Summary
Asymmetries in CNS neuroanatomy are assumed to underlie the widespread cognitive and behavioral asymmetries in vertebrates. Studies in humans have shown that the laterality of some cognitive asymmetries is independent of the laterality of the viscera; discrete mechanisms may therefore regulate visceral and neural lateralization. However, through analysis of visceral, neuroanatomical, and behavioral asymmetries in the frequent-situs-inversus (fsi) line of zebrafish, we show that the principal left-right body asymmetries are coupled to certain brain asymmetries and lateralized behaviors. fsi fish with asymmetry defects show concordant reversal of heart, gut, and neuroanatomical asymmetries in the diencephalon. Moreover, the neuroanatomical reversals in reversed fsi fish correlate with reversal of some behavioral responses in both fry and adult fsi fish. Surprisingly, two behavioral asymmetries do not reverse, suggesting that at least two separable mechanisms must influence functional lateralization in the CNS. Partial reversal of CNS asymmetries may generate new behavioral phenotypes; supporting this idea, reversed fsi fry differ markedly from their normally lateralized siblings in their behavioral response to a novel visual feature. Revealing a link between visceral and brain asymmetry and lateralized behavior, our studies help to explain the complexity of the relationship between the lateralities of visceral and neural asymmetries.
Results and Discussion
Although our understanding of the genetic mechanisms that establish visceral asymmetries has increased considerably in recent years, we still know very little about the development of neuroanatomical asymmetry [1] and its relation to functional lateralization in the CNS [2, 3]. Primarily from analysis of phenotypes of mutant zebrafish embryos, it is clear that the disruption of processes such as midline development disturbs the laterality of asymmetries both in the viscera and in the brain. However, most mutations result in randomization (heterotaxia phenotypes), rather than reversal (situs inversus phenotypes), of laterality [4, 5], and it is unclear if the events that specify the laterality of the viscera concordantly specify CNS laterality [1, 2, 6]. A hint that this might be the case has come from analysis of fish embryos; it has been proposed that Nodal-signaling-dependent events occurring in lateral-plate mesoderm might influence the laterality of the overlying CNS [7]. In this study, we explore whether there are indeed links among laterality of the viscera, neuroanatomical structures in the brain, and behavioral responses.
fsi Fry Frequently Exhibit Complete situs inversus
By screening families of fish harboring ENU-induced mutations, we identified a pair of fish that produced progeny in which heart looping was frequently reversed. The fsi line of fish derived from this pair has continued to exhibit situs inversus phenotypes over several generations. The frequency with which we observe embryos with laterality defects from sibling matings varies from about 5% to more than 25% of the clutch. Individual pairs show more consistency in the frequency of phenotypes, and laterality defects are uncommon when fsi fish are crossed to wild-type fish. These observations suggest a strong genetic influence upon the laterality phenotypes, although we have not determined why the penetrance is variable. The viability of fsi fish with laterality phenotypes does not differ noticeably from that of normally lateralized siblings.
Unlike many zebrafish laterality mutants that show heterotaxia of visceral organs and heart situs [5], fsi fry with laterality defects show complete situs inversus. For instance, cocktails of in-situ-hybridization probes showed that fsi fry with normal jogging of the heart to the left (left-hearted; LH) subsequently showed normal heart looping (morphology and cmlc2a expression, Figure 1G), right-sided positioning of the pancreas (insulin expression, n = 20; Figure 1E), and left-sided looping of the gut (foxa3 expression; n = 19; Figure 1C). In contrast, all right-hearted (RH) fsi embryos showed reversal of the heart (Figure 1H), positioning of the pancreas to the left (n = 17; Figure 1F), and right-looping of the gut (n = 19; Figure 1D). Thus, laterality of situs is concordant for heart, gut, and pancreas in fsi fry.
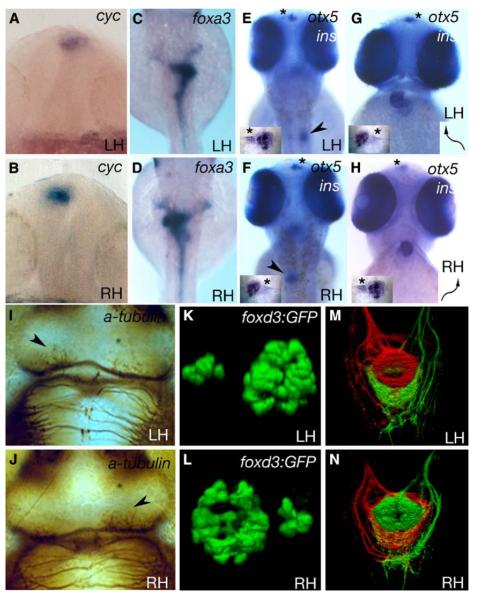
Figure 1.
Some fsi Embryos Show Complete Situs Inversus
Frontal (A, B, G, and H) and dorsal (C–F, I–N, and insets in [E]–[H]) views of LH and RH fsi fry.
(A and B) Epithalamic expression of cyc.
(C and D) Expression of foxa3 in the gut.
(E and F) insulin (pancreas, arrowheads in E,F) and otx5 expression (pineal and parapineal; inset, otx5 expression; asterisks indicate parapineal position).
(G and H) cmlc2a expression in the heart and otx5 in the pineal and parapineal nuclei (arrows indicate direction of heart looping; inset, otx5 expression; asterisks indicate the position of the parapineal nucleus). Note that the reversed position of the parapineal nucleus in E and G (LH) compared to F and H (RH) is due to different perspectives (dorsal versus frontal views). (I) and (J) show anti-acetylated tubulin labeling of the habenular neuropil (arrowheads indicate the nucleus with more robust labeling).
(K and L) 3D reconstructions of pineal (large nucleus) and parapineal (small nucleus) expression of a foxd3:GFP transgene.
In all cases, LH and RH fsi fry show opposite laterality of structures. ([A and B] 18–20 somites; [C–J] 2.5 dpf).
(M and N) Dorsal view of the intact ventral midbrain in 4 dpf LH and RH fsi fry; shown is a 3D reconstruction of projections from the left (red) and right (green) habenulae within the IPN. There is a DV inversion of projection patterns in the RH fsi fry.
fsi Fry Show Concordance between Visceral and Brain Asymmetries
We next assayed whether fsi fry show CNS asymmetry defects and, if so, whether these defects show concordance of laterality with visceral organs. The earliest known indication of CNS asymmetry is the left-sided dorsal diencephalic expression of genes functioning in the Nodal signaling pathway ([8, 9]; Figure 1A). Expression of cyc, the gene for the Nodal ligand [10], and pitx2, a gene for the Nodal-pathway target [11], was reversed in some 18- to 20-somite (18–19 hours post-fertilization [hpf]) fsi embryos (cyc: n = 26/60, Figure 1B; pitx2: n =7/18, data not shown). These data reveal early asymmetry defects in the CNS of some fsi fry, but because expression of early markers such as cyc and pitx2 ceases before heart situs can be reliably scored, a direct correlation of heart and epithalamic laterality was not possible with these markers.
Gene expression in the Nodal pathway presages later neuroanatomical asymmetries in the diencephalon; such asymmetries include the left-sided migration of the parapineal nucleus and the establishment of differences in the size, gene expression, and efferent projection pattern in left and right habenular nuclei [12-16]. The epithalamic habenular nuclei are components of the highly conserved output pathways of the limbic system [17] and have been implicated in a wide variety of functions (e.g., [18-20]). Habenular axons project to the ventral midbrain via the fasciculus retroflexus, where they innervate the interpeduncular nucleus (IPN), a structure closely associated with both serotonergic and dopaminergic circuits. In most, and perhaps all, vertebrates, asymmetries between the left and right sides of the epithalamus suggest that this region of the brain is likely to be involved in functional lateralization of the CNS [14].
To assay possible concordance between visceral and brain asymmetries, we examined the heart and brain of fsi embryos at 2.5 days post-fertilization [dpf]. In LH fsi fry, when differences were unequivocal, the left habenula showed more extensive neuropil (n = 13/25; Figure 1I; as in the wild-type, see [13]), whereas in RH fsi fry, it was the right habenula that had more pronounced neuropil labeling (8/15; Figure 1J). Neuropil labeling in all remaining LH and RH fry (12/25 LH and 7/15 RH) showed no pronounced differences between left and right sides at the stage examined. In no case did we observe an LH embryo in which the right habenular neuropil was more pronounced than the left or vice versa. Parapineal position gives a more definitive assay for diencephalic laterality, and so we assayed this by expression of otx5 [15] and a foxd3:GFP transgene [12, 21]. Expression of otx5 and foxd3:GFP in LH fsi embryos showed the parapineal nucleus to the left of the pineal nucleus in the vast majority of embryos (otx5; n = 20/20 and foxd3:GFP, n = 213/231 respectively; Figures 1E, 1G, and 1K), whereas in most RH fsi fry, the parapineal nucleus was positioned to the right (n = 17/17 and 216/241 respectively; Figures 1F, 1H, and 1L). Using combined data from foxd3:GFP and otx5 expression in RH fsi fry, we found that the overall percentage of concordance was about 91% (binomial test, z = 17.73, p << 0.001). At about 9%, the incidence of nonconcordant embryos is slightly elevated from the level of heterotaxia seen in most wild-type genetic backgrounds [5].
Recent studies have identified LR asymmetries in the efferent connectivity of the habenular nuclei with their target, the IPN [16]. In wild-type fish, the left habenula predominantly innervates the dorsal IPN, whereas the right habenula innervates the ventral IPN. We therefore assessed heart position (morphology), parapineal-nucleus position (foxd3:GFP expression) and IPN innervation patterns (diI and diASP labeling) in LH (n = 4) and RH (n = 8) fsi fry. In all cases, the concordant laterality of the heart and the parapineal nucleus predicted the dorsoventral (DV) segregation of habenular axons in the IPN (Figures 1M and 1N). Thus, reversal of laterality correlates with DV inversion of habenular axon projections in fsi fry.
Together, these results show that although the percentage of RH fsi embryos was variable for different clutches and pairs, concordance of laterality phenotypes was seen within both LH and RH groups of fsi fry. Therefore, scoring living fsi fry on the basis of heart position and/or laterality of foxd3:GFP transgene expression allows, in the great majority of cases, selection of fry showing either completely normal or completely reversed visceral and brain asymmetries.
Behavioral Analysis
Evidence from analysis of humans suggests that reversal of visceral situs is not accompanied by reversal of language and hearing lateralization or of handedness [22-24]. However, the relationship between visceral and CNS asymmetry remains poorly understood. There are very few examples in which there is a known concordance between visceral and neuroanatomical asymmetries (reviewed in [1, 2, 7]), and so fsi fish offer an excellent opportunity to explore the relationship of visceral situs, brain asymmetry, and lateralized behaviors. For these analyses, we compared groups of normal (LH) and reversed (RH) fsi fish in a variety of behavioral tests, most of which have previously been shown to reveal laterality biases in wild-type zebrafish. For instance, adult zebrafish use the right eye preferentially when controlling a response, such as approaching and biting a target [25, 26], whereas they use the left eye when assessing change from past experience, i.e., when they have seen an object or scene before [27]. At least some functional lateralization is also evident in very young fry ([28] and see below).
RH fsi Fry Show Reversal of Behavior in a Mirror-Viewing Test
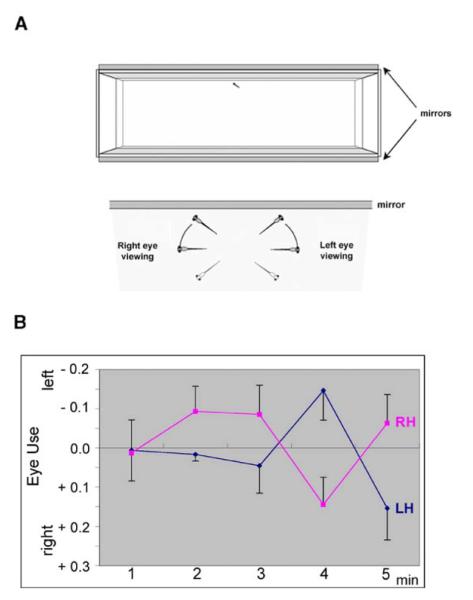
A mirror test that has been previously used for laterality studies in adult teleosts [29] was adapted for fsi fry. In this test, fish view their reflection for the first time, and this is assumed to model responses to conspecifics [30, 31]. When individual 8-day-old fry view their reflection for the first time (Figure 2A), they show changes in predominant eye usage, with a characteristic timing of shifts during the first 5 min. The most marked shift seen in group-reared wild-type fry is usually from the left to the right eye between minute four and minute five (data not shown). LH fsi fry show the same left-to-right eye shift at this time point, whereas the shift is reversed in RH fsi fry (Figure 2B). The entire pattern of shifts in eye use over the first 5 min of mirror viewing is fully reversed in RH compared to LH fsi fry. As a result, the pattern of changing eye use over time (as summarized by the shapes of the two curves) differs significantly. This is shown by the interactions involving changes over time and differences in laterality (assessment of interactions of Time by Laterality: F4,260 = 3.725, p = 0.006) and the cubic relationship between the two curves describing eye usage (F1,65 = 12.306, p = 0.001; for statistical analyses see the Supplemental Experimental Procedures available with this article online).
Figure 2.
LH and RH fsi Fry Show Opposite Eye Use While Viewing Their Own Reflection for the First Time
(A) Schematic representation of the mirror tank and scoring system used for testing.
(B) Eye use by LH and RH fsi fry during mirror viewing. For each minute of viewing, scores were calculated as the total duration of right-eye use minus the total duration of left-eye use divided by the combined total of right and left use. The mean of relative eye use and the standard error are shown.
When there is a clear bias in eye use during the first 2 or 3 min in wild-type fry, the initially preferred eye is used again in minute five (data not shown). Here, a regression analysis of minute-one scores on minute-five scores, across LH and RH fsi fry, was positive and significant (F1,65 = 4.563, p = 0.036), showing that this feature of eye use also holds for fsi fish. The reflection has novel properties, such as movement, whenever the fry moves but has no new properties at any other time. When the fish views such a reflection, it presumably calls for assessment by comparison with earlier experiences. The eye and associated central circuitry that is more appropriate for this task is presumably that which is used initially. This same circuitry is used again in the fifth minute. However, the fact that the opposite eye and associated central circuitry is consistently used in minute four may allow it, also, to play a role in assessment, despite the overall bias against its use.
Adult RH fsi Fish Show Reversal of Behavior When they Approach a Target to Bite
Adult wild-type fish show a bias for right eye use when approaching a target that they have been trained to bite, and when presented with two equivalent targets, they will prefer the one on the right [25, 26]. In the two-choice bead test, adult LH fsi fish, like wild-type fish, take a right-sided route to the target. Conversely, RH fsi fish are reversed and prefer a left-sided approach (Wilcoxon-Mann-Whitney, n1n2 = 8,10, U = 11.5, p = 0.008; see Supplemental Data). Moreover, in the same test, the laterality of selection between two presented targets is also reversed; LH fsi fish choose to bite the right target, and RH fsi fish bite the left target (Wilcoxon-Mann-Whitney, n1n2 = 8,11, U = 18, p = 0.037).
These results indicate that both for mirror viewing and for decision making with respect to biting, RH fsi fish show reversed eye use and behavior when they are compared to LH fsi and wild-type fish. Wild-type adult zebrafish preferentially use the right eye to control a planned motor response [25, 26], and so the reversals in RH fsi fish may represent a switch in the eye and associated central circuitry used for determining appropriate responses. Young fry show no schooling (or other association) behavior, and one consequence of identifying a conspecific may be that startle responses to the frequent, unpredictable movements of young fry are inhibited. One would expect such inhibition to be predominantly regulated by the system that is fed by the eye concerned with controlling response.
In other models, it has proven surprisingly difficult to correlate structural neuroanatomical and behavioral reversals of laterality. For instance, in humans with situs inversus totalis, there is reversal of certain cortical-size differences but little evidence of reversal of hand use or language localization (reviewed in [1]). However, in fsi fry, the laterality of asymmetric neural structures predicts the laterality of behavioral response.
LH and RH fsi Fry Turn in the Same Direction When They Emerge into a Novel Environment and When They Are Startled
In the tests described above, LH and RH fsi fish exhibit similar behaviors but with reversed laterality biases. However, additional tests using a multi-chambered swimway (Figure 3A) revealed that not all lateralized behaviors are reversed in RH fsi fry. In the swimway, 8-day-old fry show positive phototaxis and will therefore emerge from a darkened chamber through a narrow slit into the more brightly lit chamber. Latency of emergence, direction, and angle of turn can all be measured as the fry enters the novel environment. When wild-type zebrafish fry emerge from the start chamber under positive phototaxis, they either turn left or show no clear bias [28]. This turning bias fades after the first emergence. Surprisingly, both RH and LH fsi fry showed the same bias in direction of turn as did wild-type fry (Figure 3B; Wilcoxon signed ranks: LH, N = 60, T+ = 1191.5, z = 2.035, p = 0.042; RH, N = 25, T+ = 251, z = 2.382, p = 0.017). Indeed, there was no significant difference in behavior between RH and LH fsi fry in this test, and as with wild-type fry, the bias in turning was not significant after the first emergence.
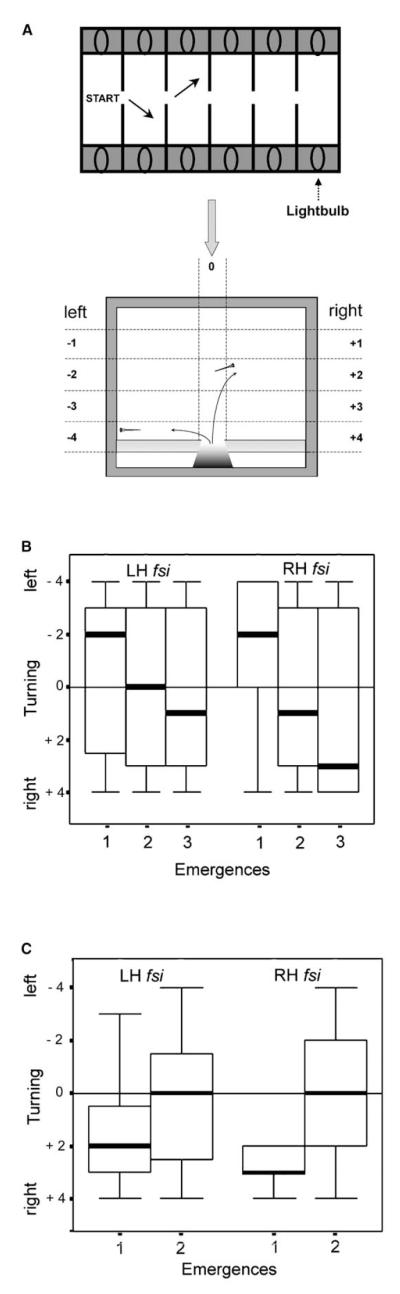
Figure 3.
LH and RH fsi Fry Show the Same Directionality Biases When Turning in a Swimway
(A) Drawing of swimway tanks (arrows indicate turning movements of fry) and scoring scheme for directionality and angle of turning upon emergence. The turning scores ranged from 1 (shallow turn) to 4 (immediately leaving the medial strip by a sharp turn toward left or right), indicated by horizontal dashed lines. Scores for left turns are negative and for right turns, positive. A score of 0 indicates fry that stayed within the medial strip from entry to the far wall (indicated by the vertical division). The panel was adapted from Watkins et al. [28].
(B) Turning scores in the first three emergences after gradual changes in illumination between chambers shows left-turn bias on first emergence for both LH and RH fsi fry. The box plots show the medians (heavy lines) and distribution across the two quartiles above and below the median representing 50% of the data. Vertical lines extend to the smallest and largest values excluding outliers.
(C) Turning scores in the first two emergences after a visual startle stimulus due to a sudden extinction of light in the compartment from which they had emerged and a sudden increase of light in the next compartment. Turning bias is to the right for both groups on first emergence, opposite to that in the no-startle-stimulus condition.
Adult fish tend to use the left eye for tasks requiring assessment of identity with a past experience [27]. It is therefore possible that fry turn toward the visual hemisphere that has been more adequately assessed by comparison with the start compartment. A complementary explanation would be that the left eye system is more effective at assessing spatial layouts. For instance, in the domestic chick, use of the left eye gives an advantage in assessing geometric layouts [32, 33] as well as degree of novelty [34], and in the rat it gives an advantage in maze learning [35].
In contrast to the behavior described above, wild-type fry preferentially turn right upon emergence when they are startled by the sudden extinction of the light in the start chamber (rather than the slow dimming used in the previous test). This bias toward turning to the right is significant in both LH and RH fsi fry at the first emergence (Figure 3C; Wilcoxon signed ranks: LH, N = 19, T+ = 151, p = 0.024; RH, N = 15, T+ = 107.5, p = 0.005). This behavioral asymmetry almost certainly depends on a mechanism different from that which causes the fish to turn left when it is not startled. Bias after a startle stimulus is likely to involve hindbrain mechanisms such as Mauthner neurons and associated reticulospinal and motor cells that initiate sudden locomotion as an escape response. In addition, in teleosts biases in the direction of escape can be imposed by visual structures, and this information is relayed via the Mauthner/reticulospinal circuits [36].
Together, these results indicate that the behavior of RH fsi fry cannot be explained by a single switch in the lateralization of CNS function because not all behavioral asymmetries are reversed in RH fsi fry. This implies that two or more mechanisms influence CNS lateralization and that not all such mechanisms are reversed in RH fsi fry.
LH and RH fsi Fry Show Different Latencies When They Confront a Novel Object upon Emergence
In all tests described so far, the behaviors exhibited by RH and LH fsi fish are qualitatively similar, albeit that in some tests there is reversal of laterality. However, given that RH fsi fish show a reassignment of some but not all functional asymmetries, one might expect that this could lead to novel behavioral responses by bringing together functions that are normally separated between the two sides of the brain. Further analysis of fsi fry behavior in a different swimway test suggests that this might indeed be the case.
In the two swimway tests described above (Figure 3A), LH and RH fsi fry did not differ in latency of emergence (data not shown). However, differences appeared when fry saw a conspicuous novel black stripe directly ahead in the compartment that they subsequently entered. In the first emergence, LH and RH fsi fry both showed the short latencies characteristic of tests without a stripe. Thereafter, LH fsi fry showed progressively increasing latencies, with most LH fry failing to emerge into the third compartment (Figure 4). In contrast, RH fsi fry showed considerably less increase in latency and so emerged much more quickly than LH fsi fry when entering the second or third compartment. The overall increase of latency in LH relative to RH fsi fry was significant in this swimway test (F1.31 = 9.008, p = 0.005). Furthermore, starting from equivalence in timing in the first emergence, the increasing rises in latency in LH relative to RH fsi fry in the second and third emergence produced significant differences in the pattern of change in behavioral responses over time (Time by Laterality: linear component, F1,31 = 6.618, p = 0.015; quadratic component, F1,31 = 5.783, p = 0.022).
Figure 4.

LH and RH fsi Fry Show Different Latencies of Emergence When Confronted with a Conspicuous Vertical Black Stripe
Graph showing latency of emergences. The first latency of emergence is similar for both LH and RH fsi fry (and similar to the emergence time in the absence of the black stripe). However, on successive emergences, latencies increase significantly for LH but not for RH fsi fry. Means and standard errors of the mean are shown.
The similar behavior of LH and RH fsi fry in the first emergence suggests that there are no differences in positive phototaxis or overall responsiveness. Rather, RH fsi fry show a stronger persistence in their initially chosen motor strategy in the course of successive emergences. This persistence in emergence of RH fsi fry despite the presence of a conspicuous novel feature may reflect competition between left-eye control of response and assessment of novelty. The failure of RH fsi fish to delay their emergence suggests interference with the examination of the compartment ahead, and hence of assessment of the novel feature.
RH fsi fish are not the only example of vertebrates with partial reversals of CNS lateralization, and indeed, human handedness shows this feature. Right-handers show a right-hemisphere advantage in visual spatial tasks and a left-hemisphere advantage in analysis required for the control of response (categorization of terms such as of “above or below”). In left-handers, categorization shifts to the right hemisphere, which retains its role in mediating spatial analysis [37]. It remains unclear whether any general effects this shift has on behavior are comparable to the changes described for the zebrafish. However, in the chimpanzee and in rhesus monkeys, left-handedness is associated with differences in nervousness and dominance [38, 39].
A Link between Visceral, Neuroanatomical, and Behavioral Lateralization
Our studies reveal a strong correlation between the laterality of the epithalamus and that of two asymmetric behaviors that involve the control of response to visual stimuli. Is it likely, therefore, that epithalamic circuits might directly be involved in these (and other) lateralized behavioral responses? The habenular nuclei are part of an evolutionarily highly conserved conduction pathway that connects telencephalic nuclei to the midbrain IPN in the limbic system [17]. Despite the evolutionary conservation of this pathway, surprisingly little is known about its specific functions. However, in mammals, the habenular nuclei are certainly involved in the control of response, at least in part through modulation of dopaminergic levels (e.g., [40]). Left- and right-sided habenular sub-nuclei in zebrafish have very discrete efferent projection patterns, making it very likely that they activate different downstream circuitry [16], and so we predict that the fish epithalamus is indeed likely to be an important regulator of lateralized CNS function.
In summary, despite the widely held opinion that functional lateralization is independent of body situs (discussed in [1, 2, 6]), our studies reveal an unequivocal link between the laterality of visceral and neuroanatomical asymmetries and behavioral lateralization in zebrafish. However, we find that not all behavioral asymmetries are concordant with visceral/neuroanatomical asymmetries. The zebrafish therefore is an excellent model system in which to begin to dissect the different components and developmental pathways that establish lateralized CNS function.
Acknowledgments
We thank members of our groups for helpful discussions, colleagues in the community for reagents, and anonymous reviewers for insightful comments and very helpful suggestions. R.J.A. was supported by the Wellcome Trust, S.W.W. by the Wellcome Trust and Biotechnology and Biological Sciences Research Council, A.M. by a Wellcome International Visiting Fellowship and the Hungarian Academy of Sciences (F01/031), and I.H.B. by a Wellcome Trust studentship.
Footnotes
Supplemental Data Supplemental Experimental Procedures are available with this article online at http://www.current-biology.com/cgi/content/full/15/9/844/DC1/.
References
- 1.Cooke J. Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biol. Rev. Camb. Philos. Soc. 2004;79:377–407. doi: 10.1017/s1464793103006298. [DOI] [PubMed] [Google Scholar]
- 2.Malashichev YB, Wassersug RJ. Left and right in the amphibian world: Which way to develop and where to turn? Bioessays. 2004;26:512–522. doi: 10.1002/bies.20036. [DOI] [PubMed] [Google Scholar]
- 3.Vallortigara G, Rogers LJ. Behavioural and Brain Sciences. Cambridge University Press; Cambridge: 2004. Survival with an Asymmetrical Brain: Advantages and Disadvantages of Cerebral Lateralization. [DOI] [PubMed] [Google Scholar]
- 4.Palmer AR. Symmetry breaking and the evolution of development. Science. 2004;306:828–833. doi: 10.1126/science.1103707. [DOI] [PubMed] [Google Scholar]
- 5.Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567–3579. doi: 10.1242/dev.127.16.3567. [DOI] [PubMed] [Google Scholar]
- 6.Levin M. Left-right asymmetry in embryonic development: A comprehensive review. Mech. Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130:2303–2316. doi: 10.1242/dev.00436. [DOI] [PubMed] [Google Scholar]
- 8.Halpern ME, Liang JO, Gamse JT. Leaning to the left: Laterality in the zebrafish forebrain. Trends Neurosci. 2003;26:308–313. doi: 10.1016/S0166-2236(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 9.Concha ML. The dorsal diencephalic conduction system of zebrafish as a model of vertebrate brain lateralisation. Neuroreport. 2004;15:1843–1846. doi: 10.1097/00001756-200408260-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- 11.Essner JJ, Branford WW, Zhang J, Yost HJ. Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development. 2000;127:1081–1093. doi: 10.1242/dev.127.5.1081. [DOI] [PubMed] [Google Scholar]
- 12.Concha ML, Russell C, Regan JC, Tawk M, Sidi S, Gilmour DT, Kapsimali M, Sumoy L, Goldstone K, Amaya E, et al. Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron. 2003;39:423–438. doi: 10.1016/s0896-6273(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 13.Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. doi: 10.1016/s0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 14.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J. Anat. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamse JT, Shen YC, Thisse C, Thisse B, Raymond PA, Halpern ME, Liang JO. Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nat. Genet. 2002;30:117–121. doi: 10.1038/ng793. [DOI] [PubMed] [Google Scholar]
- 16.Aizawa H, Bianco IH, Hamaoka T, Miyashita T, Uemura O, Concha ML, Russell C, Wilson SW, Okamoto H. A novel feature of CNS circuitry by which left-right information from the forebrain laterotopically represented along the dorso-ventral axis of a midbrain target nucleus. Curr. Biol. 2005;15:238–243. doi: 10.1016/j.cub.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland RJ. The dorsal diencephalic conduction system: A review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 18.Felton TM, Linton L, Rosenblatt JS, Morrell JI. Intact neurons of the lateral habenular nucleus are necessary for the nonhormonal, pup-mediated display of maternal behavior in sensitized virgin female rats. Behav. Neurosci. 1998;112:1458–1465. doi: 10.1037//0735-7044.112.6.1458. [DOI] [PubMed] [Google Scholar]
- 19.Valjakka A, Vartiainen J, Tuomisto L, Tuomisto JT, Olkkonen H, Airaksinen MM. The fasciculus retroflexus controls the integrity of REM sleep by supporting the generation of hippocampal theta rhythm and rapid eye movements in rats. Brain Res. Bull. 1998;47:171–184. doi: 10.1016/s0361-9230(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J. Comp. Physiol. Psychol. 1981;95:781–791. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- 21.Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34:577–588. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 22.Torgersen J. Situs inversus, asymmetry, and twinning. Am. J. Hum. Genet. 1950;2:361–370. [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy DN, O’Craven KM, Ticho BS, Goldstein AM, Makris N, Henson JW. Structural and functional brain asymmetries in human situs inversus totalis. Neurology. 1999;53:1260–1265. doi: 10.1212/wnl.53.6.1260. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Kanzaki R, Yoshibayashi M, Kamiya T, Sugishita M. Dichotic listening in patients with situs inversus: Brain asymmetry and situs asymmetry. Neuropsychologia. 1999;37:869–874. doi: 10.1016/s0028-3932(98)00144-4. [DOI] [PubMed] [Google Scholar]
- 25.Miklosi A, Andrew RJ. Right eye use associated with decision to bite in zebrafish. Behav. Brain Res. 1999;105:199–205. doi: 10.1016/s0166-4328(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 26.Miklosi A, Andrew RJ, Gasparini S. Role of right hemifield in visual control of approach to target in zebrafish. Behav. Brain Res. 2001;122:57–65. doi: 10.1016/s0166-4328(01)00167-x. [DOI] [PubMed] [Google Scholar]
- 27.Miklosi A, Andrew RJ, Savage H. Behavioural lateralisation of the tetrapod type in the zebrafish (Brachydanio rerio) Physiol. Behav. 1997;63:127–135. doi: 10.1016/s0031-9384(97)00418-6. [DOI] [PubMed] [Google Scholar]
- 28.Watkins J, Miklosi A, Andrew RJ. Early asymmetries in the behaviour of zebrafish larvae. Behav. Brain Res. 2004;151:177–183. doi: 10.1016/j.bbr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Sorvano VA, Rainoldi C, Bisazza A, Vallortigara G. Roots of brain specializations: Preferential left-eye use during mirror-image inspection in six species of teleost fish. Behav. Brain Res. 1999;106:175–180. doi: 10.1016/s0166-4328(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 30.Bisazza A, Facchin L, Vallortigara G. Heritability of lateralization in fish: Concordance of right-left asymmetry between parents and offspring. Neuropsychologia. 2000;38:907–912. doi: 10.1016/s0028-3932(00)00018-x. [DOI] [PubMed] [Google Scholar]
- 31.Bisazza A, De Santi A, Bonso S, Sovrano VA. Frogs and toads in front of a mirror: Lateralisation of response to social stimuli in tadpoles of five anuran species. Behav. Brain Res. 2002;134:417–424. doi: 10.1016/s0166-4328(02)00055-4. [DOI] [PubMed] [Google Scholar]
- 32.Tommasi L, Vallortigara G. Encoding of geometric and landmark information in the left and right hemispheres of the Avian Brain. Behav. Neurosci. 2001;115:602–613. [PubMed] [Google Scholar]
- 33.Vallortigara G, Pagni P, Sovrano VA. Separate geometric and non-geometric modules for spatial reorientation: Evidence from a lopsided animal brain. J. Cogn. Neurosci. 2004;16:390–400. doi: 10.1162/089892904322926737. [DOI] [PubMed] [Google Scholar]
- 34.Vallortigara G, Andrew RJ. Differential involvement of right and left hemispheres in individual recognition in the domestic chick. Behav Proc. 1991;33:41–59. doi: 10.1016/0376-6357(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 35.Cowell PE, Waters NS, Denenberg VH. The effects of early environment on the development of functional laterality in morris maze performance. Laterality. 1997;2:221–232. doi: 10.1080/713754274. [DOI] [PubMed] [Google Scholar]
- 36.Eaton RC, Emberley DS. How stimulus direction determines the trajectory of the Mauthner-initiated escape response in a teleost fish. J. Exp. Biol. 1991;161:469–487. doi: 10.1242/jeb.161.1.469. [DOI] [PubMed] [Google Scholar]
- 37.Jager G, Postma A. On the hemispheric specialization for categorical and coordinate spatial relations: A review of the current evidence. Neuropsychologia. 2003;41:504–515. doi: 10.1016/s0028-3932(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins WD, Bennett AJ. Handedness and approach-avoidance behavior in chimpanzees (Pan) J. Exp. Psychol. Anim. Behav. Process. 1994;20:413–418. [PubMed] [Google Scholar]
- 39.Westergaard GC, Chavanne TJ, Lussier ID, Houser L, Cleveland A, Suomi SJ, Higley JD. Left-handedness is correlated with CSF monoamine metabolite and plasma cortisol concentrations, and with impaired sociality, in free-ranging adult male Rhesus Macaques (Macaca mulatta) Laterality. 2003;8:169–187. doi: 10.1080/713754484. [DOI] [PubMed] [Google Scholar]
- 40.Lecourtier L, Kelly PH. Bilateral lesions of the habenula induce attentional disturbances in rats. Neuropsychopharmacology. 2005;30:484–496. doi: 10.1038/sj.npp.1300595. [DOI] [PubMed] [Google Scholar]