Summary
The habenulae are part of an evolutionarily highly conserved limbic-system conduction pathway that connects telencephalic nuclei to the interpeduncular nucleus (IPN) of the midbrain [1]. In zebrafish, unilateral activation of the Nodal signaling pathway in the left brain specifies the laterality of the asymmetry of habenular size [2-5]. We show “laterotopy” in the habenulo-interpeduncular projection in zebrafish, i.e., the stereotypic, topographic projection of left-sided habenular axons to the dorsal region of the IPN and of right-sided habenular axons to the ventral IPN. This asymmetric projection is accounted for by a prominent left-right (LR) difference in the size ratio of the medial and lateral habenular sub-nuclei, each of which specifically projects either to ventral or dorsal IPN targets. Asymmetric Nodal signaling directs the orientation of laterotopy but is dispensable for the establishment of laterotopy itself. Our results reveal a mechanism by which information distributed between left and right sides of the brain can be transmitted bilaterally without loss of LR coding, which may play a crucial role in functional lateralization of the vertebrate brain [6, 7].
Results and Discussion
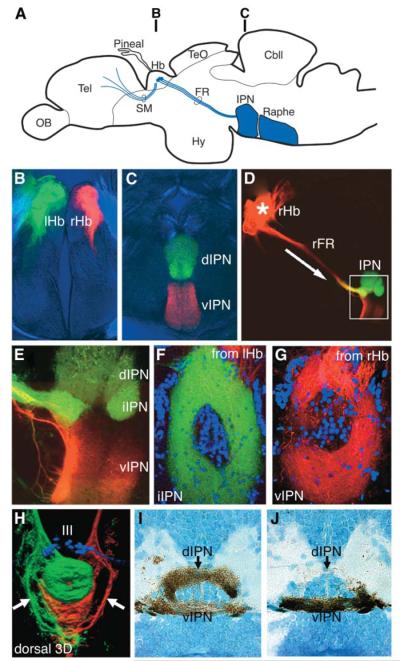
The habenula relays information from the telencephalon to the midbrain through the stria medullaris and the habenulo-interpeduncular tract (fasciculus retroflexus, FR) (Figure 1A). Analysis of the projection patterns of habenular neurons revealed that the left and right habenulae show strikingly different patterns of innervation of the IPN. In adult fish, axons from the left habenula project predominantly to the dorsal and intermediate IPN, whereas the right habenula predominantly innervates the ventral IPN (Figures 1B–1E). In the ventral and intermediate IPN, habenular axons surround IPN cell bodies that are predominantly located at the midline central core of the nucleus (Figures 1F and 1G). This organization is reminiscent of the structure of an electromagnetic coil and probably results from the spiraling of individual habenular axons at the midline [8].
Figure 1. Laterotopic Projections from the Habenular Nuclei to the IPN.
(A) Schematic illustration of a lateral view of an adult zebrafish brain showing the telencephalo-habenulo-interpeduncular pathway that connects the telencephalon with the ventral midbrain.
(B–G) Confocal images of sections of brains of adult fish in which left habenular axons are anterogradely labeled with DiO (green) and right habenular axons are labeled with DiI (red).
(B and C) Transverse sections at the level of the habenulae (B) and the IPN (C) showing left habenular axons (green) terminating in the dorsal IPN and right habenular axons (red) terminating in the ventral IPN.
(D and E) Low- (D) and high (E)-magnification views of a parasagittal section showing axonal projections from the right habenula (red) through the FR to the IPN. Within the IPN, dorsal and intermediate regions (green) are predominantly innervated by axons from the left habenula.
(F and G) Horizontal sections showing projections from the left habenula (green) terminating in the intermediate IPN (F) and projections from the right habenula (red) terminating in the ventral IPN (G). In both cases, the labeled axons surround the fluorescent Nissl-stained cell bodies (blue) at the center of the nucleus.
(H) Dorsal view of the intact ventral midbrain in a 5-day-postfertilization (dpf) larva, showing a 3D reconstruction of projections from the left (green) and right (red) habenulae. The oculomotor nucleus is visualized with a foxD3:GFP transgene (blue). Some FR axons on both left and right sides bypass the IPN and terminate more caudally (arrows).
(I and J) Transverse sections through the IPN of 4-dpf larvae showing projections from the left (I) and right (J) habenulae labeled with photoconverted DiI.
The small size of the brain in fish larvae allowed us to reconstruct the entire IPN and adjacent regions, further clarifying the projection patterns of the left and right habenulae. Consistent with analyses in adults, the dorsal IPN is innervated almost exclusively by left habenular axons, whereas the ventral IPN is innervated by right and, to a lesser extent, left habenular axons (Figures 1H–1J). Minor innervation of the ventral regions of the IPN by left habenular axons also occurs in adults (data not shown). Prior to entering the IPN, some left and right habenular axons (arrows in Figure 1H) pass lateral to the IPN and terminate in midline regions of the anterior hindbrain (Figure 1H; see Supplemental Data available with this article online), the location of the rostral subdivision of the serotonergic raphe nuclei [9]. Overall, our results reveal that the efferent circuitry of the habenular nuclei contain a conspicuous asymmetry whereby projections from the left and right sides of the brain are mapped in a laterotopic manner along the dorso-ventral (DV) axis of the IPN.
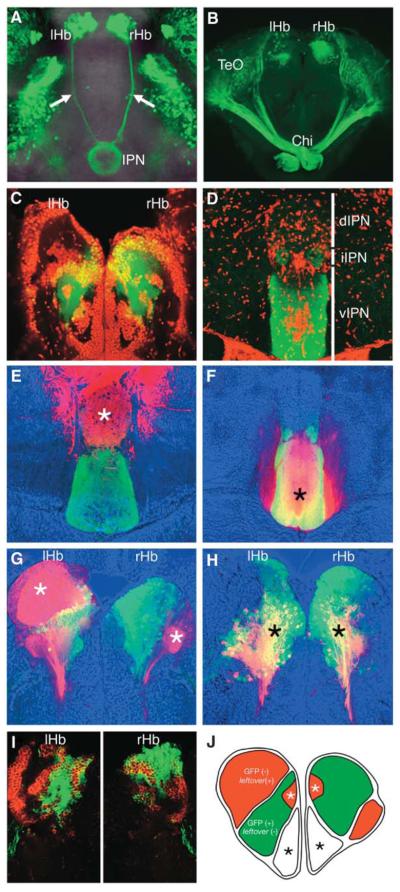
The habenulae are divided into sub-nuclei that are frequently asymmetric between left and right sides [10]. This raises the possibility that the dorso-ventrally asymmetric innervation of the IPN may reflect LR asymmetries within the habenular sub-nuclei. We addressed this hypothesis by mapping the localization within the habenulae of neurons projecting to different regions of the IPN. To facilitate this analysis, we generated a transgenic line, Tg(brn3a-hsp70:GFP), that expresses green fluorescent protein (GFP) under the control of enhancer elements of brn3a, a POU-domain transcription-factor-encoding gene expressed in the habenula (Figure 2A) [11]. In this line, GFP is expressed in sub-populations of habenular neurons and their axons (Figure 2A). GFP-positive cells occupy the medial sub-region of the habenula, and the domain of expression is larger in the right habenula than in the left (Figures 2B, 2C, and 2J). The axons from the GFP-positive neurons selectively terminate in the ventral and intermediate IPN (Figure 2D). To determine the origin of dorsal IPN innervation, we retrogradely labeled neurons from their dorsally positioned IPN terminals in Tg(brn3a-hsp70:GFP) fish (Figure 2E; n = 5). This revealed that the vast majority of neurons that innervate the dorsal IPN are located in the lateral, GFP-negative sub-region of the left habenula (Figure 2G). A small number of neurons in the lateral sub-region of the right habenula also innervate the dorsal IPN, and again these neurons are GFP negative (Figure 2G). When we labeled neurons from their ventrally positioned IPN terminals (Figure 2F; n = 5), retrogradely labeled cell bodies were confined to the medial, GFP-positive sub-nuclei on both sides (Figure 2H). The lateral sub-nuclei of the habenulae can be further distinguished by expression of leftover (lov) [4], which is largely complementary to the GFP-positive medial sub-nucleus (Figures 2I and 2J).
Figure 2. Asymmetries between Left and Right Habenular Sub-Nuclei Account for the Laterotopy of Projections to the IPN.
(A) Dorsal view of a brain of a 4-dpf Tg(brn3a-hsp70:GFP) larva showing GFP labeling of the entire length of the habenulo-interpeduncular projection (arrows).
(B) Frontal view of an adult brain from a Tg(brn3a-hsp70:GFP) fish. (C and D) Transverse sections through the habenulae (C) and the IPN (D) of an adult Tg(brn3a-hsp70:GFP) fish showing GFP expression (green) and SYTOX orange counter staining of cellular nuclei (red). (E–H) Transverse sections through the habenulae (G and H) and IPN (E and F) of adult Tg(brn3a-hsp70:GFP) fish in which DiI was applied to the dIPN (E and G) or vIPN (F and H). DiI application sites and retrogradely labeled cells are indicated by asterisks.
(I) Expression pattern of lov mRNA (red) in the habenulae of an adult Tg(brn3a-hsp70:GFP) fish.
(J) Summary schematic of habenular organization indicating brn3a-hsp70:GFP expression (green) and lov (red) in restricted sub-nuclei.
Two additional, bilaterally symmetric sub-nuclei (asterisks) are present in the dorso-medial and ventro-medial regions of the habenulae. Abbreviation: Chi, optic chiasm.
These results indicate that the habenular nuclei are subdivided into at least two asymmetric sub-nuclei that have different size ratios on the left and right sides of the brain and different targets in the IPN. The large medial habenular sub-nucleus on the right innervates the ventral and intermediate IPN together with axons from the small medial sub-nucleus on the left. Conversely, the large lateral sub-nucleus on the left is the predominant source of axons that innervate the dorsal IPN, with axons from the small lateral sub-nucleus on the right. We therefore conclude that LR asymmetries in habenular sub-nuclear organization are converted to DV asymmetries in habenular projection to the IPN.
Next, we investigated the mechanisms by which asymmetric innervation of the IPN is established. The Nodal signaling pathway regulates laterality decisions both in the viscera [12] and within the CNS [3, 13], and so this pathway is a candidate for directing laterotopic projections to the IPN. Indeed, several Nodal-pathway genes are expressed in precursors of both the left habenula and the parapineal organ on the left side of the epithalamus, and Nodal signals can act locally to influence the laterality of brain structures [3]. We therefore decided to examine habenular sub-nuclear organization and the pattern of innervation of the IPN in embryos with compromised Nodal signaling in the brain. To do this, we assayed habenular patterning and projections in mutants in which Nodal signaling is reversed, absent, or bilateral within the brain.
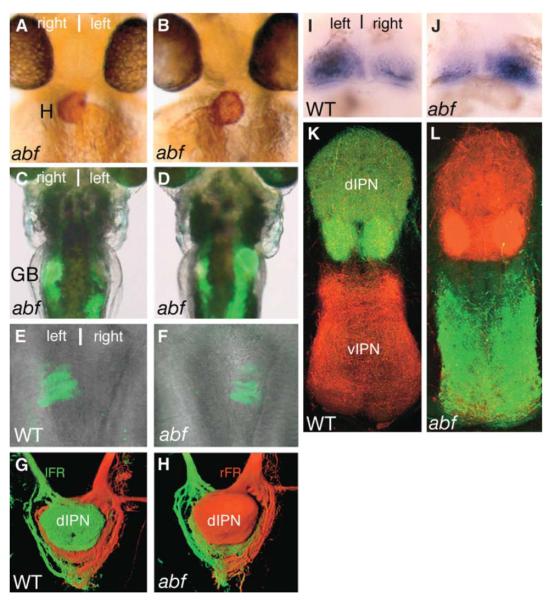
about face (abf) is a novel mutation that leads to randomized laterality of heart looping (D-loop = 50.5%, L-loop = 49.5%; n = 97; Figures 3A and 3B) and positioning of the gall bladder (right = 47.4%, left = 52.6%; n = 97; Figures 3C and 3D) in putative homozygotes. A GFP transgene that is driven by enhancer elements of the Nodal-pathway target gene lefty1 [14] and is activated on the left side of the brain in wild-type embryos (Figure 3E) shows altered expression in abf mutants with reversed heart looping. In about half of the heart-reversed abf embryos, the lefty1:GFP transgene was activated on the right side of the brain (56%, n = 16; Figure 3F), whereas expression was absent in the rest (44%; data not shown). In larvae that show reversed GFP expression, there was always complete DV inversion of habenular axon terminals in the IPN, with left neurons projecting ventrally and right neurons projecting predominantly dorsally (Figures 3G, 3H, and 4L). Furthermore, the asymmetric localization of lov expression was reversed in the majority of abf mutant larvae with reversed heart looping (87.5%; n = 48; Figures 3I and 3J). Heart-reversed abf larvae that lacked GFP expression in the brain still showed DV segregation of habenular axon terminals in the IPN, but the orientation of the laterotopic projection pattern was randomized (Figure 4L). Observations in adult abf fish are consistent with data from analysis of larvae. The right habenula showed a connectivity pattern normally associated with the left habenula in 7 of 8 adult fish presenting reversed heart looping (Figures 3K and 3L). Although the LR origin of axons innervating dorsal and ventral regions of the IPN was frequently reversed, the DV polarity and structure of the IPN appeared unaffected by the abf mutation (Figures 3K and 3L).
Figure 3. Reversal of lefty1:GFP Activation Correlates with Reversal of Habenular Laterality and Inversion of Projections in abf Fish.
(A–D) abf embryos and larvae having normal (A and C) and reversed (B and D) laterality, shown by anti-light meromyosin antibody (MF20) labeling of the heart (brown in [A] and [B], frontal views) and BODIPY FL-C5 labeling of the gall bladder (green in [C] and [D], ventral views). (A and B) 48 hr postfertilization (hpf); (C and D) 4.5 dpf.
(E and F) Dorsal views of live wild-type; Tg(lefty1:GFP) (E) and abf;Tg(lefty1:GFP) (F) 28-hpf embryos showing expression of GFP in the left epithalamus (E) and in the right epithalamus (F).
(G and H) Dorsal views of 3D reconstructions of confocal images of habenular projections in the IPN of a wild-type 5-dpf larva (G) and an abf 5-dpf larva with reversed lefty1:GFP expression (H). Axons from the left habenula have been labeled with DiA (green), and those from the right habenula have been labeled with DiI (red).
(I and J) Dorsal views of the habenulae of a wild-type (I) and a heart-reversed abf mutant (J) 4-dpf larva showing prominent lov expression in the left habenula of the wild-type and in the right habenula of the heart-reversed abf mutant.
(K and L) Confocal images of transverse sections of the IPN of a wild-type adult fish (K) and a heart-reversed abf fish (L) in which DiA (green) was applied to the left and DiI (red) to the right habenula.
Abbreviations: H, heart; GB, gall bladder; and WT, wild-type.
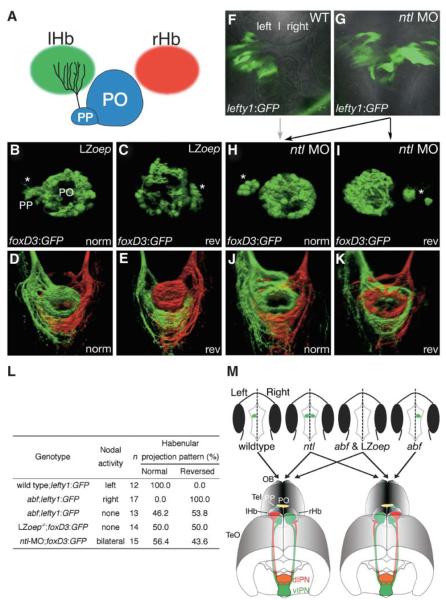
Figure 4. Laterotopic Habenular Projections Are Still Established in Embryos Laking Lateralized Nodal Activity in the Epithalamus.
(A) A cartoon viewing dorsally onto the epithalamus showing the parapineal organ innervating the left habenula. (B–K) LZoep−/−;Tg(foxD3:GFP) (B–E), wild-type (F), and ntl morphant (G–K) embryos and larvae in which Nodal-pathway activity (F and G) or parapineal (asterisks) laterality (B, C, H, I) was assessed by GFP-transgene (indicated bottom left) expression and left and right habenular projections were subsequently assessed by DiI and DiA labeling at 4 dpf (D, E, J, and K). LZoep−/− embryos show an equal number of cases of parapineal migration to the left (B) and to the right (C). Position of the parapineal is concordant with the orientation of laterotopic innervation of the IPN (D and E). The left-sided activation of lefty1:GFP in the wild-type embryo (F) is normally associated with subsequent migration of the parapineal organ to the same side of the brain (indicated by the gray arrow). lefty1:GFP is activated bilaterally in ntl morphants (G), leading to randomization of parapineal position (black arrows to [H] and [I]), which is 100% concordant with the orientation of laterotopic habenular connectivity (J and K). (L) Orientation of laterotopic habenular projections in mutants and morphants that are defective in the expression of Nodal-pathway genes in the epithalamus. Habenular projection patterns were assessed in 4-dpf larvae by carbocyanine-dye axon tracing. The orientation of laterotopy is described as “normal” when left habenular neurons innervate the dorsal IPN and as “reversed” when right-sided axons terminate in the dorsal IPN. (M) Nodal activity determines the orientation of laterotopy in habenulo-interpeduncular projections. The top panels are cartoons of dorsal views of late-somite-stage embryos showing activation of Nodal signaling (green) in the epithalamus. The bottom panels show sub-nuclear organization of the habenulae and laterotopic projections from the habenulae to the IPN in normal and reversed-laterality adult fish. In wild-type embryos, we propose that Nodal activity in the left epithalamus indirectly leads to expansion of the lateral sub-nucleus (red) in the left habenula and the medial sub-nucleus (green) in the right habenula, resulting in projection of the left habenula predominantly to the dorsal IPN and of the right habenula to the ventral IPN. This pattern of sub-nuclear organization is completely reversed (right lower panel) in those abf mutant embryos in which Nodal signaling is activated on the right side. When Nodal signaling is activated bilaterally (ntl) or is absent (abf; LZoep), habenular asymmetry is still established, but laterality of the sub-nuclear organization and consequently of the laterotopic innervation of the IPN becomes randomized.
Abbreviations: norm, normal laterality; PO, pineal organ; PP, parapineal; and rev, reversed laterality.
Analysis of abf fish shows that there is a 100% correlation between the laterality of lefty1:GFP activation and the projection pattern of habenular neurons. This is consistent with the hypothesis that Nodal signaling directly induces a pathway of sub-nuclear differentiation within the ipsilateral habenula and that this pathway leads to establishment of connectivity predominantly with dorsal IPN targets. However, laterotopic innervation of the IPN still occurred in abf embryos lacking lefty1:GFP expression, raising the possibility that Nodal signaling may not be essential for the establishment of laterotopic connectivity between the habenulae and the IPN. Such a possibility would be consistent with previous data showing that Nodal signaling is required for specifying laterality but not for establishing asymmetry of epithalamic structures [2-4].
To test the requirement for Nodal signals in the formation of laterotopic efferent connectivity with the IPN, we assessed habenular projection patterns in late-zygotic one-eyed pinhead (LZoep−/−) embryos [15] that lack Nodal activity in the brain [2, 5]. In these embryos, we scored epithalamic laterality by using a foxD3:GFP transgene [16] that labels the parapineal [3], a nucleus that is positioned adjacent to the habenula and innervates it with “left-sided” character (Figure 4A) [3, 4]. In LZoep−/−;Tg(foxD3:GFP) larvae, we observed randomization of parapineal position (50% left-sided, 50% right-sided, n = 54) and 100% correspondence between the LR laterality of the epithalamus and the DV segregation pattern of habenular axon terminals in the IPN (Figures 4B–4E and 4L). As in the abf mutant, there were no obvious alterations in the polarity and organization of the IPN of LZoep−/−;Tg(foxD3:GFP) larvae. It is therefore likely that habenular neurons are still able to differentiate and innervate the dorsal IPN in the absence of epithalamic Nodal signaling.
In wild-type and reversed abf mutants, unilateral Nodal signaling is associated with enhanced development of a lateral sub-nucleus within the ipsilateral habenula, which predominantly innervates the dorsal IPN. To assess if transient Nodal signaling is sufficient to impose connectivity to the dorsal IPN, we examined habenular connectivity in no tail (ntl) morphant Tg(foxD3:GFP) embryos in which the Nodal pathway is activated on both the left and right side of the brain (Figures 4F and 4G) [2, 3]. As in LZoep−/− embryos, parapineal position was randomized (56% left-sided, 44% right-sided, n = 55). When habenular projections were examined, we found the left and right habenulae were still asymmetric with respect to their efferent connectivity and innervated the IPN in a laterotopic manner where the DV orientation of habenular axon terminals showed 100% concordance with epithalamic laterality (Figures 4H–4L). These results indicate that transient bilateral Nodal pathway activation is not sufficient to impart the ability to innervate the dorsal IPN upon both left- and right-sided habenular neurons.
In this study, we describe lateralized neural circuits that are under genetic control. We have identified several fundamental steps in the development of this lateralized circuitry. These include the following: specification of medial and lateral habenular sub-nuclei containing distinct neuronal populations; the distinct and topo-graphic projection of axons from each sub-nucleus to either the ventral or the dorsal IPN; a role for the Nodal pathway in specifying the laterality of the asymmetric size ratio of the medial and lateral sub-nuclei and, consequently, a role in the DV innervation patterns of habenular axons in the IPN (Figure 4M). Although much remains to be learned about each of these steps, there are several obvious directions for future investigation. For instance, the establishment of stereotypic and topographic projections from different sub-groups of spinal motor neurons is dependent upon different combinations of LIM-homeodomain transcription factors [17, 18]. Transcription factors in this class are good candidates for mediating the sub-nuclear regionalization of the habenulae.
Fish are among the vertebrates that show the most pronounced asymmetries in the epithalamus [10]. However, all species show sub-nuclear regionalization of the habenulae. We show that lateralization works by regulating the reciprocal expansion or contraction of medial and lateral sub-nuclei rather than by specifying unique structures on left and right sides. This allows for variation between species, dependent upon the extent of lateralization, without the need for a complete redefinition of neuronal nuclei in lateralized and non-lateralized species. Indeed, in the less-lateralized rat epithalamus, it is likely that similar sub-nuclei with discrete IPN targets exist, albeit without pronounced size differences between left and right sides [19].
Perhaps the most novel aspect of the circuitry that we describe is the transposition of information coded on left and right sides of the brain to a bilateral representation along the DV axis of the target nucleus. This provides an elegant and very simple mechanism by which left- and right-sided brain regions can differentially activate bilaterally positioned targets. Given that functional lateralization is a very common feature of the vertebrate CNS, it will be of interest to determine if LR to DV conversion is used in other locations where there is asymmetric processing of information.
Our results show that unilateral, left-sided, transient activation of Nodal signaling is required to impart correct laterality to the medial and lateral habenular sub-nuclei and for the correct topography of the laterotopic projections from the habenulae to the IPN. This is consistent with previous studies showing that Nodal signaling specifies the laterality of epithalamic structures but does not generate asymmetry per se [2, 3, 5]. Indeed, laterotopic projections are still formed in the absence of Nodal signaling, and mutants that show right-sided Nodal-pathway activation, reversed epithalamic laterality, and inverted FR projections are viable. Why then might mechanisms exist to ensure the coordination of laterality of neuronal circuits between different individuals of the same species? One possibility is that behavioral asymmetries with consistent laterality may lead to behavioral coordination among individuals and confer a survival advantage within animal populations [20, 21]. This would support the idea that the asymmetric habenular circuitry described in this study, which shows consistent laterality in wild-type fish, influences fish social behavior.
Although a role for FR projections in lateralized behaviors has not, to date, been investigated, such a possibility would be consistent with roles for limbic-system output pathways in the modulation of many potentially emotional and/or socially based behaviors. In fish, it is likely that many social and lateralized behaviors involve preferential selection of eye-field/hemisphere selection for specific visually led tasks [22, 23], and it will be important to determine if the telencephalon-habenula-IPN pathway influences such behaviors. Analysis of the efferent connectivity of the dorsal and ventral IPN, particularly to monoaminergic nuclei in the ventral midbrain and hindbrain, which are influenced by habenular output pathways [19, 24-26], may help elucidate the neuroanatomical basis for lateralized behaviors potentially conserved throughout vertebrate evolution.
Acknowledgments
We thank members of our laboratories for discussion of this work, Christine Thisse for lefty1 probes, and Darren T. Gilmour for the Tg(foxD3:GFP) line. This research was supported by grants from RIKEN Brain Science Institute, from Core Research for Evolutional Science and Technology (CREST) (J.S.T.), from the Special Coordination Fund from the Ministry of Education, Science, Technology, Sports and Culture of Japan (H.O.), and from the Wellcome Trust, Biotechnology and Biological Sciences Research Council, and the European Community (S.W.). T.H. and T.M. were Junior Research Associates of RIKEN. M.C. is a Wellcome Trust International Research Development Award Fellow, also funded by the Fondo Nacional de Desarrollo Cientifico y Tecnológico (FONDECYT) 1020902. S.W. is a Wellcome Trust Senior Research Fellow, and I.B. is funded through a Wellcome Trust PhD program in Neuroscience.
Abbreviations
- III
oculomotor nucleus
- Cbll
cerebellum
- d
dorsal
- FR
fasciculus retroflexus
- Hb
habenula
- Hy
hypothalamus
- i
intermediate
- l
left
- OB
olfactory bulb
- r
right
- SM
stria medullaris
- Tel
telencephalon
- TeO
optic tectum
- v
ventral
Note Added in Proof
Figures 1I, 1J, and 3I have been slightly modified since the IEP version of this paper. Arrows were added to Figures 1I and 1J, and “left/right” was added to Figure 3I.
Footnotes
Supplemental Data Supplemental Experimental Procedures and an additional figure are available with this article online at http://www.current-biology.com/cgi/content/full/15/3/238/DC1/.
References
- 1.Sutherland RJ. The dorsal diencephalic conduction system: A review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 2.Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. doi: 10.1016/s0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 3.Concha ML, Russell C, Regan JC, Tawk M, Sidi S, Gilmour DT, Kapsimali M, Sumoy L, Goldstone K, Amaya E, et al. Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron. 2003;39:423–438. doi: 10.1016/s0896-6273(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 4.Gamse JT, Thisse C, Thisse B, Halpern ME. The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development. 2003;130:1059–1068. doi: 10.1242/dev.00270. [DOI] [PubMed] [Google Scholar]
- 5.Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101–5112. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- 6.Dax M. Lésions de la moitié gauche de l'encéphale coincidant avec trouble des signes de la pensée (lu au Congrès Méridional tenu à Montpelier en 1836) (2nd ser.).Gaz Hebd Méd Chir. 1865;2:259–260. [Google Scholar]
- 7.Gazzaniga MS, Bogen E, Sperry RW. Laterality effects in somesthesis following cerebral commissurotomy in man. Neuropsychologia. 1963;1:209–215. [Google Scholar]
- 8.Herrick CJ. The brain of the tiger salamander Ambystoma tigrinum. The University of Chicago Press; Chicago: 1948. [Google Scholar]
- 9.Teraoka H, Russell C, Regan J, Chandrasekhar A, Concha ML, Yokoyama R, Higashi K, Take-uchi M, Dong W, Hiraga T, et al. Hedgehog and Fgf signaling pathways regulate the development of tphR-expressing serotonergic raphe neurons in zebrafish embryos. J. Neurobiol. 2004;60:275–288. doi: 10.1002/neu.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J. Anat. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Treacy MN, Simmons DM, Ingraham HA, Swanson LW, Rosenfeld MG. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989;340:35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- 12.Hamada H, Meno C, Watanabe D, Saijoh Y. Establishment of vertebrate left-right asymmetry. Nat. Rev. Genet. 2002;3:103–113. doi: 10.1038/nrg732. [DOI] [PubMed] [Google Scholar]
- 13.Halpern ME, Liang JO, Gamse JT. Leaning to the left: Laterality in the zebrafish forebrain. Trends Neurosci. 2003;26:308–313. doi: 10.1016/S0166-2236(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 14.Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- 15.Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 16.Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34:577–588. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 18.Segawa H, Miyashita T, Hirate Y, Higashijima S, Chino N, Uyemura K, Kikuchi Y, Okamoto H. Functional repression of Islet-2 by disruption of complex with Ldb impairs peripheral axonal outgrowth in embryonic zebrafish. Neuron. 2001;30:423–436. doi: 10.1016/s0896-6273(01)00283-5. [DOI] [PubMed] [Google Scholar]
- 19.Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 20.Ghirlanda S, Vallortigara G. The evolution of brain lateralization: A game-theoretical analysis of population structure. Proc. R. Soc. Lond. B. Biol. Sci. 2004;271:853–857. doi: 10.1098/rspb.2003.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallortigara G, Rogers LJ, Bisazza A. Possible evolutionary origins of cognitive brain lateralization. Brain Res. Rev. 1999;30:164–175. doi: 10.1016/s0165-0173(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 22.Bisazza A, De santo A, Vallortigara G. Laterality and cooperation: Mosquitofish move closer to a predator when the companion is on their left side. Anim. Behav. 1999;57:1145–1149. doi: 10.1006/anbe.1998.1075. [DOI] [PubMed] [Google Scholar]
- 23.Miklosi A, Andrew RJ. Right eye use associated with decision to bite in zebrafish. Behav. Brain Res. 1999;105:199–205. doi: 10.1016/s0166-4328(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa T, Scatton B. Inhibitory influence of GABA on central serotonergic transmission. Involvement of the habenulo-raphe pathways in the GABAergic inhibition of ascending cerebral serotonergic neurons. Brain Res. 1985;331:81–90. doi: 10.1016/0006-8993(85)90717-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang RY, Aghajanian GK. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197:89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]