Abstract
The exciting and extraordinary capabilities of stem cells to proliferate and differentiate into numerous cell types not only offers promises for changing how diseases are treated, but may also impact how transfusion medicine is practiced in the future. The possibility of growing platelets in the laboratory to some day supplement and/or replace standard platelet products has clear advantages for blood bank centers and patients. Due to the high utilization of platelets by patients undergoing chemotherapy or receiving stem cell transplants, platelet transfusion has steadily increased over the past decades. This trend is likely to continue as the number of adult and pediatric patients receiving stem cell transplants is also continuously rising. As a result of increased demand coupled with the short shelf-life of platelet concentrates, providing platelets to patients can stretch the resources of most blood centers, drive donor recruitment efforts, and on occasion platelet shortages can compromise the care of thrombocytopenic patients.
The purpose of this article is to review current scientific progress to develop in vitro strategies to manufacture platelets, with an emphasis on efforts to produce functional platelets in quantities that would be required for clinical transfusion. There are a number of publications indicating that human platelets can be obtained in vitro from the controlled differentiation of hematopoietic stem cells. However, the hemostatic quality of such manufactured platelets has not been confirmed and current technologies are inadequate to ensure satisfactory expansion and platelet biogenesis on an industrial scale. Nonetheless, these studies provide proof-of-principle that developing in vitro strategies to manufacture platelets is feasible and also provide a foundation for developing more sophisticated approaches to achieve this goal.
Keywords: Megakaryocyte, Stem cells, Megakaryocytopoiesis, Thrombocytopoiesis, ex vivo cultures, Platelet, Platelet biogenesis, Hematopoiesis
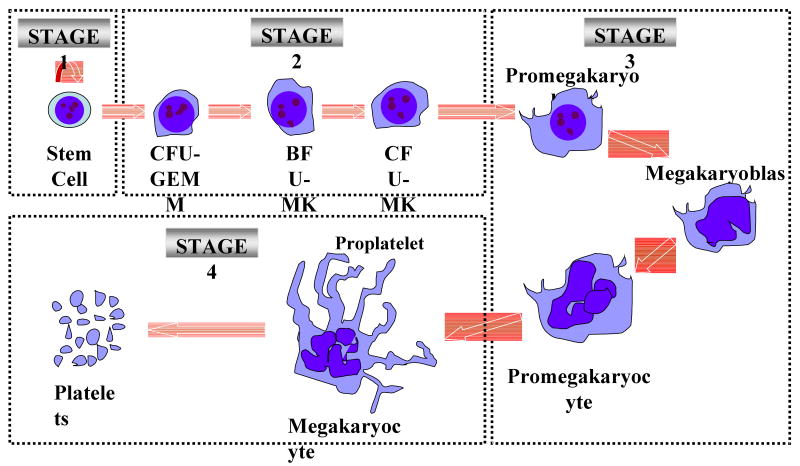
To appreciate the underlying principles and difficulties encountered when developing in vitro strategies to manufacture platelets, a brief review of the in vivo process responsible for megakaryocyte (MK) development and platelet biogenesis is presented. For additional information regarding this topic please see review references.1, 2 As shown in Figure 1 MK development and platelet biogenesis can be divided into four major sequential biological steps.1 The first stage is characterized by the self-renewal (symmetric division) of hematopoietic stem cells residing within the osteoblastic niches. In the second phase, stem cells proliferate and differentiate to amplify the number of MK progenitors, which then differentiate to become immature MKs with limited proliferation capacity. During a third phase of development, immature MKs will undergo a series of maturation events. First, they will undergo polyploidization through a process known as endomitosis in which the MK chromosomes continue to replicate, but nuclear division fails and cell division is halted due to the failure of MKs to complete cytokinesis.1, 3, 4 This stage of development will result in MKs that contain a single polylobulated nucleus with many times the normal complement of 46 chromosomes (i.e. >2N). Recent evidence now indicates that nuclear division does not always fail and that a significant proportion of MKs can contain two physically separated nuclei.4 The next maturation event that occurs during the third phase of MK development is cytoplasmic enlargement followed by cytoplasmic maturation. The processes of endomitosis and cytoplasmic enlargement/maturation are fundamental properties that are postulated to have been acquired by the MK lineage to facilitate a large production of platelets, which is reported to range from 2,000-11,000 platelets released per MK in the marrow.5 As MKs continue to mature during the third phase of development the cytoplasmic to nuclear ratio increases and a network of specialized membranes forms within the cytoplasm, termed the demarcation membranes system (DMS). The cytoplasm further organizes itself such that dense bodies, secretory vesicles and vital organelles (mitochondria, ribosomes) are found within the nascent platelets. The fourth and final stage is characterized by platelet assembly and release (i.e. thrombocytopoiesis). This stage of development involves the formation of proplatelet processes originating from the DMS that are characterized as long extensions with branching ends.6, 7 At this stage of development, maturing MKs are principally located in the so-called vascular niche in close proximity to sinusoidal bone marrow endothelial cells. Proplatelet extensions from mature MKs juxtaposed on sinusoidal bone marrow endothelial cells extend into the marrow sinusoids, where shear forces probably aid in the release of platelets from the proplatelet processes.8 Interestingly, proplatelet formation and platelet release may also be dependent upon a specialized process of cell death, referred to as localized or compartmentalized apoptosis.9, 10 Although the hypothesis of proplatelet formation and protrusion of proplatelet processes into the sinusoidal lumen is currently the most accepted hypothesis, there is also the hypothesis that global fragmentation of the MK cytoplasm can result in the formation of individual platelets.11 Support for the latter hypothesis is provided by transmission and scanning electron microscopy images of MKs in the marrow and lung capillaries that depict the global fragmentation of the MK cytoplasm into individual platelets.7, 11-13
Figure 1.
Four major sequential biological phases characterize megakaryocyte (MK) development and platelet biogenesis (for more detail see references 1, 2) Phase 1: Hematopoietic stem cells residing within the osteoblastic niches undergo self-renewal through symmetric divisions. Alternatively, stem cells undergo differentiation to produce committed MK progenitors. Phase2: A hierarchy of MK progenitors (i.e. colony forming units-granulocytes, erythrocytes, monocytes and megakaryoyctes (CFU-GEMM), burst forming unit-megakaryoycytes (BFU-MK) and colony forming units-megakaryoyctes (CFU-MK)) capable of undergoing extensive rounds of mitotic divisions in response to mitogenic factors are responsible for amplifying MK numbers. Phase 3: CFU-MK progenitors further differentiate to become immature MKs of limited proliferation capacities (i.e. promegakaryoblast, megakaryoycte and promegakaryocyte), which undergo further maturation to become polyploidy cells. As MKs continue to mature they form the demarcation membranes system (DMS), a continuous network of specialized membranes within the cytoplasm. Phase 4: A series of proplatelets processes with branching ends form from the DMS on mature MKs, nascent platelets are released from the proplatelets and the MK undergoes compartmentalized apoptosis.
In Vitro Strategies to Produce Megakaryocytes and Platelets
There are basically two strategies under investigation to use hematopoietic stem cells to augment and/or replace standard platelet products. Some investigators are developing in vitro strategies to expand MK progenitors for co-infusion with unmanipulated hematopoietic stem cells for the purpose of shortening periods of thrombocytopenia following stem cell transplants.14 The hypothesis is that terminal differentiation and thrombocytopoiesis will occur in vivo, resulting in bridging the gap until hematopoietic stem cells can engraft and newly formed MKs can mature. A second in vitro strategy is based on the hypothesis that clinical quantities of functional platelets can be produced in vitro from pluripotent stem cells. This approach requires the development of methods to not only produce mature culture-derived MKs, but also a means to facilitate the release and collection of functional platelets from culture-derived MKs. The latter of the two strategies is the focus of this review.
Prior to the cloning thrombopoietin (TPO), the principal cytokine that regulates megakaryocytopoiesis and thrombocytopoiesis, it was recognized that after culturing normal peripheral blood mononuclear cells with serum from aplastic dogs that human MKs could be isolated15 and that various cytokine combinations could enhance MK development.16, 17 However, it was not until recombinant TPO became available that reasonable expansions were achieved to obtain highly enriched populations of MKs that exhibited normal structural features specific to MK development and maturation (Table 1).14, 18-26 After expanding CD34+ cells from various sources (i.e mobilized peripheral blood stem cells (PB), bone marrow (BM), and umbilical cord blood (UCB)); 3-99% of culture-derived MKs were reported to express platelet-specific receptors that play essential roles in hemostasis such as GPIIb, GPIIIa, GPIbα (Table 1).27-29
Table 1.
Megakaryocytes produced per input cellsa
| Cell Source | Input Cell Phenotype | Serum | Culture Additions | Culture Time (days) | a MK yield per input cell | Reference |
|---|---|---|---|---|---|---|
| PB | MNCs | 20% human serum | Aplastic canine serum (Meg-CSA) | 12-14 | 0.04 | [15] |
| BM & PB | Lineage Negative | No | IL3, IL6, kit-ligand, mpl-ligand | 10-12 | 10 | [18] |
| BM & UCB | CD34+ | 1.5% BSA | MGDF | 14 | 27 UCB 14 BM | [19] |
| PB | CD34+ | No | MGDF, SCF, IL3, IL6, IL11, FL, MIP-1α | 7 | 2 | [14] |
| BM | CD34+/CD38lo | No | IL3, IL6, SCF, TPO | 10 | 7 | [20] |
| PB & UCB | CD34+ | No | SCF, FL, IL6, IL11, TPO | 10 | 15 UCB 6 PB | [21] |
| UCB | CD34+ | 10% FCS | IL3, IL6, SCF, TPO | 28 | 30 | [24] |
| UCB | CD34+ | 10% FCS | IL6, SCF, FL, TPO | 56 | 1,580 | [25] |
| hESCs | H9 | Yes | Co-culture with OP9+TPO | 15-17 | 0.1-0.4 | [26] |
MK yields were estimated based on results presented. For more details, readers are invited to consult the original paper. Due to the high number of published studies, only selected studies are presented in this table. The authors would like to apologize to other non-cited authors.
PB=Peripheral blood; MNC=Mononuclear cells; BM=Bone marrow; UCB=Umbilical cord blood; hESCs: human embryonic stem cells; IL= Interleukin; MGDF=Megakaryocyte growth and development factor; SCF=Stem cell factor
Gaur et al.26 also showed that MKs can be derived from human embryonic stem cells (hESCs) by culturing undifferentiated hESCs in the presence of sub-confluent OP9 stromal cell monolayers (Table 1). This study was consistent with other studies that achieved significant, but varying levels of MK production from murine ESCs30 and hESCs31, 32 when co-cultured on OP9 or other stromal cells.
When reviewing the yield of MKs produced per input cell relative to the length of the culture period for the studies listed in Table 1, one can appreciate that MK yields increase as the culture period was extended; 0.04-170 MKs versus 30-1,580 MKs per input cell for cultures maintained for 7-21 days and 3-8 weeks, respectively.25
The first demonstration that functional human platelets could be generated in vitro was reported by Choi et al. using a 2-step strategy (Table 2).27 MK formation was first promoted using peripheral blood CD34+ cells cultured with aplastic canine serum. MKs were isolated from the cultures after 11-12 days and replated with fresh media supplemented with human AB plasma without aplastic canine serum. At the peak of proplatelet formation, an average of 40% of the MKs exhibited proplatelets and platelet-sized fragments and the platelet sized fragments aggregated in the presence of agonists (i.e. thrombin and ADP). Subsequent investigators reported the in vitro production of functional platelets using TPO treated human stem cells from different sources including PB, BM, UCB and hESCs (Table 2).23, 30, 31, 33-38 Among these studies were two reports suggesting that it may be possible to generate human platelets on a large enough scale for clinical use.36, 37
Table 2.
Summary of articles reporting in vitro production of human platelets from hematopoietic stem/progenitors.
| Cell Source | Serum | Number of Culture Steps | Total Culture Period (days) | MK yield per CD34+ cellsa | Plt yield per input CD34 | Functional Characterization | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | CD62p and/or PAC-1b | α-granule releaseb | EMc | Aggregation | ||||||
| PB CD34+ | yes | 10% aplastic canine serum; 8-12 days | 10%huAB plasma: 2-3 days | N/A | 11-15 | n.i.d | n.i.d | yes | yes | yes | yes | [27] |
| PB &BM CD34+ | no | IL3, PEG-rHuMGDF; | N/A | N/A | 11-14 | 18 | 300e | yes* | n.i.d | yes | n.i. | [33] |
| UCB CD34+ | no | SCF, IL3, Tpo; 7 days | Tpo only; 14 days | N/A | 21 | n.i.d | n.i.d | yes | n.i.d | n.i.d | n.i.d | [34] |
| UCB CD34+ | no | IL6,SCF, , FL,TPO; 7 days | SCF, TPO, FL, IL6; 10 days | N/A | 17 | 135 | 142g | n.i.d | n.i.d | n.i.d | n.i.d | [23] |
| PB CD34+ | yes | IL1β, IL6, SCF, Tpo | N/A | N/A | 21 | n.i.d | n.i.d | yes | yes | yes | yes | [35] |
| BM CD34+/CD38lo | no | IL3, IL6, SCF, Tpo; 10 days | Tpo, SU6656; 6days | N/A | 16 | n.i.d | n.i.d | n.i.d | n.i.d | yes | yes | [38] |
| UCB CD34+ | no | Co-culture with hTERT stroma plus SCF, TPO, FL; 14 days | Co-culture with hTERT stroma plus SCF, TPO, FL, IL11; 14 days | SCF, TPO, FL, IL11; 5 days | 33 | 500 | 42,000 f | yes | yes | yes | yes | [36] |
| UCB CD34+ | yes | SCF, FL, TPO, IL6; 2-3 days | TPO, FL; 7 days | TPO, SCF, IL6, IL9, SU6656 >11 days | >21 | n.i.d | 20e | yes* | n.i.d | n.i.d. | yes | [37] |
| UCB CD34+ | no | TPO, SCF, FL; 4 days | TPO, SCF, IL6, IL9, 10 days | N/A | 14 | 170 | 29f | yes | yes | yes | yes | [39] |
MK and Plt yield were estimated based on results presented. For more details, readers are invited to consult the original paper.
yield per seeded CD34+ cells
tested by thrombin activation and flow cytometry with CD62p or Pac-1 antibody
platelet morphology performed by scanning or transmission electron microscopy
not indicated
total platelet like particles (CD41a+ and CD41a-PLPs)
CD41a+CD42b+platelet like particles
portion of platelets were activated in the absence of thrombin
n.i.=not indicated; N/A=not applicable
PB=Peripheral blood; BM=Bone marrow; UCB=Umbilical cord blood
The first report to demonstrate that large quantities of platelets could be theoretically produced from a limited number of CD34+ employed a three step in vitro culture system to more closely mimic the in vivo process of MK/platelet development (Table 2).36 First, CD34+ cells were expanded for 14 days to amplify hematopoietic stem/progenitor cells (1st step). Next, the cells were transferred to a culture environment to differentiate and expand MKs for another 14 days (2nd step). This was followed by a 5 day culture period to support the maturation of MKs to produce platelets that exhibited normal morphology and function (3rd step). However, major problems associated with this approach were the low levels of MKs obtained during the 2nd step of the culture (i.e. <1%), and an inability to scale up the culture system. Thus, these investigators were unable to determine actual yields and ended up reporting estimated platelet yields.
Sullenbarger and co-workers were responsible for a second report that provided evidence that it may be possible to produce platelets in quantities needed for clinical transfusion.37 In their system, UCB CD34+ cells were expanded for three days prior to placement in a 3-dimensional bioreactor continuously perfused with media and free of stromal cells. One of the attractive aspects of their system was that it permitted the continuous collection of platelets, while allowing for an independent control of media and gas flow. However, they found that a majority of the manufactured platelets were activated in the absence of agonists, and that some platelets showed abnormal morphology. Nonetheless, these studies supported the concept that developing in vitro strategies to manufacture platelets was feasible.
As indicated in Table 2, the majority of culture systems use at least a 2-step culture strategy to generate platelets and both the Matsunaga36 and Sullenbarger37 studies use a 3-step culture approach. For most of the studies using a 2-step strategy, the first step was to amplify stem and progenitors followed by a second step to differentiate MKs and support platelet biogenesis. For the studies using a 3-step strategy, the first step was to amplify the progenitors, the second step was to support MK differentiation, and the third step was to promote platelet biogenesis. Thus, the progression of the in vitro culture strategy from 2-steps to 3-steps moved closer to reproducing the 4-phases of MK/platelet development as previously described: 1) stem cell self-renewal; 2) MK progenitor amplification; 3) MK differentiation/maturation; and 4) platelet release (Fig 1.).
For the strategies outlined in table 2, it is worth noting that relatively short culture periods for the first step were used to amplify progenitors (i.e. <14 days) (Table 2) Our group has found (JAR) that if culture conditions are implemented to maximize stem cell self-renewal and an amplification of MK progenitors before an induction of MK differentiation/maturation and platelet biogenesis there is a greater likelihood of achieving an industrial scale production of platelets. For example, after red cell depletion and CD34+ cell enrichment 1.85-2.59×106 UCB CD34+ cells are recovered in a single UCB unit. Using a culture strategy which employs gas permeable teflon bags containing X-vivo 10 supplemented with IL-6 (10 ng/ml), SCF (50 ng/ml), TPO (50 ng/ml) and FL (50 ng/ml) plus 10% fetal calf serum, at the end of 7-8 weeks of culture, there are ∼1,600 MKs produced per input CD34+ cell (unpublished data).25 Based on this yield, a total of 2.9-4.1×109 CD41+ cells could be produced from a single unit of UCB. This means that only ∼13-19 platelets need to be generated per MK to obtain a single standard platelet unit containing ∼5.5×1010 platelets. However, given that 2,000-11,000 platelets are reported to be released per MK in vivo,5 if culture strategies are discovered that allow thrombocytopoiesis to proceed as efficiently ex vivo as in vivo, then a single UCB unit could possibly produce 5.8×-1012-4.5×1013 platelets or ∼100-800 units of standard platelets in a 7-8 week period.
Quality of Platelets Produced Ex Vivo
Platelets are very sensitive to their environment reacting rapidly to agonists to initiate thrombus formation and coagulation. However, this property which is vital for hemostasis makes it significantly more difficult to produce functional platelets in an artificial environment. Indeed, some studies have reported that a large proportion of platelets produced ex vivo are already in a semi-activated state as determined by the level of the P-selectin (CD62p) present on the surface of platelet-size-particles harvested from cultures.35, 37 However, recently obtained results by one of the authors (NP) suggest that this is in large part reversible. It appears that partially activated manufactured platelets as well as normal blood platelets return to a non-activated state following an overnight incubation at 37°C.39
A number of studies indicate that platelets produced in vitro have for the most part morphological and functional characteristics similar to blood-derived platelets. This has been demonstrated through the use of several in vitro assays that include α- and dense granule release assays, fibrinogen binding, shape change and spreading, electron microscopy and aggregation assays (Table 2).29, 30, 32, 35-40 However, not all particles found in cultures with a size comparable to platelets are bona fide platelets. Moreover, although some reports demonstrate that manufactured platelets are functional as measured by single or multiple in vitro assays, the in vivo hemostatic function of culture-derived platelets still remains to be evaluated.
Indeed, platelet-like-particles produced in MK cultures are quite heterogeneous in shape, size and in phenotype.29-31, 33, 37, 39 Importantly, a considerable proportion of these particles share the same size of normal blood-derived platelets, but lack detectable GPIIb (CD41a) and/or GPIbα (CD42a). These platelet-size-particles can be readily observed in most studies that show non-gated flow cytometry data of size as well as granularity and expression of GPIIb and GPIbα.23, 29–32, 36, 37, 39 Though such entities have not yet been fully characterized, transmission electron microscopy and cytometry analyses suggest that they are likely fragments from MK, non-MK cells and proplatelets, activated platelets and platelet remnants.27, 29, 30, 32, 37, 39 In addition, it has been widely reported that platelets produced ex vivo are often larger and highly heterogeneous in size compared to blood platelets.29-32, 37,39 This latter observation supports the hypothesis that shear stress may be an important regulator of platelet size in vivo, and that platelets produced in static culture systems lack such factors to enable them to reach normal size.
Human platelets have a limited life span of 10 days in vivo and when stored ex vivo progressively lose their function due to biochemical and morhphological changes caused by aging and changes in their environment such as pH (reviewed in41). Platelets produced ex vivo are, unfortunately, not protected from loss of function. Indeed, several investigators, including one of the authors (NP), have noted the rapid loss of several receptors that are required for normal platelet function.32, 39 Chief amongst these is GPIbα, which appears to correlate directly with the normal function of ex vivo produced platelets from UCB.32, 39 GPIbα is lost in platelet concentrates and culture derived platelets due to ectodomain shedding mediated mostly by metalloproteinases.32, 42, 43 The work of Nishikii et al. recently demonstrated that GPIbα, GPV and GPVI on ex vivo produced platelets were also sensitive to such degradation, while other common receptors such as the fibrinogen receptor composed of GPIIb and GPIIIa were resistant.32 These results have important repercussions on the functional property of platelets because GPVI is the major collagen receptor for platelet activation,44 whereas, GPIbα and GPV form with GPIbβ and GPIX to form the major receptor for von Willebrand factor.45 Strikingly, loss of GPIbα due to metalloproteinases occurred at a much greater rate in culture-derived platelets.39 Fortunately, this process can be efficiently inhibited by broad range metalloproteinase inhibitors, such as GM6001.32, 39, 46 These observations demonstrate that the addition of small molecular weight inhibitors, such as GM6001, may be an efficient way to prevent loss of function of newly derived platelets, but also emphasizes that platelet production estimates based solely on size and GPIIb expression are probably not the best indicators for the production of functional platelets.
Important Consideration for Developing in Vitro Strategies to Produce Megakaryocytes and Platelets for Clinical Transfusion
Despite the fact that a complete understanding about the complex network of extrinsic and intrinsic regulatory events that control MK development and platelet biogenesis from hematopoietic stem cells are not fully elucidated, recreating the appropriate cues/factors needed by cells to promote platelet biogenesis are gradually being revealed.
Stem/Progenitor Source
A major factor that can greatly impact overall platelet yields is the choice of stem/progenitor cells used as the culture inoculum. As mentioned previously and summarized in Tables 1 and 2, MKs and platelets have been produced from various stem cell sources with the majority of the early studies conducted with adult-derived progenitor cells. However, the most recent reports seem to favor the use of UCB CD34+ cells, perhaps due to the ease of collection, and the findings that UCB CD34+ cells may be a better source of cells than marrow19 or peripheral blood21 for producing MKs. A major advantage of UCB CD34+ is their increased mitotic potential, which will invariably result in the production of more MKs than their adult counterparts (i.e. PB and BM).47-50 However, the extent to which UCB MKs undergo maturation seems to be precluded relative to marrow and peripheral blood MKs, which raises the possibility that UCB MKs may produce fewer platelets on a cell per cell basis than their adult counterparts.47, 49, 50 For instance, the majority of MKs derived in culture from UCB stem cells remain diploid or in a low ploidy class (<8N), while the majority of adult-derived MKs become polyploidy and can reach ploidy levels as high as 64N.47 Thus, it remains unclear as to which source of HSCs is better suited for the in vitro production of platelets in culture. No study to date has examined whether platelets produced from either source are functionally equivalent, even though existing results would suggest that they are.
Recent advances in hESC culture protocols have now paved the way for the development of platelet production processes using an almost unlimited source of stem cells.30-32 However, many challenges remain. For example, very little is known about the ability of embryonic derived platelets to function normally in patients. Additionally, the culture process required for platelet production from hESC is relatively complex and the final platelet yields at this time are within the same range reported for adult stem cells. Nonetheless, the possibility of producing platelets from ESCs is probably the most exciting scenario for the future of platelet production ex vivo. Indeed, autologous platelet concentrates could, perhaps, be produced by transforming skin fibroblast cells from the patient into pluripotent stem cells (i.e. induced pluripotent stem cells (iPS) 51, 52) that could be greatly expanded for the production of platelets following induction of MK differentiation. However, this has not yet been formally demonstrated and will certainly require major development and optimization efforts.
Media Formulation and Growth Conditions
Cytokines remain the best tool to expand and control the differentiation of uncommitted CD34+ cells. Typically, serum deprived media supplemented with different cytokines are used. As mentioned, TPO is primarily responsible for the growth and differentiation of MKs.53-57 Unlike other lineage-specific cytokines, TPO also plays a vital role in maintaining the hematopoietic stem cell population (reviewed in 58, 59). TPO synergizes in vitro with multiples cytokines including IL-348, 60, 61, stem cell factor (SCF/also known as kit ligand),60, 62 IL-6,23, 60 IL-9,62, 63 and IL-11,21, 36, 64 each of which can increase the number of CFU-MKs and/or MKs.
There also appears to be another set of cytokines that synergize with TPO to support the proliferation of immature progenitors, mainly IL-3, Flt-3 Ligand (FL) 21, 36, 64-66 and SCF.67, 68 The combination of FL and TPO has been shown to be a powerful combination for expanding SCID-Repopulating Cells derived from umbilical cord blood.69 The fact that TPO has biological activity for both primitive progenitors/stem cells as well as MKs, suggests that other factors present in the marrow milieu (i.e. cell-cell and cell-to-extracellular matrix) may play a major role in determining the balance between proliferation and differentiation. It has also been shown in vivo that there are two sets of chemokines, stromal-derived factor-1 (SDF-1) and fibroblast growth factor 4 (FGF-4) that can promote thrombopoiesis in the absence of TPO or c-Mpl. These two chemokines facilitate the migration of MKs toward bone marrow sinusoidal endothelial, which promote their maturation and release of platelets.70 Conversely, other growth factors are known to inhibit megakaryocytopoiesis, these include the cytokines TGF-β1, platelet factor 4 (PF4), and IFN-α.60, 71-74 Additionally, although IL-3 synergizes with TPO to support the proliferation of immature progenitors, IL-3 has also been reported to inhibit the maturation of MKs.60
Besides cytokines, small molecules or mimetics are also reported to modulate the growth and development of MKs and platelet biogenesis. Some of these are TPO receptor agonists, such as AKR-501 (YM477), 75 AMG531, 76 and a fungal nuclear migration protein, hNUDC.77 There is also the Src kinase inhibitor, SU6656, which can induce TPO-dependent polyploidization of MKs.38, 78 In fact, SU6656 was employed in the Sullenbarger study to facilitate an in vitro production of platelets.37
Although existing evidence indicates that current media formulations are conducive for MK development, proplatelet formation, and release of particles into the media with platelet function, the ex vivo production of platelets are arguably 100- to 1000-fold less efficient than reported in vivo. The inefficiency of this process raises the issue as to whether there are cytokines yet to be discovered, and/or whether cell-to-cell and cell-to-extracellular matrix interactions need to be incorporated into culture strategies.
Clearly, there are a myriad of possible permutations that can be constructed to recreate the in vivo microenvironment using an in vitro approach. Direct contact of hematopoietic stem cells to accessory cells and/or extracellular matrix proteins, such as fibronectin,79 arranged in a 3-dimensional microenvironment may be required as they are known to play important roles in regulating the proliferation, differentiation, migration, and apoptosis of developing blood cells.80-82 As a result, it may be necessary to construct synthetic scaffolds that provide architectural cues to modulate the bioavailability of cytokines and to organize cell-cell and cell-extracellular matrix interactions.
Nutrient requirements
Altogether, there are a whole host of variables that can be exploited to optimize cell culture conditions to maximize platelet production. For example, based on solid experimental evidence, physical parameters such as oxygen pressure (pO2),83-85 pH,86 and temperature87, 88 can be adjusted to maximize MK and platelet production. For instance, elevating pO2 conditions can increase MK expansion and accelerate MK differentiation, maturation and proplatelet formation.83-85 Conversely, Proulx et al. also demonstrate that mild hyperthermia (i.e. 39°C) can be used to favor and accelerate MK differentiation of UCB CD34+ cells.87, 88 Together these studies demonstrate that careful modulation of such variables can be exploited at little cost to increase MK development.
Cultures which are set up and not fed on a regular basis are referred to as batch cultures which over time, due to the changing biochemical environment, accumulate metabolic waste, which can inhibit cell growth. To overcome inhibitory effects by metabolic waste products, culture media is changed on a regular basis (batch-fed) or a perfusion culture system is used so that fresh media is continually supplied while depleted media is removed. Perfusion bioreactors offer more control over such variables as oxygen, glucose and glutamine levels as well as the metabolite accumulation of ammonia and lactic acid.
Shear stress
Our current understanding on how platelets are formed and released from MKs in vivo has greatly been enhanced in recent years. If the primary mechanism of platelet release is via utilization of mechanical forces to sever platelets from proplatelet processes, 8, 89, 90 then low yields of platelets from culture-derived MKs are not surprising given the static nature of the majority of the current culture protocols that are being explored. Likewise, if an explosive and global fragmentation of the MK cytoplasm is the primary means responsible for producing platelets, 7, 11, 12 then current liquid culture systems are inferior and need to be re-evaluated.
Even though the precise mechanism used by MKs to sense and respond to shear forces is not clearly understood, shear force can potentially be used to promote in vitro platelet production. This is evident from recent work which shows that when mature human MKs are exposed to high shear forces proplatelet processes become apparent and platelets are released within 20 minutes. But, when left in static cultures often no platelets are generated or it takes several hours for platelets to be released.91
Concluding Remarks
The possibility of using human stem/progenitor cells to manufacture platelets to supplement and/or replace standard platelet products for clinical transfusion will help to alleviate a reliance on blood donors, avert platelet shortages that may compromise the care of thrombocytopenic patients, and possibly improve product safety. Over the last decade significant progress has been made to produce platelets in vitro. However, the number of platelets produced in vitro per MK are orders of magnitude lower than what occurs in vivo and the quality of the platelets generated is unclear. The current inefficiency of this process raises issues of whether cytokines are delivered at the appropriate time, sequence, concentration, in the right combination and/or whether there are cytokines that are yet to be discovered. Additionally, because MK development is also governed by immobilized matrices, cell-to-cell interactions, and various other physical cues (i.e. shear stress), the task to recreate the appropriate signals required by stem/progenitor cells, maturing MKs and terminally differentiated MKs to produce ex vivo functional platelets is monumental.
To achieve the goal of manufacturing platelets for clinical transfusion will require a series of technological advancements that will foster a development of bioreactors that maximize yields at each of the four biological phases of in vivo MK/platelet development (Fig 1.) These advancements will need to incorporate new measurement devices that precisely control culture environments, non-invasive ways to monitor the composition of cells within a bioreactor, and a way to capture and maintain functional platelets as they are generated.
Although there are still many unanswered questions that need to be resolved before platelets can be successfully manufactured, the potential benefits of such approaches are immeasurable. Due to the complex nature of the task at hand, success will be achieved only through the collaborative efforts of bioengineers, scientists and clinicians. In the end the innovations that are made could revolutionize patient care.
Acknowledgments
This work was supported in part by the Puget Sound Blood Center and NIH grant P50 HL081015.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Long MW, Hoffman R. Thrombocytopoiesis. USA: Churchill Livingstone; 2000. Hematology Basic Principles and Practice 3rd Edition; pp. 245–260. [Google Scholar]
- 2.Battinelli EM, Hartwig JH, Italiano JE., Jr Delivering new insight into the biology of megakaryopoiesis and thrombopoiesis. Curr Opin Hematol. 2007;14:419–426. doi: 10.1097/MOH.0b013e3282bad151. [DOI] [PubMed] [Google Scholar]
- 3.Vitrat N, Cohen-Solal K, Pique C, et al. Endomitosis of human megakaryocytes are due to abortive mitosis. Blood. 1998;91:3711–3723. [PubMed] [Google Scholar]
- 4.Lordier L, Jalil A, Aurade F, et al. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood. 2008;112:3164–3174. doi: 10.1182/blood-2008-03-144956. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman RM, Airo R, Pollack S, Crosby WH. Circulating megakaryocytes and platelet release in the lung. Blood. 1965;26:720–731. [PubMed] [Google Scholar]
- 6.Italiano JE, Jr, Shivdasani RA. Megakaryocytes and beyond: The birth of platelets. J Thromb Haemost. 2003;1:1174–1182. doi: 10.1046/j.1538-7836.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 7.Becker RP, De Bruyn PP. The transmural passage of blood cells into myeloid sinusoids and the entry of platelets into the sinusoidal circulation; A scanning electron microscopic investigation. Am J Anat. 1976;145:183–205. doi: 10.1002/aja.1001450204. [DOI] [PubMed] [Google Scholar]
- 8.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 9.De Botton S, Sabri S, Daugas E, et al. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 10.Clarke MC, Savill J, Jones DB, et al. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J Cell Biol. 2003;160:577–587. doi: 10.1083/jcb.200210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zucker-Franklin D, Philipp CS. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol. 2000;157:69–74. doi: 10.1016/S0002-9440(10)64518-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zucker-Franklin D, Petursson S. Thrombocytopoiesis--analysis by membrane tracer and freeze-fracture studies on fresh human and cultured mouse megakaryocytes. J Cell Biol. 1984;99:390–402. doi: 10.1083/jcb.99.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosaki G. In vivo platelet production from mature megakaryocytes: does platelet release occur via proplatelets? Int J Hematol. 2005;81:208–19. doi: 10.1532/IJH97.04177. [DOI] [PubMed] [Google Scholar]
- 14.Bertolini F, Battaglia M, Pedrazzoli P, et al. Megakaryocytic progenitors can be generated ex vivo and safely administered to autologous peripheral blood progenitor cell transplant recipients. Blood. 1997;89:2679–2688. [PubMed] [Google Scholar]
- 15.Mazur EM, Basilico D, Newton JL, et al. Isolation of large numbers of enriched human megakaryocytes from liquid cultures of normal peripheral blood progenitor cells. Blood. 1990;76:1771–1782. [PubMed] [Google Scholar]
- 16.Teramura M, Katahira J, Hoshino, et al. Clonal growth of human megakaryocyte progenitors in serum-free cultures: effect of recombinant human interleukin 3. Exp Hematol. 1988;16:843–848. [PubMed] [Google Scholar]
- 17.Bruno E, Hoffman R. Effect of interleukin 6 on in vitro human megakaryocytopoiesis: its interaction with other cytokines. Exp Hematol. 1989;17:1038–1043. [PubMed] [Google Scholar]
- 18.Guerriero R, Testa U, Gabbianelli M, et al. Unilineage megakaryocytic proliferation and differentiation of purified hematopoietic progenitors in serum-free liquid culture. Blood. 1995;86:3725–3736. [PubMed] [Google Scholar]
- 19.Tao H, Gaudry L, Rice A, Chong B. Cord blood is better than bone marrow for generating megakaryocytic progenitor cells. Exp Hematol. 1999;27:293–301. doi: 10.1016/s0301-472x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 20.Shim MH, Hoover A, Blake N, Drachman JG, Reems JA. Gene expression profile of primary human CD34+CD38lo cells differentiating along the megakaryocyte lineage. Exp Hematol. 2004;32:638–648. doi: 10.1016/j.exphem.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 21.De Bruyn C, Delforge A, Martiat P, Bron D. Ex vivo expansion of megakaryocyte progenitor cells: cord blood versus mobilized peripheral blood. Stem Cells Dev. 2005;14:415–424. doi: 10.1089/scd.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 22.Su RJ, Li K, Yang M, et al. Platelet-derived growth factor enhances ex vivo expansion of megakaryocytic progenitors from human cord blood. Bone Marrow Transplant. 2001;27:1075–1080. doi: 10.1038/sj.bmt.1703042. [DOI] [PubMed] [Google Scholar]
- 23.Proulx C, Boyer L, Hurnanen DR, Lemieux R. Preferential ex vivo expansion of megakaryocytes from human cord blood CD34+-enriched cells in the presence of thrombopoietin and limiting amounts of stem cell factor and Flt-3 ligand. J Hematother Stem Cell Res. 2003;12:179–188. doi: 10.1089/152581603321628322. [DOI] [PubMed] [Google Scholar]
- 24.Bruno S, Gunetti M, Gammaitoni L, et al. In vitro and in vivo megakaryocyte differentiation of fresh and ex-vivo expanded cord blood cells: rapid and transient megakaryocyte reconstitution. Haematologica. 2003;88:379–387. [PubMed] [Google Scholar]
- 25.Sun SYR, Reems JA. Large scale production of megakaryocytes. Exp Hematol. manuscript in preparation. [Google Scholar]
- 26.Gaur M, Kamata T, Wang S, et al. Megakaryocytes derived from human embryonic stem cells: a genetically tractable system to study megakaryocytopoiesis and integrin function. J Thromb Haemost. 2006;4:436–442. doi: 10.1111/j.1538-7836.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85:402–413. [PubMed] [Google Scholar]
- 28.Debili N, Wendling F, Katz A, et al. The Mpl-ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors. Blood. 1995;86:2516–2525. [PubMed] [Google Scholar]
- 29.Cramer EM, Norol F, Guichard J, et al. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 1997;89:2336–2346. [PubMed] [Google Scholar]
- 30.Fujimoto TT, Kohata S, Suzuki H, Miyazaki H, Fujimura K. Production of functional platelets by differentiated embryonic stem (ES) cells in vitro. Blood. 2003;102:4044–4051. doi: 10.1182/blood-2003-06-1773. [DOI] [PubMed] [Google Scholar]
- 31.Takayama N, Nishikii H, Usui J, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–5306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- 32.Nishikii H, Eto K, Tamura N, et al. Metalloproteinase regulation improves in vitro generation of efficacious platelets from mouse embryonic stem cells. J Exp Med. 2008;205:1917–1927. doi: 10.1084/jem.20071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norol F, Vitrat N, Cramer E, et al. Effects of cytokines on platelet production from blood and marrow CD34+ cells. Blood. 1998;91:830–843. [PubMed] [Google Scholar]
- 34.Sun L, Tan P, Yap C, et al. In vitro biological characteristics of human cord blood-derived megakaryocytes. Ann Acad Med Singapore. 2004;33:570–575. [PubMed] [Google Scholar]
- 35.Ungerer M, Peluso M, Gillitzer A, et al. Generation of functional culture-derived platelets from CD34+ progenitor cells to study transgenes in the platelet environment. Circ Res. 2004;95:e36–44. doi: 10.1161/01.RES.0000141700.96085.2e. [DOI] [PubMed] [Google Scholar]
- 36.Matsunaga T, Tanaka I, Kobune M, et al. Ex vivo large-scale generation of human platelets from cord blood CD34+ cells. Stem Cells. 2006;24:2877–2887. doi: 10.1634/stemcells.2006-0309. [DOI] [PubMed] [Google Scholar]
- 37.Sullenbarger B, Bahng JH, Gruner R, et al. Prolonged continuous in vitro human platelet production using three-dimensional scaffolds. Exp Hematol. 2009;37:101–110. doi: 10.1016/j.exphem.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi MJ, Drachman JG, Reems JA, Thorning D, Lannutti BJ. A novel strategy for generating platelet-like fragments from megakaryocytic cell lines and human progenitor cells. Blood Cells Mol Dis. 2005;35:70–73. doi: 10.1016/j.bcmd.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Robert A, Boyer, Lucie, Pineault N. Glycoprotein Iba receptor integrity is essential for functional properties of platelet-like particles produced from cord blood CD34+ enriched cells in liquid culture. Transfusion. submitted for publication. [Google Scholar]
- 40.Choi ES, Hokom M, Bartley T, et al. Recombinant human megakaryocyte growth and development factor (rHuMGDF), a ligand for c-Mpl, produces functional human platelets in vitro. Stem Cells. 1995;13:317–322. doi: 10.1002/stem.5530130313. [DOI] [PubMed] [Google Scholar]
- 41.Seghatchian J, Krailadsiri P. The platelet storage lesion. Transfus Med Rev. 1997;11:130–144. doi: 10.1053/tm.1997.0110130. [DOI] [PubMed] [Google Scholar]
- 42.Bergmeier W, Piffath CL, Cheng G, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates GPIbalpha shedding from platelets in vitro and in vivo. Circ Res. 2004;95:677–683. doi: 10.1161/01.RES.0000143899.73453.11. [DOI] [PubMed] [Google Scholar]
- 43.Rabie T, Strehl A, Ludwig A, Nieswandt B. Evidence for a role of ADAM17 (TACE) in the regulation of platelet glycoprotein V. J Biol Chem. 2005;280:14462–8. doi: 10.1074/jbc.M500041200. [DOI] [PubMed] [Google Scholar]
- 44.Kehrel B, Wierwille S, Clemetson KJ, et al. Glycoprotein VI is a major collagen receptor for platelet activation: It recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa, and von Willebrand factor do not. Blood. 1998;91:491–499. [PubMed] [Google Scholar]
- 45.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–34. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 46.Bergmeier W, Burger PC, Piffath CL, et al. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro-aged or -injured mouse platelets. Blood. 2003;102:4229–4235. doi: 10.1182/blood-2003-04-1305. [DOI] [PubMed] [Google Scholar]
- 47.Bornstein R, Garcia-Vela J, Gilsanz F, Auray C, Cales C. Cord blood megakaryocytes do not complete maturation, as indicated by impaired establishment of endomitosis and low expression of G1/S cyclins upon thrombopoietin-induced differentiation. Br J Haematol. 2001;114:458–465. doi: 10.1046/j.1365-2141.2001.02954.x. [DOI] [PubMed] [Google Scholar]
- 48.van den Oudenrijn S, von dem Borne AE, de Haas M. Differences in megakaryocyte expansion potential between CD34(+) stem cells derived from cord blood, peripheral blood, and bone marrow from adults and children. Exp Hematol. 2000;28:1054–1061. doi: 10.1016/s0301-472x(00)00517-8. [DOI] [PubMed] [Google Scholar]
- 49.Mattia G, Vulcano F, Milazzo L, et al. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–897. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- 50.Miyazaki R, Ogata H, Iguchi T, et al. Comparative analyses of megakaryocytes derived from cord blood and bone marrow. Br J Haematol. 2000;108:602–609. doi: 10.1046/j.1365-2141.2000.01854.x. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 53.Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- 54.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 55.Kaushansky K, Broudy V, Lin N, et al. Thrombopoietin, the Mpl ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci, USA. 1995;92:3234–3238. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaushansky K. Thrombopoietin: The primary regulator of platelet production. Blood. 1995;86:419–431. [PubMed] [Google Scholar]
- 57.Lok S, Kaushansky K, Holly RD, et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–8. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- 58.Kaushansky K. Thrombopoietin and hematopoietic stem cell development. Ann N Y Acad Sci. 1999;872:314–319. doi: 10.1111/j.1749-6632.1999.tb08475.x. [DOI] [PubMed] [Google Scholar]
- 59.Drachman JG. Role of thrombopoietin in hematopoietic stem cell and progenitor regulation. Curr Opin Hematol. 2000;7:183–190. doi: 10.1097/00062752-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Dolzhanskiy A, Basch RS, Karpatkin S. The development of human megakaryocytes: III. Development of mature megakaryocytes from highly purified committed progenitors in synthetic culture media and inhibition of thrombopoietin-induced polyploidization by interleukin-3. Blood. 1997;89:426–434. [PubMed] [Google Scholar]
- 61.Schattner M, Lefebvre P, Mingolelli SS, et al. Thrombopoietin-stimulated ex vivo expansion of human bone marrow megakaryocytes. Stem Cells. 1996;14:207–214. doi: 10.1002/stem.140207. [DOI] [PubMed] [Google Scholar]
- 62.Cortin V, Garnier A, Pineault N, Lemieux R, Boyer L, Proulx C. Efficient in vitro megakaryocyte maturation using cytokine cocktails optimized by statistical experimental design. Exp Hematol. 2005;33:1182–1191. doi: 10.1016/j.exphem.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 63.Fujiki H, Kimura T, Minamiguchi H, et al. Role of human interleukin-9 as a megakaryocyte potentiator in culture. Exp Hematol. 2002;30:1373–1380. doi: 10.1016/s0301-472x(02)00966-9. [DOI] [PubMed] [Google Scholar]
- 64.Feng Y, Zhang L, Xiao ZJ, et al. An effective and simple expansion system for megakaryocyte progenitor cells using a combination of heparin with thrombopoietin and interleukin-11. Exp Hematol. 2005;33:1537–1543. doi: 10.1016/j.exphem.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Shaw PH, Gilligan D, Wang XM, Thall PF, Corey SJ. Ex vivo expansion of megakaryocyte precursors from umbilical cord blood CD34 cells in a closed liquid culture system. Biol Blood Marrow Transplant. 2003;9:151–156. doi: 10.1053/bbmt.2003.50013. [DOI] [PubMed] [Google Scholar]
- 66.Sigurjonsson OE, Gudmundsson KO, Haraldsdottir V, Rafnar T, Agnarsson BA, Gudmundsson S. Flt3/Flk-2 ligand in combination with thrombopoietin decreases apoptosis in megakaryocyte development. Stem Cells Dev. 2004;13:183–191. doi: 10.1089/154732804323046783. [DOI] [PubMed] [Google Scholar]
- 67.Sitnicka E, Lin N, Priestley GV, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998–5005. [PubMed] [Google Scholar]
- 68.Kobayashi M, Laver JH, Kato T, Miyazaki H, Ogawa M. Thrombopoietin supports proliferation of human primitive hematopoietic cells in synergy with steel factor and/or interleukin-3. Blood. 1996;88:429–436. [PubMed] [Google Scholar]
- 69.Piacibello W, Sanavio F, Severino A, et al. Ex vivo expansion of cord blood progenitors. Vox Sang. 1998;74:457–462. doi: 10.1111/j.1423-0410.1998.tb05456.x. [DOI] [PubMed] [Google Scholar]
- 70.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 71.Majka M, Baj-Krzyworzeka M, Kijowski J, Reca R, Ratajczak J, Ratajczak MZ. In vitro expansion of human megakaryocytes as a tool for studying megakaryocytic development and function. Platelets. 2001;12:325–332. doi: 10.1080/09537100120068152. [DOI] [PubMed] [Google Scholar]
- 72.Mazur EM, Cohen JL, Newton J, et al. Human serum megakaryocyte colony-stimulating activity appears to be distinct from interleukin-3, granulocyte-macrophage colony-stimulating factor, and lymphocyte-conditioned medium. Blood. 1990;76:290–297. [PubMed] [Google Scholar]
- 73.Gewirtz AM, Calabretta B, Rucinski B, Niewiarowski S, Xu WY. Inhibition of human megakaryocytopoiesis in vitro by platelet factor 4 (PF4) and a synthetic COOH-terminal PF4 peptide. J Clin Invest. 1989;83:1477–1486. doi: 10.1172/JCI114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffin CG, Grant BW. Effects of recombinant interferons on human megakaryocyte growth. Exp Hematol. 1990;18:1013–1018. [PubMed] [Google Scholar]
- 75.Fukushima-Shintani M, Suzuki K, Iwatsuki Y, et al. AKR-501 (YM477) in combination with thrombopoietin enhances human megakaryocytopoiesis. Exp Hematol. 2008;36:1337–1342. doi: 10.1016/j.exphem.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 76.Broudy VC, Lin NL. AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl. Cytokine. 2004;25:52–60. doi: 10.1016/j.cyto.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Wei MX, Yang Y, Ge YC, Xu P. Functional characterization of hNUDC as a novel accumulator that specifically acts on in vitro megakaryocytopoiesis and in vivo platelet production. J Cell Biochem. 2006;98:429–439. doi: 10.1002/jcb.20803. [DOI] [PubMed] [Google Scholar]
- 78.Lannutti BJ, Blake N, Gandhi MJ, Reems JA, Drachman JG. Induction of polyploidization in leukemic cell lines and primary bone marrow by Src kinase inhibitor SU6656. Blood. 2005;105:3875–3878. doi: 10.1182/blood-2004-10-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han P, Guo XH, Story CJ. Enhanced expansion and maturation of megakaryocytic progenitors by fibronectin. Cytotherapy. 2002;4:277–283. doi: 10.1080/146532402320219790. [DOI] [PubMed] [Google Scholar]
- 80.Verfaillie CM. Adhesion receptors as regulators of the hematopoietic process. Blood. 1998;92:2609–2612. [PubMed] [Google Scholar]
- 81.Rafii S, Shapiro F, Pettengell R, et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. [PubMed] [Google Scholar]
- 82.Schattner M, Green D, Cohen I. Stromal-conditioned medium synergizes with thrombopoietin in stimulating megakaryocytopoiesis. Stem Cells. 1998;16:61–5. doi: 10.1002/stem.160061. [DOI] [PubMed] [Google Scholar]
- 83.LaIuppa JA, Papoutsakis ET, Miller WM. Oxygen tension alters the effects of cytokines on the megakaryocyte, erythrocyte, and granulocyte lineages. Exp Hematol. 1998;26:835–843. [PubMed] [Google Scholar]
- 84.Mostafa SS, Miller WM, Papoutsakis ET. Oxygen tension influences the differentiation, maturation and apoptosis of human megakaryocytes. Br J Haematol. 2000;111:879–889. [PubMed] [Google Scholar]
- 85.Mostafa SS, Papoutsakis ET, Miller WM. Oxygen tension modulates the expression of cytokine receptors, transcription factors, and lineage-specific markers in cultured human megakaryocytes. Exp Hematol. 2001;29:873–883. doi: 10.1016/s0301-472x(01)00658-0. [DOI] [PubMed] [Google Scholar]
- 86.Yang H, Miller WM, Papoutsakis ET. Higher pH promotes megakaryocytic maturation and apoptosis. Stem Cells. 2002;20:320–328. doi: 10.1634/stemcells.20-4-320. [DOI] [PubMed] [Google Scholar]
- 87.Proulx C, Dupuis N, St-Amour I, Boyer L, Lemieux R. Increased megakaryopoiesis in cultures of CD34-enriched cord blood cells maintained at 39 degrees C. Biotechnol Bioeng. 2004;88:675–680. doi: 10.1002/bit.20288. [DOI] [PubMed] [Google Scholar]
- 88.Pineault N, Boucher JF, Cayer MP, et al. Characterization of the effects and potential mechanisms leading to increased megakaryocytic differentiation under mild hyperthermia. Stem Cells Dev. 2008;17:483–493. doi: 10.1089/scd.2007.0149. [DOI] [PubMed] [Google Scholar]
- 89.Scurfield G, Radley JM. Aspects of platelet formation and release. Am J Hematol. 1981;10:285–296. doi: 10.1002/ajh.2830100308. [DOI] [PubMed] [Google Scholar]
- 90.Hagiwara T, Nagasawa T, Nagahisa H, et al. Expression of adhesion molecules on cytoplasmic processes of human megakaryocytes. Exp Hematol. 1996;24:690–695. [PubMed] [Google Scholar]
- 91.Dunois-Larde C, Capron C, Fichelson S, et al. Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood. 2009 doi: 10.1182/blood-2009-03-209205. Epub ahead of print. [DOI] [PubMed] [Google Scholar]