSK3β/axin/APC- and β-catenin-independent, but share a function for Dsh with the Wnt/β-catenin pathway. Depending on the context, activation of β-catenin-independent Wnt signaling can involve intracellular calcium release, small GTPases of the Rho family, and activation of the JNK signaling cascade. All of these events ultimately affect the cytoskeletal architecture and the establishment of cell polarity and/or cell behavior. In vertebrates, noncanonical Wnt signaling has been most closely studied with respect to its role in modulating the convergence and extension (CE) movements of mesodermal cells that shape the embryo during gastrulation.
The vertebrate genome encodes many Wnt ligands, which, in most cases, show preferential activation of either β-catenin-dependent or β-catenin-independent pathways (Veeman et al., 2003). It is unclear how the specificity of each ligand for one or other branch of the Wnt pathway is accomplished; it is also largely unclear whether different Wnts have specific Fz partners, and if so, whether this could confer specificity in their signaling activity.
A widely favored model of early neural plate patterning postulates that a gradient of Wnt/β-catenin activity specifies different regional fates with high levels of signaling promoting more caudal neural identities (Kiecker and Niehrs, 2001; Yamaguchi, 2001). This leads to establishment of more localized sources of Wnts and Wnt antagonists that subsequently refine regional patterning (Wilson and Houart, 2004). Within the forebrain, evidence from studies in mice, chicks, and fish supports the idea that Wnt/β-catenin signaling promotes caudal diencephalic identity and that raised levels of signaling can suppress more rostral forebrain fates. For instance, in zebrafish, establishment of telencephalic identity requires the suppression of high levels of Wnt/β-catenin signaling (Houart et al., 2002), whereas establishment of diencephalic identity is promoted by high levels of Wnt/β-catenin signaling (Heisenberg et al., 2001; Kim et al., 2002).
The role of Wnt signaling during early stages of eye formation is uncertain. Genetic studies of masterblind (mbl; Heisenberg et al., 2001) and headless (hdl; Kim et al., 2000) mutants in zebrafish, which affect the components of the Wnt/β-catenin pathway, axin and TCF3a, respectively, suggest that enhanced Wnt/β-catenin signaling suppresses eye formation. However, overexpression of Fz receptors in both Xenopus (Rasmussen et al., 2001) and in zebrafish (FC, FCB, MT, and SW; unpublished data and see Figure S1 in the Supplemental Data available with this article online) can lead to induction of ectopic eyes. Therefore, although modulation of the Wnt pathway affects formation of the eyes, the mechanisms underlying this activity are still unclear.
In this study, we explore the mechanisms by which Wnt signaling regulates early stages in eye field development. We find that two branches of the Wnt pathway have very different effects on eye formation. High levels of Wnt/β-catenin signaling are required for the acquisition of caudal diencephalic fate and antagonize eye induction. In contrast, Wnt11 signaling within the eye field promotes eye formation, at least partially, by antagonizing the Wnt/β-catenin pathway. In addition, Wnt11 signaling promotes coherence of eye field cells, potentially contributing to the coordinated morphogenetic behaviors of cells in the nascent eye field. Each branch of the Wnt pathway appears to be activated by a different Wnt/Fz combination in the nascent forebrain. We propose that Wnt/β-catenin signaling is activated by Wnt8b and Fz8a, whereas noncanonical Wnt signaling is activated by Wnt11 and Fz5. These results allow us to present a simple model in which the integration of Wnt11, Fz5, and Wnt/β-catenin signaling coordinates fate determination and morphogenesis of the nascent eye field.
Results
Several Wnts Are Expressed in the Anterior Neural Plate
During late gastrulation wnt1 and wnt10b (Lekven et al., 2003) are expressed in bilateral bands of ANP cells around six to ten cell diameters away from the posterior boundary of the eye field (detected by the expression of the rx3 gene [Chuang et al., 1999; Figure 1D and data not shown]), while wnt8b is expressed in a broader domain with a rostral boundary only a few cell diameters caudal to the eye field (Figure 1E). Finally, wnt11 is expressed in a broad ANP domain (Heisenberg et al., 2000) overlapping with the posterior/lateral region of the eye field (Figure 1F). The expression of all these wnts is first detected at 75%–80% epiboly (Heisenberg et al., 2000; Lekven et al., 2003; and data not shown), coinciding with initiation of the expression of rx3, the earliest known marker for the eye field in zebrafish (Figure 1A). Other Wnts, including wnt4, wnt3a, and wnt8-ORF2, are also expressed in the ANP (Ungar et al., 1995; Buckles et al., 2004; Lekven et al., 2001), but the timing and/or localization of their expression makes it unlikely that they directly influence eye field cells. To address whether these different Wnt ligands could affect eye formation, we localized overexpression within the eye field by transplanting wnt-expressing cells within the ANP at early to midgastrula stages, just before eye field specification takes place.
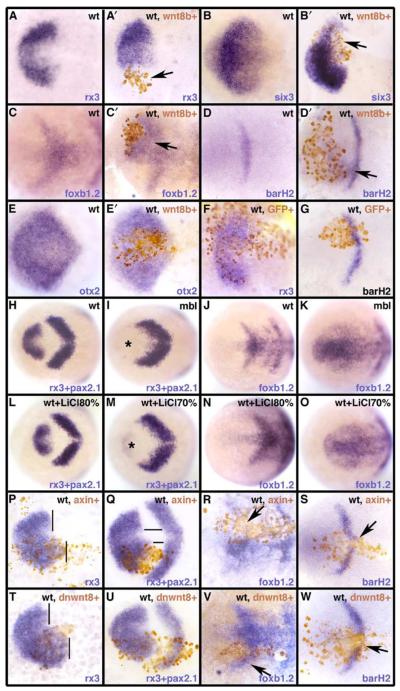
Figure 1. Several Wnts Are Expressed Adjacent to the Eye Field.
Dorsal views of the anterior neural plate (A–F) and forebrain (G–I) showing expression of genes identified in each panel (lower right; text color matches expression domain color) at stages shown (bottom left). In this and all subsequent figures, panels are dorsal views with the anterior to the left; genotype and treatments performed on embryos or proteins expressed by the transplanted cells (brown) are indicated top right and transplanted cells are marked in brown. (A–F) Prospective telencephalon (t) is shown by emx1 expression (A); eye field (ef), by rx3 expression (A–F); and prospective diencephalon (d), by foxb1.2 expression (C). Panels (D)–(F) show expression of Wnt genes in relation to the eye field.
(G–I) Host brains/eyes containing transplants of wild-type GFP+ control cells (G), wnt8b+ cells (H), and wnt11+ cells (I) at 24 hr postfertilization. Wnt8b interferes with eye formation ([H], asterisk), while Wnt11 induces a bigger misshapen eye ([I], asterisk).
Exogenous Wnt8b and Wnt11 Have Opposing Effects on Eye Formation
Transplants of cells overexpressing wnt1 or wnt8b inhibit eye formation (Figure 1H), a result consistent with previous data (Houart et al., 2002) and with the idea that high levels of Wnt activity suppress the specification of anterior neural fates (Wilson and Houart, 2004). In contrast, transplants of cells overexpressing wnt11 lead to the development of bigger misshapen eyes (Figure 1I).
Wnt11 is thought to activate a signaling pathway related to the PCP pathway of flies (Heisenberg et al., 2000; Tada and Smith, 2000), whereas Wnt8b and Wnt1 activate β-catenin-dependent signaling (Cui et al., 1995; Moon et al., 1993; Veeman et al., 2003). We therefore hypothesized that the activation of different branches of the Wnt signaling pathway might account for the phenotypic differences resulting from overexpression of these proteins within the eye field.
Wnt/β-Catenin Activity Defines Eye Field versus Posterior Diencephalic Identity
Cells overexpressing either wnt8b or wnt1 (wnt8b+/wnt1+) locally suppressed the expression of eye field markers in nearby ANP cells (rx3 and six3 [Seo et al., 1998]; Figures 2A′ and 2B′). This loss of eye field markers was accompanied by an expansion of posterior diencephalic markers (foxb1.2, Odenthal and Nusslein-Volhard, 1998; barH2, Figures 2C′ and 2D′). otx2, which is expressed throughout the forebrain- and midbrain-forming regions of the ANP (Li et al., 1994), was unaffected by the transplants (Figure 2E′), confirming that exogenous Wnt8b/Wnt1 affected regional patterning within the ANP, but not formation of this region of the CNS. Next, we asked if Wnt1 and Wnt8b were acting through the Wnt/β-catenin branch of the Wnt signaling pathway.
Figure 2. Wnt/ß-Catenin Signaling Suppresses the Eye Field and Promotes Caudal Diencephalic Fates.
Dorsal views of the anterior neural plate at tailbud, showing expression of genes indicated (bottom right of each panel).
(A–G) Transplants of wnt8b+ cells (A′,B′,C′, D′, and E′) suppress rx3 ([A′], arrow) and six3 ([B′], arrow) and expand foxb1.2 ([C′], arrow) and barH2 ([D′], arrow) while having no effect on otx2 expression (E′). (A)–(E) show wild-type controls and (F)–(G) show control transplants of GFP+ cells. (H–K) mbl−/− embryos lose expression of rx3 ([I], asterisk) and show expansion of foxb1.2 expression (K) compared to wild-type (H and J). (L–O) Expression of eye field ([L and M], asterisk) and diencephalic (N and O) markers in embryos treated with LiCl at 70% epiboly (M and O) or 80% epiboly (L and N). (P–W) Transplants of axin1+ cells (P–S) or dnwnt8+ cells (T–W) expand rx3 expression caudally (P and Q, T and U; vertical bars in [P] and [T] delimit the posterior boundary of the eye field) and suppress foxb1.2 (arrows in [R] and [V]) and barH2 (arrows in [S] and [W]) expression. Note the narrowing of the prospective diencephalic domain in (Q) (horizontal bars). Abbreviations: mbl, masterblind mutant; wt, wild-type.
Activation of the Wnt/β-catenin pathway by different means leads to the same fate transformations, as with wnt8b+/wnt1+ transplants. mbl−/− mutants, which have disrupted function of Axin1 (Heisenberg et al., 2001), a negative modulator of the Wnt/β-catenin pathway (Ikeda et al., 1998; Kishida et al., 1998), lack expression of eye field markers and show a rostral expansion of posterior diencephalic markers (Figures 2H–2K; see also Heisenberg et al., 2001). Exposing embryos to lithium chloride (LiCl) activates the Wnt/β-catenin pathway (Hedgepeth et al., 1997; Klein and Melton, 1996) and, when done at midgastrula stages prior to the induction of the eye field, leads to transformation of the eye field into diencephalon (Figures 2M and 2O). Treatment at later stages, when the eye field has already been specified, can reduce the size of this territory, but does not interfere with its specification (Figures 2L and 2N) (Kim et al., 2002). Together, these results show that activation of Wnt/β-catenin signaling promotes expression of diencephalic markers and suppresses expression of eye field markers.
These results led us to predict that suppression of Wnt/β-catenin signaling in the diencephalon should lead to caudal expansion of the eye and suppression of diencephalic identity. Consistent with this idea, downregulation of Wnt/β-catenin activity in the posterior diencephalon suppressed diencephalic gene expression and expanded the eye field. Thus, overexpression of either axin1 or a dominant negative form of Wnt8 (dnwnt8; Hoppler et al., 1996) in the diencephalon induced rx3 and suppressed expression of diencephalic marker genes (Figures 2P–2W). In some dnwnt8+ transplants, diencephalic and midbrain markers shifted caudally, consistent with an overall caudalization of the anterior neural plate (Figures 2U and 2V, arrow).
Wnt11 Directly Influences Cell Movements in the ANP
In contrast to wnt8b+/wnt1+ cells, transplants of wnt11+ cells led to the development of bigger eyes (Figure 1I) and, at tailbud, they distorted and expanded rx3 and six3 domains laterally or posteriorly, depending on the position of the transplant (Figures 3A and 3B and data not shown). The caudal expansion of eye field markers was not obviously accompanied by suppression of other anterior neural fates, since the prospective telencephalic, diencephalic, and midbrain markers emx1, foxb1.2, and pax2.1 were still expressed, albeit with distorted expression domains that accommodated the deformed eye fields (Figures 3C and 3D and data not shown). These results are consistent with a role for Wnt11 in modulating morphogenetic cell movements, analogous to the role documented for this protein in the regulation of CE movements of mesendodermal cells that contribute to shaping the embryo during gastrulation (Heisenberg et al., 2000; Tada and Smith, 2000).
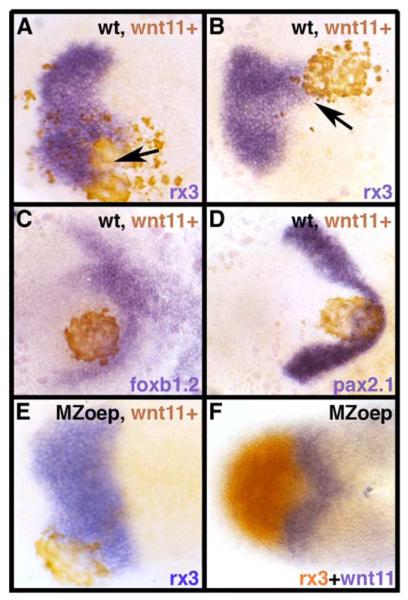
Figure 3. Wnt11 Affects the Shape of Expression Domains in the ANP.
Dorsal views of the ANP at tailbud, showing expression of genes indicated (bottom right of panels).
(A–E) Transplants of wnt11+ cells lead to expanded and misshapen rx3 expression domains both in wild-type ([A and B], arrows) and MZoep mutants (E). foxb1.2 (C) and pax2.1 (D) expression domains are also perturbed by wnt11+ transplants.
(F) wnt11 (purple) and rx3 (red) expressions in an MZoep embryo, showing that wnt11 is still expressed in the mutant.
The ability of Wnt11 to disrupt morphogenesis in the eye field could be due to Wnt11 acting either directly on neural plate cells or indirectly through its ability to disrupt mesodermal cell movements that subsequently affect overlying neural tissues (Heisenberg and Nusslein-Volhard, 1997). To determine whether Wnt11 can directly affect neural plate cells, we assessed whether it could disrupt eye field morphogenesis in the absence of underlying mesendoderm. Mutants lacking both maternal and zygotic activity of the nodal pathway protein Oep (MZoep mutants) lack all mesendoderm in anterior regions (Gritsman et al., 1999). MZoep cells expressing Wnt11 transplanted into MZoep mutants still induced distortion and expansion of the eye field (Figure 3E), indicating that Wnt11 can elicit these phenotypes in the absence of mesendodermal signals.
These experiments revealed an ability of Wnt11 to influence the shape of gene expression domains in the ANP, most likely by affecting the movements of eye field cells. Indeed, cell movement defects were evident when comparing the distribution of the transplanted wnt11+ and wild-type cells in the host neural plate: wild-type donor cells interspersed widely with host wild-type cells (e.g., Figure 2F), whereas wnt11+ cells usually remained more coherent and tightly clustered (e.g., Figure 3C). To begin to address the events that lead to these different cell distributions, we performed time lapse analysis on transplants of wild-type and wnt11+ cells. In the first few minutes following transplantation, both wild-type and wnt11+ cells integrated into the host neural plate and interspersed with wild-type host cells (Movies S1 and S2and data not shown). However, subsequent to this, wild-type and wnt11+ cells showed different behaviors.
Wild-type cells (or cells expressing dnwnt8, Movie S3) showed variable trajectories with respect to the position of transplantation: some moved toward the center of the transplant; some moved away; and some moved parallel to it (Figures 4A and 4C: paths 1–3, paths 4–7, and paths 8–9, respectively). This is consistent with the cells responding to directional movement cues originated in the host tissue that are independent of the transplant. In contrast, wnt11+ cells were less influenced by gastrulation movements in the host tissue; they moved coherently such that, over time, they tended to converge toward the center of the transplant, thus conferring the well-circumscribed shape to the cluster of transplanted cells by the end of gastrulation (Figures 4B and 4D).
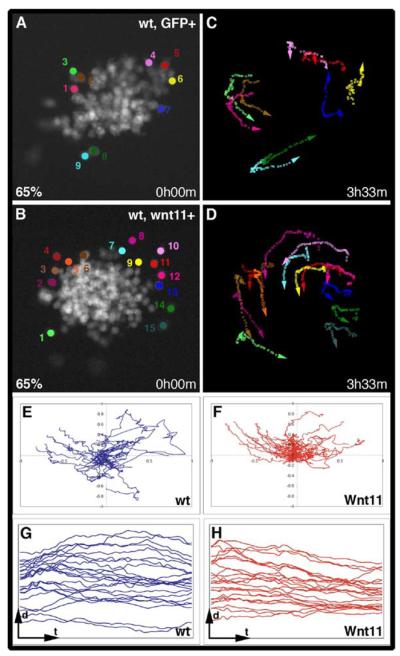
Figure 4. Wnt11 Affects the Behavior of ANP Cells.
(A and B) Time point 0:00 hr of a GFP+ (A) and a wnt11+ (B) transplant corresponding to Movies S1 and S2; the cells tracked in these experiments have been marked by colored dots.
(C and D) Cell paths for the movie of wild-type GFP+ cells (C) and wnt11+ cells (D) at the end of the time-lapse.
(E and F) The cell paths of 25 wild-type GFP+ (E) and 30 wnt11+ cells (F) have been plotted on an x-y graph to represent the direction of their movement relative to the center of the transplant (see Results and Experimental Procedures). The origin of all of the paths is x0:y0, and the center of the transplant is represented as x0:y1.
(G and H) Distance of GFP+ (G) and wnt11+ (H) cells to the center of the corresponding transplant (d), represented over time (t).
To quantitate the behaviors of wild-type and wnt11+ cells, we analyzed cell trajectories of approximately 25 to 30 cells from several representative wild-type and wnt11+ transplants and compared individual cell movements relative to the central position of the transplant. We represented the paths of the cells on a graph with the origin of each cell movement at x0:y0 and the center of the transplant being a fixed reference at x0:y1 (see Experimental Procedures). The paths of wild-type cells (Figure 4E) distributed in all quadrants of the graph, suggesting no common directionality in their movement relative to the center of the transplant. In contrast, the paths of wnt11+ cells were not independent of the position of the transplantation, with most cell paths falling within the two quadrants directed toward the center of the transplant (Figure 4F). Indeed, the distance of the wnt11+ cells to the center of the transplant considerably decreased over time (Figure 4H), which was not observed for wild-type cells (Figure 4G; the trajectories of wild-type versus wnt11+ cells over time with respect to the center of the transplant differs significantly, p = 0.0016). In summary, these results indicate that a source of Wnt11 is able to influence the movement of cells in its vicinity and suggest a direct requirement for Wnt11 in the regulation of morphogenetic movements in the ANP.
Wnt11 Signaling Contributes to Eye Field Formation
In gain-of-function experiments, Wnt11 disrupts formation of the eye field; so we next assessed whether embryos with compromised Wnt11 signaling showed an eye field phenotype. silberblick (slb−/−) embryos lack Wnt11 activity (Heisenberg et al., 2000) and exhibit partial fusion of the eyes, a phenotype thought to be due to defective signaling from the underlying axial mesendoderm (Heisenberg and Nusslein-Volhard, 1997; Marlow et al., 1998). However, early stages in eye field formation have not previously been analyzed in slb−/− embryos.
The eye field in slb−/− mutants is smaller compared to that in wild-type embryos (Figures 5B and 5E). ANP cell movements are probably affected in slb−/− mutants (Heisenberg and Nusslein-Volhard, 1997), and this may be contributing to the eye field phenotype. However, the defects in the eye field are more pronounced than in other regions of the ANP, since other ANP regional markers that were analyzed showed only very mild alterations (Figures 5B, 5C, 5E, and 5F, and Figure S2). These results suggest that the eye phenotype in slb−/− may result from a combination of defects in ANP morphogenesis and a local requirement for Wnt11 in the eye field.
Figure 5. Wnt11 Signaling Is Required for Correct Formation of the Eye Field.
(A–F) Wild-type (A–C) and slb−/− (D–F) embryos, showing expression of genes indicated (bottom right of panels) at stages indicated (bottom left of panels).
(G) A transplant of wnt11+ cells rescues the size and shape of the rx3 expression domain in a slb−/− mutant.
(H and I) Transplants of dsh-DEP++ cells show normal otx2 (I) expression, but express only very low levels of rx3 (H).
A specific role for Wnt11 signaling in eye field development is supported by the observation that the slb−/− eye phenotype is evident from midgastrulation (rx3, Figures 5A and 5D and opl/zic1, [Varga et al., 1999], data not shown) prior to the morphogenetic movements that occur in anterior regions during late gastrula stages. Transplants of wnt11+ cells in the ANP of slb−/− embryos partially rescued the shape and size of the eye field (Figure 5G), whereas transplants of cells expressing a dominant-negative form of Wnt11 (Tada and Smith, 2000) suppressed eye field markers (data not shown). These observations further support the idea that the slb−/− eye phenotype is due to a lack of Wnt11 locally acting in the eye field from middle to late stages of gastrulation.
In mediating mesodermal cell movements, Wnt11 signals through a β-catenin-independent pathway (Veeman et al., 2003), so we asked if embryos more severely compromised in their ability to activate noncanonical Wnt signaling also showed defects in eye field formation. Indeed, transplants of cells expressing a truncated form of Dsh (Dsh-DEP+) that specifically interferes with noncanonical Wnt signaling (Tada and Smith, 2000) led to a severe reduction of eye field marker gene expression (Figure 5H and data not shown) without compromising the general ANP marker otx2 (Figure 5I).
Altogether, these results suggest that Wnt11 activity functioning through a noncanonical branch of the Wnt signaling pathway is required at an early stage of eye formation to promote rx3 expression and that this requirement may, at least in part, be independent of this pathway's role in the regulation of cell movements during gastrulation.
Fz8a Facilitates Wnt8b Signaling
The very different activities of and requirements for Wnt11 and Wnt8b/Wnt1 signaling in early eye development suggest activation of different signaling cascades by these two classes of Wnts. We next asked whether differences in receptor usage by Wnt11 and Wnt8b/Wnt1 might contribute to the differences in activity. Fz proteins are Wnt receptors (Veeman et al., 2003), but the in vivo specificity/affinity of different Wnts for different Fz receptors is largely unknown. Indeed, it is likely that there is promiscuity between ligands and receptors (Logan and Nusse, 2004; He et al., 2004). Nevertheless, we reasoned that looking for genetic interactions between ligands and receptors would be a good way to identify likely in vivo interactions. The only Fz gene with characterized expression in the ANP during late stages of gastrulation is fz8a (Kim et al., 1998; Kim et al., 2002); to analyze fz8a function, we abrogated its activity by injection of antisense morpholinos (Mo) (Nasevicius and Ekker, 2000).
In support of the hypothesis that Fz8a acts as a receptor for Wnt8b (Kim et al., 2002), we observed a genetic interaction between Fz8a and Wnt8b. Thus, while neither fz8a nor wnt8b morphants showed obvious defects in the formation of the eye field at the Mo concentrations used (Figures 6D and 6E; Table S2; and Kim et al., 2002), wnt8b/fz8a double morphants showed a striking expansion of this territory (Figure 6F). This result is in agreement with the idea that Wnt/β-catenin activity specifies diencephalon and suppresses eye field formation. In contrast, fz8a Mo injections in slb/wnt11−/− mutants did not enhance the small eye field phenotype characteristic of slb−/− embryos (data not shown).
Figure 6. Fz5 and Fz8a Facilitate Wnt11 and Wnt8b Signaling in the ANP.
All of the embryos are at tailbud stage and show the expression of the genes indicated (bottom right of panels).
(A–C) fz5 expression (blue) and comparison with emx1 ([B], red) and foxb1.2 ([C], red).
(D–F) Wild-type embryos injected with 1 pmol/embryo of fz8a Mo (D), 0.5 pmol/embryo of wnt8b Mo (E), or coinjected with 1 pmol/embryo of fz8a Mo and 0.5 pmol/embryo of wnt8b Mo (F).
(G–I) rx3+pax2.1 (G), emx1+foxb1.2 (H), and barH2 (I) expression domains in fz5 morphants (0.8 pmol/embryo).
(J–M) Interaction of Fz5 with Wnt11 and Wnt8b. (J–L) the slb+/− (J) and wild-type embryos treated with 0.4 pmol/embryo of fz5 Mo (K) are phenotypically wild-type; the slb+/− embryo treated with 0.4 pmol/embryo of fz5 Mo (L) shows a small-eye phenotype. (M) An embryo coinjected with fz5 Mo and wnt8b Mo shows an essentially wild-type eye field.
(N–O) fz5 morphants (0.8 pmol/embryo, N) and fz5 morphants (0.8 pmol/embryo) coinjected with DshΔN mRNA (O).
Table S2 details the number of embryos analyzed for each condition, as well as the proportion of embryos displaying the phenotypes shown in this figure and in Figure 7. p, pmol/embryo.
Fz5 Facilitates Wnt11 Signaling in the Eye Field
In situ hybridization analysis of a gene encoding a Fz receptor most similar to mammalian Fz5 (accession number AF117387) showed expression exclusively within the eye field during late gastrula stages (Figures 6A and 6C).
fz5 morphants displayed a reduction of the eye field (Figure 6G) similar to that observed in slb−/− mutants (compare Figure 6G with Figure 5E). Consistent with the restricted expression of fz5 in the eye field, other territories of the ANP were essentially normal in fz5 morphants (Figures 6H and 6I). These observations suggest that Fz5 may be a receptor for Wnt11, thus contributing to the activation of Wnt11 signaling within the eye field.
Supporting the idea that Fz5 is a Wnt11 receptor, we found a genetic interaction between fz5 and wnt11 through injection of very low concentrations of fz5 Mo in heterozygous slb+/− embryos that are normally phenotypically wild-type (Figure 6J). At these low concentrations (0.2-0.4 pmol/embryo), the fz5 Mo does not cause a phenotype in wild-type embryos (Figure 6K). However, heterozygous slb+/− embryos injected with low doses of fz5 Mo displayed a phenotype (Figure 6L) very similar to that of embryos homozygous for the slb mutation or injected with a large amount of fz5 Mo (0.8 pmol/embryo). These results indicate a synergistic effect of Fz5 and Wnt11 on the formation of the eye field. In contrast, coinjection in wild-type embryos of 0.5 pmol/embryo of wnt8b Mo together with low concentrations of fz5 Mo (0.2–0.4 pmol/embryo) led to no enhancement of the wnt8b morphant phenotype (Figure 6M).
The mesodermal cell movement defects caused by loss of Wnt11 activity can be rescued by overexpression of a truncated form of Dsh (Dsh-ΔN) that activates Wnt/noncanonical, but not Wnt/β-catenin, signaling (Heisenberg et al., 2000; Tada and Smith, 2000). Indeed, coinjection of RNA encoding Dsh-ΔN together with fz5 Mo was able to reduce the frequency of embryos displaying a severe reduction of the eye field from 80% (Figure 6N and Table S2) to only 21%, with the remaining embryos exhibiting an essentially wild-type eye field (Figure 6O and Table S2). Altogether, these results suggest that at early stages of development, Fz5 transduces Wnt11/noncanonical signals in the forming eye field.
Wnt11 and Fz5 Signaling Suppresses Wnt/β-Catenin Activity within the Eye Field
Our results indicate that ligands and receptors acting in both β-catenin-dependent and β-catenin independent branches of the Wnt signaling pathway influence formation of the eye field. These two branches share some intracellular components, such as Dsh, and various assays have suggested molecular interactions between the pathways (reviewed in Veeman et al., 2003). However, it remains unclear whether such interactions occur during normal development. To address this issue in the context of eye formation, we manipulated either Wnt8b/Fz8/β-catenin or Wnt11 and Fz5 activity in embryos sensitized for signaling via the other branch of the pathway. Our first approach was to compare the effects of progressively depleting activity of the Headless/Tcf3a transcriptional repressor of Wnt/β-catenin signaling in wild-type and slb−/− embryos.
Abrogation of Wnt11 activity sensitized the eye field to activation of Wnt/β-catenin signaling. Thus, while injection of 0.02 pmol of tcf3a Mo in wild-type embryos did not have any effect, injection of the same amount of Mo in slb−/− embryos led to a reduction in the size of the eye field (Figures 7B and 7F), suggesting that slb−/− mutants are more sensitive to activation of the Wnt/β-catenin pathway than are wild-type embryos. Injection of greater amounts of tcf3a Mo in wild-type embryos (0.04 –0.1 pmol) led to progressively more severe suppression of anterior structures (Figures 7C and 7D); in all cases, the suppression was more severe when equivalent amounts of tcf3 Mo were injected in slb−/− embryos (Figures 7G and 7H).
Figure 7. Wnt11 and Fz5 Antagonize Canonical Wnt/β-Catenin Signaling in the Eye Field.
All embryos are at tailbud stage and show expression of genes indicated (bottom right of panels).
(A–H) tcf3a Mo injections in wild-type (A–D) and slb−/− (E–H) embryos. Injection of 0.02 pmol/embryo of tcf3a Mo in slb−/− background severely compromises eye field formation (F), while in wild-type it does not have any obvious effect (B); injection of higher amounts of tcf3a Mo (0.04 pmol/embryo, [C] and [G]; 1 pmol/embryo, [D] and [H]) also leads to more severe effects in slb−/− mutants than in wild-type embryos (compare the extent of the eye field and the expansion of midbrain markers in slb−/− ([G] and [H]) with wild-type ([C] and [D]) embryos).
(I–K) Gsk3-DN-expressing cells do not have any effect on rx3 expression when transplanted into a wild-type host (I), but suppress rx3 (J) and induce ectopic foxb1.2 ([K], arrow) when transplanted into a slb−/− host.
(L) rx3 expression, which is downregulated in tcf3a Mo-injected embryos (0.04 pmol/embryo, [C]), is partially rescued by a transplant of wnt11+ cells ([L], arrows).
(M–P) Abrogation of Fz8a activity (M–O) rescues the small-eye phenotype of fz5 morphants (P). p, pmol/embryo.
We found a similar result when we examined the ability of cells sensitized to Wnt/β-catenin activity to contribute to the eye field in slb−/− embryos. We activated the Wnt/β-catenin pathway by transplanting cells over-expressing a dominant negative form of Gsk3β (Gsk3-DN; Yost et al., 1998). At the concentrations used, gsk3-DN+ transplants occasionally show a slight downregulation of rx3 in wild-type embryos, but leave eye field formation largely unaffected (Figure 7I). In contrast, equivalent transplants in slb−/− embryos led to suppression of rx3 expression (Figure 7J); moreover, the expression of diencephalic markers such as foxb1.2 was weakly activated in the transplanted cells (Figure 7K), a phenotype never observed when transplants were performed in a wild-type background (data not shown). Again, these results suggest that Wnt11 normally buffers the eye field to prevent activation of Wnt/β-catenin signaling.
Next, we asked whether activation of Wnt11 signaling could suppress the effects of overactivation of Wnt/β-catenin signaling. To do this, we transplanted wnt11+ cells in embryos injected with low amounts of tcf3a Mo (0.04 pmol/embryo) that reduce the eye field, but do not completely abrogate it (Figure 7C). Consistent with an antagonistic role for these pathways, we found that activation of the Wnt11/noncanonical pathway partially rescued expression of eye field markers in embryos with increased activity of the Wnt/β-catenin pathway. In tcf3a morphants, tcf3a Mo cells expressing Wnt11 can rescue the expression of rx3, albeit at a low frequency (Figure 7L; Table S1), indicating that activation of Wnt11 signaling can partially alleviate the effects of moderate overactivation of Wnt/β-catenin signaling.
Finally, we addressed the consequences of suppressing activity of both branches of the pathway by simultaneous abrogation of both Fz8a and Fz5. To do this, we performed a series of injections with a fixed amount of fz5 Mo and increasing amounts of fz8a Mo. While the proportion of embryos with an obviously smaller eye field in fz5 morphants was 80%, coinjection of fz8a Mo led to only 25% of the embryos displaying an obviously smaller eye field (Table S2; Figures 7M–7P show the most frequent phenotype displayed in each condition). Similarly, the expansion of the eye field induced when Wnt/β-catenin signaling is severely abrogated (by coinjection of fz8a and wnt8b Mo) is strikingly suppressed by coinjection of fz5 Mo in the wnt8b/fz8a double morphants (Table S2). Thus, all four approaches in which we have simultaneously manipulated both Wnt/β-catenin signaling and Wnt11 or Fz5 activity support the idea that there are antagonistic interactions between these branches of the Wnt pathway during formation of the eye field.
Discussion
Our results reveal an essential role for Wnt11 and Fz5 signals in the coordination of eye field specification and morphogenesis during the early stages of forebrain development. Activation of Wnt11 signaling within the eye field promotes eye formation, at least partially by antagonizing Wnt/β-catenin signaling, which otherwise promotes posterior diencephalic identity. In addition, Wnt11 signaling promotes cohesion of eye field cells, potentially contributing to the different morphogenetic behaviors of eye field cells compared to those of neighboring neural plate tissue. Transduction of signaling by each class of Wnt in the forebrain shows different receptor requirements. Our results suggest that Fz8a preferentially responds to Wnt8b, whereas Fz5 preferentially responds to Wnt11. These receptors show different expression patterns in the ANP, allowing more precise spatial control of activation of each pathway. Thus, the coordination of eye field specification and morphogenesis during the early stages of forebrain development relies on the combined activities of Wnt11, Fz5, and Wnt/β-catenin signaling.
Wnt/β-Catenin Signaling through Wnt8b and Fz8a Regulates the Allocation of Eye Field and Diencephalic Domains
Our data add to the body of evidence (Wilson and Houart, 2004) that Wnt/β-catenin signaling regulates the regionalization of the forebrain. We, and others, have shown that overactivation of Wnt/β-catenin signaling promotes posterior diencephalic fates and suppresses anterior telencephalic and eye field identities (our results and previous data reviewed in Wilson and Houart, 2004). Here, we further show that local suppression of Wnt/β-catenin signaling can expand eye field markers caudally into the posterior diencephalon. There are at least three Wnts potentially involved in this process: Wnt1, Wnt10b, and Wnt8b. However, a number of results argue in favor of Wnt8b being the one most likely involved in the regionalization of the forebrain. While wnt8b is expressed in the posterior diencephalon, wnt1 and wnt10b are expressed more posteriorly. Moreover, wnt1/wnt10b double mutants/morphants do not show an obvious patterning defect in the forebrain (Lekven et al., 2003), and the slight posterior expansion of the eye field found in wnt8b morphants (Kim et al., 2002) is not significantly enhanced in the wnt8b/wnt10b/wnt1 triple morphants (unpublished observations).
Our results strengthen the hypothesis that Fz8a is the receptor responsible for transducing the Wnt8b signal (Kim et al. 2002). fz8a is expressed in a broad domain within the ANP (Kim et al., 2002), consistent with the entire prospective forebrain being susceptible to reception of Wnt8b signals in a graded posterior/high to anterior/low fashion. Still, it is unclear whether Wnts can exert their action at a distance or can act only locally. We favor a scenario in which Wnt8b would be working as a short-range signal, as Wnt8b is required for the formation of diencephalon and midbrain (Kim et al., 2002), the main territories where it is expressed, and to establish the posterior boundary of the eye field (this study), which is located no more than a few cell rows away from the anterior boundary of the wnt8b domain. Specification of the eye field more anteriorly requires local suppression of Wnt/β-catenin signaling, but as yet, there is no evidence that Wnts signaling through the β-catenin branch of the pathway significantly encroach throughout the eye field during gastrula stages of normal development.
Similar to the eye field, induction of the telencephalon also requires suppression of Wnt/β-catenin signals (Houart et al., 2002). What, then, might specify the difference between eye field and telencephalon? Slight differences in the level of Wnt signaling may be enough to effect the separation of these two territories. Alternatively, additional signals, such as those coming from the margin of the neural plate, may also be required for this patterning process. For instance, early-acting BMP signals promote telencephalic gene expression (Barth et al., 1999), but can suppress specification of eye field gene expression (Barth et al., 1999; Hammerschmidt et al., 2003).
Wnt11 Signaling Directly Affects Behavior of Eye Field Cells
Transplants of wnt11+ cells severely disrupt the organization of gene expression domains in the ANP. This is due to a direct effect on ectodermal cells, since the same disruptions occur when these transplants are generated in an MZoep background, where a role for the mesendoderm can be ruled out. The phenotype induced by wnt11+ transplants could be due to complex alterations in regional patterning and/or alterations in cell behavior/morphogenesis.
Cell behavior/morphogenesis is affected by Wnt11, as revealed by the differing abilities of wnt11+ and wild-type cells to intersperse in the host tissue and by in vivo observations indicating that wnt11+ cells move coherently and tend to converge and cohere over time. Ectopic Wnt11 sources also disturb the movements of cells surrounding them, an effect very evident in the disruption of the pattern of eye field, diencephalic, and midbrain markers. These results suggest a role for this pathway in maintaining coherent behavior of eye field cells.
What might be the molecular mechanisms by which Wnt11 influences eye field cell behavior? One hypothesis is that it maintains the integrity of the eye field by establishing repulsion boundaries between this territory and the adjacent prospective telencephalon anteriorly and the diencephalon posteriorly. Eph/ephrin interactions underlie segregation of domains of cells in other potentially similar processes, such as boundary formation in the zebrafish hindbrain and somites (reviewed in Poliakov et al., 2004). In these situations, a complementary pattern of expression of ephrins and Ephs restricts intermingling of adjacent cell populations. In zebrafish, several components of the Eph/ ephrin family are expressed in complementary domains in the forebrain (Chan et al., 2001; Xu et al., 1996; Zebrafish Information Network, ZFIN), and Eph/Ephrin signaling has been proposed to influence the integrity of the forming eye both in fish (Xu et al., 1996) and in frog (Moore et al., 2004). Thus, it will be of interest to determine if Wnt11 signaling affects the behavior of ANP cells by regulating the expression or activity of different Eph/ephrins in this territory.
Given the pronounced effect of wnt11+ transplants on cell behavior and morphogenesis, it is difficult to assess the extent to which these manipulations affect regional patterning. Certainly, following transplantation of wnt11+ cells the eye field was larger in most cases compared to that of wild-type embryos, but we were unable to distinguish the relative contribution of fate changes, morphogenesis, and other factors, such as proliferation, to this phenotype (see for instance, Van Raay et al., 2005). Given our data that Wnt11 and Fz5 signaling can antagonize Wnt/β-catenin signaling, one might suspect that some of the expansion of the eye field induced by a local source of Wnt11 may be due to partial suppression of Wnt/β-catenin signaling. The fact that other domains of the neural plate are still present in these experiments suggests that there are not dramatic fate changes, but more subtle alterations would be difficult to assess. wnt11+ transplants, however, are not equivalent to dnwnt8+ transplants in terms of cell behavior, since dnwnt8+ cells show behavior similar to that of wild-type cells. Thus, the role of Wnt11 in controlling cell behavior and morphogenesis is likely to be independent of the role of this molecule in antagonizing Wnt/β-catenin signaling.
Wnt11 and Fz5 Promote Formation of the Eye Field
In slb−/− embryos, the eye field is significantly smaller compared with that in wild-type embryos. This may be due, in part, to complementary overactivation of Wnt/β-catenin signaling, which would tend to suppress eye field-specific genes (see below). However defective cell movements and coherence could also potentially contribute to the small eye field phenotype. Cells that fail to properly incorporate into the eye field due to defective morphogenesis are unlikely to subsequently maintain expression of eye field genes, as has been demonstrated during the refinement of expression domains in many other scenarios (e.g., see Cooke and Moens, 2002). Our results do not clarify whether there are coherence defects in slb−/− embryos, since any cell that “drops out” of the eye field would stop expressing eye field markers and would not be detectable by the analysis that we have performed; in the future, it would be interesting to follow the development of such cells by labeling them with stable markers.
The localized expression of fz5 within the nascent eye field supports a local function for Wnt11 and Fz5 signaling in promoting eye field formation. The possibility that Fz5 directly transduces Wnt11 signals is supported by the small-eye phenotype of fz5 morphants, reminiscent of slb−/− embryos; the synergistic effect on the eye field of simultaneous downregulation of Wnt11 and Fz5 activities; and the rescue of fz5 morphants by activation of the Wnt/noncanonical pathway downstream of the receptor. In contrast to this conclusion, it has recently been proposed that at later stages of eye development, Xenopus Fz5 activates canonical Wnt signaling (Van Raay et al., 2005). Indeed, in gain-of-function experiments, zebrafish Fz5 does appear to be able to activate Wnt/β-catenin signaling (see Supplementary Data). If the interpretation of data in both our study and the Van Raay study is correct, the intriguing possibility emerges that the specificities of Wnt/Fz interactions are highly context-dependent.
Although we focus on a role for Wnt11 within the eye field, it is likely to have activity beyond this area; certainly, other, more caudally located neural plate cells can respond to exogenous Wnt11, and this does not require Fz5, since exogenous Wnt11 does not induce fz5 expression (data not shown). This suggests the existence of other receptors that are able to respond to Wnt11 in the ANP. Indeed, by in situ hybridization analysis, the onset of expression of fz5 in the eye field at 90% epiboly (data not shown) is slightly later than the initial phenotypic defects in eye field formation in slb−/− embryos. Rasmussen and colleagues (Rasmussen et al., 2001) have suggested that Fz3 may activate a non-canonical Wnt pathway during eye field formation in Xenopus, so orthologs of this receptor are candidates for mediating early Wnt11 signaling. However, very recent data suggest that Fz3 might interact with Wnt4 to promote transcriptional elongation (Maurus et al., 2005), a process not previously suspected to be regulated by Wnt signaling and very unlikely to be related to the activities of β-catenin-dependent and -independent Wnt pathways analyzed in our study. Clearly, further research is required to determine the full extent of Wnt/Fz interactions and the nature of the signaling cascades activated during different stages of eye development.
Wnt11 and Fz5 Signaling Antagonizes the Effects of Wnt/β-Catenin Signaling in the Forming Eye
Overactivation of Wnt/β-catenin signaling may contribute to the small eye field phenotype displayed by slb−/− mutants and fz5 morphants. We present several lines of evidence supporting a role for Wnt11 and Fz5 as antagonists of Wnt/β-catenin signaling in the nascent eye field. Thus, both high levels of Wnt/β-catenin activity and low levels of Wnt11 or Fz5 activity compromise the formation of the eye field; reciprocally, downregulating Wnt/β-catenin activity or upregulating either Wnt11 or Fz5 activity can increase the size of the eye. In addition, mild overactivation of the Wnt/β-catenin pathway in the ANP has more dramatic caudalizing effects in embryos compromised for Wnt11 signaling (such as slb−/− mutants) than in a wild-type background. Consistent with this, the posteriorizing effect of overactivating the Wnt/β-catenin pathway can be partially suppressed by overactivation of Wnt11 activity. Not only does overactivation of both pathways negate each other's effect, but this is also true for simultaneous downregulation of both pathways (as in fz5/fz8a double morphants or fz5/fz8a/wnt8b triple morphants). All of this data is consistent with the results of other, mostly in vitro, assays suggesting antagonism between different branches of the Wnt signaling cascade (Veeman et al., 2003).
In recent years, two different molecular mechanisms have been proposed to explain how β-catenin-independent Wnt signaling could counteract Wnt/β-catenin activity (Veeman et al., 2003). Ishitani and colleagues (Ishitani et al., 2003a; Ishitani et al., 2003b) proposed a model in which noncanonical Wnt signaling induces the phosphorylation of LEF/TCF factors and interferes with their DNA binding ability, thus interfering with the transcriptional activation function of the β-catenin/LEF/TCF complex. Topol and colleagues (Topol et al., 2003) instead favor a model in which noncanonical Wnt signaling induces β-catenin degradation in a Gsk3-independent manner. These mechanisms are not exclusive, and Wnt11 may utilize a combination of different mechanisms to antagonize Wnt/β-catenin signaling. Indeed, this may be the case during regulation of CE movements in the gastrulating Xenopus embryo, where antagonism between β-catenin-dependent and β-catenin-independent Wnt pathways at different levels has been proposed (Kuhl et al., 2001). Our results show that levels of Wnt11 and Fz5 can influence Wnt/β-catenin signaling when this branch of the pathway is activated/ suppressed at the level of ligand (Wnt8b), receptor (Fz8a), Gsk3β, or in the nucleus (Tcf3a). Furthermore, the Wnt/β-catenin pathway has a regulatory feedback role in the ANP (e.g., see Houart et al., 2002), so modulation of downstream components of the pathway can lead to changes in expression of upstream components. Given these complexities, it will be a challenge to resolve, in vivo, the relative contribution of different proteins to the crosstalk between Wnt11, Fz5, and Wnt/β-catenin pathways.
Summary: A Model for Activity of Wnt Signaling during the Early Stages of Eye Field Development
During formation of the eye, nascent eye field cells must be specified to acquire eye field identity and must undergo a program of morphogenesis quite distinct from that of adjacent forebrain territories. In this study, we show that a noncanonical Wnt pathway activated by Wnt11 in the eye field helps to coordinate these two events. Wnt11 function may direct the morphogenesis of the eye field by maintaining the coherence of this territory. Simultaneously, noncanonical Wnt activity would consolidate the extent of the territory defined as eye field by keeping it refractory to any residual Wnt/β-catenin signals encroaching from more posterior domains. Thus, through the coordinated antagonism of signals that suppress retinal identity and promotion of cell coherence, Wnt11 and Fz5 signaling would link induction and morphogenesis during the early stages of eye development.
Experimental Procedures
Fish Lines
Zebrafish strains were maintained and bred according to standard procedures (Westerfield, 1995). AB and tupl wild-type lines and masterblind (mbltm213) and silberblick (slbtx22b) mutant lines were used.
Cloning and Synthesis of mRNA
To clone the fz5cDNA (accession number AF117387), the following primers were designed:
5′fz5 (ATGAGGAAACCTGCAGACGAGCATC) and 3′fz5 (TCAG ACATGTGATGAGGGTGCTG).
The Fz receptor corresponding to accession number AF117387 has previously been published as Fz8c and submitted to Genbank as fz2 (Momoi et al., 2003); however, due to its expression, mainly restricted to the eye field from late stages of gastrulation, as is the case for Xenopus fz5 (Sumanas and Ekker, 2001) and mouse fz5 (Borello et al., 1999), and due to the fact that phylogenetic trees place it closer to Xfz5 than to Xfz8 (data not shown), we consider it to be the true fz5 ortholog.
All of the cDNAs used in this work for RNA synthesis were cloned in pCS2+. Capped mRNA was generated using a Message Machine RNA synthesis kit (Ambion) according to the manufacturer's instructions.
Antibody Labeling and In Situ Hybridization Procedures
Anti-sense RNA probes were generated using digoxigenin/fluorescein RNA labeling kits (Boheringer-Mannheim). GFP protein was detected by anti-GFP antibody (Torrey Pines Biolabs). Whole-mount in situ hybridization and antibody detection were performed with standard procedures (Shanmugalingam et al., 2000).
Morpholino Experiments
The following Mos (Genetools) were used in this study: wnt8b Mo and fz8a Mo (Kim et al., 2002); wnt1 Mo and wnt10b Mo (Lekven et al., 2003); and tcf3a Mo (Dorsky et al., 2003). A fz5 Mo was designed against the first 25 nucleotides of the fz5 ORF (GAT GCTCGTCTGCAGGTTTCCTCAT). A number of controls have been performed to confirm the specificity of the fz5 morphant phenotype: a fz5 Mo with five mismatches in the sequence (GATc CTCcTCTcCAGcTTTgCTCAT) does not display any phenotype (data not shown); a fz5 Mo against the 5′UTR (ACTGGAAAGCTAT GAATTTCAATT) displays the same phenotype as fz5 Mo, albeit requiring higher concentrations (Figure S3B). In addition, the secondary axes induced by the ubiquitous overexpression of fz5 mRNA (Figure S3D) can be rescued by coinjection of fz5 Mo (Figure S3E), but not by coinjection of fz5 mismatch Mo (Figure S3F).
In all cases, the injections were done at concentrations between 0.2 mM and 1 mM; the amount of Mo injected in each experiment (expressed in pmol/embryo [p/emb]) is provided in the text and in Table S2.
Cell Transplantation and Lithium Chloride Treatment Experiments
Embryos coinjected with gfp mRNA (20–40 pg/emb) and wnt8b (20–40 pg/emb), wnt1 (50 pg/emb), wnt11 (40 pg/emb), axin1 (80–100 pg/ emb), gsk3-DN (200 pg/emb), Xdnwnt8 (150 pg/emb), Xdnwnt11 (150 pg/emb), or Xdsh-DEP (150–175 pg/emb) were used as donors at the midblastula stage. Around 30 to 40 cells from the apical region of the donor embryos were transplanted to early gastrula stage hosts (55%–65% epiboly) in the region fated to become the eye field (Woo and Fraser, 1995). Cells derived from donor embryos injected with gfp mRNA alone were used for control transplants. For the transplantation procedure, embryos were mounted in 3% methylcellulose in fish water and viewed with a fixed-stage compound microscope (Nikon Optiphot). Cells were moved by suction using an oil-filled manual injector (Sutter Instrument Company). The number of transplants analyzed for each condition and the phenotypes observed are summarized in Table S1.
The expression of chordin, a landmark for the future dorsal side of the embryo, is activated by β-catenin at the late blastula stage (40% epiboly; Heasman et al., 2000); therefore we used it as a control to analyze the effect of the introduced reagents on the activity of the canonical Wnt/β-catenin pathway in the donor embryos (Figure S4). wnt8b and gsk3DN overexpression induced ectopic chordin expression ( the same result obtained by overexpressing a constitutively activated form of β-catenin (Figures S4C and S4D; Domingos et al., 2001), consistent with the activation of the Wnt/β-catenin pathway. axin1 overexpression blocks chordin expression (Figures S4I and S4J), a result in agreement with its role as a negative modulator of the canonical pathway. Wnt/noncanonical components do not affect chordin expression or the establishment of the DV axis (Figures S4K–S4P). Instead, analysis of embryos over-expressing DNwnt11 and dsh-DEP shows phenotypes compatible with defects in convergence and extension movements (Figures S4S and S4T; Veeman et al., 2003). Lithium chloride (LiCl) treatments were performed as described in Kim et al. (Kim et al., 2002).
Time-Lapse Analysis and Cell Tracking
Five control transplants expressing gfp mRNA alone were time-lapsed from 65% epiboly for approximately 3.5 hr at 28°C and compared to six wnt11+ transplants. Single-channel time-lapse images were acquired using custom-made routines written in Openlab (Improvision).
Cell tracking was performed using the tracking module of Openlab. Wild-type cells (25) and wnt11+ cells (30), from two representative wild-type and three representative wnt11+ transplants, were manually tracked, and the paths were generated using Openlab. To track the central position of the transplant, its outline was manually traced and the centroid was calculated at each time point, using the tracking module of Volocity (Improvision). All further calculations were performed in Microsoft Excel. To follow the distance of each cell to the center of the transplant during the course of the time-lapse, the position of each cell at all time points was recalculated relative to the center of the transplant. The relative paths followed by the cells were represented on an x-y graph, with the origin of each cell movement at x0:y0 and the center of the transplant as a fixed reference at x0:y1. In addition, the distances of the cells to the center of the transplant were represented over time. For statistical analysis, a two-tailed Student's t-Test was applied.
Acknowledgments
We thank Lila Solnica-Krezel, Randy Moon, and other colleagues, for providing reagents used in this study; Tetsu Kudoh and other members of our group, for helpful discussions; Carole Wilson and her team, for care of the fish; and Gabriel Arias, for help with the cell tracking analysis. This work was supported by a European Community Marie Curie Fellowship and a Human Frontiers Program Fellowship to F.C. and by grants from the Wellcome Trust, BBSRC, and European Community to S.W.W. F.C.B. was supported by Programa Gulbenkian de Doutoramento em Biologia e Medicina; R.M.Y. was supported by a Fondecyt 2010058 Grant, a Company of Biologists Travel Fellowship, and a Boehringer Ingelheim Travel Allowance; M.L.A. is funded by FONDECYT Chile (1020902) and Wellcome Trust; M.L.A. was supported by Fondecyt 1031003, ICM P02-050, ICGEB; M.T. had a Career Development Fellowship of the MRC; and S.W.W. was a Wellcome Trust Senior Research Fellow.
Footnotes
Supplemental Data Supplemental data include four figures, two tables, and three movies and are available with this article online at http://www.neuron.org/cgi/content/full/47/1/43/DC1/.
References
- Barth KA, Kishimoto Y, Rohr KB, Seydler C, Schulte-Merker S, Wilson SW. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–4987. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- Borello U, Buffa V, Sonnino C, Melchionna R, Vivarelli E, Cossu G. Differential expression of the Wnt putative receptors Frizzled during mouse somitogenesis. Mech. Dev. 1999;89:173–177. doi: 10.1016/s0925-4773(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Buckles GR, Thorpe CJ, Ramel MC, Lekven AC. Combinatorial Wnt control of zebrafish midbrain-hindbrain boundary formation. Mech. Dev. 2004;121:437–447. doi: 10.1016/j.mod.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Chan J, Mably JD, Serluca FC, Chen JN, Goldstein NB, Thomas MC, Cleary JA, Brennan C, Fishman MC, Roberts TM. Morphogenesis of prechordal plate and notochord requires intact Eph/ephrin B signaling. Dev. Biol. 2001;234:470–482. doi: 10.1006/dbio.2001.0281. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Raymond PA. Embryonic origin of the eyes in teleost fish. Bioessays. 2002;24:519–529. doi: 10.1002/bies.10097. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Mathers PH, Raymond PA. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech. Dev. 1999;84:195–198. doi: 10.1016/s0925-4773(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Cooke JE, Moens CB. Boundary formation in the hindbrain: Eph only it were simple. Trends Neurosci. 2002;25:260–267. doi: 10.1016/s0166-2236(02)02134-3. [DOI] [PubMed] [Google Scholar]
- Cui Y, Brown JD, Moon RT, Christian JL. Xwnt8b: a maternally expressed Xenopus Wnt gene with a potential role in establishing the dorsoventral axis. Development. 1995;121:2177–2186. doi: 10.1242/dev.121.7.2177. [DOI] [PubMed] [Google Scholar]
- Domingos PM, Itasaki N, Jones CM, Mercurio S, Sargent MG, Smith JC, Krumlauf R. The Wnt/beta-catenin pathway posteriorizes neural tissue in Xenopus by an indirect mechanism requiring FGF signaling. Dev. Biol. 2001;239:148–160. doi: 10.1006/dbio.2001.0431. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Itoh M, Moon RT, Chitnis A. Two tcf3 genes cooperate to pattern the zebrafish brain. Development. 2003;130:1937–1947. doi: 10.1242/dev.00402. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Kramer C, Nowak M, Herzog W, Wittbrodt J. Loss of maternal Smad5 in zebrafish embryos affects patterning and morphogenesis of optic primordia. Dev. Dyn. 2003;227:128–133. doi: 10.1002/dvdy.10281. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel anti-sense approach. Dev. Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Nusslein-Volhard C. The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev. Biol. 1997;184:85–94. doi: 10.1006/dbio.1997.8511. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, et al. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppler S, Brown JD, Moon RT. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- Houart C, Caneparo L, Heisenberg C, Barth K, Take-Uchi M, Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–265. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol. 2003a;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol. Cell. Biol. 2003b;23:1379–1389. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/ beta-catenin signaling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Park HC, Yeo SY, Hong SK, Choi JW, Kim CH, Weinstein BM, Huh TL. Characterization of two frizzled8 homologues expressed in the embryonic shield and prechordal plate of zebrafish embryos. Mech. Dev. 1998;78:193–201. doi: 10.1016/s0925-4773(98)00137-3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Shin J, Park HC, Yeo SY, Hong SK, Han S, Rhee M, Kim CH, Chitnis AB, Huh TL. Specification of an anterior neuroectoderm patterning by Frizzled8a-mediated Wnt8b signaling during late gastrulation in zebrafish. Development. 2002;129:4443–4455. doi: 10.1242/dev.129.19.4443. [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J. Biol. Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl M, Geis K, Sheldahl LC, Pukrop T, Moon RT, Wedlich D. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/beta-catenin and Wnt/Ca2+ signaling. Mech. Dev. 2001;106:61–76. doi: 10.1016/s0925-4773(01)00416-6. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Buckles GR, Kostakis N, Moon RT. Wnt1 and wnt10b function redundantly at the zebrafish midbrain-hindbrain boundary. Dev. Biol. 2003;254:172–187. doi: 10.1016/s0012-1606(02)00044-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Allende ML, Finkelstein R, Weinberg ES. Expression of two zebrafish orthodenticle-related genes in the embryonic brain. Mech. Dev. 1994;48:229–244. doi: 10.1016/0925-4773(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Marlow F, Zwartkruis F, Malicki J, Neuhauss SC, Abbas L, Weaver M, Driever W, Solnica-Krezel L. Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev. Biol. 1998;203:382–399. doi: 10.1006/dbio.1998.9032. [DOI] [PubMed] [Google Scholar]
- Maurus D, Heligon C, Burger-Schwarzler A, Brandli AW, Kuhl M. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. EMBO J. 2005;24:1181–1191. doi: 10.1038/sj.emboj.7600603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momoi A, Yoda H, Steinbeisser H, Fagotto F, Kondoh H, Kudo A, Driever W, Furutani-Seiki M. Analysis of Wnt8 for neural posteriorizing factor by identifying Frizzled 8c and Frizzled 9 as functional receptors for Wnt8. Mech. Dev. 2003;120:477–489. doi: 10.1016/s0925-4773(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Moon RT, Christian JL, Campbell RM, McGrew LL, DeMarais AA, Torres M, Lai CJ, Olson DJ, Kelly GM. Dissecting Wnt signaling pathways and Wnt-sensitive developmental processes through transient misexpression analyses in embryos of Xenopus laevis. Dev. Suppl. 1993;120:85–94. [PubMed] [Google Scholar]
- Moore KB, Mood K, Daar IO, Moody SA. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev. Cell. 2004;6:55–67. doi: 10.1016/s1534-5807(03)00395-2. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Nusslein-Volhard C. fork head domain genes in zebrafish. Dev. Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rasmussen JT, Deardorff MA, Tan C, Rao MS, Klein PS, Vetter ML. Regulation of eye development by frizzled signaling in Xenopus. Proc. Natl. Acad. Sci. USA. 2001;98:3861–3866. doi: 10.1073/pnas.071586298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HC, Drivenes O, Ellingsen S, Fjose A. Expression of two zebrafish homologues of the murine Six3 gene demarcates the initial eye primordia. Mech. Dev. 1998;73:45–57. doi: 10.1016/s0925-4773(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Shanmugalingam S, Houart C, Picker A, Reifers F, Macdonald R, Barth A, Griffin K, Brand M, Wilson SW. Ace/Fgf8 is required for forebrain commissure formation and patterning of the telencephalon. Development. 2000;127:2549–2561. doi: 10.1242/dev.127.12.2549. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Ekker SC. Xenopus frizzled-5: a frizzled family member expressed exclusively in the neural retina of the developing eye. Mech. Dev. 2001;103:133–136. doi: 10.1016/s0925-4773(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar AL, Kelly GM, Moon RT. Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mech. Dev. 1995;52:153–164. doi: 10.1016/0925-4773(95)00386-f. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Wegner J, Westerfield M. Anterior movement of ventral diencephalic precursors separates the primordial eye field in the neural plate and requires cyclops. Development. 1999;126:5533–5546. doi: 10.1242/dev.126.24.5533. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) Third Edition University of Oregon Press; Eugene, OR: 1995. [Google Scholar]
- Wilson SW, Houart C. Early steps in the development of the forebrain. Dev. Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K, Fraser SE. Order and coherence in the fate map of the zebrafish nervous system. Development. 1995;121:2595–2609. doi: 10.1242/dev.121.8.2595. [DOI] [PubMed] [Google Scholar]
- Xu Q, Alldus G, Macdonald R, Wilkinson DG, Holder N. Function of the Eph-related kinase rtk1 in patterning of the zebrafish forebrain. Nature. 1996;381:319–322. doi: 10.1038/381319a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr. Biol. 2001;11:R713–R724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Yost C, Farr GH, 3rd, Pierce SB, Ferkey DM, Chen MM, Kimelman D. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell. 1998;93:1031–1041. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]