Abstract
Most human cells can only replicate a limited number of times in cultures before they lose the ability to divide, a phenomenon known as replicative senescence, which seems to play a role in aging at the organismal level. Recent studies have shown that culture in low magnesium (Mg) accelerates the senescence of human endothelial cells and fibroblasts. Given the numerous critical roles of Mg, it seems likely that Mg inadequacy would interfere with cellular metabolism, which could affect the senescence process. Since i) several pieces of evidence link low Mg to aging and age-related diseases and ii) the Occidental diet is relatively deficient in Mg, we propose that broadly correcting nutritional intakes of Mg might contribute to healthier aging and the prevention of age-related diseases.
Keywords: magnesium, aging, senescence, endothelium, fibroblasts
Aging is characterized by the progressive deterioration of physiological functions. This complex process has been proposed to be under the influence of both genetic and environmental (nutrition, lifestyle) influence at a ratio of approximately 30:70 [1], not counting stochastic variation. Several pieces of evidence link low magnesium (Mg) to aging and age-related diseases. In turn, it is noteworthy that aging itself constitutes a risk factor for Mg deficit [2, 3], thus creating a vicious circle that drives the clinical patterns seen in aging. Interestingly, a long-term moderate Mg-deficient diet aggravates cardiovascular risk associated with aging in rats by significantly increasing blood pressure and inflammatory markers [4]. In addition, low Mg is associated with age-associated memory decline [5], neurodegenerative disease [6], decreased muscle performance [7], insulin-resistance [8], osteoporosis [9], and development of some cancers [10] in human studies. All these data underscore the contribution of Mg deficiency to aging in vivo. The cellular mechanisms that drive the aging phenotype are not fully characterized, but growing evidence supports the involvement of senescent cells in these processes. Given the numerous critical roles of Mg, it seems likely that Mg inadequacy would interfere with cellular metabolism, which could affect the senescence process.
Only recently, some light has been shed on the cellular and molecular mechanisms involved, as it has been demonstrated that culture in low Mg promotes cellular senescence of human endothelial cells and fibroblasts [11, 12].
Cellular senescence
Reminiscent of aging at the organismal level, normal human diploid cells in culture divide only for a limited number of times before entering cellular senescence. As cell doublings reach their limit, changes occur in hundreds of variables from the molecular to the whole cell and these changes are analogous to those seen in humans and animals as they age [13]. The senescence theory of aging proposes that gradual accumulation of senescent cells in adult organisms contributes to the aging phenotype by depleting the renewal capacity of the tissue and by interfering with tissue homeostasis [14]. Indeed, senescent cells cease to proliferate and undergo dramatic changes in gene expression, probably because of the reorganization of chromatin structure [15]. Replicative senescence in human cells can invariably be induced by repeated serial passaging in vitro, which leads to telomere shortening. Alternatively, various types of stressful conditions, such as DNA damage and oxidative stress, can induce premature senescence [15]. Multiple molecular pathways leading to senescence exist, which seem to converge on the tumor suppressors p53 and pRb. One of the targets of p53 is p21, an inhibitor of the cyclin dependent kinase (Cdk)-E, whose increased expression is mediated by activated p53 upon DNA damage and/or telomere shortening [16]. p21 has been shown to play a role in senescence since it is strongly increased in senescent human fibroblasts and endothelial cells [17]. The pRb pathway, instead, seems to mediate premature senescence induced by specific stresses that activate the Cdk4-Cdk6 inhibitor p16 [15]. The emerging notion is that the p53/p21 pathway is primarily responsible for senescence induced by oxidative stress and/or telomerase shortening, whereas p16-pRb pathway mediates premature senescence induced by other stresses.
The relationship of Mg deficiency to senescence
Mg, the second most abundant intracellular cation after potassium, plays an essential role in fundamental cellular reactions. Indeed, several in vitro studies have provided evidence for the interactions of Mg with phospholipids, proteins and nucleic acids [18]. In particular, Mg influences the catalytic activity of various enzymes and this is well exemplified by the ability of Mg to promote trans-phosphorylation reactions through the formation of ATP-Mg complexes which anchor substrates to the active sites of enzymes [18]. In addition, Mg stabilizes DNA, promotes DNA replication and transcription, influences RNA translation, induces ribosome assembly and regulates the opening-closure of ion channels [18]. Mg deficiency also induces oxidative stress in various cell types including human [19], which might promote cellular senescence by promoting the shortening of telomeres. Increased oxidative stress may result, in part, from alteration of mitochondrial function, as adequate Mg is essential for normal mitochondrial function [20]; mitochondrial dysfunction is a frequently observed phenomenon in aging. Low Mg might therefore accelerate cellular senescence by compromising DNA stability, protein synthesis and cell energy metabolism.
Evidence suggests that the “Occidental or Westernized diet” is relatively deficient in Mg because of a preference for calorie-rich, micronutrient-poor foods, low Mg content in water and soil, and the processing of many food items, whereas the “Oriental diet” which is characterized by a greater intake of fruits and vegetables, is rich in Mg [21, 22]. This different dietary intake of the cation may contribute to explaining the lower incidence of cardiovascular diseases in Oriental than in Western populations. Moreover, it should be recalled that Mg deficiency is a significant clinical complication arising in patients with diabetes mellitus, in nephropathics, in individuals treated with some classes of diuretics or anticancer drugs, as well as in alcoholics.
Mg deficiency and endothelial senescence
Like most normal eukaryotic cells, endothelial cells have a finite capacity to replicate and function whether they are cultured in vitro or transplanted as grafts in vivo. We have shown that short-term [11] and long-term (unpublished results) exposure to low Mg promotes the acquisition of some features that are typically associated with endothelial senescence. Indeed, under Mg deficiency, cultured human endothelial cells became enlarged, elongated and vacuolated, and expressed senescence-associated β-galactosidase activity. They also upregulated p21, which induced premature senescence associated with cell dysfunction [23]. In low Mg, endothelial cells also overexpressed interleukin 1α (IL-1α) which is considered a marker of endothelial senescence, because silencing of IL-1α extended the lifespan of endothelial cells [24–26]. Additionally, exposure to low Mg upregulated plasminogen activator inhibitor-1 (PAI-1) [24]. PAI-1 is considered not merely a marker of senescence, since it is both necessary and sufficient for the induction of replicative senescence downstream of p53 [27]. The upstream events through which low Mg promotes endothelial senescence remain unclear. Recently, Mg deficiency has been shown to induce a transient oxidative stress which leads to oxidative DNA damage in endothelial cells [28]. It is therefore reasonable to propose that Mg deficit initiates stress-induced premature senescence. To this end, it is noteworthy that we have observed that endothelial cells overexpress hsp70 in low Mg (unpublished results), a result which is in agreement with the evidence that hsp70 levels are increased in senescent cells under basal, unstimulated conditions [29]. Interestingly, direct evidence has also been reported that endothelial oxidative stress increases with aging in humans [30]. It is also possible that low Mg might shorten telomeres, destabilize chromatin and induce the expression of “senescence genes”.
Mg deficiency and fibroblast senescence
Primary diploid fibroblasts are a commonly used culture model for studies on cellular senescence. Several short-term studies have previously demonstrated that low Mg affects fibroblast growth [31–33]. More recently, Killilea and Ames demonstrated that long-term culturing of human fibroblasts in moderately deficient Mg conditions resulted in accelerated cellular senescence [12]. Similar to findings with endothelial cells, fibroblasts cultured in low Mg responded with a dose-dependent increase in cell size and senescence-associated β-galactosidase activity. Cultures from Mg deficient conditions also exhibited increased expression of p16 and p21, which could explain the loss of replicative capacity in this cell population. Concomitantly, telomere lengths were shorter in cultures from low Mg conditions compared to standard Mg cultures from the same passaging time. Greater telomere shortening could also have contributed to accelerated replicative senescence in the fibroblasts maintained in low Mg, although the relationship between telomere loss and senescence is complex. Increases in telomere attrition in fibroblasts from Mg deficient conditions may have been driven by other upstream stimuli, such as oxidative stress. In fact, Wright et al. using the same fibroblast cell line as Killilea and Ames previously reported that population senescence appeared to be governed more by oxidative stress levels than telomere attrition per se [34]. One possible cause of increased oxidative stress levels in cells is aberrant mitochondrial homeostasis, which is well-established in aging cells and has been documented in other models of micronutrient deficiencies [20, 35]. Perhaps it is not surprising then that Killilea and Ames have also observed evidence of mitochondrial dysfunction in fibroblast cultures maintained in low compared to standard Mg conditions (unpublished data). Other reports have shown that Mg deficiency affects mitochondrial metabolism and bioenergetics [36]. However, the initial metabolic events triggered by exposure to low Mg that result in accelerated cellular senescence are still being elucidated.
Consequences of Mg deficiency and cellular senescence – age-related diseases
Counter to initial impressions, cellular senescence is not a completely unwanted process; current understanding suggests that this process is a protective response that causes growth arrest in cells which have acquired harmful levels of damage or mutations [37]. However, the other side of the coin is that senescent cells - particularly senescent stromal fibro-blasts, epithelial and endothelial cells - secrete factors that can disrupt tissue architecture. Accumulating senescent cells within the tissues has been proposed to be one of the key etiological events that drives age-related diseases. It is interesting to note that endothelial cells with senescence-associated phenotypes are present in human atherosclerotic lesions and that endothelial senescence has been shown to contribute to atherogenesis [38]. Senescent fibroblasts have also been associated with aging-related morbidities, including reduced wound-healing capacity in some models [39], but not others [40]. The preponderance of the evidence links low Mg, cellular senescence, and age-related diseases.
Consequences of Mg deficiency and cellular senescence – cancer
Cellular senescence is a potent tumor suppressive mechanism that irreversibly arrests proliferation to attenuate neoplastic transformation. It has been illustrated that senescent cells can modify the tissue environment in a way that synergizes with oncogenic mutations to promote the progression of age-related cancers [37, 41]. It is possible that Mg deficiency could accelerate the senescent phenotype of cells in vivo, which could be permissive to cancer growth. Mg has a complex role in many biological aspects of cancer, from neoplastic transformation to tumor growth and progression [10]. It is noteworthy that in an experimental murine model, prolonged consumption of a Mg deficient diet inhibited primary tumor growth both directly (by inhibiting tumor cell proliferation) and indirectly (by inhibiting angiogenesis and promoting an inflammatory response), whereas it also seems to favour metastasis [42–43]. It is tempting to speculate that the angiostatic effect of low Mg in vivo might be due to the acquisition of a senescent-like phenotype in the endothelial cells, while the increased metastases could be linked to the remodelling of the stroma by senescent cells which release molecules that generate a pro-invasive microenvironment. A supporting observation from several culture studies is that transformed cells have the ability to proliferate in lower Mg than primary cell counterparts [12, 31–33]. Thus in a moderately Mg deficient environment (the likely scenario in the human population eating a Western diet), there may be a growth advantage to the tumor cell if enough Mg is present to still permit tumor cell processes. Yet, the questions about the role of Mg deficiency in cancer remain: friend or foe?
Concluding remarks
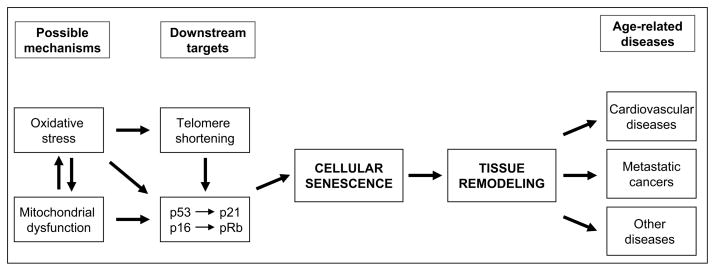
More studies are necessary to unravel the details connecting low Mg and cellular senescence, however figure 1 summarizes the currently known pathways through which Mg inadequacy might influence cellular senescence. The relevance of cellular aging to aging at the organismal level is still controversial, but has growing support. If there is a causal relationship, the cellular senescence-promoting effects of low Mg may have clinical relevance.
Figure 1.
Schematic of the cellular pathways that are activated during magnesium deficiency that can promote age-related diseases.
Aging is not only an important biological issue, but is also an important socioeconomical, geopolitical, and emotional issue that affects industrialized nations with a rapidly growing number of elderly citizens. Based on epidemiological, cellular and molecular evidences, we propose that broadly correcting the nutritional intake of Mg might be a simple and inexpensive solution that contributes to healthier aging and the prevention of age-related diseases.
Footnotes
Presented in part at the European Meeting on Magnesium, EuroMag, Paris, May 15–17, 2008.
References
- 1.Brack C, Lithgow G, Osiewacz H, Toussaint O. EMBO WORKSHOP REPORT: Molecular and cellular gerontology. EMBO J. 2000;19:1929–34. doi: 10.1093/emboj/19.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durlach J, Bac P, Durlach V, Rayssiguier Y, Bara M, Guiet-Bara A. Magnesium status and ageing: an update. Magnes Res. 1998;11:25–42. [PubMed] [Google Scholar]
- 3.Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56:65–80. doi: 10.1093/gerona/56.suppl_2.65. [DOI] [PubMed] [Google Scholar]
- 4.Adrian M, Chanut E, Laurant P, Gaume V, Berthelot A. A long-term moderate magnesium-deficient diet aggravates cardiovascular risks associated with aging and increases mortality in rats. J Hypertens. 2008;26:44–52. doi: 10.1097/HJH.0b013e3282f09f68. [DOI] [PubMed] [Google Scholar]
- 5.Billard JM. Ageing, hippocampal synaptic activity and magnesium. Magnes Res. 2006;19:199–215. [PubMed] [Google Scholar]
- 6.Lemke MR. Plasma magnesium decrease and altered calcium/magnesium ratio in severe dementia of the Alzheimer type. Biol Psychiatry. 1995;37:341–3. doi: 10.1016/0006-3223(94)00241-T. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez LJ, Barbagallo M, Lauretani F, Bandinelli S, Bos A, Corsi AM, Simonsick EM, Ferrucci L. Magnesium and muscle performance in older persons: the InCHI-ANTI study. Am J Clin Nutr. 2006;84:419. doi: 10.1093/ajcn/84.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bo S, Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol. 2008;19:50–6. doi: 10.1097/MOL.0b013e3282f33ccc. [DOI] [PubMed] [Google Scholar]
- 9.Kitchin B, Morgan SL. Not just calcium and vitamin D: other nutritional considerations in osteoporosis. Curr Rheumatol Rep. 2007;9:85–92. doi: 10.1007/s11926-007-0027-9. [DOI] [PubMed] [Google Scholar]
- 10.Wolf FI, Maier JA, Nasulewicz A, Feillet-Coudray C, Simonacci M, Mazur A, Cittadini A. Magnesium and neoplasia: from carcinogenesis to tumor growth and progression or treatment. Arch Biochem Biophys. 2007;458:24–32. doi: 10.1016/j.abb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Ferré S, Mazur A, Maier JAM. Low-magnesium induces senescent features in cultured human endothelial cells. Magnes Res. 2007;20:66–71. [PubMed] [Google Scholar]
- 12.Killilea DW, Ames BN. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc Natl Acad Sci USA. 2008;105:5768–73. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayflick L. Aging and the genome. Science. 1999;283:2019. doi: 10.1126/science.283.5410.2017f. [DOI] [PubMed] [Google Scholar]
- 14.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbours. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H. Molecular signaling and genetic pathways of senescence: its role in tumorigenesis and aging. J Cell Physiol. 2007;210:567–74. doi: 10.1002/jcp.20919. [DOI] [PubMed] [Google Scholar]
- 16.Herbig U, Wei W, Dutriaux A, Jobling WA, Sedivy JM. Real-time imaging of transcriptional activation in live cells reveals rapid up-regulation of the cyclin-dependent kinase inhibitor gene CDKN1A in replicative cellular senescence. Aging Cell. 2003;2:295–304. doi: 10.1046/j.1474-9728.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 17.Wagner M, Hampel B, Bernhard D, Hala M, Zwerschke W, Jansen-Durr P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp Gerontol. 2001;37:41–55. doi: 10.1016/s0531-5565(01)00105-x. [DOI] [PubMed] [Google Scholar]
- 18.Wolf FI, Cittadini A. Chemistry and biochemistry of magnesium. Mol Aspects Med. 2003;24:3–9. doi: 10.1016/s0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 19.Belin RJ, He K. Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnes Res. 2007;20:107–29. [PubMed] [Google Scholar]
- 20.Ames BN, Atamna H, Killilea DW. Mineral and vitamin deficiencies can accelerate the mitochondrial decay of aging. Mol Aspects Med. 2005;26:363–78. doi: 10.1016/j.mam.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Seelig MS. Cardiovascular consequences of magnesium deficiency and loss: pathogenesis, prevalence and manifestations--magnesium and chloride loss in refractory potassium repletion. Am J Cardiol. 1989;63 doi: 10.1016/0002-9149(89)90213-0. (4G21G) [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133:2879–82. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 23.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–20. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maier JAM, Malpuech C, Zimowska W, Rayssiguier Y. Mazur. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 2004;1689:13–21. doi: 10.1016/j.bbadis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Maier JAM, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin 1α antisense oligomer. Science. 1990;249:1570–4. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- 26.Mariotti M, Castiglioni S, Bernardini D, Maier JAM. Interleukin 1α is a marker of endothelial cellular senescence. Immun Ageing. 2006;3:4. doi: 10.1186/1742-4933-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–84. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf FI, Trapani V, Simonacci M, Ferré S, Maier JAM. Magnesium deficiency and endothelial dysfunction: is oxidative stress involved? Magnes Res. 2008;21:58–64. [PubMed] [Google Scholar]
- 29.Njemini R, Lambert M, Demanet C, Kooijman R, Mets T. Basal and infection-induced levels of heat shock proteins in human aging. Biogerontology. 2007;8:353–64. doi: 10.1007/s10522-006-9078-y. [DOI] [PubMed] [Google Scholar]
- 30.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–66. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 31.Rubin H. Effect of Magnesium content on density-dependent regulation of the onset of DNA synthesis in transformed 3T3 cells. Cancer Res. 1982;42:1761–6. [PubMed] [Google Scholar]
- 32.McKeehan WL, Ham RG. Calcium and magnesium ions and the regulation of multiplication in normal and transformed cells. Nature. 1978;275:756–8. doi: 10.1038/275756a0. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro SM, Armelin HA. Ca2+ and Mg2+ requirements for growth are not concomitantly reduced during cell transformation. Mol Cell Biochem. 1984;59:173–81. doi: 10.1007/BF00231313. [DOI] [PubMed] [Google Scholar]
- 34.Forsyth NR, Evans AP, Shay JW, Wright WE. Developmental differences in the immortalization of lung fibro-blasts by telomerase. Aging Cell. 2003;2:235–43. doi: 10.1046/j.1474-9728.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 35.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci USA. 2006;103:17589–94. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch Biochem Biophys. 2007;458:90–102. doi: 10.1016/j.abb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Campisi J. Cancer and aging: rival demons? Nat Rev Cancer. 2003;3:339–49. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 38.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial senescence in human atherosclerosis. Circulation. 2002;105:1541–6. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 39.Stanley A, Osler T. Senescence and the healing rates of venous ulcers. J Vasc Surg. 2001;33:1206–11. doi: 10.1067/mva.2001.115379. [DOI] [PubMed] [Google Scholar]
- 40.Liu D, Hornsby PJ. Fibroblast stimulation of blood vessel development and cancer cell invasion in a subrenal capsule xenograft model: stress-induced premature senescence does not increase effect. Neoplasia. 2007;9:418–26. doi: 10.1593/neo.07205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–7. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasulewicz A, Wietrzyk J, Wolf FI, Dzimira S, Madej J, Maier JAM, Rayssiguier Y, Mazur A, Opolski A. Magnesium deficiency inhibits primary tumor growth but favors metastasis in mice. Biochim Biophys Acta. 2004;1739:26–32. doi: 10.1016/j.bbadis.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Maier JAM, Nasulewicz-Goldeman A, Simonacci M, Boninsegna A, Mazur A, Wolf FI. Insights into the mechanisms involved in magnesium-dependent inhibition of primary tumor growth. Nutr Cancer. 2007;59:192–8. doi: 10.1080/01635580701420624. [DOI] [PubMed] [Google Scholar]