Abstract
A survey conducted as part of an International Workshop on Genotoxicity Testing (IWGT) has identified a number of compounds that appear to be more readily detected in vivo than in vitro. The reasons for this property varies from compound to compound and includes metabolic differences; the influence of gut flora; higher exposures in vivo compared to in vitro; effects on pharmacology, in particular folate depletion or receptor kinase inhibition. It is possible that at least some of these compounds are detectable in vitro if a specific in vitro test is chosen as part of the test battery, but the ‘correct’ choice of test may not always be obvious when testing a compound of unknown genotoxicity. It is noted that many of the compounds identified in this study interfere with cell cycle kinetics and this can result in either aneugenicity or chromosome breakage. A decision tree is outlined as a guide for the evaluation of compounds that appear to be genotoxic agents in vivo but not in vitro. The regulatory implications of these findings are discussed.
Keywords: IWGT, Genotoxicity tests, In vivo, Rodent bone marrow, Micronucleus test, In vivo-only positive compounds, ADME, In vitro versus in vivo metabolism, Sex-specific metabolism, Influence of gut flora, Pharmacological mechanisms, Kinase inhibitors, Regulatory implication, Pharmaceuticals, Cosmetics, Food additives, In vitro-only genotoxicity test batteries
1. Introduction
In vivo genotoxicity tests are included in most regulatory batteries for two purposes. The first is to put any positive results obtained in vitro into perspective, i.e., to determine if the genotoxic potential observed in vitro is realised in vivo. The second is to ensure that genotoxic carcinogens that are not detected or are difficult to detect in vitro, but do cause detectable genotoxic damage in the tissues of an intact animal, are recognised. This later issue is addressed in the ICH guideline for pharmaceuticals (S2B—Genotoxicity: A Standard Battery for Genotoxicity Testing of Pharmaceuticals) which states that “An in vivo test for genetic damage should usually be a part of the test battery to provide a test model in which additional relevant factors (absorption, distribution, metabolism, excretion) that may influence the genotoxic activity of a compound are included”. As a result, in vivo tests permit the detection of some additional genotoxic agents. Note 5 of the guideline states that, “There are a small but significant number of genotoxic carcinogens that are reliably detected by bone marrow tests for chromosomal damage that have yielded negative/weak/conflicting results in the pairs of in vitro tests outlined in the standard battery options … Carcinogens such as procarbazine, hydroquinone, urethane and benzene fall into this category”.
An IWGT working group was formed to examine the published data supporting the existence of genotoxic agents only detectable in vivo and through the use of a questionnaire, determine if there are further unpublished data on additional compounds that may fall into this category [1].
This paper does not review all possible ‘unique’ in vivo-positive compounds from the literature but is focussed on previously unpublished data obtained from company archives via the IWGT questionnaire (Appendix I). The exceptions include urethane, salicylazosulfapyridine, sulfapyridine and morphine. Urethane is regarded by the working group as a well-studied example of an in vivo-only positive. Data on the other compounds were brought to our attention in response to the IWGT questionnaire.
2. Analysis of possible in vivo-only positives from the literature
2.1. Urethane (ethyl carbamate) and benzene
Urethane has been recognised as a carcinogen since the 1940s and induces a variety of tumours in rodents, including tumours of the lung (alveolar/bronchiolar adenoma or carcinoma) and liver (hemangioma or angiosarcoma) [2]. In addition, lymphomas, melanomas, and vascular tumours have been reported [3]. Urethane has been extensively tested in a variety of in vitro tests for genotoxicity. There are sporadic reports of positive results for urethane in in vitro tests (usually in the presence of rat liver S9) [4–6], but only when tested at concentrations above internationally agreed-upon limits for relatively non-toxic compounds, i.e., 5 mg/plate in the Ames test and 10 mM in cultured mammalian cell tests. At or below these limits, the compound is uniformly negative. Negative results have been reported for the Ames assay, the human lymphoblastoid TK6 TK mutation test, chromosome aberration tests in a variety of cell lines, and the in vitro UDS test in primary rat hepatocytes [7].
The situation is very different in vivo. Urethane gives strongly positive results in mouse bone marrow micronucleus tests, as has been shown by many laboratories. In a typical study using CBA mice given 900 mg/kg (i.p. or oral), up to 30-fold increases in micronucleated polychromatic erythrocytes (PCEs) were observed relative to concurrent control values [8] (Table 1). In addition, weak, but statistically significant, positive results have been detected in the Muta™ Mouse following single i.p. dosing with 900 mg/kg, where increases in mutant frequency in lung and liver (equivocal results in bone marrow and spleen) were seen [9]. Similar results have also been obtained in an evaluation of mutant frequencies of chemically induced tumours and normal tissues in lambda/cII transgenic mice treated with urethane [10]. Urethane-associated adducts are formed in the DNA of lung and liver cells from exposed mice (the principal sites for urethane-induced carcinogenesis) [11].
Table 1.
Activity of urethane in the mouse bone marrow micronucleus test
| Agent | Dose (mg/kg) | Route | PCE/NCE ratio (±S.D.) | MNPCE/1000 PCE (±S.D.) |
|---|---|---|---|---|
| Vehicle control | – | Oral | 1.1 (0.05) | 2.6 (1.6) |
| – | i.p. | 1.05 (0.08) | 2.9 (0.9) | |
| Urethane | 900 | Oral | 0.66 (0.09) | 62.2 (10.8) |
| 900 | i.p. | 0.55 (0.05) | 67.9 (11.0) |
Data taken from Ashby et al. [8]. Assay was carried out in male CBA mice; bone marrow was sampled 24 h after dosing. i.p.: intraperitoneal; PCE: polychromatic erythrocyte; NCE: normochromatic erythrocyte; MNPCE: micronucleated PCE.
The most widely accepted hypothesis for the discrepancy between the in vitro and in vivo genotoxicity profiles of urethane is that the S9 used for metabolic activation in many in vitro assays is deficient in the specific cytochromes P450 (CYPs) and possibly other enzymes, necessary to metabolize urethane to its ultimate genotoxic metabolites; by contrast, these metabolites are readily formed in vivo. Urethane metabolism is known to require CYP2E1 and carboxylesterase isozyme hydrolase A [12]. The metabolic activation route is thought to involve C-hydroxylation to form vinyl carbamate, which is then converted to an epoxide that can interact with nucleic acids [13]. This scheme is supported by the observation that urethane-induced bone marrow micronucleus frequencies are reduced in CYP2E1-null mice [14]. Attempts have been made to detect urethane in vitro using rat liver S9 from animals pre-treated with CYP2E1 inducers such as ethanol, but these were unsuccessful [15].
Another important example is benzene, a known human carcinogen that gives a strong in vivo response whilst being weak or negative in in vitro assays, benzene undergoes complex metabolism in vivo, which may be difficult to reproduce in vitro. The precise metabolites involved in carcinogenicity or indeed the mechanism of carcinogenicity induced by this molecule or its metabolites are not known, but may involve inhibition of DNA topoisomerase II [16].
2.2. Salicylazosulfapyridine and sulfapyridine
The mutagenicity profile of salicylazosulfapyridine (SASP), an anti-inflammatory drug used for over 50 years, is detailed by Bishop et al. [17]. Ames tests were negative at concentrations up to 5 mg/plate. Similarly CHO chromosomal aberration (and sister-chromatid exchange [SCE]) studies were negative at concentrations up to 1 mg/ml. However, 20–160 (µg/ml produced positive responses in human lymphocyte chromosomal aberration (and SCE) assays.
Bone marrow micronucleus tests in male B6C3F1 mice using single oral doses of SASP of up to 1000 mg/kg were negative; but weak, statistically significant and dose-related increases were seen when animals received 500, 1000 or 2700 mg/kg for 3 days. Micronucleus tests in peripheral blood erythrocytes from male and female B6C3F1 mice were clearly positive at doses of 675, 1350 or 2700 mg/kg given orally for 90 days (Table 2). Sulfapyridine (SP), a major metabolite of SASP, was subsequently shown to have the same profile, i.e., negative for chromosomal aberrations (although SCEs were induced) in CHO cells, but positive in the mouse bone marrow micronucleus test [18]. Further work showed that both SASP and SP are strong inducers of kinetochore-positive micronuclei in vivo. Although small increases in kinetochore-negative micronuclei also were observed in SP treated mice, as well as in mice receiving the highest test dose of SASP (Table 3), the results suggest that both chemicals are predominantly aneugens [19]. Increases in micronucleus and SCE frequencies have been reported in patients treated with SASP for inflammatory bowel diseases (IBD) for one or more months, although there were confounding factors in these studies [20].
Table 2.
Mouse peripheral blood micronucleus test of salicylazosulfapyridine (SASP)
| Agent | Dose (mg/kg) | MNPCE/1000 PCE (±S.D.) | MNNCE/1000 NCE (±S.D.) |
|---|---|---|---|
| Vehicle control | – | 1.71 (0.32) | 1.07(0.08) |
| SASP | 675 | 2.27 (0.42) | 2.46a(0.17) |
| 1350 | 3.42a (0.45) | 2.94a (0.22) | |
| 2700 | 3.66a (0.41) | 2.78a (0.20) |
Data from male B6C3F1 mice (five per group); peripheral blood was obtained at the termination of a 90-day oral gavage NTP toxicity study. At least 2000 PCEs and 10,000 NCEs from each animal were scored for micronuclei [17]. PCE: polychromatic erythrocyte; NCE: normochromatic erythrocyte; MNPCE: micronucleated PCE; MNNCE: micronucleated NCE.
p <0.01, Cochran–Armitage test.
Table 3.
Mouse bone marrow micronucleus tests of SASP and SP with kinetochore (KC) staining
| Agent | Treatment (mg/kg) | MNPCE/1000 PCE (±S.D.) |
||
|---|---|---|---|---|
| Total MN | KC− | KC+ | ||
| Vehicle control | – | 1.6 (0.19) | 1.6 (0.19) | 0.0 |
| SASP | 1875 | 4.5 (0.52) | 2.2a (0.20) | 2.3 (0.44) |
| 2721 | 5.3 (0.34) | 2.8a (0.34) | 2.5 (0.27) | |
| 3649 | 6.3 (0.68) | 2.1a (0.29) | 4.2 (0.58) | |
| SP | 2083 | 7.8 (0.88) | 4.2 (0.58) | 3.6 (0.37) |
| 2721 | 9.5 (0.42) | 3.8 (0.25) | 5.7 (0.37) | |
| 3472 | 14.2 (1.56) | 5.7 (1.33) | 8.5 (0.65) | |
| TEM (positive control) | 1.0 | 100.2 (4.94) | 96.3 (4.68) | 3.9 (0.58) |
| VCR (positive control) | 0.125 | 83.0 (2.91) | 6.7 (0.94) | 76.3 (3.00) |
Data from Witt et al. [19]. Five animals per dose group; 2000 PCEs scored per animal; vehicle control was corn oil. PCE: polychromatic erythrocyte; MNPCE: micronucleated PCE; MN: micronuclei; SASP: salicylazosulfapyridine; SP: sulfapyridine; TEM: triethylenemelamine; VCR: vincristine sulfate; KC−: kineticore negative; KC+: kineticore positive.
Not statistically significant, otherwise all pairwise comparisons of dosed groups to control were statistically significant at p < 0.01.
Bishop et al. [17] suggest that the results they observed in vivo could have been due to the induction or exacerbation of folate deficiency. Folate deficiency is known to cause chromosomal aberrations and fragile-site expression. Sulfa drugs,as a class, are known to inhibit p-aminobenzoic acid uptake and to inhibit the formation of folic acid by gut flora. SASP therapy is associated with impaired folic acid absorption, although serum folate levels in IBD patients were low, they were still in the normal range when tested [21]. It has now been established that SASP does depress folate levels significantly in treated patients [22,23]. Humans are particularly sensitive to micronucleus induction due to limiting folate or B12 levels [24,25].
Reticulocytosis was observed in a 90-day study of SASP in mice [26], which also suggests that SASP may have a rebound erythropoietic effect following a haemotoxic insult, which may also contribute to induction of micronuclei in bone marrow cells (see accompanying paper, this issue).
SASP treatment increases the number of urinary bladder tumours in F344 rats and liver tumours in B6C3F1 mice, when the animals are maintained under ad libitum (AL) feeding conditions; under a feed restriction (FR) regimen, these tumours were not increased [23]. With regard to the etiology of the bladder tumours, SASP caused intraluminal bladder changes in the rat (especially males) consisting of chronic urothelial stimulation, concretions, and hyperplasia, which resulted in neoplasia. With regard to the mouse liver tumours, chronic hepatocellular toxicity was observed, resulting in preneoplasia and neoplasia within 2 years. Thus, it is probable that these rodent tumours are not induced as a consequence of the direct genotoxicity of the test agent.
To follow up this work, SASP and its two major metabolites, 5-aminosalicylic acid (ASA) and SP, were tested for the induction of micronuclei in mouse bone marrow, with or without pre-treatment with folate, and for the formation of DNA adducts in rat and mouse liver and urinary bladder [27]. None of the compounds exhibited genotoxicity or DNA reactivity under the protocols used. However, the authors of this paper stated that, without folate supplementation, SASP is an aneugen, and thus the folate deficiency associated with SASP administration is probably responsible for its in vivo genotoxicity in lymphocytes and erythrocytes.
From the genotoxicity profile above, it is clear that SASP is detected as a genotoxic agent in vitro if chromosome damage is measured in human peripheral lymphocytes, but not if Chinese hamster cells are used. Thus, if SASP were a new drug candidate and the testing laboratory concerned routinely used Chinese hamster cells, this activity would have been missed. Why is there a difference between these two cell types for the genotoxicity of SASP? SASP undergoes acetylation in vivo, and CHO cells are known to be poor acetylators [28], whilst acetylated metabolites of SASP have been shown to induce chromosome damage [29]. It is possible that human lymphocytes are better or more rapid acetylators than Chinese hamster cells and that this is responsible for the differences in ability to detect SASP as a genotoxic agent. In terms of the differences in the in vitro and in vivo genotoxicity profiles, Bishop et al. [17] conclude that the differences in distribution and metabolism of SASP, its cleavage into SP and 5-ASA metabolites by gut flora, the absorption of these metabolites, their acetylation and/or hydroxylation in the liver, their reaction with macromolecules, and their subsequent elimination from the body are all factors for understanding differences between species and cell types in the amounts and types of chromosome damage induced by SASP.
2.3. Morphine
According to the Physicians Desk Reference (PDR) [30], no formal studies to assess the mutagenic potential of morphine have been conducted for the FDA. However, literature studies are cited and include results showing that morphine was non-mutagenic in the Drosophila melanogaster sex-linked recessive lethal mutation assay and produced no evidence of chromosomal aberrations when incubated with murine splenocytes. However, as described in the PDR, morphine increased DNA fragmentation when incubated in vitro with a human lymphoma cell line, and in vivo, morphine produced an increase in the frequency of micronuclei in bone marrow cells and in immature red blood cells in the mouse micronucleus test, and induced chromosomal aberrations in murine lymphocytes and spermatids. The product labelling states that some of the in vivo clastogenic effects reported with morphine in mice may be directly related to increases in glucocorticoid levels produced by morphine in this species.
Morphine has been reported to produce apoptosis in human peripheral lymphocytes [31,32], and thus the DNA fragmentation in the human lymphoma cell line reported in the product labelling may be attributed to apoptosis. The induction of micronuclei in vivo has also been discussed in the literature [33], and it was concluded that the genotoxic response is opioid receptor-mediated because it was abolished in adrenalectomized animals. Further, plasma from morphine-treated animals also induced micronuclei in naive lymphocytes in vitro; this response was blocked by inclusion of the steroid antagonist RU 486 in the incubation mixture. Despite this hypothesis for the responses in the in vivo micronucleus test, others [34] have concluded that, although basal levels of glucocorticosteroids are required for induction of micronuclei by morphine in murine splenocytes, activation of the hypothalamo-pituitary-adrenal (HPA) axis by morphine does not contribute to the observed response. This is based on studies with N-methylmorphine, which did not stimulate the release of corticosterone from adrenal glands, yet induced micronuclei in splenocytes. Also metyrapone, an inhibitor of corticosterone biosynthesis, blocked the morphine-induced increase in corticosterone secretion, but had no effect on the frequency of micronuclei.
An alternative explanation for the in vivo micronucleus effect is that it is a consequence of hypothermia, which is caused in rodents by morphine [30,35] (see accompanying paper). Since the data from the chromosomal aberration effects in mouse lymphocytes has not been evaluated independently, it is not clear whether morphine is a unique in vivo positive, but this is worthy of further study. Increases in DNA fragmentation (Comet assay) and HPRT mutations have been reported in the human HUT-78 cell line exposed to morphine in vitro [36].
3. Candidate in vivo-only positives identified by the questionnaire
3.1. Alimta® (pemetrexed)
Alimta® is indicated for the treatment of malignant pleural mesothelioma in combination with cisplatin. It also is indicated for the treatment of locally advanced or metastatic non-small cell lung cancer after prior chemotherapy. Alimta® is an antifolate agent that exerts its action by disrupting folate-dependent metabolic processes essential for cell replication. No carcinogenicity tests have been carried out. However, Alimta® administered at i.v. doses of 0.1 mg/(kg day) or greater to male mice (about 1/1666 the recommended human dose on a mg/m2 basis) reduced fertility, and induced hypospermia and testicular atrophy.
Ames tests were negative up to 250 µg/plate, which was the lowest precipitating concentration. Alimta® also was negative in chromosome aberration and Hprt mutation assays using CHO cells at concentrations up to 2099 µg/ml (limited by solubility). Two bone marrow micronucleus studies were carried out on Alimta® in ICR mice. Both sexes were dosed intravenously with 393, 787, and 1574 mg/kg. Two doses were given 24 h apart, with the animals killed 24 h after the last dose. Bone marrow cells were stained with Acridine Orange. The ratio of PCEs to normochromatic erythrocytes (NCEs) was not affected at any dose, but Alimta® was positive in both sexes in both tests, with significant differences in two-tailed trend tests and also in pairwise comparisons between individual doses and the controls (Table 4).
Table 4.
Mouse bone marrow micronucleus test of Alimta®
| Agent | Dose (mg/kg) | PCE/NCE ratio (±S.D.) | MNPCE/1000 PCE (±S.D.) |
|---|---|---|---|
| Vehicle control | – | 1.2 (0.3) | 1.2 (1.1) |
| Alimta® | 393.5 | 1.1 (0.4) | 5.0 (2.4) |
| 787.1 | 0.7 (0.2) | 4.8 (2.2) | |
| 1574.1 | 0.8 (0.1) | 4.3a (2.2) | |
| Cyclophosphamide (positive control) | 25.0 | 1.0 (0.2) | 16.4 (1.5) |
Two equal i.v. doses were given 24 h apart to ICR mice, with harvest 24 h after the second treatment. Male mouse data shown (five per group); similar results obtained in female mice. PCE: polychromatic erythrocyte; NCE: normochromatic erythrocyte; MNPCE: micronucleated PCE.
p = 0.02, treated groups were significantly increased relative to the vehicle control as determined by a two-tailed trend test.
The pharmacology of the test compound indicates that Alimta® causes a decrease in thymidine levels along with a concomitant increase in uridine levels, which may have disrupted normal DNA replication. Why this should affect micronucleus induction in cells in vivo, and not in vitro, is unclear.
3.2. Pfizer and AstraZeneca MEK kinase inhibitors
The genotoxicities of two MEK kinase (Mitogen Extracellular Kinase kinase that activates mitogen-activated protein kinase) inhibitors of the same structural class developed by Pfizer were studied in vitro and in vivo. In mini-Ames tests, both were negative at concentrations up to 5 mg/plate. In vitro micronucleus tests using CHO WBL cells also were negative using a concentration range of 32–125 µg/ml (+/− S9); the highest concentration was limited by cytotoxicity. Similarly chromosome aberration tests using human peripheral lymphocytes (3 h exposure +/− S9 and 24 h −S9) were negative (no increases in chromosome aberrations or polyploidy) when tested at concentrations inducing up to 50% inhibition of the mitotic index (300–400 µg/ml).
The first compound, MEK1, was tested in a rat bone marrow micronucleus test in males and females. The compound was administered once daily for 2 days by oral gavage at doses of 0.3, 1.0 and 3.0 mg/kg and the bone marrow harvested 24 h after the second dose. Increases were seen at the highest dose indicative of clastogenicity, although the effects seen were within the historical control range for this laboratory (Tables 5a and 5b). An in vivo metaphase analysis test was carried out in bone marrowcells of Sprague–Dawley rats (Tables 6a and 6b). The compound was administered at the same doses as the micronucleus test, using the same dosing regimen. Although increases were seen in the highest dose group in both sexes, possibly indicating a weak clastogenic effect, the increases were not significant. No aberrations were observed in male control animals whereas at 3 mg/kg MEK1, 2.6% aberrant cells were observed; in females 0.2% aberrant cells were observed in the cells of the negative controls and 1.0% aberrant cells were seen at 3.0 mg/kg MEK1.
Table 5.
| Table 5a Rat bone marrow micronucleus test of Pfizer MEK kinase inhibitor 1 (MEK1) in male animals | |||
|---|---|---|---|
| Agent | Dose (mg/kg) | PCE/NCE ratio | MNPCE/1000 PCE (±S.D.) |
| Vehicle control | –a | 7.3 | 2.0 (0.82) |
| MEK1 | 0.3 | 11.5 | 1.8 (0.84) |
| 1.0 | 8.1 | 1.8 (0.45) | |
| 3.0 | 10.0 | 3.2 (1.3) | |
| Cyclophosphamide (positive control) | 20.0 | 5.3 | 5.8 (1.1) |
| Table 5b Rat bone marrow micronucleus test of Pfizer MEK kinase inhibitor 1 (MEK1) in female animals | |||
|---|---|---|---|
| Agent | Dose (mg/kg) | PCE/NCE ratio | MNPCE/1000 PCE (±S.D.) |
| Vehicle control | –a | 5.7 | 2.4 (1.1) |
| MEK1 | 0.3 | 6.1 | 2.2 (0.8) |
| 1.0 | 5.3 | 2.8 (0.45) | |
| 3.0 | 5.3 | 5.4b (1.1) | |
| Cyclophosphamide (positive control) | 20.0 | 2.1 | 10.8b (2.4) |
Doses were administered to groups of five Sprague–Dawley rats once daily for 2 days by oral gavage and the bone marrow harvested 24 h after the final dose. PCE: polychromatic erythrocyte; NCE: normochromatic erythrocyte; MNPCE: micronucleated PCE.
0.5% methylcellulose/0.2% Tween 80 in water.
Statistically significant, p < 0.05.
Table 6.
| Table 6a In vivo metaphase analysis of rat bone marrow cells from male animals dosed with Pfizer MEK1 inhibitor | |||
|---|---|---|---|
| Agent | Dose (mg/kg) | % Mitotic index | % Cells with chromosomal aberrations (±S.D.) |
| Vehicle control | –a | 5.9 | 0.0 |
| MEK1 | 0.3 | 4.5 | 0.6 (0.4) |
| 1.0 | 6.7 | 0.6 (0.4) | |
| 3.0 | 4.2 | 2.6 (7.0) | |
| Cyclophosphamide (positive control) | 60.0 | 0.9 | 62.4b (5.15) |
| Table 6b In vivo metaphase analysis of rat bone marrow cells from female animals dosed with Pfizer MEK1 inhibitor | |||
|---|---|---|---|
| Agent | Dose (mg/kg) | % Mitotic index | % Cells with chromosomal aberrations (±S.D.) |
| Vehicle control | –a | 7.2 | 0.2 (0.2) |
| MEK1 | 0.3 | 6.7 | 0.2 (0.2) |
| 1.0 | 7.6 | 0.4 (0.24) | |
| 3.0 | 3.6 | 1.0 (0.77) | |
| Cyclophosphamide (positive control) | 60.0 | 1.0 | 89.8b (4.8) |
Doses were administered to groups of five Sprague–Dawley rats once daily for 2 days (except for the positive control which was administered only once, 24 h before bone marrow harvest) by oral gavage and the bone marrow harvested 24 h after the final dose.
0.5% methylcellulose/0.2% Tween 80 in water.
Statistically significant, p < 0.05.
MEK2 was tested in the rat bone marrow micronucleus test in males and females. Animals were given two consecutive doses of 50, 100, and 300 mg/kg and the bone marrows were sampled 24 h after the last dose. Significant increases in micronucleated PCE were observed in males at 100 and 300 mg/kg, 2.7- and 4.0-fold relative to the negative control (Table 7), whilst significant increases in micronucleated PCEs were seen in females at all doses (3.4- to 6.4-fold relative to the control). A second test was carried out in males with 4 weeks of dosing with lower doses (10, 25 and 50 mg/kg). This test was negative (data not shown) establishing ‘no-detectable effect’ levels.
Table 7.
Rat bone marrow micronucleus test of Pfizer MEK kinase inhibitor 2 (MEK2)
| Agent | Dose (mg/kg) | PCE/NCE ratio | MNPCE/1000 PCE (±S.D.)a |
|---|---|---|---|
| Vehicle | –b | 2.7 | 4.4 (0.6) |
| MEK2 | 50 | 4.0 | 4.2 (1.2) |
| 100 | 2.3 | 11.8 (2.8) | |
| 300 | 1.0 | 17.5 (2.4) | |
| Cyclophosphamide (positive control) | 20 | 1.9 | 13.0 (1.1) |
Doses were administered to groups of five Sprague–Dawley rats once daily for 2 days by oral gavage and the bone marrow harvested 24 h after the final dose. Data from male animals shown; female animals gave comparable results. PCE: polychromatic erythrocyte; NCE: normochromatic erythrocyte; MNPCE: micronucleated PCE.
Statistical analysis not provided.
0.5% methylcellulose/0.2% Tween 80 in water.
AstraZeneca also provided genotoxicity data for a selective inhibitor of human MEK kinase with an in vitro IC50 of ~10 nmol/l. The compound was negative in a standard regulatory Ames test and a mouse lymphoma Tk assay, but the compound was clearly positive in two independent mouse bone marrow micronucleus tests (Table 8). In the second of these, up to 82% of the micronuclei contained centromere-staining material, indicating a primarily aneugenic mode of action. In vitro micronucleus tests in mouse lymphoma cells were inconclusive. MEK is necessary for normal mitotic spindle function [37,38], so it is not surprising that aneugenicity was observed in micronucleus tests. It is possible that the regulatory functions controlled by MEK and MAPK become altered in established cell lines. It would be of interest to determine if MEK inhibitors induce aneugenicity in primary cells, such as cultured human peripheral lymphocytes.
Table 8.
Mouse (ICR) bone marrow micronucleus tests of the AstraZeneca MEK kinase inhibitor
| Agent | Dose (mg/kg) | MNPCE/1000 PCE |
% Centromere positive MN from experiment 2 |
||
|---|---|---|---|---|---|
| Experiment 1 (males) | Experiment 1a (females) | Experiment 2b (males) | |||
| Vehicle control | 0 | 0.4 | 0.5 | 1.15 | 10 |
| Test agent | 500 | 0.3 | 0.5 | 2.55c | 82 |
| 1000 | 0.7 | 0.5 | 3.28c | 74 | |
| 2000 | 2.5c | 2.9c | 2.72c | 39 | |
| CPA (positive control) | 50 | 19.3 | 21.5 | – | – |
| 65 | – | 27.5 | – | ||
All data is from bone marrows sampled 24 h after oral dosing (no effect at 48 h). PCE: polychromatic erythrocyte; MNPCE: micronucleated PCE; MN: micronuclei; CPA: cyclophosphamide.
Erythrocytes stained with May–Gruenwald–Giemsa.
Erythrocytes stained with Acridine Orange.
p < 0.05.
3.3. Merck compound B (a kinase inhibitor)
Data on this compound were obtained via the IWGT questionnaire and were previously unpublished. The compound was negative in Ames tests up to 6 mg/plate, and CHO cell chromosome aberration assays were negative when tested to 100 µM. Micronucleus tests also were conducted in CHO cells, and one assay showed a slight increase at a single concentration and sampling time (1.1–2.6%) that was not reproduced in second assay when tested up to 120 µM. As is normal practise at Merck, alkaline elution tests for DNA breakage were carried out in primary rat hepatocytes (at concentrations up to 40 µM); these tests were also negative.
Compound B produced strong positive responses in the in vivo mouse bone marrow micronucleus test at 50, 100, 200 mg/kg (a no-effect level was not achieved). A marked suppression in the PCE/NCE ratio was observed at all the test doses, indicating bone marrow toxicity (Table 9). No mechanistic studies, e.g., kinetochore staining, were done to determine if the micronuclei observed were chromosome fragments or whole chromosomes.
Table 9.
Mouse micronucleus assay of Merck compound B kinase inhibitor
| Agent | Dose (mg/kg) | 24 h |
48 h |
||
|---|---|---|---|---|---|
| PCE/NCE ratio | MNPCE/1000 PCE | PCE/NCE ratio | MNPCE/1000 PCEa | ||
| Solvent control | 0 | 0.73 | 1.7 | 0.96 | 1.5 |
| Compound B | 50 | 0.30 | 23.0 | 0.05 | 19.3 |
| 100 | 0.13 | 37.9 | 0.06 | 53.2 | |
| 200 | 0.13 | 29.8 | 0.05 | 57.4 | |
| Mitomycin C (positive control) | 2.0 | 0.90 | 59.5 | – | – |
Mice were dosed orally in groups of five males; one set of groups was killed 24 h after dosing, the second set after 48 h. 2000 PCEs scored for micronuclei. PCE: polychromatic erythrocyte; MNPCE: micronucleated PCE.
Statistical analysis not provided.
There was no evidence for a mouse-specific metabolite or evidence for in vivo metabolites not seen in vitro. It is possible that the in vivo micronucleus induction was related to the high levels of bone marrow toxicity seen at all test doses (some apoptotic nuclei were observed).
A limited bone marrow micronucleus test (single rat per dose level) was subsequently carried out in rats (3 daily oral gavage doses, sacrificed 6 h after the last dose, 2000 PCEs scored/rat). Whereas firm conclusions cannot be drawn from this study, it is of interest to note that no effects were seen at doses of compound B below 100 mg/kg, but sharp increases in micronuclei were observed at both 100 and 200 mg/kg at relatively moderate levels of bone marrow suppression as measured by the PCE/NCE ratio (Table 10).
Table 10.
Bone marrow micronucleus assay of compound B kinase inhibitor in the rat
| Compound B dose (mg/(kg day)) | MNPCE/1000 PCE’s | PCE/NCE ratioa |
|---|---|---|
| 0 (vehicle control, 3 rats) | 1.0 | 1.20 (range 0.93–1.50) |
| 12.5 | 1.5 | 0.46 |
| 25 | 1.0 | 1.10 |
| 50 | 1.5 | 0.89 |
| 100 | 10.5 | 0.27 |
| 200 | 6.5 | 0.43 |
Three daily oral gavage doses, sacrificed 6 h after last dose; except as noted, single rat per dose level; 2000 PCEs scored per rat. PCE: polychromatic erythrocyte; MNPCE: micronucleated PCE.
Statistical analysis not provided.
Merck has conducted genotoxicity assays on three other kinase inhibitors from the same series; two produced the same profile as compound B, i.e., negative in vitro and positive in vivo, whilst the third was negative in all the tests (Sheila Galloway, pers. commun.).
3.4. Pharmacia example
Data on this example, a receptor kinase inhibitor, were received through the IWGT questionnaire. The Ames test was negative at concentrations up to 317 µg/plate (+/− S9). Chromosome aberration tests using human peripheral lymphocytes were negative up to the relatively low concentrations of 3 µg/ml (−S9) and 10 µg/ml (+S9); the test concentrations were limited by toxicity.
The test compound produced a weak, but statistically positive response in a rat bone marrow micronucleus test conducted at doses up to 1000 mg/kg (1.1 MNPCE/1000 PCE in the negative control, maximum 2.5 MNPCE/1000 PCE in treated animals) (Table 11). Further studies on this compound are planned, including a rat bone marrow metaphase analysis to help determine the mechanism of chromosome damage (clastogenicity versus aneugenicity).
Table 11.
Rat bone marrow micronucleus test with pharmacia compound X
| Treatment | Dose | MNPCE/1000 PCE (±S.D.) | PCE:NCE (±S.D.) |
|---|---|---|---|
| Vehicle | Methylcellulose | 1.1 (0.02) | 0.98 (0.07) |
| Cyclophosphamide (positive control) | 60 mg/kg | 21.7a (0.24) | 0.79 (0.06) |
| Compound X | 250 mg/(kg day) | 1.8a (0.02) | 1.06 (0.04) |
| 500 mg/(kg day) | 2.2a (0.03) | 1.14 (0.06) | |
| 1000 mg/(kg day) | 2.5a (0.02) | 1.04 (0.06) |
PCE: polychromatic erythrocyte; NCE: normochromatic erythrocyte; MNPCE: micronucleated PCE.
Significantly greater than corresponding vehicle control, p < 0.05.
3.5. Roche example 1
Data on this compound were received through the IWGT questionnaire. Example 1 is a tubulin synthesis inhibitor developed for anti-cancer chemotherapy. Ames tests were negative up to 5 mg/plate. The compound was highly toxic in the mouse lymphoma Tk assay (maximum tested concentrations −S9: 0.02 µg/ml for 3 h treatment, 0.004 µg/ml for 24 h treatment; +S9: 3 µg/ml for both 3 and 24 h treatments). No clear treatment-related increase in mutant frequency was detected.
A mouse bone marrow micronucleus test was carried out by dosing animals i.v. with 1, 2, and 4 mg/kg of example 1, and assaying the animals 24 h later. Non-dose-related increases were observed at all doses, up to a maximum eight-fold increase in MNPCEs (at the lowest dose). There was a marked dose-related reduction of the PCE/NCE ratio, indicating bone marrow toxicity (Table 12). The response was similar to the colcemid positive control.
Table 12.
Mouse bone marrow micronucleus test of Roche tubulin inhibitor, compound 1
| Agent | Dose (mg/kg) | PCE/NCE ratio | MNPCE/1000 PCE |
|---|---|---|---|
| Vehicle control | 0 | 1.82 | 1.0 |
| Compound 1 | 1.0 | 0.50 | 8.5a |
| 2.0 | 0.42 | 5.0a | |
| 4.0 | 0.18 | 5.0a | |
| Colcemid (positive control) | 2.5 | 0.75 | 10.7a |
Mice were given single i.v. doses (apart from the positive control which was dosed orally), and the bone marrow harvested 24 h later. PCE: polychromatic erythrocyte; NCE: normochromatic erythrocyte; MNPCE: micronucleated PCE.
p < 0.01.
Further analyses indicated that the toxicity to the bone marrow was progressive with time in that the PCE/NCE ratio declined a further 4.5-fold in next 24 h.
In vitro micronucleus tests of intermediates in the chemical synthesis of example 1, which are structurally related to the parent compound, were clearly positive; thus, it is possible that the parent compound itself may have produced positive responses in vitro if such a test had been used in addition to the mouse lymphoma test.
3.6. AstraZeneca examples
Data on these compounds were received through the IWGT questionnaire and have not been published elsewhere. Compounds A–C are compounds from the same chemical series and have similar pharmacological profiles; B and C are very closely related in both structure and pharmacology. All three compounds were completely negative in regulatory Ames tests and either a mouse lymphoma Tk assay or an in vitro cytogenetics assay using lymphocytes in whole blood.
Compounds A and B were tested in chronological order; bone marrow micronucleus tests were negative using male rats treated up to the maximum tolerated doses (MTDs) of each compound, 1000 mg/kg A and 250 mg/kg B. In marked contrast, compound C was positive in the rat micronucleus test at doses from 25 mg/kg to the MTD, 760 mg/kg (Table 13). Metabolism data then showed that there was less extensive metabolism in female rats than in males, and little metabolism in any in vitro system. Therefore, compound C was tested in the bone marrow micronucleus test in female rats, with negative results up to the MTD, 380 mg/kg (Table 14).
Table 13.
Bone marrow micronucleus tests of AstraZeneca compound C in male rats
| Compound C oral dose (mg/kg) | MNPCE/1000 PCE |
||
|---|---|---|---|
| Test 1 | Test 2 | Test 3 | |
| 0.0 (vehicle control) | 0.7 | 0.7 | 1.1 |
| 0.8 | 0.9 | ||
| 2.5 | 0.5 | ||
| 8 | 1.5a | 0.9 | |
| 25 | 2.4a | ||
| 76 | 1.8a | 2.7a | |
| 380 | 2.5a | ||
| 760 | 2.4a | ||
All results 24 h after oral dosing (2000 PCE scored); no increases seen after 48 h. No evidence of aneugenicity (increased kinetochore-staining micronuclei). PCE: polychromatic erythrocyte; MNPCE: micronucleated PCE.
p < 0.001.
Table 14.
Bone marrow micronucleus test of AstraZeneca compound C in female rats
| Oral dose (mg/kg) | MNPCE/1000 PCE |
|---|---|
| 0 (vehicle control) | 1.1 |
| 25 | 2.0a |
| 76 | 1.7 |
| 380 | 1.2 |
Results 24 h after dosing (2000 PCEs scored). PCE: polychromatic erythrocyte; MNPCE: micronucleated PCE.
p < 0.05.
Subsequently, compound C was tested in an in vitro Comet assay using hepatocytes from male and female rats. Consistent with the in vivo responses, clearly positive results were obtained in male, but not female hepatocytes. If the sex difference in the responses in rats is due to metabolism, metabolism by CYP2C11 is an obvious candidate. Limited DMPK data, and a structural similarity to sildenafil (Viagra) [39] for which CYP2C11-mediated N-demethylation of a piperazine ring is an important route of metabolism, are consistent with CYP2C11 metabolism being involved in the genotoxic activation of compound C.
4. Discussion
The working group concluded that some compounds are much more easily detected as genotoxic agents with in vivo tests than with in vitro tests. It is noted that many of the compounds identified in this study interfere with cell cycle kinetics and this can result in either aneugenicity or chromosome breakage. For some of these compounds, variations of the in vitro methodology or cell system can detect genotoxic activity, but for an unknown compound, the variations are not always obvious. The pharmacology of the test material may be important in limiting in vitro responses, e.g., depletion of nucleotide pools and/or related effects on folate metabolism (e.g., Alimta®, SASP and SP). In these cases, in vitro detection may be possible if chromosome damage is assessed in human peripheral lymphocytes rather than in Chinese hamster cells.
There may be differences between in vitro and in vivo metabolism, as is the case for urethane. Some compounds may be metabolized by gut flora in vivo (e.g., SASP) to release genotoxic metabolites. Higher exposures may be possible in vivo than in vitro (Roche example 1, Pharmacia example). Certain genotoxic receptor kinases appear to be difficult to detect as genotoxic agents in vitro (in established cell lines), but are clearly active in rodent bone marrow micronucleus tests (Pfizer, Merck, Pharmacia and AstraZeneca examples). From the research done at AstraZeneca with their example, it appears that such compounds may be aneugens. Further research using different primary or near-primary cells may provide a means of detecting these compounds in vitro. It is likely that these compounds have been selected as kinase inhibitors in in vitro model systems. It is feasible that the cells used may provide models where genotoxicity could be detected in vitro. If these compounds still prove difficult to detect in vitro, prudence dictates that bone marrow micronucleus tests be performed early in the toxicological programme for future drug candidates in this pharmacological class.
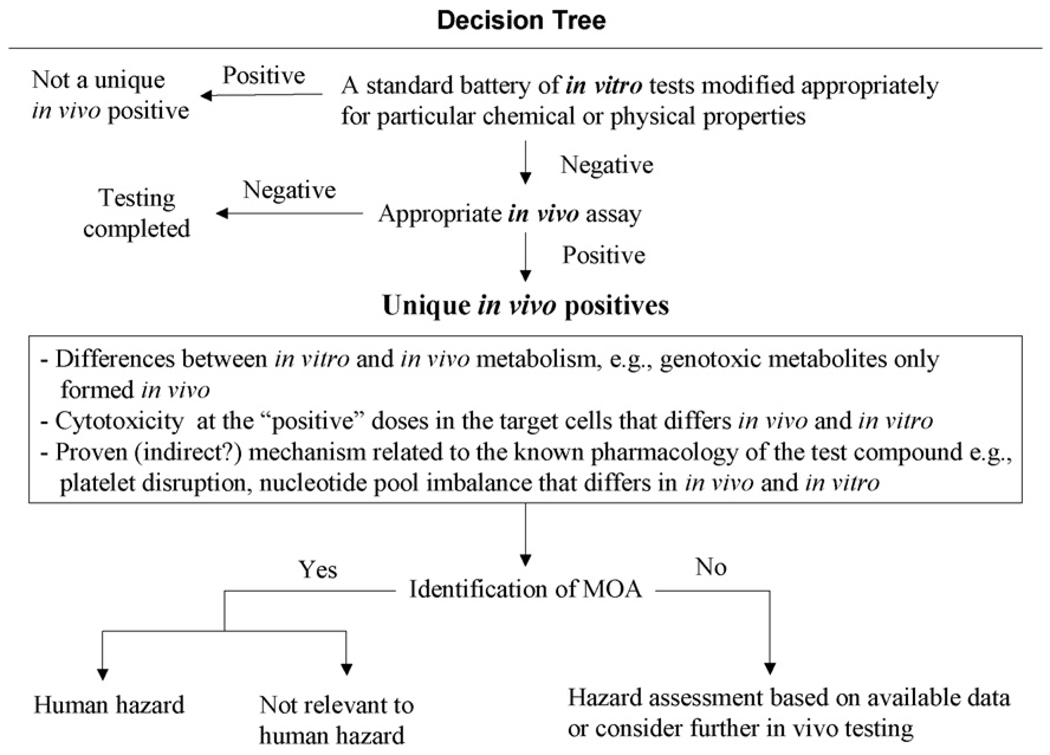
A decision tree is provided as a guide for the evaluation of compounds that appear to be genotoxic agents in vivo but not in vitro (Fig. 1).
Fig. 1.
Decision tree for evaluating possible ‘unique’ in vivo-positive compounds.
5. Implications for different product classes
5.1. Pharmaceuticals
The ICH M3 guideline ‘Non-clinical safety studies for the conduct of human clinical trials for pharmaceuticals’ states that ‘Prior to first human exposure, in vitro tests for the evaluation of mutations and chromosomal damage are generally needed … The standard battery of tests for genotoxicity should be completed prior to the initiation of Phase II studies’. Based on the evidence above, the IWGT working group advises that the entire standard battery should be completed before the initiation of Phase I human studies provided that the characteristics of the compound under question imply certain mechanisms of metabolic activation, receptor interaction or pivotal cell cycle targets that were not fully taken into account under in vitro genotoxicity testing conditions. For pharmaceutical candidate compounds, such information is generally available from the standard metabolism and receptor panel studies normally carried out prior to initiating clinical trials.
5.2. Cosmetics
A European Union Directive prohibits the use of animal tests for the development of new cosmetics starting in 2009 (2003/15/EC). The working group feels that reliance on in vitro-only test batteries may run the risk of missing some important genotoxic agents. Given the nature of cosmetics, these risks may be lower than for pharmaceuticals. Cosmetics are normally much less well characterized than pharmaceuticals regarding their effects on biological systems. A conscious decision not to do in vivo genotoxicity tests can be made for low exposures, knowledge about closely related compounds, etc. However, for novel cosmetic chemicals the information gained from in vivo genotoxicity tests may be necessary for a comprehensive risk assessment. This is supported by two additional considerations: (a) such compounds are normally not tested for carcinogenicity in animals (as opposed to pharmaceuticals) and (b) novel chemicals being used in some cosmetic formulations are being designed that may have a pharmacological mechanism of action. In such cases, and when there is significant use/systemic exposure, etc. more comprehensive testing than is provided by in vitro genotoxicity tests may be needed.
The analysis below also suggests that in those situations where there may be such a risk, such chemicals could be avoided as development compounds for cosmetics.
5.3. Industrial chemicals
Industrial chemicals, like cosmetics are normally much less well characterized than pharmaceuticals regarding their effects on biological systems. A conscious decision not to do in vivo genotoxicity tests can be made on absence of or low exposure, protection measures to limit exposure, knowledge about closely related compounds, etc. Where the potential for human exposure is high, the level of concern may drive the need for in vivo tests despite negative in vitro tests, again as for cosmetics carcinogenicity tests are not normally carried out for these chemicals, so additional genotoxicity testing may be required.
5.4. Food additives
There is a potential for long-term exposure to compounds in this product class; at present in vivo data on key compounds is often lacking. Again, a small subset of compounds with negative in vitro data may be worthy of in vivo testing.
6. In vitro-only test batteries
The testing of compounds with both in vitro and in vivo assays indicates that there are small subsets of compounds where conventional in vitro test batteries may miss inherent genotoxicity. Therefore, there is a case for including in vivo tests in regulatory test guidelines to ensure that the genotoxicity of such compounds is evaluated in an adequate manner. However, it can be envisioned that most compounds from these subsets could be identified prior to in vivo testing. Thus, for compounds that are negative in vitro, but are suspected of having activity that may be more easily detected in vivo (i.e., where there is prior knowledge that metabolism is likely to be different in vitro and in vivo; where the compound is likely to affect nucleotide pools through folate disruption; where metabolism by gut flora is suspected; where receptor kinase inhibitors are to be tested; where cytotoxicity at the ‘positive’ doses in the target cells differs in vivo and in vitro such that higher exposures may be achievable in vivo), it is prudent to conduct in vivo tests. The working group points out that modified in vitro test batteries may be capable of identifying many of those compounds currently shown to be positive only in vitro, and notes that reliance solely on in vitro tests may be acceptable in some cases of limited exposure.
Whilst the discussion above is relevant to the sensitivity of current genotoxicity test batteries, the problem remains of the poor specificity of currently used in vitro test batteries for finding non-carcinogens negative [40]. At present, in vivo tests are often used to determine if the genotoxic potential seen in vitro is realised in the whole animal. All-in vitro test batteries would result in many compounds that in fact do not pose a genotoxic hazard in the whole animal or man being discarded or labelled as genotoxic agents. Thus, there is a major challenge to develop new in vitro assays with higher overall accuracy for resolving genotoxic carcinogens from non-genotoxic non-carcinogens or to modify existing assays to improve specificity.
Acknowledgements
The workgroup is grateful to all the companies and individuals that supplied data to the IWGT questionnaire on ‘unique’ in vivo positives or supplied data and analyses from their Institutes. Apart from some of the authors of this paper and their Institutes, these include: Todd Bunch (ex Pharmacia); Sheila Galloway (Merck); Mike Garriott (Eli Lilly); Elmar Gocke (Hoffmann-La Roche); Warren Ku (Pfizer); Jim MacGregor (Toxicology Consulting Services) and Veronique Thybaud (sanofi-aventis).
Appendix A. IWGT questionnaire: examples of ‘unique’ in vivo positives
Do you have data on unequivocal in vivo genotoxic agents, where all standard in vitro tests are negative? If so, please proceed to the next questions.
- Please give details of:
- Chemical structure (if not confidential);
- Relevant mode of action if known, e.g., topoisomerase inhibitor; spindle poison; alkylating agent, etc.
Please list in vitro tests used in primary screening for each chosen compound.
Please submit summary test data for primary in vitro tests, i.e., test concentrations; mean plate counts for each strain (Ames test); mean Tk mutant frequencies and RTG scores for each concentration and time point (mouse lymphoma assay); mean aberration counts and mitotic index scores (chromosome aberration assays) for each concentration and time point, etc.
Please submit summary test data for primary positive in vivo tests, e.g., PCE/NCE ratios and mean micronucleated PCE counts for each time point and dose (in vivo micronucleus test), etc.
If further testing was carried out to explore differences between in vitro and in vivo test results, e.g., qualitative and quantitative comparisons of metabolites in vitro and in vivo, please give summary details and outcome of test.
If further testing was carried out in vitro, e.g., variations in composition and/or nature of the metabolic activation system; tests on isolated metabolites; use of different test systems, etc., please provide details and summary data as above.
References
- 1.Müller L, Blakey D, Dearfield KL, Galloway S, Guzzie P, Hayashi M, Kasper P, Kirkland D, MacGregor JT, Parry JM, Schechtman L, Smith A, Tanaka N, Tweats D, Yamasaki H. Strategy for genotoxicity testing and stratification of genotoxicity test results–report of the initial activities of the IWGT Expert Group. Mutat. Res. 2003;540:177–181. doi: 10.1016/j.mrgentox.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Inai K, Arihiro K, Takeshima Y, Yonehara S, Tachiyama Y, Khatum N, Nishisaka T. Quantitative risk assessment of carcinogenicity of urethane (ethyl carbamate) on the basis of long-term oral administration to B6C3F1 mice. Jpn.J.Cancer Res. 1991;82:380–385. doi: 10.1111/j.1349-7006.1991.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC, Urethane, IARC Monograph. Some Anti-thyroid and Related Substances, Nitrofurans and Industrial Chemicals. 1974;vol. 7:111–140. [Google Scholar]
- 4.Anderson D, Styles JA. An evaluation of 6 short-term tests for detecting organic chemical carcinogens. Appendix 2. The bacterial mutation test. Br. J. Cancer. 1974;37:924–930. doi: 10.1038/bjc.1978.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishidate M, Jr., Harnois MC, Sofuni T. A comparative analysis of data on the clastogenicity of 951 chemical substances tested in mammalian cell cultures. Mutat. Res. 1988;195:151–213. doi: 10.1016/0165-1110(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 6.Hubner P, Groux PM, Weibel B, Sengstag C, Horlbeck J, Leong-Morgenthaler P-M, Luthy J. Genotoxicity of ethyl carbamate (urethane) in Salmonella, yeast and human lymphoblastoid cells. Mutat. Res. 1997;390:11–19. doi: 10.1016/s0165-1218(96)00160-7. [DOI] [PubMed] [Google Scholar]
- 7.Sotomayor RE, Collins TFX. Mutagenicity, metabolism and DNA interactions of urethane. Toxicol. Ind. Health. 1990;6:71–108. doi: 10.1177/074823379000600106. [DOI] [PubMed] [Google Scholar]
- 8.Ashby J, Tinwell H, Callander RD. Activity of urethane and N,N′ -dimethylurethane in the mouse bone marrow micronucleus assay; equivalence of oral and intraperitoneal routes of exposure. Mutat. Res. 1990;245:227–230. doi: 10.1016/0165-7992(90)90055-o. [DOI] [PubMed] [Google Scholar]
- 9.Williams CV, Fletcher K, Tinwell H, Ashby J. Weak mutagenicity of ethyl carbamate to Lac-Z transgenic mice. Mutagenesis. 1998;13:133–137. doi: 10.1093/mutage/13.2.133. [DOI] [PubMed] [Google Scholar]
- 10.Mirsalis JC, Shimon JA, Johnson A, Fairchild D, Kanazawa N, Nguyen T, de Boer J, Glickman B, Winegar RA. Evaluation of mutant frequencies of chemically induced tumors and normal tissues in lambda/cII transgenic mice. Environ. Mol. Mutagen. 2005;45:17–35. doi: 10.1002/em.20084. [DOI] [PubMed] [Google Scholar]
- 11.Fernando RC, Nair J, Barbin A, Miller JA, Bartsch JA. Detection of 1,N6-ethenodeoxyadenosine and 3,N4-ethenodeoxycytidine by immunoaffinity/32P-postlabelling in liver and lung DNA of mice treated with ethyl carbamate (urethane) or it’s metabolites. Carcinogenesis. 1996;17:1711–1718. doi: 10.1093/carcin/17.8.1711. [DOI] [PubMed] [Google Scholar]
- 12.Forkert PG, Lee RP. Metabolism of ethyl carbamate by pulmonary cytochrome P450 and carboxylesterase isozymes: involvement of CYP2E1 and hydrolase A. Toxicol. Appl. Pharmacol. 1997;146:245–254. doi: 10.1006/taap.1997.8233. [DOI] [PubMed] [Google Scholar]
- 13.Guegerich FP, Kim D-H. Enzymatic oxidation of ethyl carbamate to vinyl carbamate and its role as an intermediate in the formation of 1,N6-ethenoadenosine. Chem. Res. Toxicol. 1991;4:413–421. doi: 10.1021/tx00022a003. [DOI] [PubMed] [Google Scholar]
- 14.Hoffler U, Dixon D, Peddada S, Ghanayem BI. Inhibition of urethane-induced genotoxicity and cell proliferation in CYP2E1-null mice. Mutat. Res. 2005;572:58–72. doi: 10.1016/j.mrfmmm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Burke DA, Wedd DJ, Herriott D, Bayliss MK, Spalding DJ, Wilcox P. Evaluation of pyrazole and ethanol induced S9 fraction in bacterial mutagenicity testing. Mutagenesis. 1994;9:23–29. doi: 10.1093/mutage/9.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Whysner J, Reddy MV, Ross PM, Mohan M, Lax EA. Genotoxicity of benzene and its metabolites. Mutat. Res. 2004;566:99–130. doi: 10.1016/s1383-5742(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 17.Bishop JB, Witt KL, Gulati DK, MacGregor JT. Evaluation of the mutagenicity of the anti-inflammatory drug salicylazosul-fapyridine. Mutagenesis. 1990;5:549–554. doi: 10.1093/mutage/5.6.549. [DOI] [PubMed] [Google Scholar]
- 18.Witt KL, Bishop JB, McFee AF, Kumaroo V. Induction of chromosomal damage in mammalian cells in vitro and in vivo by sulfapyridine or 5-aminosalicylic acid. Mutat. Res. 1992;283:59–64. doi: 10.1016/0165-7992(92)90122-x. [DOI] [PubMed] [Google Scholar]
- 19.Witt KL, Gudi R, Bishop JB. Induction of kinetochore positive and negative micronuclei in mouse bone marrow cells by salicylazosulfapyridine and sulfapyridine. Mutat. Res. 1992;283:53–57. doi: 10.1016/0165-7992(92)90121-w. [DOI] [PubMed] [Google Scholar]
- 20.Eskine IA, Mackay JM, Fox DP. Monitoring patients on long-term drug therapy for genotoxic effects. Basic Life Sci. 1984;29:895–905. doi: 10.1007/978-1-4684-4892-4_29. [DOI] [PubMed] [Google Scholar]
- 21.Fox DP, Mackay JM, Brunt PW, Hawksworth GM, Brown J. 5-ASA: The State of the Art, The Medicine Publishing Foundation Symposium, Series 21. Toronto: MES Medical Education Services; 1987. Abnormal chromosomes and sulfasalazine therapy; pp. 15–28. [Google Scholar]
- 22.Pironi L, Cornia GL, Ursitti MA, Dallasta MA, Miniero R, Fasano F, Miglioli M, Barbara L. Evaluation of oral administration of folic and folinic acid to prevent folate deficiency in patients with inflammatory bowel disease treated with salicylazosulfapyridine. Int. J. Clin. Pharmacol. Res. 1988;8:143–148. [PubMed] [Google Scholar]
- 23.Krogh Jensen M, Ekelund S, Svendsen L. Folate and homocysteine status and haemolysis in patients treated with sulphasalazine for arthritis. Scand. J. Clin. Lab. Invest. 1996;56:421–429. doi: 10.3109/00365519609088797. [DOI] [PubMed] [Google Scholar]
- 24.NTP. NTP toxicology and carcinogenesis studies of salicylazo-sulfapyridine (CAS no. 599-79-1) in F344/N rats and B6C3F1 mice (Gavage Studies) Natl. Toxicol. Program Tech. Rep. Ser. 1997;457:1–327. [PubMed]
- 25.Everson RB, Wehr CM, Erexson GL, MacGregor JT. Association of marginal folate depletion with increased human chromosomal damage in vivo: demonstration by analysis of micronucleated erythrocytes. J. Natl. Cancer Inst. 1988;80:525–529. doi: 10.1093/jnci/80.7.525. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor JT, Wehr CM, Hiatt RA, Peters B, Tucker JD, Langlois R, Jacob R, Jensen R, Yager JW, Shigenaga MK, Frei B, Eynon B, Ames BN. “Spontaneous” genetic damage in man: evaluation of interindividual variability, relationship among markers of damage, and influence of nutritional status. Mutat. Res. 1997;377:125–135. doi: 10.1016/s0027-5107(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 27.Iatropoulos MJ, Williams GM, Abdo KM, Kari FW, Hart RW. Mechanistic studies on genotoxicity and carcinogenicity of salicylazosulfapyridine an anti-inflammatory medicine. Exp. Toxicol. Pathol. 1997;49:15–28. doi: 10.1016/S0940-2993(97)80052-8. [DOI] [PubMed] [Google Scholar]
- 28.Heflich RH, Djuric Z, Zhou Z, Fullerton NF, Casciano DA, Beland FA. Metabolism of 2-acetylaminofluorene in the Chinese hamster ovary cell mutation assay. Environ. Mol. Mutagen. 1988;11:167–181. doi: 10.1002/em.2850110203. [DOI] [PubMed] [Google Scholar]
- 29.Mackay JM, Fox DP, Brunt PW, Hawksworth GM, Brown JE. In vitro induction of chromosome damage by sulphasalazine in human lymphocytes. Mutat. Res. 1989;222:27–36. doi: 10.1016/0165-1218(89)90032-3. [DOI] [PubMed] [Google Scholar]
- 30.PDR 2005 Physicians Desk Reference. NJ: Medical Economics Montvale; 2005. [Google Scholar]
- 31.Nair MP, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC. Immunoregulatory effects of morphine on human lymphocytes. Clin. Diagn. Lab. Immunol. 1997;4:127–132. doi: 10.1128/cdli.4.2.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singhal P, Kapasi A, Reddy K, Franki N. Opiates promote T cell apoptosis through JNK and caspase pathway. Adv. Exp. Med. Biol. 2001;493:127–135. doi: 10.1007/0-306-47611-8_15. [DOI] [PubMed] [Google Scholar]
- 33.Couch DB, Sawant SG. The clastogenicity of morphine sulfate in vivo. Adv. Exp. Med. Biol. 1995;373:123–129. doi: 10.1007/978-1-4615-1951-5_17. [DOI] [PubMed] [Google Scholar]
- 34.Sawant SG, Kozlowski RS, Couch DB. The role of adrenal corticosteroids in induction of micronuclei by morphine. Mutat. Res. 2001;498:129–133. doi: 10.1016/s1383-5718(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 35.Baker AK, Meert TF. Functional effects of systemically administered agonists and antagonists of mu, delta, and kappa opioid receptor subtypes on body temperature in mice. Pharmacol. Exp. Therap. 2002;302:1253–1264. doi: 10.1124/jpet.102.037655. [DOI] [PubMed] [Google Scholar]
- 36.Schafer DA, Xie Y, Falek A. Detection of opiate-enhanced increases in DNA damage, HPRT mutants and the mutation frequency in human HUT-78 cells. Environ. Mol. Mutagen. 1994;23:37–44. doi: 10.1002/em.2850230107. [DOI] [PubMed] [Google Scholar]
- 37.Willard FS, Crouch MF. MEK, ERK, and p90RSK are present on mitotic tubulin in Swiss 3T3 cells: a role for the MAP kinase pathway in regulating mitotic exit. Cell. Signal. 2001;13:653–664. doi: 10.1016/s0898-6568(01)00185-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhang WL, Huitorel P, Glass R, Fernandez-Serra M, Arnone MI, Chiri S, Picard A, Ciapa B. A MAPK pathway is involved in the control of mitosis after fertilization of the sea urchin egg. Dev. Biol. 2005;282:192–206. doi: 10.1016/j.ydbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Walker DK, Ackland MJ, James GC, Muirhead GJ, Rance DJ, Wastall P, Wright PA. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 40.Kirkland D, Aardema M, Henderson L, Müller L. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens. I. Sensitivity, specificity and relative predictivity. Mutat. Res. 2005;584:1–256. doi: 10.1016/j.mrgentox.2005.02.004. [DOI] [PubMed] [Google Scholar]