Summary
In this study, we elucidate the roles of the winged-helix transcription factor Foxa2 in ventral CNS development in zebrafish. Through cloning of monorail (mol), which we find encodes the transcription factor Foxa2, and phenotypic analysis of mol-/- embryos, we show that floorplate is induced in the absence of Foxa2 function but fails to further differentiate. In mol-/- mutants, expression of Foxa and Hh family genes is not maintained in floorplate cells and lateral expansion of the floorplate fails to occur. Our results suggest that this is due to defects both in the regulation of Hh activity in medial floorplate cells as well as cell-autonomous requirements for Foxa2 in the prospective laterally positioned floorplate cells themselves.
Foxa2 is also required for induction and/or patterning of several distinct cell types in the ventral CNS. Serotonergic neurones of the raphé nucleus and the trochlear motor nucleus are absent in mol-/- embryos, and oculomotor and facial motoneurones ectopically occupy ventral CNS midline positions in the midbrain and hindbrain. There is also a severe reduction of prospective oligodendrocytes in the midbrain and hindbrain. Finally, in the absence of Foxa2, at least two likely Hh pathway target genes are ectopically expressed in more dorsal regions of the midbrain and hindbrain ventricular neuroepithelium, raising the possibility that Foxa2 activity may normally be required to limit the range of action of secreted Hh proteins.
Keywords: Midline development, Hedgehog signalling, Zebrafish
Introduction
The floorplate is a band of non-neuronal cells in the ventral neural tube that acts as an organising centre to pattern the ventral CNS (Strähle et al., 2004). At early stages, the floorplate is a source of secreted Hedgehog (Hh) proteins that establish a gradient within the ventral neural tube (Jacob and Briscoe, 2003; Ruiz i Altaba et al., 2003). As a consequence, different classes of neurone and glial cells are established at discrete dorsoventral (DV) positions depending upon the level of Hh activity to which precursor cells are exposed. Hh signals also have important roles in mediating cell proliferation and perhaps survival of at least some CNS cells (Thibert et al., 2003; Ruiz i Altaba et al., 2002a; Ruiz i Altaba et al., 2002b). At later developmental stages, the floorplate mediates axon guidance through the expression of a variety of secreted guidance cues that direct both DV and longitudinal trajectories of axons (Kaprielian et al., 2001; Charron et al., 2003).
The floorplate of both tetrapods and teleosts has distinct lateral and medial subdivisions that are distinguished by expression of different repertoires of genes and may have different embryonic origins (Odenthal et al., 2000; Charrier et al., 2002; Strähle et al., 2004). In zebrafish, the two subdivisions of the floorplate are named medial floorplate (MFP) and lateral floorplate (LFP) (Odenthal et al., 2000). Expression of genes including sonic hedgehog (shh), tiggywinkle hedgehog (twhh) and the transcription factor encoding foxa1 (previously forkhead7) are restricted to the MFP, whereas foxa2 (axial, hnf3b) and foxa (forkhead4, fkh4, pintallavis) are expressed in both MFP and LFP cells (Odenthal et al., 2000; Odenthal and Nüsslein-Volhard, 1998).
In zebrafish, it is the Nodal signalling pathway that specifies MFP identity. Mutations affecting the Nodal ligand Cyclops (Cyc) (Hatta et al., 1991; Sampath et al., 1998; Rebagliati et al., 1998; Tian et al., 2003), the EGF-CFC protein One-eyed-pinhead (Oep) (Strähle et al., 1997a; Schier et al., 1997; Zhang et al., 1998) and the transcriptional effector of Nodal signalling FoxH1/Schmalspur (Sur) (Pogoda et al., 2000; Sirotkin et al., 2000) all show defects in MFP induction. Furthermore, analysis of enhancer elements of several genes expressed in the floorplate show dependence on Nodal signals for their activity (Müller et al., 1999; Müller et al., 2000; Rastegar et al., 2002). These observations support a two-step model of floorplate formation (Odenthal et al., 2000; Albert et al., 2003), whereby Nodal activity induces the MFP, after which Hh signals from the MFP and/or underlying axial mesendodermal tissues induce LFP cells.
In mouse, one of the targets of Hh signalling within prospective floorplate tissue is Foxa2, which encodes a winged-helix transcription factor. Foxa2 expression is absent from the ventral CNS of mouse mutants lacking activity of the Hh transcriptional effector Gli2, and exogenous Shh can induce ectopic Foxa2 expression (reviewed by Strähle et al., 2004). Reciprocally, Foxa2 consensus binding sites are present within the enhancers of the mouse and zebrafish shh genes (Chang et al., 1997; Müller et al., 1999; Epstein et al., 1999; Jeong and Epstein, 2003) and ectopic Foxa2 activity can induce shh expression (Hynes et al., 1995). These studies suggest that Hh and Foxa2 act in a positive feedback loop, thereby explaining the homeogenetic inductive properties of floorplate tissue. It has proven difficult to test this model so far, as Foxa2 knockout mice fail to form a node and the consequent absence of axial tissues has precluded analysis of the direct role of this gene in floorplate development (Ang and Rossant, 1994; Weinstein et al., 1994).
In zebrafish, LFP-specific expression of foxa2 is absent in Hh pathway mutants, suggesting dependence on Hh activity for transcription in this region (Odenthal et al., 2000; Schauerte et al., 1998). However, within the MFP, it appears that foxa2 is induced and functions downstream of the Nodal pathway. For example, cell-autonomous activation of Nodal signalling leads to induction of foxa2 expression (Müller et al., 2000), and exogenous Foxa2 can rescue medial floorplate gene expression in Nodal pathway mutants (Rastegar et al., 2002). These and other observations have led to the suggestion that during floorplate induction in fish, Foxa2 functions downstream of Nodal signals (Rastegar et al., 2002; Strähle et al., 2004), analogous to the role proposed for Foxa2 acting downstream of Hh activity in mammals.
In this study, we elucidate the role of Foxa2 in patterning the ventral CNS through phenotypic analysis of mol-/- mutant zebrafish embryos that carry mutations in the foxa2 gene. mol-/- embryos show a fully penetrant and fully expressed phenotype in which a floorplate forms but fails to expand or differentiate. Our results suggest that Nodal-dependent induction of floorplate occurs in the absence of Foxa2 activity but that subsequent steps in floorplate development depend heavily upon functional Foxa2. We also show that absence of Foxa2 function leads to defects in the development of oligodendrocytes, the serotonergic raphé nucleus and several cranial motor nuclei.
Materials and methods
Mutant/gene nomenclature
smu is the slow muscle omitted mutation, which disrupts the smoothened gene; syu is the sonic-you mutation, which disrupts the sonic hedgehog gene.
Fish stocks
Embryos were obtained by natural spawnings from wild-type and monorail (mol)tv53a (Brand et al., 1996), monorail (mol)st20, smoothened (smu)b641 (Barresi et al., 2000; Varga et al., 2001) and sonic-you (syu)tq252 (Schauerte et al., 1998) fish. mol-/- fish expressing the isl1:GFP transgene were obtained from parents generated by crossing a heterozygous moltv53a carrier with a homozygous Tg(isl1:GFP) fish (Higashijima et al., 2000). mol-/-;syu-/- double mutant fish were generated by crossing fish heterozygous for both moltv53a and syutq252. The moltv53a and molst20 alleles have very similar phenotypes and moltv53a mutants were used for most of the phenotypic characterisation.
Pharmacological blockade of Hh signalling
For inhibition of Hh pathway signalling, embryos were incubated in 100 μM cyclopamine (diluted in fish water from 20 mM stock in ethanol) between 4 hours and the time of fixation for analysis of floorplate differentiation or between 36 hours and 48 hours for experiments analysing ectopic ntn1 and nk2.2a expression (see Results). Treatment was terminated by three washes in fish water followed by fixation. To exclude the possibility that observed defects were due to ethanol treatment, control embryos were incubated in an equivalent concentration of ethanol without cyclopamine. Furthermore, cyclopamine treatment gave rise to phenotypes well established as being due to abrogation of Hh activity, such as partial cyclopia and U-shaped somites.
Microinjection and rescue experiments
foxa2 morpholino (5′-CCTCCATTTTGACAGCACCGAGCAT-3′, Gene-Tools) was diluted in 1 × Danieau buffer [58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca (NCO3)2, 5.0 mM HEPES pH 7.6] and stored at −20°C. Synthetic mRNAs were synthesised using the SP6 mMessage mMachine transcription kit (Ambion) according to the manufacturer’s protocol. Embryos were injected at the single cell stage with either 4 ng/μl morpholino, or with between 50 ng/μl and 100 ng/μl mRNA. Rescue experiments were performed using mRNA transcribed from a pCS2MT:foxa2-ER plasmid (see Rastegar et al., 2002). Protein activity was initiated at between 50% epiboly and 70% epiboly by treatment with 10−7 M βEstradiol (Sigma). Control experiments were performed using mRNA transcribed from a pCS2MT:hER plasmid containing no foxa2 sequence. shh RNA was injected at the single cell stage at a concentration of 50 ng/μl and injected embryos were left to develop for up to three days before fixation.
In situ hybridisation and immunohistochemistry
In situ hybridisation was carried out as described previously (Macdonald et al., 1994) and standard antibody protocols were used (Wilson et al., 1990). The following mRNA in situ hybridisation probes were used: foxa2 (Strähle et al., 1993), foxa1 and foxa (Odenthal et al., 1998); shh (Krauss et al., 1993); twhh (Ekker et al., 1995); nk2.2a (Barth and Wilson, 1995); col2a1 (Lele and Krone, 1997); min1 and min2 (Higashijima et al., 1997); tphR (Teraoka et al., 2004); th (Holzschuh et al., 2001); dbh (Guo et al., 2000); ntn1 (Strähle et al., 1997b); ctgf (Dickmeis et al., 2004); arx (Miura et al., 1997); her9 (Leve et al., 2001); olig2 (Park et al., 2002); nkx2.2b (Schäfer et al., 2004); mbp and plp (Brösamle and Halpern, 2002). In some cases, stained embryos were embedded in gelatin and 25 μm sections cut using a vibratome (Leica). Anti-GFP antibody (AMS Biotechnology) was used at 1:1000, with anti-rabbit IgG secondary antibody (Sigma) at 1:200. Anti-acetylated tubulin antibody (Wilson et al., 1990) (Sigma) was used at 1:1000 with an anti-mouse IgG secondary (Sigma). Antibody staining was visualised with diaminobenzidine (DAB).
Linkage analysis, genetic mapping, cloning and sequencing
The moltv53A locus was mapped using F2 offspring of a Tübingen×WIK reference cross (Rauch et al., 1997) with a panel of simple sequence length polymorphism (SSLP) markers (Knapik et al., 1996) on pools of 48 mutants and 48 wild-type siblings and localised to linkage group 17. Linkages were confirmed and refined by genotyping single mutant and sibling embryos. To confirm that foxa2 is tightly linked to the moltv53A mutation, a polymorphism within the foxa2 gene was scored by cleavage with the enzyme Tsp509 I. To identify the lesion in foxa2, RNA pools from 20 mol-/- and 20 wild-type embryos at 24 hpf were reverse transcribed. The entire coding sequence of the foxa2 gene was amplified by PCR using the forward and reverse primers TTCCAGGATGCTCGGTGCTGTCAAAATGG and GTCACAAGGTCCAAGAGAGTTTAGGAAG. The product was cloned into the TOPO TA vector (Invitrogen) for sequence analysis. Automated fluorescent sequencing was carried out using an ABI 373A sequencer. Internal foxa2 primers were designed and 17 moltv53A and seven wild-type samples analysed. Sequence analysis was also carried out on PCR products from single embryo genomic DNA from five moltv53A, three wild-type and nine siblings. The molst20 locus was mapped with a panel of SSLP markers (Knapik et al., 1996) using progeny obtained from crosses of F2 founder fish heterozygous for molst20. Initial mapping of the molst20 locus to LG 17 was performed on mutant and wild-type DNA pools obtained from 20 embryos per pool. Linkages were confirmed and refined by genotyping single mutant and wild-type sibling embryos. The mutation was identified by amplifying and sequencing foxa2 exons with template DNA prepared from tail fins of heterozygous molst20 fish. The part of the second exon containing the mutation was amplified and sequenced from the 5′ and 3′ direction using the primers CAGCACACCCTGACATTTCTTT and GTGATTGAACGAGTAGTGATGTT, respectively. The presence of the mutation was confirmed in 108 single molst20 embryos by PCR with the primers TACCATGAGCCCAATGGCAG and CGAGTGGCGGATAGAGTTT, and subsequent cleavage of the PCR product with the restriction enzyme MseI.
Transplantation experiments
Mosaic analysis was carried out between wild-type and mutant embryos at different stages. Biotin (1%)-injected donor cells were taken from dome stage embryos and placed into mol-/- or wild-type hosts at the shield stage. Cells were placed in the shield, some of the cells of which are destined to form floorplate (Woo et al., 1995). Embryos were left to develop until 32 hours of development and were then fixed for analysis. At this time point, it was possible to score genotype of donor and host embryos by morphology. Embryos were stained for foxa gene expression by in situ hybridisation and position of transplanted cells was determined by revealing the biotin using the Vectastain Elite kit. In some cases, embryos from these and other experiments were embedded into JB4 medium (Polysciences) and sectioned at 10 μm for further analysis.
Results
monorail encodes foxa2
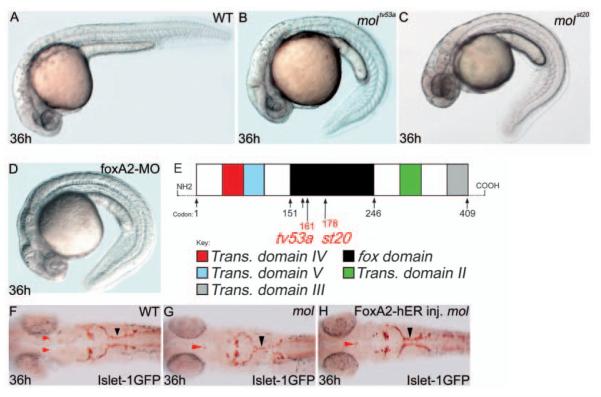
We identified monorail (moltv53a) as a locus important for patterning of axons that cross the midline in the midbrain and hindbrain through screening existing lines of fish for mutations affecting midline axon guidance (Fig. 1F,G; data not shown). mol-/- embryos were originally identified by their curled down tail and lethality by 5 days post-fertilisation (Fig. 1A,B) (Brand et al., 1996). A second allele (molst20) was isolated in a screen for mutations affecting myelin basic protein (mbp) expression, and this allele shows a similar phenotype (Fig. 1C).
Fig. 1.
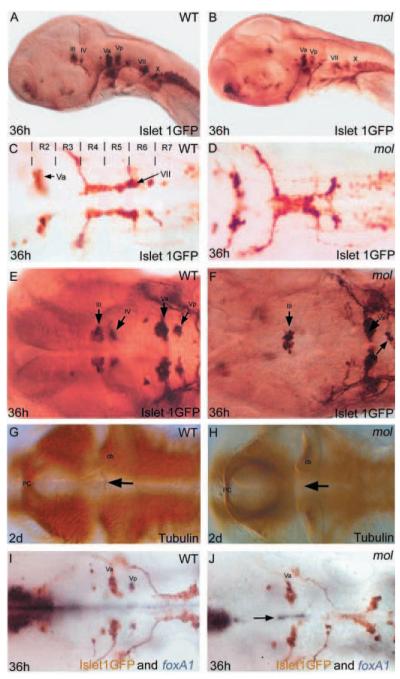
The mol locus encodes Foxa2. (A-D) Lateral views of 36 hpf wild-type (A), moltv53a (B), molst20 (C) and foxa2 morphant (D) embryos. (E) Structure of the Foxa2 protein showing the positions of the likely truncations within the forkhead domain caused by the moltv53a and molst20 mutations. moltv53a results in the complete loss of transactivating domains II and III, leaving a C-terminally truncated Foxa2 protein. molst20 disrupts the forkhead box DNA-binding domain, downstream of moltv53a. Trans. domain, transactivating domain; fox domain, forkhead box DNA-binding domain. (F-H) Dorsal views of brains of a wild-type (F) and two moltv53a mutant (G,H) embryos labelled to reveal the cranial motor nuclei. The fusion of the facial motor nucleus (black arrowheads) is rescued in the moltv53a embryo expressing functional Foxa2 (H). The embryo in H is a homozygous moltv53a mutant as it still shows midline fusion of a reduced oculomotor nucleus (red arrowhead).
To elucidate the role of the mol gene product, we cloned the gene affected by the mutations. Mapping and sequence analysis revealed linkage to, and mutations within, the conserved forkhead box of foxa2 in both alleles (Fig. 1E; see Fig. S1 in the supplementary material). Furthermore, injection of foxa2 morpholino antisense oligonucleotides phenocopies the curly tail phenotype of mol-/- embryos (Fig. 1D). To confirm that the defects in mol-/- embryos are due to a loss of Foxa2 function, we attempted to rescue the phenotype by expressing functional Foxa2 in mutant embryos. Embryos were injected with RNA encoding a 17β-estradiol inducible Foxa2-ER construct (Rastegar et al., 2002). To assess phenotypic rescue, we focused upon the organisation of the facial motor nucleus (CNVII), which is fused at the midline in mol-/- embryos (Fig. 1F,G). Activation of the Foxa2-ER fusion protein during gastrulation rescued this phenotype in 48% of mutant embryos (Fig. 1H). Altogether, these data allow us to conclude that the gene mutated in mol-/- embryos is foxa2.
mol-/- embryos possess a floorplate that lacks lateral cells
Previous studies have suggested that Foxa2 mediates floorplate development downstream of Hh signalling in amniotes (Sasaki and Hogan, 1994) and downstream of Nodal signalling in fish (Rastegar et al., 2002). To investigate if this is indeed the case, we assessed floorplate induction and differentiation in mol-/- embryos. To our surprise, slightly enlarged cells with typical cuboidal floorplate morphology (Odenthal et al., 2000; Hatta et al., 1991) were evident along the entire axis of the CNS in moltv53a embryos (Fig. 2A,B). We therefore conclude that Foxa2 is not essential for induction of a floorplate.
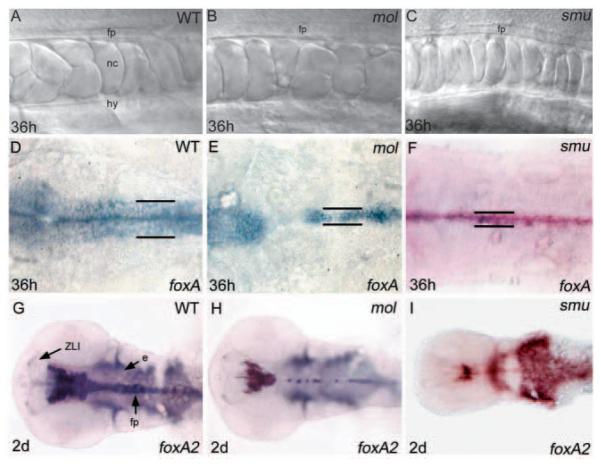
Fig. 2.
The floorplate is reduced in width in both mol-/- and smu-/- embryos. (A-C) Lateral views of the floorplate in the trunk of living wild-type, mol-/- and smu-/- embryos. Cuboidal floorplate cells are present in all embryos, although the cells are increased in size in mol-/-. (D-F) Dorsal views of foxa expression in the hindbrain floorplate. In the mol-/- and smu-/- mutants, expression is reduced from a six cell wide band to a one to three cell wide band (black lines). Additionally, gaps of expression are present in the mol-/- mutant. (G-I) Dorsal views of foxa2 expression in the head. Midline expression is narrow in both the mol-/- and the smu-/- mutant, and additionally is patchy in the midbrain and hindbrain in the mol-/- mutant. e, endoderm; fp, floorplate; hy, hypochord; nc, notochord; ZLI, zona limitans intrathalamica.
Subsequent to its initial induction during gastrulation, the floorplate laterally expands such that it eventually encompasses MFP and LFP cell populations. We next assessed whether the floorplate of mol-/- embryos expands laterally. In wild-type embryos, both foxa and foxa2 are expressed in the hindbrain in a band of cells six or seven cell diameters wide that encompasses the MFP and LFP (Fig. 2D,G). Expression of both genes is reduced to a one to three cell wide row in mol-/- embryos (Fig. 2E,H). From this, we conclude that the floorplate in mol-/- embryos lacks laterally positioned cells. This could be due to loss of MFP and/or loss of LFP components of the floorplate. The narrow floorplate phenotype is similar to smu-/- embryos that lack zygotic Hh signalling and possess MFP but not LFP cells (Chen et al., 2001; Varga et al., 2001) (Fig. 2C,F,I). However, unlike smu-/- mutants, mol-/- embryos show gaps of foxa and foxa2 expression at stereotypic positions in the posterior midbrain and hindbrain, and reduced levels of expression of these genes in the spinal cord (Fig. 2E,H and see below).
Expression of regulatory genes is not maintained in the floorplate of mol-/- embryos
Gene expression analysis revealed no obvious defects in induction of the early floorplate markers tiggywinkle hedgehog (twhh; Fig. 3A), her9 (Latimer et al., 2005) (Fig. 3B), ntn1 (Strähle et al., 1997b) (Fig. 3C), foxa1 and shh (data not shown) in mol-/- embryos, suggesting that by early somite stages, induction of floorplate has occurred.
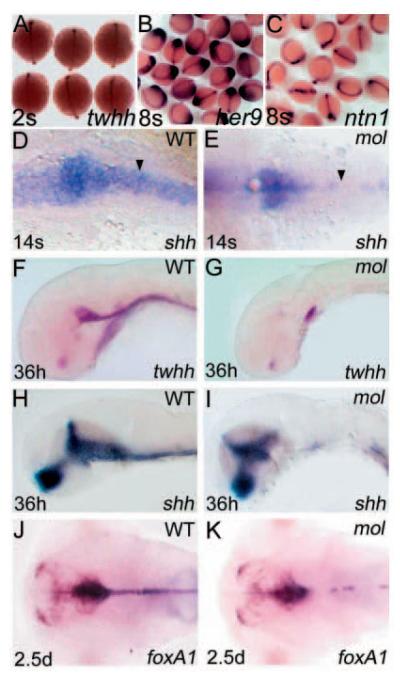
Fig. 3.
Expression of regulatory genes expressed in the MFP of wild-type embryos is not maintained in mol-/- mutants. Dorsal (A-E,J,K) and lateral (F-I) views of wild-type (D,F,H,J) and mol-/- embryos (E,G,I,K), at ages shown in the bottom left-hand corner, analysed for expression of various genes (indicated in the bottom right-hand corner). (A-C) Clutches of embryos from parents heterozygous for the moltv53a mutation. No obvious differences in twhh, her9 or ntn1 expression in prospective floorplate between wild-type and mol-/- mutants is evident at these early stages. The arrowheads in D,E show gaps in floorplate shh expression in the mol-/- embryo compared with wild type.
Although initial steps of floorplate development occur in mol-/- embryos, gaps soon start to appear in the ventral midbrain and hindbrain expression domains of shh and other floorplate markers (Fig. 3D,E; data not shown). At later developmental stages, all early markers of floorplate analysed, including foxa1, shh and twhh, show reduced and patchy expression in the hindbrain and reduced or absent expression further caudally (Fig. 3F-K). Expression of these genes in the forebrain showed no major changes in mol-/- embryos (data not shown). TUNEL analysis of mol-/- embryos did not reveal any increase in apoptosis of floorplate cells, suggesting that midline cells were still present even though gene expression is reduced (data not shown). These results indicate that Foxa2 is required for the maintenance of expression of regulatory genes in the floorplate.
Development of floorplate in mol-/- embryos does not depend on Hh activity
We next performed experiments to assess whether mol-/- floorplate shares features of wild-type MFP or LFP. Hh signalling is essential for induction of LFP but is not required for development of MFP (Odenthal et al., 2000; Schauerte et al., 1998). Therefore, if the floorplate remaining in mol-/- embryos is LFP, abrogating Hh activity should abolish its development. To reduce Hh activity, we treated wild-type and mol-/- embryos with the Hh pathway inhibitor cyclopamine (Incardona et al., 1998). In the trunk, mol-/- embryos still possessed a morphologically distinct floorplate following cyclopamine treatment (Fig. 4B).
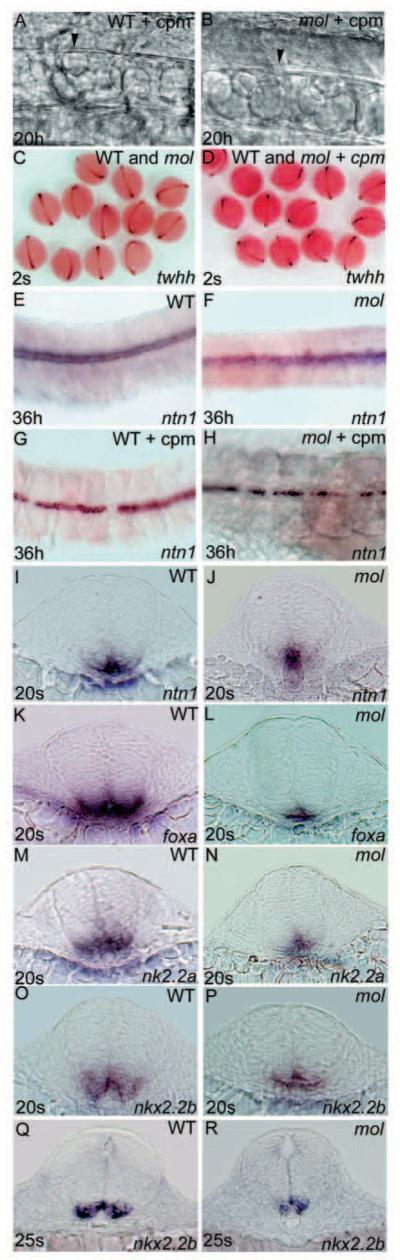
Fig. 4.
Floorplate develops in mol-/- embryos independently of Hh activity. Lateral views (A,B), dorsal views (C-H) and transverse sections (I-R) of wild-type (A,C,E,G,I,K,M,O,Q) and mol-/- (B,D,F,H,J,L,N,P,R) embryos with (A,B,D,G,H) and without (C,E,F,I-R) cyclopamine treatment. (A,B) Both the wild-type and the mol-/- embryo treated with cyclopamine have cuboidally shaped floorplate cells (arrowhead) at 20 hpf. (C,D) Clutches of embryos from parents heterozygous for the moltv53a mutation. Treatment with cyclopamine between the 4-hour and 2-somite stages did not appear to affect expression of twhh in either wild-type or mol-/- mutants. (E-H) Wild-type and mol-/- embryos showing patchy ntn1 expression after treatment with cyclopamine. (I-R) Transverse sections through the spinal cord of wild-type and mol-/- embryos showing changes in floorplate expression of various genes in the mutants. cpm, cyclopamine.
Early markers of floorplate such as twhh (Ekker et al., 1995), ntn1 (Strähle et al., 1997b) and her9 (Latimer at al., 2005) are predominantly or exclusively expressed in the MFP of wild-type embryos. The initial retention of expression of these markers in mol-/- embryos (Fig. 3A-C) suggests that at early stages, the floorplate of mol-/- embryos is similar to the MFP of wild-type embryos. Furthermore, expression of twhh, ntn1 and other early floorplate markers is retained in mol-/- (and wild-type) embryos following cyclopamine treatment (Fig. 4C-H; data not shown). Unlike other floorplate markers, ntn1 expression is retained in the spinal cord midline at later stages in mol-/- embryos, albeit at reduced levels.
The observations that a floorplate still forms in mol-/- embryos with compromised Hh signalling and, at early stages, expresses markers restricted to the MFP of wild-type embryos indicates that the floorplate of mol-/- embryos shares, at least at early stages, some features of the MFP of wild-type embryos.
The absence of markers that clearly and exclusively label the LFP of wild-type embryos made it more difficult for us to assess if the residual floorplate of mol-/- embryos shares features with wild-type LFP. Markers of both MFP and LFP in wild-type embryos, such as foxa, are expressed in a single row of midline cells in the trunk spinal cord of mol-/- embryos (Fig. 4K,L). Nkx2.2 genes may initially be expressed throughout the ventral most spinal cord but over time, expression is lost in midline floorplate cells. Both nk2.2a (Barth and Wilson, 1995) and nkx2.2b (Schäfer et al., 2004) are expressed in a narrower band of cells in the spinal cord of mol-/- embryos compared with wild-type embryos (Fig. 4M-R). The absence of midline expression evident in wild-type embryos was less apparent in mol-/- embryos (Fig. 4O-R). This suggests that at late somite stages, the residual floorplate in mol-/- embryos expresses markers that would be localised to LFP in wild-type embryos. Together, these observations suggest that at late somite stages, the expression profile of the residual floorplate of mol-/- embryos matches neither MFP nor LFP of wild-type embryos.
Floorplate fails to differentiate in mol-/- embryos
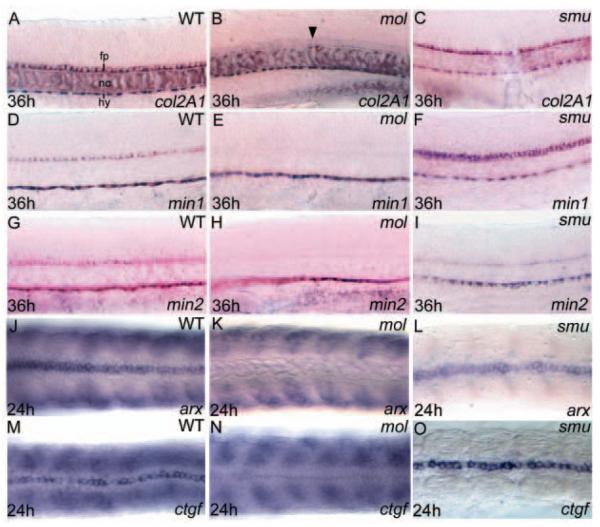
The results described above show that although medially positioned floorplate cells with typical cuboidal morphology are present in mol-/- embryos, these cells fail to maintain the expression of several key regulatory signals and transcription factors. We next asked what the consequences of these defects are upon the differentiated character of the floorplate. Mature medial floorplate expresses the extracellular matrix protein Col2a1 (Lele and Krone, 1997), the secreted proteins Mindin1 and Mindin2 (Min1, Min2) (Higashijima et al., 1997), Connective tissue growth factor (Ctgf) (Dickmeis et al., 2004), and the homeodomain protein Arx (Miura et al., 1997).
Expression of all these markers of floorplate differentiation is severely reduced or absent in the spinal cords of mol-/- embryos and reduced/patchy further rostrally (Fig. 5B,E,H,K,N; data not shown). Other sites of expression, such as notochord or hypochord, are not obviously affected by the mol-/- mutation. This failure in floorplate differentiation is in striking contrast to the situation in smu-/- embryos, which maintain MFP cells (Chen et al., 2001; Varga et al., 2001). In smu-/- embryos, expression of all medial floorplate differentiation markers is very similar to wild type (Fig. 5C,F,I,L,O). As virtually all Hh signalling is absent in smu-/- embryos, these data indicate that Foxa2 mediates floorplate differentiation independent of the Hh pathway.
Fig. 5.
Floorplate differentiation fails in mol-/- but not in smu-/- embryos. Lateral (A-I) and dorsal (J-O) views of the trunk floorplate in 36 hpf (A-I) and 24 hpf (J-O) wild-type, mol-/- and smu-/- embryos. (A-O) Expression of floorplate differentiation markers col2a1, min1, min2, arx and ctgf is similar in wild-type and smu-/- embryos but expression is severely reduced or absent in the mol-/- embryos (despite the presence of floorplate cells, arrowhead in B). Although floorplate expression is retained, the somitic expression of arx and ctgf is lost in the smu-/- mutants (L,O). fp, floorplate; hy, hypochord; nc, notochord.
The floorplate defects in mol-/- embryos are not only due to reduced levels of Hh activity
Given that Hh signalling is not required for expression of MFP differentiation markers, the loss of such markers in mol-/- embryos is unlikely to be a consequence of the failure to maintain Hh gene expression in the floorplate. However, as Hh signals are required for LFP formation, then reduced Hh activity in mol-/- embryos could contribute to the loss of lateral cells in the mol-/- floorplate. To address this issue, we overexpressed shh in mol-/- embryos and assessed consequences upon floorplate-specific gene expression.
Injection of shh RNA leads to ectopic dorsal expansion of the MFP/LFP marker foxa in the midbrain of both wild-type and mol-/- embryos (Fig. 6A,B) (Schauerte et al., 1998; Odenthal et al., 2000). However, in mol-/- embryos, exogenous Shh failed to restore the normal width of the floorplate (Fig. 6B). This suggests that Foxa2 is required downstream of Shh to promote the expression of foxa. As expected, shh injections had no effect upon the differentiated MFP marker col2a1 in either wild-type or mol-/- embryos (Odenthal et al., 2000) (data not shown).
Fig. 6.
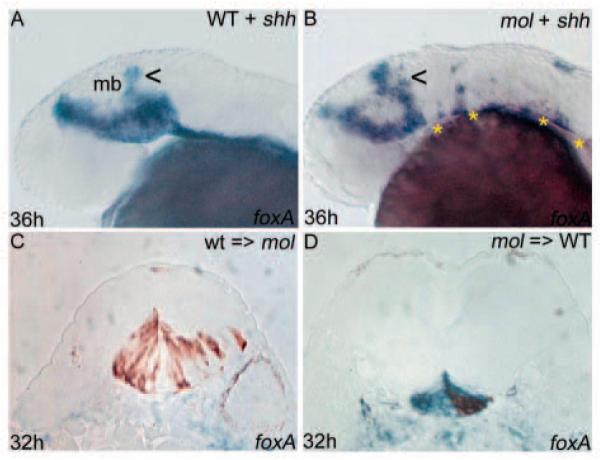
Floorplate phenotypes in mol-/- embryos are not only due to reduced Hh activity. (A,B) Lateral views of wild-type and mol-/- embryos following overexpression of exogenous shh. There is some dorsal expansion (arrowheads) of foxa expression in the midbrain of both the wild-type and the mol-/- embryo; however, foxa expression remains discontinuous and narrow along the CNS midline of the mol-/- embryo (asterisks in B). (C,D) Transverse sections of embryos in which wild-type cells were transplanted into the prospective floorplate of a mol-/- embryo (C) or mol-/- cells were transplanted into the prospective floorplate of a wild-type embryo (D). foxa is shown in blue and transplanted cells are stained brown.
To address if the reduced levels of Hh activity do contribute to the floorplate defects in mol-/- embryos, we transplanted wild-type cells into the prospective floorplate of mol-/- hosts (n=9). In some cases, wild-type cells expressed the floorplate marker foxa (not shown), whereas in others they did not (Fig. 6C). As wild-type cells should possess all the proteins required for floorplate differentiation, the most likely interpretation of this result is that the mol-/- environment fails to provide cell non-autonomous signals (such as Hh proteins) to either induce or maintain floorplate marker expression in the wild-type cells. Further supporting this conclusion, mol-/- cells transplanted into a wild-type environment (n=9) often maintained expression of foxa in lateral regions of the floorplate (Fig. 6D). This indicates that there is not an absolute requirement for Foxa2 in the maintenance of foxa expression and that non-autonomous signals arising from a wild-type environment can alleviate the mol-/- floorplate phenotype.
Altogether, these results provide evidence that Foxa2 functions both upstream of Hh signalling (in the regulation of Hh gene expression) and downstream of Hh activity (in the induction/maintenance of expression of various floorplate markers).
ntn1 and nk2.2a are ectopically expressed in the midbrain and hindbrain of mol-/- embryos
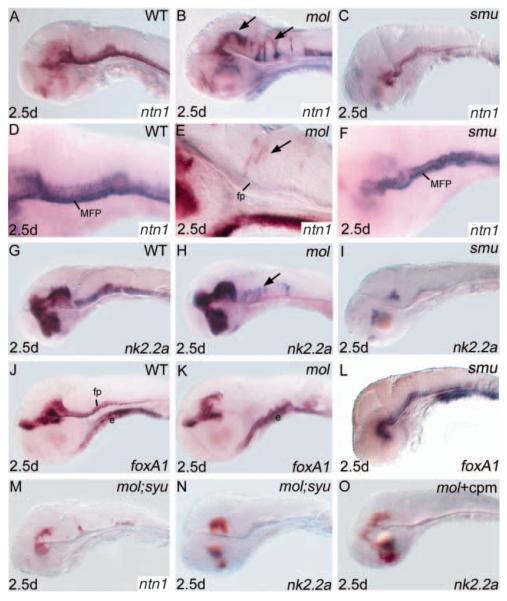
Consistent with other markers of floorplate maturation, reduced levels of expression of ntn1 are retained in the spinal cord floorplate of mol-/- embryos (Fig. 4F). However, with this marker, we found a more complex phenotype in the midbrain and hindbrain. Transcripts are absent from the most ventral CNS cells. However, ectopic patches of expression are observed in ventricular zone cells positioned more dorsally (Fig. 7B,E). The extent of mis-expression varied from a few cells to large clusters of cells. Similarly, analysis of the homeobox gene nk2.2a showed that like ntn1, ventricular zone expression in the hindbrain is more dorsally positioned in mol-/- embryos than in wild type (Fig. 7H). Unlike other mol-/- phenotypes, the dorsal expansion of nk2.2a and ntn1 expression shows highly variable expressivity.
Fig. 7.
Expression of nk2.2a and ntn1 is expanded dorsally in the midbrain and hindbrain neural tube of mol-/- embryos, but is reduced in smu-/- embryos. Lateral views of brains of wild-type (A,D,G,J), mol-/- (B,E,H,K), smu-/- (C,F,I,L) and mol-/-;syu-/- double mutant (M,N) embryos and mol-/- embryos treated with cyclopamine (O) (cpm) analysed for expression of genes indicated bottom right. Arrows indicate ectopic dorsal expression of ntn1 (B,E) and nk2.2a (H) in mol-/- embryos. cpm, cyclopamine; e, endoderm; fp, floorplate; MFP, medial floorplate.
As nk2.2a expression is dependent on Hh signals (e.g. Barth and Wilson, 1995; Varga et al., 2001), we wondered whether the ectopic dorsal expression of ntn1 and nk2.2a depends upon Hh signals acting through transcription factors other than Foxa2. smu-/- embryos show no ectopic dorsal expression of either ntn1 or nk2.2a (Fig. 7C,F,I); however, the ectopic expression may be dependent upon loss of Foxa2 activity. We therefore analysed mol-/-;syu-/- embryos that have reduced Hh activity. In these embryos, the ectopic dorsal expression of ntn1 and nk2.2a was considerably reduced (Fig. 7M,N). Similarly, mol-/- embryos treated with cyclopamine between 36 and 48 hpf show no ectopic ntn1 and nk2.2a expression (Fig. 7O; data not shown). Together, these results suggest that Hh activity is required for the ectopic expression of ntn1 and nk2.2a in mol-/- embryos. However, we never observed ectopic expression of any Fox genes (foxa, foxa1, foxa2, Fig. 7K and data not shown) or indeed Hh genes (data not shown) in a pattern similar to that of ectopic ntn1 or nk2.2a expression. Altogether, these results indicate that in the absence of Foxa2 function, Hh activity dependent ectopic expression of ntn1 and nk2.2a occurs via unknown transcription factors.
Foxa2 is required for formation of the trochlear nuclei and for bilateral separation of the facial and oculomotor nuclei
In order to analyse the induction and patterning of neurones adjacent to the floorplate in mol-/- embryos, we crossed a GFP transgene driven by isl1 regulatory elements into the mol-/- line (Fig. 8B). This transgene labels cranial motoneurones from soon after their birth (Higashijima et al., 2000).
Fig. 8.
mol-/- embryos exhibit cranial motoneurone induction and patterning defects. Lateral (A,B) and dorsal (C-J) views of wild-type (WT) and mol-/- embryos labelled with various antibodies and RNA probes (indicated bottom right) to reveal cranial motoneurones and their axons. The unlabelled arrow in F shows a few Vp neurones in the mol-/- embryo. Arrows in G and H show the trochlear decussation in wild type (G) and its absence in mol-/- (H) embryos. The arrow in J indicates foxa1 expression retained between the two bilateral Va nuclei of the mol-/- mutant. III, oculomotor nucleus; IV, trochlear motor nucleus; Va and Vp, anterior and posterior components of the trigeminal motor nucleus; VII, facial motor nucleus; X, vagal nucleus cb, cerebellum; pc, posterior commissure; R, rhombomere.
A prominent phenotype in mol-/- embryos is that the facial (cranial motor nucleus VII) and oculomotor (III) neurones form single nuclei at the midline rather than bilateral pairs of nuclei (Fig. 8C,D). Although the number of facial motoneurones is not obviously reduced in mol-/- embryos, the oculomotor nucleus has fewer neurones than in wild type (Fig. 8E,F). There is a similar reduction in posterior trigeminal motoneurones (Vp) and the trochlear nucleus (IV) is completely absent in mol-/- embryos (Fig. 8E,F), which consequently lack a trochlear decussation (Fig. 8G,H). Whereas the facial nucleus is positioned at the midline in mol-/- embryos, more rostral trigeminal motoneurones almost always remain as bilateral nuclei. One potential reason for this is the consistent retention of expression of other Fox family genes in floorplate cells between the two trigeminal motor nuclei (Fig. 8I,J). Complementing the changes in positioning of neurones, axons in the medial longitudinal fasciculi were closer together or occasionally fused in the region of the facial nucleus but remained normally positioned lateral to the floorplate at all other positions (data not shown). These results indicate that Foxa2 activity within the floorplate is required for the induction of specific cranial motor nuclei and for the mediolateral positioning of other motoneurones and axon pathways.
Foxa2 is required for formation of the serotonergic raphé nucleus
The hindbrain raphé is the major location of serotonergic neurones in the brain. Because these neurones differentiate close to the floorplate (Ye et al., 1998; Pattyn et al., 2003), we examined their development and differentiation in mol-/- embryos. To do this, we analysed expression of a tryptophan hydroxylase encoding gene (tphR; tph2 – Zebrafish Information Network) (Teraoka et al., 2004) that encodes a key enzyme in the synthetic pathway for serotonin production.
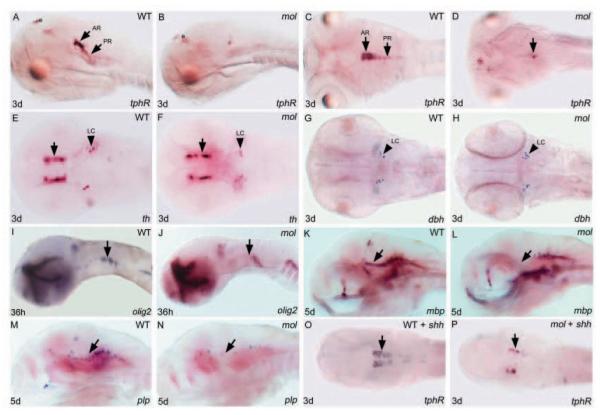
By 3 days of development in wild-type embryos, serotonergic raphé neurones are positioned in anterior and posterior clusters that probably correspond to the dorsal and ventral subdivisions of the mature nucleus (Fig. 9A,C) (Bellipanni et al., 2002). By contrast, mol-/- embryos are virtually devoid of tphR-expressing cells with the few remaining neurones located caudally within the raphé (Fig. 9B,D). tyrosine hydroxylase (th)-expressing neurones in the diencephalon and other catecholaminergic neurones all appear to differentiate as normal in mol-/- embryos (Fig. 9E-H).
Fig. 9.
Both serotonergic raphé neurones and oligodendrocyte precursor cells are severely depleted in mol-/- embryos. Lateral (A,B,I-N) and dorsal (C-H,O,P) views of brains of wild-type and mol-/- embryos and wild-type and mol-/- embryos overexpressing shh (WT + shh; mol + shh) analysed for genes indicated bottom right. (A-D) tphR expression reveals serotonergic neurones. Arrows indicate anterior (AR) and posterior (PR) raphé neurones. Unlabeled arrow in D indicates a few residual tphR + neurons. (E-H) th reveals dopaminergic diencephalic neurones (arrows in E,F); noradrenergic neurones of the LC are labelled both with th and dbh probes. (I-N) olig2 labels oligodendrocyte precursor cells and mbp and plp expression marks nascent oligodendrocytes. Arrows indicate areas of reduced olig2, mbp and plp expression in mol-/- mutant embryos. (O,P) Arrows indicate increased number of tphR-expressing serotonergic neurones in the wild-type embryo and a rescue of serotonergic neurones in the mol-/- embryo following shh injection. e, epiphysis; LC, locus coeruleus.
At least two possibilities could explain the loss of serotonergic neurones in mol-/- embryos. The first is that there is an intrinsic requirement for Foxa2 in serotonergic neurones or their precursors. Alternatively, the failure in differentiation of floorplate tissue may secondarily lead to a failure in production of signals such as Shh, important for induction or differentiation of raphé neurones (Ye et al., 1998; Teraoka et al., 2004). Supporting the possibility that loss of Hh signalling activity leads to loss of raphé neurones in mol-/- embryos, expression of exogenous shh restored tphR expression (Fig. 9O,P; 43% of mutant embryos). This result suggests that foxa2 is not autonomously required in these neurones, and that their absence is due to reduced Hh signalling.
mol-/- embryos show defective oligodendrogenesis in the midbrain and hindbrain
In a screen for zebrafish mutants that show myelination defects (H.-M.P., B.D. and W.S.T., unpublished), a second allele of mol-/- (molst20) was isolated. Olig2 is a basic helix-loop-helix transcription factor expressed in precursors of both motoneurones and oligodendrocytes (Park et al., 2002) and myelin basic protein (mbp) and proteolipid protein (plp) both mark myelinating cells (Brösamle and Halpern, 2002). By 36 hours, there is a reduction of olig2 expression in the hindbrain (Fig. 9I,J) and during later development, there is a reduction of both mbp (Fig. 9K,L) and plp (Fig. 9M,N) in the midbrain and hindbrain, suggesting a loss of myelin forming cells in these areas.
Discussion
In this study, we have characterised the zebrafish mol-/- mutant phenotype and show that mol encodes foxa2. A floorplate is induced in mol-/- embryos but fails to maintain expression of various Fox family transcription factors and Hh signals. Subsequently, the floorplate fails to expand laterally and fails to differentiate. Surprisingly, despite the severity of these defects, cells with floorplate morphology remain viable through the first few days of development. In addition to the role in floorplate development, Mol/Foxa2 is required for differentiation and patterning of various ventral CNS cells, including serotonergic raphé neurones, cranial motoneurones and oligodendrocytes. Our results suggest that defects in these cells in mol-/- embryos are probably due at least in part to regulation of Hh and possibly other signals by Foxa2. Curiously, we observe ectopic activation of Hh target genes in dorsally positioned ventricular cells of the midbrain and hindbrain. This phenotype suggests that Foxa2 may help limit the range of activity of Hh proteins. Altogether, our data allow us to propose a revised model for midline signalling and floorplate development in zebrafish (Fig. 10).
Fig. 10.
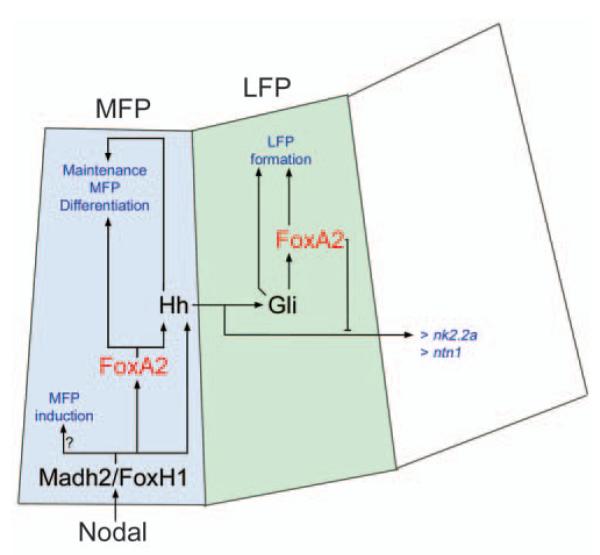
Summary of proposed role for Foxa2, Nodal and Hh signalling pathways in ventral CNS development. The model is based upon data in this paper and in other papers cited in the text. Nodal signalling induces MFP through Madh/Smad and FoxH1/Fast/Sur transcription factors. This occurs in the absence of Foxa2 function, implicating other factors downstream of Nodal in the earliest induction of floorplate identity. Nodal signalling induces foxa2 expression in MFP and Foxa2 function is required for differentiation of these cells. Hh genes are among those requiring Foxa2 function for maintained expression. However, as Hh genes are initially expressed in mol-/- embryos, we suggest that other transcription factors, including Madh2/FoxH1, contribute to the presence of Hh proteins in the ventral neural tube. The only known role for Hh proteins in the MFP is to maintain expression of differentiation markers. Hh signals working through Gli proteins induce foxa2 expression in the LFP. Foxa2 activity is required for proper formation of the LFP and this is likely to be due both to non-autonomous roles (e.g. regulation of Hh production in the MFP), and activity within the LFP itself. Hh activity also spreads further to induce and pattern adjacent ventral CNS cell types, including cranial motoneurones and serotonergic raphé neurones. In the absence of Foxa2 function, Hh activity leads to ectopic dorsal expression of several Hh pathway target genes. This implies that Foxa2 may also negatively regulate the dorsal spread of Hh activity within the ventral CNS.
Foxa2 is not required for floorplate induction in zebrafish
Our analysis of mol-/- embryos shows that floorplate is induced at all axial levels in the absence of normal Foxa2 function. Even in the midbrain and hindbrain, where expression of floorplate markers is severely reduced, floorplate cells are still recognisable by their cuboidal morphology. Furthermore, the medial longitudinal fasciculi are displaced towards the midline in mutant embryos but rarely fuse, as they do in Nodal mutants, suggesting that at least some of the signalling properties of the floorplate cells are retained. From these observations, we conclude that initial floorplate induction in zebrafish can occur independently of Foxa2 function.
Foxa2 knockout mice fail to form a node and all its derivatives, making it impossible to assess the requirement for Foxa2 in floorplate formation (Ang and Rossant, 1994; Weinstein et al., 1994). However, the observations that exogenous Foxa2 can induce floorplate markers (Sasaki and Hogan, 1994; Ruiz i Altaba, 1995; Rastegar et al., 2002), and that Foxa2 binding sites are present in the regulatory regions of floorplate specific genes such as shh and ntn1 (Chang et al., 1997; Epstein et al., 1999; Jeong and Epstein, 2003) has led to the suggestion that Foxa2 mediates floorplate induction (reviewed by Strähle et al., 2004). However, all these data are also consistent with the primary role of Foxa2 being maintenance of expression of floorplate-specific markers and differentiation of the floorplate.
If zebrafish Foxa2 mediates floorplate expansion and differentiation (see below), then what might be the identity of the transcription factors required for floorplate induction? Other Foxa family genes are clear candidates, but morpholino knock-down of foxa or foxa1 alone produces no obvious phenotype (Rastegar et al., 2002). Alternatively, there could be cooperation and redundancy between related Fox family members. Although our data do not address this issue with respect to floorplate induction, there is very little redundancy at later stages. Foxa2 is required to maintain expression of other Foxa genes and so all Foxa activity is severely compromised following loss of Foxa2 activity alone.
In zebrafish, floorplate induction initially requires Nodal signalling. Transcription factors directly downstream of this signalling cascade such as Madh2/Smad2 and Foxh1/Sur/Fast1 induce floorplate markers cell autonomously most probably by directly binding to the promoter/enhancer elements of floorplate-specific genes (Müller et al., 2002). However, the widespread requirement for these components of the Nodal pathway in the development of other cell types (e.g. Kunwar et al., 2003) indicates that, alone, they are unlikely to confer specificity to the induction of floorplate markers. Other transcription factor encoding genes may play a role in the initial steps in induction of the floorplate, including her9 (Latimer et al., 2005), tbx (Amacher et al., 2002) and homeobox (Jeong and Epstein, 2003) genes. Indeed, given the complexity of the regulatory regions of floorplate-specific genes (discussed in Strähle et al., 2004), cooperation between many of these proteins may be required to induce floorplate identity.
In contrast to mice lacking Foxa2 activity (Ang and Rossant, 1994; Weinstein et al., 1994; Hallonet et al., 2002) mol-/- embryos do not appear to have any major mesodermal, endodermal or anterior CNS defects. This may reflect significant differences in the developmental cascades used in mice and fish. Alternatively, it may be due to different Fox family genes mediating similar events in different species. Zebrafish frequently have additional homologues of mammalian genes, most probably owing to a genome duplication event in the lineage leading to teleosts (Postlethwait et al., 1998; Woods et al., 2000). When such duplicated genes are retained, the original roles of the ancestral gene are predicted, in some cases, to be divided between the duplicates (Force et al., 1999). Although we do not know if this is the reason for the difference in phenotypes between mice and fish lacking Foxa2 function, the expression of other Foxa genes in mesendodermal derivatives in fish (Odenthal et al., 1998) is consistent with the possibility that such genes play an equivalent role to Foxa2 in mice.
Foxa2 is required for floorplate differentiation
Although cuboidally shaped cells with typical floorplate morphology are present in mol-/- embryos, they fail to maintain expression of Foxa and Hh family genes and lack expression of markers normally restricted to the differentiated MFP of wild-type embryos. Our favoured interpretation of this phenotype is that Foxa2 regulates floorplate differentiation. The requirement of Foxa2 for maintained expression of floorplate regulatory genes is unequivocal. However, our interpretation of a requirement for Foxa2 during floorplate differentiation is based upon the assumption that the floorplate of mol-/- embryos shares identity with the MFP of wild-type embryos (and that loss of MFP differentiation markers therefore reflects a failure in floorplate differentiation). If instead, Foxa2 mediates induction of MFP, then the absence of MFP differentiation markers would reflect the absence of the MFP rather than a failure in differentiation.
There are two lines of evidence that make us favour the idea that Foxa2 mediates floorplate differentiation rather than MFP induction. The first is the absence of severe floorplate defects in mol-/- embryos until mid-somite stages (for example, the MFP markers twhh, her9 and ntn1 are expressed as in wild type). This suggests that a structure equivalent to the MFP of wild-type embryos is initially present in mol-/- embryos. Second, disrupting Hh activity in mol-/- embryos fails to completely abolish the floorplate that remains. As LFP induction in zebrafish is reliant on Hh pathway signalling (Odenthal et al., 2000; Etheridge et al., 2001), then if the residual floorplate in mol-/- embryos was equivalent to LFP of wild-type embryos, we would expect that abrogating Hh activity should result in complete loss of floorplate identity. As this does not happen, then we think that the mol-/- floorplate is more similar to the Nodal pathway-induced MFP than the LFP of wild-type embryos. Together, these observations support the conclusion that floorplate induction occurs in mol-/- embryos but differentiation fails.
Although we suggest that the floorplate in mol-/- derives from MFP precursors, it does appear to abnormally express markers normally restricted to LFP. nk2.2a and nkx2.2b have both been considered as LFP markers as their expression is excluded from MFP (Strähle et al., 2004; Schäfer et al., 2004) and both genes show some patchy and/or reduced expression in the ventral midline spinal cord of mol-/- embryos. Similarly, in late stage cyc-/- mutant embryos, floorplate tissue expresses a combination of MFP and LFP markers (Albert et al., 2003). Ventral CNS Nk2 genes are regulated between thresholds of Hh activity (e.g. Jacob and Briscoe, 2003) and the altered spatial expression of nk2.2a and nkx2.2b in mol-/- embryos may reflect the lowered levels of Hh activity in the mutants. Taken together, our results suggest that the floorplate in mol-/- embryos is derived from MFP, but exhibits some gene expression characteristics of the LFP of wild-type embryos.
The mechanisms by which Foxa2 regulates floorplate differentiation are likely to be both directly through binding to the regulatory regions of floorplate differentiation genes and indirectly through the transcriptional control of regulatory genes (e.g. Chang et al., 1997; Müller et al., 1999; Rastegar et al., 2002; Epstein et al., 1999). For example, the reduction of foxa2 expression in mol-/- mutants suggests that positive autoregulation by Foxa2 activity is required to maintain foxa2 expression. Analysis of the expression of other class 1 Fox family members (foxa, foxa1) revealed a similar dependence on Foxa2 activity and so all these transcription factors, and probably others, may mediate Foxa2-dependent floorplate differentiation. Although Foxa2 regulates expression of Hh genes, these are unlikely to play a significant role in MFP differentiation as this occurs normally in embryos with severely reduced Hh signalling (Etheridge et al., 2001; Chen et al., 2001; Varga et al., 2001).
Foxa2 functions in the lateral expansion of the floorplate
Although a floorplate forms in mol-/- mutants, it never expands laterally to acquire the full width of floorplate tissue of wild-type embryos. The lateral expansion of the floorplate is a hh-dependent process in zebrafish (Odenthal et al., 2000) and probably in chick (Charrier et al., 2002) and other vertebrates. Various studies have suggested that Foxa2 (and/or highly related genes) functions in a positive regulatory loop with Hh genes, whereby Hh gene activity induces foxa2 expression and Foxa2 activity promotes hh expression (reviewed by Strähle et al., 2004). This model was originally proposed to explain how Hh signals from the notochord could induce foxa2 in the floorplate that would in turn induce hh expression in floorplate cells. In zebrafish, the initial induction of foxa2 in the prospective floorplate is dependent upon Nodal, rather than Hh, activity (discussed in Strähle et al., 2004), but as we discuss below, the regulatory loop between Hh and Foxa2 proteins may contribute to the failure of lateral expansion of floorplate tissue in mol-/- embryos.
The absence of lateral cells with floorplate identity in mol-/- suggests that Foxa2 usually has a role in both MFP and in LFP precursors. We suggest that Nodal signals initially induce Foxa2 expression but in the absence of Foxa2 activity, there is a progressive loss of Hh expression in the floorplate. As a consequence, the reduced levels of Hh activity in the MFP may be insufficient to induce (or maintain) LFP identity. However, the absence of laterally positioned floorplate cells in mol-/- embryos is unlikely to be due solely to reduced Hh activity within the floorplate. For example, at early stages, transcription of hh genes appears to be normal in mol-/- embryos. Indeed, LFP cells can still form in cyc mutant embryos that lack MFP, suggesting that Hh signals from other sources are sufficient to induce LFP (e.g. Albert et al., 2003). Furthermore, overexpression of shh fails to restore the full width of the floorplate in mol-/- mutant embryos suggesting that Foxa2 function is required within LFP cells for these cells to differentiate with floorplate identity.
Foxa2 activity may limit the range of Hh activity
Within the midbrain and hindbrain, loss of Foxa2 activity leads to reduction of ntn1 and nk2.2a expression in ventral CNS cells, but surprisingly there is variable ectopic activation of both genes in dorsal cells close to the ventricle. This implies that the normal activity of Foxa2 is required to limit the activation of these genes to cells at, or close to, the midline. Both nk2.2a (e.g. Barth and Wilson, 1995; Varga et al., 2001) and ntn1 (e.g. Müller et al., 2000) are regulated by Hh activity and so we predict that the ectopic expression of these genes is due to ectopic activation of the Hh signalling pathway. This idea is supported by the observation that abrogation of Shh activity in mol-/- embryos reduces the ectopic nk2.2a and ntn1 expression. Although a dependence on Hh signalling is evident, all our other analyses suggest that the level of Hh signalling is considerably reduced in the ventral CNS. How, then, could reduced levels of Hh activity ventrally lead to ectopic Hh signalling dorsally?
Hh signalling can negatively regulate Hh target genes through the induction of genes that limit the range/efficiency of Hh signalling. For example, the transmembrane protein Patched is induced by Hh signalling and appears to sequester Hh protein, thereby limiting its range of action. This was elegantly demonstrated by showing that Hh signals spread more efficiently across clones of cells expressing a modified form of Patched that fails to bind Hh proteins (Briscoe et al., 2001). Other proteins such as the EXT family members, Toutvelu, Brother of tout-velu and Sister of tout-velu also regulate the range of activity of Hh signals (Han et al., 2004; Takei et al., 2004). Therefore one possibility is that Foxa2 is required to induce proteins that subsequently limit the range of Hh activity (as expected, patched expression is severely reduced in mol-/- embryos, data not shown). Although this is an attractive possibility, the source of Hh proteins that lead to ectopic activation of nk2.2a and ntn1 is not obvious. There is very little hh transcription in floorplate cells of older mol-/- embryos, and so one possibility is that Hh proteins may come from underlying tissues such as the notochord or even gut endoderm as has been proposed in other situations (Wijgerde et al., 2002). An alternative possibility is that Hh proteins come from other cells in the CNS. hh expression is unaffected in the diencephalon and anterior midbrain of mol-/- embryos. Although these hh expressing cells are some distance from the site of ectopic expression, the third ventricle provides a route by which secreted Hh proteins could potentially move along the AP axis of the CNS. Indeed, the ectopic expression of nk2.2a and ntn1 is tightly restricted to cells adjacent to the ventricles.
Foxa2 and Hh signalling regulates induction and patterning of ventral CNS cell populations
mol-/- embryos exhibit a variety of defects in ventrally positioned cells adjacent to the floorplate, including a severe reduction in the serotonergic raphé neurones and prospective oligodendrocytes. There are two possible mechanisms by which Foxa2 could be involved in the development of these cell types. Specification may be due either to a direct requirement for Foxa2 within the precursors of the cell groups or, alternatively/additionally, Foxa2 may regulate signals that influence cell specification.
Overexpression of exogenous Shh restores some tphR-expressing prospective raphé neurones in mol-/- embryos, implying that there probably is not a cell-autonomous requirement for Foxa2 in the specification of these neurones. Hh signalling is required for specification of raphé neurones (Ye et al., 1998; Teraoko et al., 2004) and so the simplest explanation of the serotonergic neurone phenotype of mol-/- embryos is that the reduced levels of Hh activity lead to a failure in induction of this cell type. It is perhaps surprising that injection of shh RNA can rescue the raphé neurones given the short lifetime of the injected RNA and the relatively late appearance of serotonergic neurones. However, it is currently unknown when serotonergic neurone precursors are first specified, nor is it known at what stage in the specification/differentiation of these cells that Hh signalling is required.
The reduction of expression of mbp, olig2 and plp expression in the hindbrain of mol-/- embryos provides the first evidence that Foxa2 is required for oligodendrocyte development and consequently myelin formation. It is not known if the oligodendrocyte precursors express Foxa2 and we have been unable to determine if the loss of these cells in mol-/- embryos is due to reduced Hh activity (data not shown). Further work will be required to determine how Foxa2 mediates the development of the oligodendrocyte lineage.
Unlike prospective oligodendrocytes and serotonergic neurones, most cranial motoneurones are specified normally in mol-/- embryos. This suggests that Foxa2 is not essential for specification of most motoneurones and that levels of Hh activity are still sufficiently high in mol-/- embryos at the stages at which the motoneurones are induced. The anterior motor nuclei, however, are reduced or absent in mol-/- mutants. We have not resolved whether there is a cell-autonomous requirement for Foxa2 in the specification of these cells.
A revised model of floorplate formation in zebrafish
Taken together, our analyses of the mol-/- mutant and other studies allow us to revise existing models of floorplate formation in zebrafish (Fig. 10). Nodal signalling induces MFP (and floorplate-specific genes such as shh and foxa2) through Madh/Smad and FoxH1/Fast1/Sur transcription factors. This occurs in the absence of Foxa2 function, implicating other transcription factors, perhaps including Her9 (Latimer et al., 2005), downstream of Nodal in the earliest induction of floorplate identity. Downstream of Nodal activity, Foxa2 function is required for maintained expression of Fox and Hh family genes and for differentiation of the floorplate. Hh signals produced in the MFP subsequently contribute to the induction of foxa2 (and foxa) expression in more lateral cells. Foxa2 activity is required for lateral expansion of the floorplate, probably owing both to non-autonomous roles (e.g. regulation of Hh production in the MFP) and to activity within the LFP itself. Hh signalling also spreads further dorsally to induce and pattern adjacent ventral CNS cell types, including cranial motoneurones, serotonergic raphé neurones and oligodendrocytes. In the absence of Foxa2 function, Hh activity leads to ectopic dorsal expression of several Hh pathway target genes, implying that Foxa2 also negatively regulates Hh activity within the ventral CNS by an unknown mechanism.
Acknowledgments
We thank Matthias Schäfer and other colleagues for reagents, Jon Clarke and members of our groups for discussions, and Marika Kapsimali, Juliette Mathieu, Gillian Brunt and Filippo Del Bene for comments on the manuscript. This work was supported by grants from the Wellcome Trust to S.W.W. and M.R.; from the BBSRC, Royal Society and EC to S.W.W.; from the German Human Genome project to R.G.; from the Japanese Ministry of Education, Science, Sports and Culture to H.T.; from the Deutsche Forschungsgemeinschaft by stipend DFG P807/1-1 to H.-M.P.; and from the National Multiple Sclerosis Society and Wadsworth Foundation to W.S.T. S.W.W. was a Wellcome Trust Senior Research Fellow.
Footnotes
Supplementary material Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/132/4/645/DC1
References
- Albert S, Müller F, Fischer N, Biellmann D, Neumann C, Blader P, Strähle U. Cyclops-independent floor plate differentiation in zebrafish embryos. Dev. Dyn. 2003;226:59–66. doi: 10.1002/dvdy.10211. [DOI] [PubMed] [Google Scholar]
- Amacher SL, Draper BW, Summers BR, Kimmel CB. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development. 2002;129:3311–3323. doi: 10.1242/dev.129.14.3311. [DOI] [PubMed] [Google Scholar]
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Barresi MJ, Stickney HL, Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–2199. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- Barth KA, Wilson SW. Expression of zebrafish nk2.2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development. 1995;121:1755–1768. doi: 10.1242/dev.121.6.1755. [DOI] [PubMed] [Google Scholar]
- Bellipanni G, Rink E, Bally-Cuif L. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Gene Expr. Patterns. 2002;2:251–256. doi: 10.1016/s1567-133x(02)00056-x. [DOI] [PubMed] [Google Scholar]
- Brand M, Heisenberg CP, Warga RM, Pelegri F, Karlstrom RO, Beuchle D, Picker A, Jiang YJ, Furutani-Seiki M, van Eeden FJ, et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–142. doi: 10.1242/dev.123.1.129. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Chen Y, Jessell TM, Struhl G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol. Cell. 2001;7:1279–1291. doi: 10.1016/s1097-2765(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Brösamle C, Halpern ME. Characterization of myelination in the developing zebrafish. Glia. 2002;39:47–57. doi: 10.1002/glia.10088. [DOI] [PubMed] [Google Scholar]
- Chang BE, Blader P, Fischer N, Ingham PW, Strähle U. Axial (HNF3beta) and retinoic acid receptors are regulators of the zebrafish sonic hedgehog promoter. EMBO J. 1997;16:3955–3964. doi: 10.1093/emboj/16.13.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier JB, Lapointe F, le Douarin NM, Teillet MA. Dual origin of the floor plate in the avian embryo. Development. 2002;129:4785–4796. doi: 10.1242/dev.129.20.4785. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen Sonic Hedgehog is an axonal chemoattractant that collaborates with Netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chen W, Burgess S, Hopkins N. Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development. 2001;128:2385–2396. doi: 10.1242/dev.128.12.2385. [DOI] [PubMed] [Google Scholar]
- Dickmeis T, Plessy C, Rastegar S, Aanstad P, Herwig R, Chalmel F, Fischer N, Strähle U. Expression profiling and comparative genomics identify a conserved regulatory region controlling midline expression in the zebrafish embryo. Genome Res. 2004;14:228–238. doi: 10.1101/gr.1819204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr. Biol. 1995;1:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, McMahon AP, Joyner AL. Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development. 1999;126:281–292. doi: 10.1242/dev.126.2.281. [DOI] [PubMed] [Google Scholar]
- Etheridge LA, Wu T, Liang JO, Ekker SC, Halpern ME. Floor plate develops upon depletion of tiggy-winkle and sonic hedgehog. Genesis. 2001;30:164–169. doi: 10.1002/gene.1056. [DOI] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Yamaguchi Y, Schilbach S, Wada T, Lee J, Goddard A, French D, Handa H, Rosenthal A. A regulator of transcriptional elongation controls vertebrate neuronal development. Nature. 2000;408:366–369. doi: 10.1038/35042590. [DOI] [PubMed] [Google Scholar]
- Hallonet M, Kaestner KH, Martin-Parras L, Sasaki H, Betz UA, Ang SL. Maintenance of the specification of the anterior definitive endoderm and forebrain depends on the axial mesendoderm: a study using HNF3beta/Foxa2 conditional mutants. Dev. Biol. 2002;243:20–33. doi: 10.1006/dbio.2001.0536. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signaling and gradient formation. Development. 2004;131:1563–1575. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Hatta K, Kimmel CB, Ho RK, Walker C. The cyclops mutation blocks specification of the floor plate of the zebrafish central nervous system. Nature. 1991;350:339–341. doi: 10.1038/350339a0. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Nose A, Eguchi G, Hotta Y, Okamoto H. Mindin/F-spondin family: novel ECM proteins expressed in the zebrafish embryonic axis. Dev. Biol. 1997;192:211–227. doi: 10.1006/dbio.1997.8760. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J. Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech. Dev. 2001;101:237–243. doi: 10.1016/s0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron. 1995;15:35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- Jacob J, Briscoe J. Gli proteins and the control of spinal-cord patterning. EMBO Rep. 2003;4:761–765. doi: 10.1038/sj.embor.embor896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Epstein DJ. Distinct regulators of Shh transcription in the floor plate and notochord indicate separate origins for this tissue in the mouse node. Development. 2003;130:3891–3902. doi: 10.1242/dev.00590. [DOI] [PubMed] [Google Scholar]
- Kaprielian Z, Runko E, Imondi R. Axon guidance at the midline choice point. Dev. Dyn. 2001;221:154–181. doi: 10.1002/dvdy.1143. [DOI] [PubMed] [Google Scholar]
- Knapik EW, Goodman A, Atkinson OS, Roberts CT, Shiozawa M, Sim CU, Weksler-Zangen S, Trolliet MR, Futrell C, Innes BA, et al. A reference cross DNA panel for zebrafish (Danio rerio) anchored with simple sequence length polymorphisms. Development. 1996;123:451–460. doi: 10.1242/dev.123.1.451. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Kunwar PS, Zimmerman S, Bennett JT, Chen Y, Whitman M, Schier AF. Mixer/Bon and FoxH1/Sur have overlapping and divergent roles in Nodal signaling and mesendoderm induction. Development. 2003;130:5589–5599. doi: 10.1242/dev.00803. [DOI] [PubMed] [Google Scholar]
- Latimer AJ, Shin J, Appel B. her9 promotes floor plate development in zebrafish. 2005. (in press) [DOI] [PubMed]
- Lele Z, Krone PH. Expression of genes encoding the collagen-binding heat shock protein (Hsp47) and type II collagen in developing zebrafish embryos. Mech. Dev. 1997;61:89–98. doi: 10.1016/s0925-4773(96)00626-0. [DOI] [PubMed] [Google Scholar]
- Leve C, Gajewski M, Rohr KB, Tautz D. Homologues of c-hairy1 (her9) and lunatic fringe in zebrafish are expressed in the developing central nervous system, but not in the presomitic mesoderm. Dev. Genes Evol. 2001;211:493–500. doi: 10.1007/s00427-001-0181-4. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Xu Q, Barth KA, Mikkola I, Holder N, Fjose A, Krauss S, Wilson SW. Regulatory gene expression boundaries demarcate sites of neuronal differentiation in the embryonic zebrafish forebrain. Neuron. 1994;13:1039–1053. doi: 10.1016/0896-6273(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Miura H, Yanazawa M, Kato K, Kitamura K. Expression of a novel aristaless related homeobox gene ‘Arx’ in the vertebrate telencephelon, diencephalon and floor plate. Mech. Dev. 1997;65:99–109. doi: 10.1016/s0925-4773(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Müller F, Chang B, Albert S, Fischer N, Tora L, Strähle U. Intronic enhancers control expression of zebrafish sonic hedgehog in floor plate and notochord. Development. 1999;126:2103–2116. doi: 10.1242/dev.126.10.2103. [DOI] [PubMed] [Google Scholar]
- Müller F, Albert S, Blader P, Fischer N, Hallonet M, Strähle U. Direct action of the nodal-related signal cyclops in induction of sonic hedgehog in the ventral midline of the CNS. Development. 2000;127:3889–3897. doi: 10.1242/dev.127.18.3889. [DOI] [PubMed] [Google Scholar]
- Müller F, Blader P, Strähle U. Search for enhancers: teleost models in comparative genomic and transgenic analysis of cis regulatory elements. BioEssays. 2002;24:564–572. doi: 10.1002/bies.10096. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Nusslein-Volhard C. fork head domain genes in zebrafish. Dev. Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Odenthal J, van Eeden FJ, Haffter P, Ingham PW, Nüsslein-Volhard C. Two distinct cell populations in the floor plate of the zebrafish are induced by different pathways. Dev. Biol. 2000;219:350–363. doi: 10.1006/dbio.1999.9589. [DOI] [PubMed] [Google Scholar]
- Park HC, Mehta A, Richardson JS, Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. 2002;248:356–368. doi: 10.1006/dbio.2002.0738. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Vallstedt A, Dias JM, Samad OA, Krumlauf R, Rijli FM, Brunet JF, Ericson J. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003;17:729–737. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda HM, Solnica-Krezel L, Driever W, Meyer D. The zebrafish forkhead transcription factor FoxH1/Fast1 is a modulator of nodal signaling required for organizer formation. Curr. Biol. 2000;10:1041–1049. doi: 10.1016/s0960-9822(00)00669-2. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Yan YL, Gates MA, Horne S, Amores A, Brownlie A, Donovan A, Egan ES, Force A, Gong Z, et al. Vertebrate genome evolution and the zebrafish gene map. Nat. Genet. 1998;18:345–349. doi: 10.1038/ng0498-345. [DOI] [PubMed] [Google Scholar]
- Rastegar S, Albert S, le Roux I, Fischer N, Blader P, Muller F, Strähle U. A floor plate enhancer of the zebrafish netrin1 gene requires Cyclops (Nodal) signalling and the winged helix transcription factor Foxa2. Dev. Biol. 2002;252:1–14. doi: 10.1006/dbio.2002.0837. [DOI] [PubMed] [Google Scholar]
- Rauch G-J, Granato M, Haffter P. A polymorphic zebrafish line for genetic mapping using SSLPs on high-percentage agarose gels. Tech. Tips Online. 1997:T01208. [Google Scholar]
- Rebagliati MR, Toyama R, Haffter P, Dawid IB. cyclops encodes a nodal-related factor involved in midline signaling. Proc. Natl. Acad. Sci. USA. 1998;95:9932–9937. doi: 10.1073/pnas.95.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Jessell TM, Roelink H. Restrictions to floor plate induction by hedgehog and winged-helix genes in the neural tube of frog embryos. Mol. Cell Neurosci. 1995;6:106–121. doi: 10.1006/mcne.1995.1011. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat. Rev. Cancer. 2002a;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat. Rev. Neurosci. 2002b;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Nguyen V, Palma V. The emergent design of the neural tube: prepattern, SHH morphogen and GLI code. Curr. Opin. Genet. Dev. 2003;13:513–521. doi: 10.1016/j.gde.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL. HNF-3 beta as a regulator of floor plate development. Cell. 1994;76:103–115. doi: 10.1016/0092-8674(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Schäfer M, Kinzel D, Neuner C, Schartl M, Volff J-N, Winkler C. Hedgehog and retinoid signalling confines nkx2. 2b expression to the lateral floor plate of the zebrafish trunk. Mech. Dev. 2004 doi: 10.1016/j.mod.2004.09.002. (in press) [DOI] [PubMed] [Google Scholar]
- Schauerte HE, van Eeden FJ, Fricke C, Odenthal J, Strähle U, Haffter P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Sirotkin HI, Gates MA, Kelly PD, Schier AF, Talbot WS. Fast1 is required for the development of dorsal axial structures in zebrafish. Curr. Biol. 2000;10:1051–1054. doi: 10.1016/s0960-9822(00)00679-5. [DOI] [PubMed] [Google Scholar]
- Strähle U, Blader P, Henrique D, Ingham PW. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 1993;7:1436–1446. doi: 10.1101/gad.7.7b.1436. [DOI] [PubMed] [Google Scholar]
- Strähle U, Jesuthasan S, Blader P, Garcia-Villalba P, Hatta K, Ingham PW. one-eyed pinhead is required for development of the ventral midline of the zebrafish (Danio rerio) neural tube. Genes Funct. 1997a;1:131–148. doi: 10.1046/j.1365-4624.1997.00010.x. [DOI] [PubMed] [Google Scholar]
- Strähle U, Fischer N, Blader P. Expression and regulation of a netrin homologue in the zebrafish embryo. Mech. Dev. 1997b;62:147–160. doi: 10.1016/s0925-4773(97)00657-6. [DOI] [PubMed] [Google Scholar]
- Strähle U, Lam CS, Ertzer R, Rastegar S. Vertebrate floor-plate specification: variations on common themes. Trends Genet. 2004;20:155–162. doi: 10.1016/j.tig.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Russell C, Regan J, Chandrasekhar A, Concha ML, Yokoyama R, Higashi K, Take-uchi M, Dong W, Hiraga T, Holder N, Wilson SW. Hedgehog and Fgf signaling pathways regulate the development of tphR-expressing serotonergic raphe neurons in zebrafish embryos. J. Neurobiol. 2004;60:275–288. doi: 10.1002/neu.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibert C, Teillet MA, Lapointe F, Mazelin L, le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;8:843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- Tian J, Yam C, Balasundaram G, Wang H, Gore A, Sampath K. A temperature-sensitive mutation in the nodal-related gene cyclops reveals that the floor plate is induced during gastrulation in zebrafish. Development. 2003;130:3331–3342. doi: 10.1242/dev.00544. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Amores A, Lewis KE, Yan YL, Postlethwait JH, Eisen JS, Westerfield M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–3509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE., Jr The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Wijgerde M, McMahon JA, Rule M, McMahon AP. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002;16:2849–2864. doi: 10.1101/gad.1025702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SW, Ross LS, Parrett T, Easter SS., Jr The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990;108:121–145. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- Woo K, Shih J, Fraser SE. Fate maps of the zebrafish embryo. Curr. Opin. Genet. Dev. 1995;5:439–443. doi: 10.1016/0959-437x(95)90046-j. [DOI] [PubMed] [Google Scholar]
- Woods IG, Kelly PD, Chu F, Ngo-Hazelett P, Yan YL, Huang H, Postlethwait JH, Talbot WS. A comparative map of the zebrafish genome. Genome Res. 2000;10:1903–1914. doi: 10.1101/gr.10.12.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–251. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]