Abstract
Ceramide synthases are highly conserved transmembrane proteins involved in the biosynthesis of sphingolipids, which are essential structural components of eukaryotic membranes and can act as second messengers regulating tissue homeostasis. However, the role of these enzymes in development is poorly understood due to the lack of animal models. We identified schlank as a new Drosophila member of the ceramide synthase family. We demonstrate that schlank is involved in the de novo synthesis of a broad range of ceramides, the key metabolites of sphingolipid biosynthesis. Unexpectedly, schlank mutants also show reduction of storage fat, which is deposited as triacylglyerols in the fat body. We found that schlank can positively regulate fatty acid synthesis by promoting the expression of sterol-responsive element-binding protein (SREBP) and SREBP-target genes. It further prevents lipolysis by downregulating the expression of triacylglycerol lipase. Our results identify schlank as a new regulator of the balance between lipogenesis and lipolysis in Drosophila. Furthermore, our studies of schlank and the mammalian Lass2 family member suggest a novel role for ceramide synthases in regulating body fat metabolism.
Keywords: body fat metabolism, ceramide synthases, Lass 2, schlank, SREBP
Introduction
In all animals energy homeostasis is under tight control of evolutionarily conserved nutrient-sensing systems. These include the target of rapamycin (TOR) pathway (Martin and Hall, 2005) and several families of secreted peptide hormones, which regulate and fine-balance carbohydrate and lipid metabolism to match energy requirements. Particularly during growth, a coordinated regulation of the lipid metabolism is important on both the cellular and organismal level. On the cellular level, sterols in mammalian cells or phosphatidylethanolamine, the major phospholipid in Drosophila, control the release of sterol-regulatory element-binding protein (SREBP) from cell membranes, exerting feedback control on the synthesis of fatty acids (FAs) and phospholipids (Dobrosotskaya et al, 2002; Seegmiller et al, 2002; Kunte et al, 2006). SREBPs are membrane-bound transcription factors that monitor cell membrane composition and adjust lipid synthesis accordingly. The de novo synthesis of sphingolipids is also pivotal since sphingolipids are essential structural components of eukaryotic membranes and play important roles as second messengers regulating apoptosis, survival and differentiation (Spiegel and Milstien, 2000; Hannun et al, 2001; Acharya and Acharya, 2005). Misregulation of the sphingolipid metabolism is involved in the aetiology and pathology of a number of human diseases, including neurodegeneration, cancer, immunity, cystic fibrosis, emphysema, diabetes and sepsis (Kolter and Sandhoff, 2006; Lahiri and Futerman, 2007). The enzymes of the sphingolipid pathway are conserved in all genetically studied eukaryotes (Jiang et al, 1998; Hannun et al, 2001; Acharya and Acharya, 2005). However, in vivo information on how these enzymes are regulated in response to growth requirements or during starvation, is either limited by early lethality of the knockout animals reflecting the fundamental necessity of these enzymes (Li et al, 2002; Hojjati et al, 2005; Mizugishi et al, 2005) or by the lack of mutants. The latter includes mutants for key enzymes of the pathway such as ceramide synthases, which produce the precursor metabolites for all sphingolipids. Mammals contain six family members of the ceramide synthase family, also called Lass (longevity assurance homologue-1 of yeast Lag1) proteins. Their function has mainly been studied in tissue culture cells since knockout models are not available yet, and it was shown that they control the synthesis of different ceramides (for reviews see Pewzner-Jung et al, 2006; Teufel et al, 2009).
On the organismal level, energy homeostasis depends on the ability to control the balance between lipid synthesis, storage and lipid mobilization during conditions of energy abundance or deprivation, respectively (Hay and Sonenberg, 2004; Zechner et al, 2005). Storage fat is deposited as triacylglycerols (TAGs), in intracellular lipid droplets (Martin and Parton, 2006), which accumulate in specialized organs such as mammalian adipose tissue or the fat body of flies (Canavoso et al, 2001; Rosen and Spiegelman, 2006). Lipolysis of TAGs is induced by lipases and leads to release of free FAs or sn-1,2-diacylglycerol in mammals and insects, respectively, into the circulatory system (Arrese and Wells, 1997; Gibbons et al, 2000; Patel et al, 2005). Chronic dysregulation of the balance between lipolysis and lipogenesis may lead to metabolic abnormalities such as obesity, lipodystrophy syndromes or insulin resistance in humans (Kahn et al, 2006; Simha and Garg, 2006).
We have identified a new regulator of growth and lipid homeostasis in Drosophila, the schlank gene. We show that schlank encodes a Drosophila member of the Lass/ceramide synthase family required for de novo synthesis of ceramides. Unexpectedly, schlank is also involved in the regulation of body fat metabolism and we show that it regulates the balance between lipogenesis and lipolysis during larval growth.
Results
Identification and molecular characterization of schlank
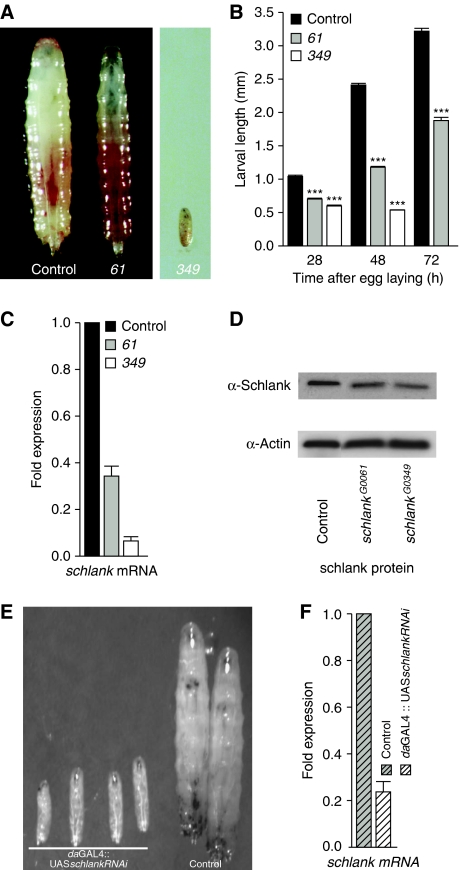
Wild-type larvae hatch after embryogenesis and pass through three larval instar stages until puparium formation, which occurs at about 96–120 h after hatching. During larval development, the animals increase their body size by about 200-fold. In a search for genes controlling larval growth in Drosophila, we screened the Göttingen P-element collection (Peter et al, 2002) and identified four P-element insertion lines affecting a single gene locus on the X-chromosome, which we had previously named Drosophila longevitiy assurance gene-1 homologue (DLag1) due to its sequence homology to the yeast LAG1 ceramide synthase (for review see D'mello et al, 1994; Teufel et al, 2009). Since we now show that the P-element insertions into the DLag1 locus cause defects in larval growth and fat metabolism (see below), we propose to rename the gene schlank (‘slim' in German) following the Drosophila nomenclature in which genes are named according to the phenotype of the respective mutants.
The schlank gene locus maps to the X-chromosome (Supplementary Figure S1A). Hemizygous schlank mutants show a delay of larval development and pronounced growth defects, which depend on the strength of the alleles (Figure 1A–C). Mutants carrying the stronger schlankG0349 allele fail to grow in the larval stages, although they feed as determined by feeding assays. After about 3days, these animals die as small larvae, which morphologically correspond in size to first instar larvae (Figure 1A–C). In contrast, hemizygous animals carrying the weaker schlankG0061 allele are developmentally delayed; however, they reach the third instar larval stage and a fraction thereof even pupariates. Quantitative real-time PCR (qRT–PCR) experiments reveal that the level of schlank transcripts is reduced to less than 40% in hemizygous schlankG0061 larvae and to less than 10% in hemizygous schlankG0349 larvae as compared with control animals (Figure 1C). However, using an antibody against the C-terminus of the schlank protein (Supplementary Figure S4), we found that the level of schlank protein is only reduced by about 20% in the schlankG0061 and by 40% in the schlankG0349 mutants (Figure 1D), indicating a maternal supply of the gene products and/or an enhanced stability of the protein. Consistently, we found that schlank mRNA and protein are highly abundant in oocytes and during early embryogenesis (Supplementary Figure S5A and B). In order to generate a ‘null' situation, we largely eliminated both schlank mRNA and protein expression by generating germline clones using the schlankG0349 allele. When both the zygotic and maternal contributions of schlank are largely eliminated, we did not obtain any eggs. This demonstrates that schlank has both a maternal and a zygotic supply and explains the residual mRNA and protein activity in schlankG0349-mutant animals. Furthermore, this is consistent with a fundamental function of schlank in the lipid metabolism (see below) and unfortunately impedes to work with complete schlank-null animals in Drosophila.
Figure 1.
Schlank is essential for larval growth. (A) Larval growth of schlankG0061 (61) mutants compared with wild-type w1118 larvae (control) at late L3 stage. schlankG0349 (349) hatch as first instar larvae and die after about 3 days as morphological first instar larvae. Food intake in mutants was controlled by feeding red-coloured yeast. (B) Average length of control [w1118] (n=123, n=81, n=48), schlankG0061 (n=54, n=187, n=63) and schlankG0349 (n=14, n=106) larvae after 24–25, 48–49 and 72–73 h after egg laying. (C) Reduced schlank mRNA expression in schlank mutants as compared with that in w1118 controls. (D) Determination of residual schlank protein (predicted size of about 46 kDa) in schlank mutants; reduction to 80% in schlankG0061 and 60% in schlankG349 mutants as compared with that in wild-type controls (100%; w1118) using schlank antibody (Supplementary Figure S4). An actin antibody was used to determine the loading control. (E,F) schlank knockdown using schlank RNAi (UASschlankRNAi) in combination with the daughterless-GAL4 (daGAL4) driver line phenocopies the schlank larval growth phenotype (E). Quantification of mRNA levels by qRT–PCR in panel F. Control: daGAL4∷w1118. Asterisks in panel B indicate significant differences to the wild type (P<0.001). (B–G) Error bars indicate s.e.m.
To further provide evidence that the lethality and the growth phenotypes of the schlank P-alleles are caused by downregulation of the schlank gene, we used RNAi-mediated knockdown. Expression of a UASschlankRNAi transgene in combination with the ubiquitous daughterless-GAL4 (daGAL4) driver line phenocopied the growth defects observed in the schlank-mutant alleles (Figure 1E and F). Furthermore, transgenic flies carrying the full-length schlank cDNA were able to rescue schlankG0349-mutant animals to the third larval instar stage and some of the animals even to the pupal stage (Supplementary data and Supplementary Figure S1C). Together, molecular analysis of the schlank alleles, reversion of the phenotype by perfect excision of the P-elements (Supplementary data from Fly strains, and data not shown), RNAi-mediated knockdown and genetic rescue experiments demonstrate that the lethality and the growth phenotype of the schlank alleles are linked to the schlank gene function.
Schlank encodes the Drosophila homologue of the Lass/ceramide synthase family
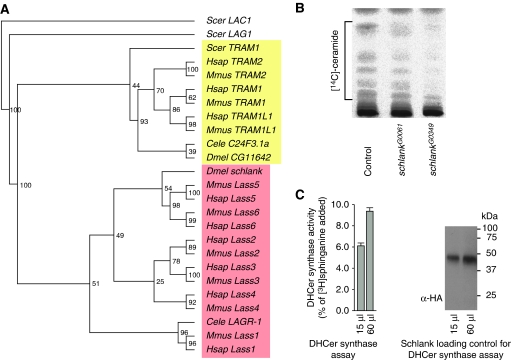
The schlank gene locus codes for two transcripts, which differ in their 5′ UTR; however, they encode the same open reading frame (ORF; Supplementary Figure S1A). The schlank ORF codes for a transmembrane protein with high homology to ceramide synthases and a phylogenetic analysis indicates that schlank is closely related to the mammalian Lass family members both in sequence and protein domain structure (Figure 2A; Supplementary Table and Supplementary Figure 1B).
Figure 2.
Schlank is involved in de novo ceramide synthesis. (A) Phylogenetic tree of Lass family members and TRAM proteins. Protein sequences were derived from the Ensembl database. If more than one protein variant existed, the version with all domain features was used (for accession numbers see Supplementary Table SI). Sequences were aligned using EMBL-EBI ClustalW2 online service using standard settings. The alignment file was used to generate 100 bootstrapped data sets with seqboot (PHYLIP 3.68 package; see also Felsenstein, 1989). The output was analysed using maximum likelihood (proml, PHYLIP package). An unrooted consensus tree was generated with consense (PHYLIP package). Bootstrap values are marked above each branch. The ceramide synthase family is highlighted in red and the TRAM family in yellow. Note that schlank falls into the Lass family and CG11642 into the TRAM family. (B) Biosynthesis of ceramides is significantly reduced in first instar schlankG0061 and schlankG0349 larvae as compared with that in w1118 wild-type controls. Sphingolipids were labelled by feeding larvae with radiolabelled L-[3-14C]-serine for 12 h. After lipid extraction equal amounts of radioactivity were applied to TLC plates, developed with chloroform/ methanol/ glacial acetic acid (190:9:1) and quantified. The total ceramide content in schlankG0061- and in schlankG0349-mutant larvae was reduced to 89 and 60%, respectively, as compared with that in the wild-type (100%). (C) The dose-dependent increase in the expression of immunoreactive schlankHA product correlates with an increase in ceramide synthase activity. Different amounts of eluted fractions after binding to a HA affinity matrix were used for immunoblotting with an HA antibody or determination of ceramide synthase activity (see also Supplementary data).
Ceramide synthases use long-chain bases, sphinganine or sphingosine, and FA-CoAs with varying chain length to produce (dihydro)ceramide, which is a precursor metabolite for all sphingolipids. Sphingolipids are structural components of most cellular membranes and can act as signalling molecules in cell growth, differentiation and apoptosis (Spiegel and Milstien, 2000; Hannun et al, 2001; Acharya and Acharya, 2005). Lass family members contain four to seven predicted transmembrane domains (Venkataraman and Futerman, 2002), a catalytic Lag1 motif and most an N-terminal domain showing sequence homology to DNA-binding homeodomains (Hox domain) (Gehring et al, 1994; Venkataraman and Futerman, 2002). Structure predictions indicate that the putative schlank protein contains six transmembrane domains, a Lag1 motif and a Hox domain, which are highly conserved (Supplementary Figures 1B and 2C). The Lag1 motif of Lass proteins, which are found in organisms ranging from yeast to mammals, is functionally required for ceramide synthesis and is contained within a stretch of 52 amino acids (Pewzner-Jung et al, 2006; Spassieva et al, 2006). The Lag1 motif of schlank is highly conserved and shows sequence identity to the Lag1 consensus motif of 82.3% (Supplementary Figure S2A). Furthermore, it contains all of the conserved amino acids that were shown to be crucial for the catalytic function of Lag1 domains in ceramide synthesis (Spassieva et al, 2006 and Supplementary Figure S2A). In addition, schlank contains a putative homeodomain, which is also found in most vertebrate Lass proteins (Supplementary Figures S1B and S2C), but not in yeast, worms and plants (Venkataraman and Futerman, 2002). The function of Lass homeodomains is unknown. In addition to schlank, a second gene (CG11642) with some homology to Lass/ceramide synthase family members was identified in the Drosophila genome by sequence similarity searches (Acharya and Acharya, 2005). However, the sequence similarity of the putative Lag1 motif of the CG11642 gene product is much lower as compared with schlank, and it is of note that most of the amino acids, which were shown to be crucial for the function of the Lag1 motif in ceramide synthesis (Spassieva et al, 2006), are not conserved and changed in the CG11642 gene product (Supplementary Figure S2B). Rather, the gene product of CG11642 seems more homologous to members of the translocating chain-associated membrane (TRAM) protein family (Figure 2A), which are not involved in ceramide synthesis (Jiang et al, 1998; Winter and Ponting, 2002; Spassieva et al, 2006). This is further supported by RNAi-mediated knockdown of CG11642 showing no phenotype in ceramide synthesis (Supplementary Figure S3B). Together, these data suggest that schlank may encode the only ceramide synthase family member in Drosophila.
Schlank is involved in de novo ceramide synthesis in Drosophila
Since schlank appears to be a member of the Lass/ceramide synthase family, we first tested whether schlank is involved in ceramide metabolism and found that the total ceramide content in schlankG0061 and in schlankG0349-mutant larvae was significantly reduced as compared with wild-type animals (Figure 2B). To specifically address whether de novo synthesis of ceramides is affected in the mutant animals, we fed schlankG0061 and schlankG0349-mutant larvae for 12 h with radiolabelled L-[3-14C]-serine, a precursor of sphingolipid biosynthesis, and we analysed the incorporation of this label into de novo generated ceramide. schlankG0061 and schlankG0349 mutants as well, as the wild-type controls, incorporated the same amount of total radioactivity per dry weight (schlankG0349 19 882 cpm/mg, schlankG0061 18 930 cpm/mg and wild-type controls 20 685 cpm/mg; s.e.m.±5%). After the extraction of lipids, ceramides were separated by thin-layer chromatography (TLC) and identified and quantified using commercially available reference standards (Supplementary Figure S3A and Supplementary data). We found in our metabolic labelling studies that the levels of de novo generated ceramides were significantly decreased in schlankG0061 (69.25%) and schlankG0349 (39%) mutants as compared with wild-type controls (100%; Figure 2B), in line with the reduced schlank protein levels in the mutants (Figure 1D).
To further establish a role of schlank in the de novo synthesis of ceramides, we generated transgenic flies expressing a C-terminally HA-tagged, full-length schlank protein (UASschlankHA). We induced the tagged protein in larvae using a heat shock-GAL4 (hsGAL4) driver line, prepared extracts to purify it partially (Materials and methods) and performed standard in vitro ceramide synthase assays (Wang and Merrill, 1999). We found that increasing amounts of purified schlank led to a dose-dependent increase in ceramide synthase activity and increased ceramide levels (Figure 2C).
Manipulation of schlank activity in loss- and gain-of-function experiments
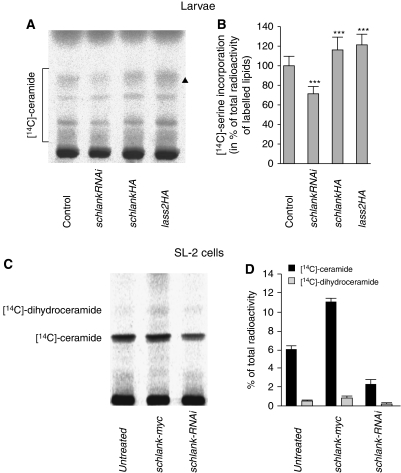
To further support a role of schlank as ceramide synthase, we reduced or elevated its activity in loss- and gain-of-function experiments and analysed the effects on ceramide levels. To this end, we fed radiolabelled L-[3-14C]-serine to larvae carrying transgenic UASschlankRNAi or UASschlankHA in combination with the hsGAL4 driver line. Upon a short heat shock (1 h) to induce schlank RNAi knockdown or schlank overexpression, we observed a decrease or increase of ceramide levels, respectively (Figure 3A and B). Most interestingly, overexpression of the murine Lass2 homologue for which ceramide synthase activity has been shown previously (Mizutani et al, 2005), resulted in a similar increase in ceramide levels (Figure 3A and B). In contrast, an increase in ceramide levels could not be observed when we overexpressed a schlank protein variant, schlankH215D (Supplementary Figure S3B), which contains a point mutation in the Lag1 motif shown to inhibit ceramide synthase function in Lass1 and 5 (Spassieva et al, 2006; change of a highly conserved histidine at position 215 into glutamate; Supplementary Figure S2A).
Figure 3.
Modulation of schlank activity correlates with the rate of ceramide de novo synthesis (A, B) Larvae carrying hsGAL4 and either UASschlankRNAi (schankRNAi), UASschlankHA (schlankHA) or UASlass2HA (lass2HA) were heat shocked for 1 h and subsequently fed L-[3-14C]-serine. Its incorporation of L-[3-14C]-serine into de novo ceramide was analysed in larvae 12 h after heat shock by TLC with chloroform/methanol/glacial acetic acid (190:9:1) (A) and quantified (B). Asterisks in panel B indicate significant differences to wild-type controls [hsGAL4∷w1118] (P<0.001). (C, D) Treatment of SL-2 cells in the presence of [14C]serine with schlank dsRNA or schlank overexpression after transfection of SL-2 cells shows significant downregulation or upregulation of (dihydro)ceramide, respectively. Ceramides and dihydroceramides were separated on TLC plates impregnated with borate with chloroform/methanol 9:1. The main (dihydro)ceramide bands depicted in panel C correspond to the main ceramide marked with an asterisk in panel A (see also Supplementary Figure S2A). Error bars indicate s.d.
To further test whether the effects on larval lipid composition were due to the influence of schlank on the metabolism and catabolism of endogenous lipids, rather than, for example, on an impaired uptake from the yeast food, we also analysed de novo synthesis of ceramide in SL-2 cells. Upon RNAi-mediated knockdown of schlank (dihydro)ceramide levels were significantly downregulated, whereas they were upregulated upon schlank overexpression (Figure 3C and D), consistent with the results obtained in larvae. Together, the analysis of schlank mutants, knockdown animals and overexpression studies in vivo and in vitro strongly support a role of schlank as a ceramide synthase in Drosophila.
TAG levels are reduced in schlank mutants
While hemizygous animals carrying the stronger schlankG0349 allele fail to grow in the larval stages and die with a morphology of first instar larvae, the schlankG0061 mutants are developmentally delayed; however, some of them pass through the third instar larval stage and die later after eclosion. When analysing third instar larvae of schlankG0061 mutants, we noticed that they appeared much slimmer and somewhat transparent, indicating loss of storage fat in the fat body (Figure 1A). A function in regulating organismal fat storage or mobilization has previously not been observed for Lass/ceramide synthase family members due to lack of animal models. We, therefore, investigated this phenotype in more detail.
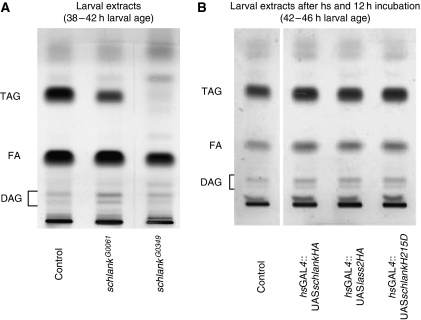
The larval period is critical for the control of animal growth. Regulation of larval growth by fat body has been demonstrated previously (Britton et al, 2002; Colombani et al, 2005). The larval stage is characterized by extensive feeding, which supports rapid growth of the animal and allows accumulation of energy stores, primarily in the larval fat body (Aguila et al, 2007). Immunohistochemical analysis indicates that schlank is strongly expressed in the fat-body cells (Supplementary Figure S5C–F). To study whether schlank may play a role in fat metabolism, we analysed TAG, diacylglycerol (DAG) and FA levels in schlankG0061- and schlankG0349-mutant larvae of the same developmental stage and compared them with those in wild-type animals. Both schlankG0061 and schlankG0349 mutants showed significantly reduced TAG levels to 63 and 13%, and FA and DAG levels were also altered (Figure 4A; Table I for quantification). The specificity of this phenotype was further confirmed by induction of schlank RNAi knockdown showing also strong TAG reduction (Supplementary Figure S3C). In contrast, we observed an increase of TAG, DAG and FA levels upon overexpression of schlank and the murine Lass2 homologue (Figure 4B; Table I for quantification), suggesting a conserved role for Lass proteins in lipid homeostasis. When we overexpressed the schlankH215D variant containing a mutation, which severely affects ceramide synthase activity (Supplement Figure S3B; Materials and methods), we observed a similar increase of TAG, DAG and FA levels (Figure 4B and Table I). This effect was comparable to the increase seen when overexpressing wild-type schlank or murine Lass2, suggesting that there are also effects of schlank on fat metabolism that may be independent of its ceramide synthase function. In summary, these data indicate an important role of schlank in regulating TAG levels and body fat metabolism, consistent with the strong expression of schlank protein in the larval fat body. To study how this effect occurs, we analysed the expression of key regulators of lipogenesis and lipolysis in schlank mutants and in animals overexpressing schlank.
Figure 4.
A role for schlank in TAG regulation. (A) Comparison of in vivo TAG levels in 38- to 42-h-old schlankG0061- and schlankG0349-mutant animals of the same age with w1118 controls. In both mutants TAG and FA levels were reduced to a different extent correlating well with the severity of the mutant schlank alleles. (B) TAG and FAs are elevated in 42- to 46-h-old larvae upon overexpression of either schlankHA (hsGAL4∷UASschlankHA), murine lass2HA (hsGAL4∷UASlass2HA) or schlankH215D (hsGAL4∷UASschlankH215D), which cannot upregulate ceramide synthesis (see Supplementary Figure S3B). Triacylglycerol (TAG), diacylglycerol (DAG), fatty acids (FA). For photodensitometric quantification, see Table I.
Table 1.
Quantification of TAG, FA and DAG in schlank-mutants (Figure 4A)
| TAG | FA | DAG | |
|---|---|---|---|
| Controla | 100.0 | 100.0 | 100.0 |
| schlankG0061a | 63.41 | 98.06 | 139.57 |
| schlankG0349a | 13.40 | 73.25 | 105.36 |
| Controlb | 100.0 | 100.0 | 100.0 |
| UAS-schlankHAb | 109.0c | 121.8 | 116.4 |
| murine UAS-lass2b | 106.6c | 114.6c | 120.0c |
| UAS-schlankH215Db | 106.8 | 106.5 | 117.4c |
| aRelative lipid content of TAGs, FAs and DAGs in 38–42 h schlank-mutant larvae (Figure 4A) compared to w1118 larvae as control. | |||
| bRelative lipid content of TAGs, FAs and DAG (Figure 4B) after heat shock (for 1 h at 37°C) followed by 12 h incubation at 20°C to exclude further induction by the heat shock promoter. Larval age was 42 to 46 h; w1118 was used as control. | |||
| cP<0.05. | |||
Schlank negatively regulates the expression of lipases in the fat body, downregulating lipolysis
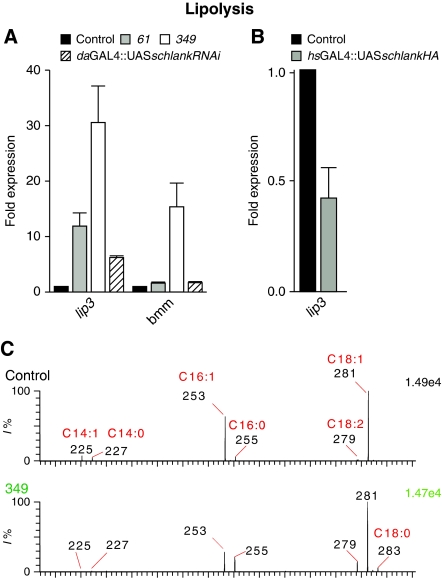
Lipolysis in response to fasting is accompanied by upregulation of lipases, the rate-limiting enzymes regulating TAG mobilization in insect fat body cells (Arrese and Wells, 1997; Grönke et al, 2005). As schlank is highly expressed in the larval fat body and TAG levels were reduced in schlankG0349 and schlankG0061 animals, we first asked whether lipases are misregulated in schlank mutants. Using quantitative RT–PCR we found that both mRNA levels of brummer encoding an ATGL lipase (Grönke et al, 2005; Haemmerle et al, 2006), and of lipase3 (lip3), which encodes a TAG lipase (Zinke et al, 2002; Fuss et al, 2006), were upregulated (Figure 5A) in hemizygous schlankG0349 and schlankG0061 mutants, fitting well with the decreased TAG levels in these animals (Figure 4A and Table I). Likewise, upon RNAi-mediated knockdown of the schlank gene, the mRNA levels of both lipases were also increased (Figure 5A). A detailed analysis by ESI-MS of first instar schlankG0349 animals demonstrated that particularly myristic acid (C14:0), myristoleic acid (C14:1) and palmitic acid (C16:1) were reduced (Figure 5C and Table II). In contrast, the mRNA level of lipase3 was decreased upon ubiquitous expression of schlank in larvae with the hsGAL4 driver (Figure 5B).
Figure 5.
Schlank affects lipolysis. (A) Transcript levels of lip3 and bmm lipases are upregulated in first instar schlankG0061 and schlankG0349 mutants and upon schlank knockdown using a daGAL4 driver line, as quantified by qRT–PCR. schlank mutants were compared with wild-type w1118 larvae, and schlankRNAi was compared with daGAL4∷w1118) (B) Overexpression of UASschlankHA using a heat shock-GAL4 (hsGAL4) driver line decreases lip3 transcript levels (hsGAL4∷w1118 were used as control). (C) Analysis and quantification of FAs in 38- to 42-h-old w1118 control and schlankG0349 mutants. Quantification of FAs was performed by ESI-MS using an internal standard (Table I and Supplementary data). Error bars indicate s.e.m.
Table 2.
ESI-MS quantification of FAs in first instar schlankG0349 mutants (see Figure 4A)
| FA | Control | schlank G0349 | Ratio (349/control) (%) |
|---|---|---|---|
| (pmol/1 mg dry weight) | |||
| C14:0 | 27.87 | 8.56 | 30.7 |
| C14:1 | 9.64 | 1.32 | 13.7 |
| C16:0 | 48.35 | 21.31 | 44.1 |
| C16:1 | 38.74 | 11.94 | 30.8 |
| C18:0 | 21.48 | 10.67 | 49.6 |
| C18:1 | 40.59 | 17.29 | 42.6 |
| For the quantitative MS analysis of fatty acids we used 1 μg 18,18,18-D3-stearic acid as internal standard and lipid extract from 100 or 200 μg dry larvae. Fatty acids were identified by their m/z values [M-H]− and their relative concentrations were calculated on the basis of peak intensity; w1118 was used as control. Values represent means (n=2); s.d. was in the range of 10 to 15%. | |||
We next addressed the question whether schlank is also functionally required in the fat body. Therefore, we expressed UASschlankHA under the control of the fat body-specific driver line pumpless-GAL4 (pplGal4; Zinke et al, 2002) in the background of hemizygous schlankG0349 mutants. We found that schlankG0349 larvae, which normally die as small morphologically first instar larvae, were now rescued. A couple of these rescued animals generated a fat body, showed an increase of TAG, DAG and FA levels (Supplementary Figure S3D and E), and grew to the second or third larval instar stage. Some of these animals even pupariated and showed a phenotype similar to the weaker schlankG0061 mutants (Supplementary Figure S1C). Noteworthy, expression of the murine Lass2 in the fat body of schlankG0349 mutants led also to rescue of the small schlankG0349 mutant larvae to the second and third larval instar stages (Supplementary Figure S1C). However, when we expressed the schlankH215D variant in schlankG0349 mutant larvae no rescue could be observed (Supplementary Figure S1C).
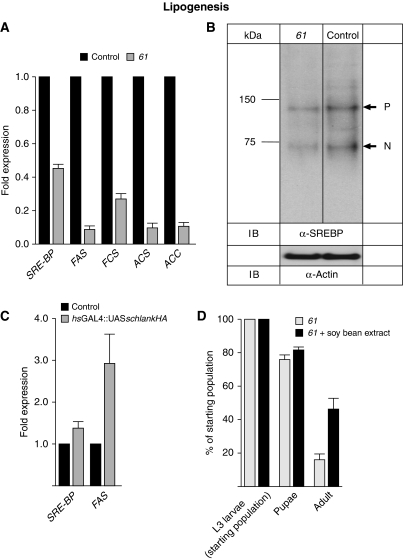
Schlank can act as a positive regulator of SREBP-dependent lipogenesis in Drosophila larvae
Since TAG levels were increased upon overexpression of schlank (Figure 4B and Table I), we studied its putative role in lipogenesis in more detail. Lipid homeostasis is controlled in all metazoans studied by a regulatory pathway involving the SREBP protein. SREBPs are transcription factors required for synthesis of cholesterol and unsaturated FAs in mammals and saturated FAs in Drosophila (Sato et al, 1994; Seegmiller et al, 2002). SREBPs are generated as precursors containing two membrane-spanning helices inserted into ER membranes. In response to cellular lipid needs, SREBP exits the ER and travels to the Golgi apparatus, where it is subject to sequential cleavages. A transcriptionally active domain is then released from the membrane, enabling it to travel to the nucleus and activate the transcription of target genes required for FA synthesis. Since schlankG0349 mutants die as first instar larvae, we analysed schlankG0061 mutant larvae in which the fat body, the storage organ for lipid droplets, is well developed. Furthermore, using feeding assays, we made sure that schlankG0061-mutant larvae feed properly. We found that in schlankG0061 hemizygous animals the level of SREBP mRNA is markedly decreased (Figure 6A) and accompanied by reduced SREBP protein level of both the membrane-bound precursor protein and the transcriptionally active nuclear protein (Figure 6B). Consistently, transcription of the SREBP-target genes (Seegmiller et al, 2002) acetyl-CoA synthetase (ACS), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), encoding enzymes of FA biosynthesis, and fatty acyl-CoA synthetase (FCS), encoding an enzyme involved in synthesis of phospholipids, are strongly reduced (Figure 6A).
Figure 6.
Schlank is a positive regulator of lipogenesis. (A) Reduced mRNA expression of SREBP, FAS, FCS, ACS as well as ACC in schlankG0061 as compared with w1118 control. Quantification by qRT–PCR. (B) Immunoblot analysis of schlankG0061 (61) and w1118 (control) whole-fly lysates (60 μg/lane) shows reduction in SREBP protein levels in schlankG0061 mutants. The blot was probed with monoclonal antibody against the NH2-terminal fragment of dSREBP (IgG-3B2; Seegmiller et al, 2002). The membrane was then stripped and reprobed with anti-actin antibody used as loading control (lower panel). (C) Increased dSREBP and FAS transcript levels in larvae overexpressing UASschlankHA in combination with an hsGAL4 driver line after 1 h of heat shock. (D) Soy lipid extract can rescue schlankG0061-mutant animals to adulthood while the percentage of larvae and pupae is not altered. Error bars indicate s.e.m.
In contrast, overexpression of schlank in larvae using transgenic UASschlank effector lines and hsGAL4 driver lines led to an increase of SREBP transcript levels and of SREBP-dependent target gene expression, including FAS (Figure 6C). These data are in agreement with the observed downregulation of TAG and FA levels in schlank-mutant larvae and elevated levels of TAG and FAs in larvae overexpressing schlank (Figures 4A and B; Table I).
Data from mutant and overexpression suggest that schlank may act as a positive regulator of SREBP transcript levels and of SREBP-dependent FA and phospholipid synthesis. This is consistent with the similarity of the phenotypes found in dSREBP and schlank mutants, which both show growth and metabolic defects, including reduction of the FA levels (Kunte et al, 2006). Additional evidence for a link between SREBP and schlank was provided by a dietary supplementation assay. For SREBP mutants it was shown that they suffer from FA deficiency due to a transcriptional deficit in genes needed for lipid synthesis, and that addition of soy lipids to the food improved their survival (Kunte et al, 2006). Similarly, feeding schlank mutants with yeast paste supplemented with soy lipids led to significant increase of schlankG0061 mutant animals surviving to adulthood (Figure 6D). These results further indicate that SREBP is downstream of schlank and regulated by schlank activity.
Discussion
Our results provide strong evidence that schlank is a Drosophila member of the highly conserved Lass/ceramide synthase family and is involved in the synthesis of a broad range of ceramides, key metabolites of the de novo biosynthesis of sphingolipids. Although ceramide synthases are highly conserved in all eukaryotes, their role in development and growth is poorly understood due to the lack of animal models.
Mutants of the Drosophila Lass homologue schlank show severe growth defects
Genetic experiments and our mRNA and protein expression studies indicate that schlank is provided both maternally and zygotically, reflecting the importance of the enzyme for cellular metabolism and survival. We found that zygotic mutants of the stronger schlankG0349 allele still have considerable amount of schlank protein, although mRNA levels are strongly reduced. This reflects the maternal supply of the protein and its stability, and it explains the residual ceramide synthase activity found in the zygotic schlankG0349 mutants. When we eliminated both the zygotic and the maternal contributions of schlank to obtain a null situation (in schlankG0349 germline clones), oogenesis failed to occur and no embryos could be obtained for further studies. These data also indicate that the CG11642 gene, which is the only other Drosophila gene showing some sequence homology to Lass family members (Acharya and Acharya, 2005), cannot compensate for the loss of schlank and further suggest that schlank most likely encodes the only ceramide synthase family member in Drosophila. The CG11642 gene seems more homologous to members of the TRAM protein family (Figure 2A and Supplementary Figure 2B), which are not involved in ceramide synthesis.
Both schlankG0349and schlankG0061 mutants show delay of larval development and pronounced growth defects depending on the strength of the respective allele. Determination of the ceramide synthase activity in both schlank-mutant alleles, in RNAi-knockdown animals, in tissue culture cells and in animals overexpressing schlank demonstrate that it is involved in the synthesis of a rather broad spectrum of ceramides (Figure 3A). In contrast, the six known members of the mammalian Lass family each seem to possess characteristic substrate specificity (Mizutani et al, 2005; Laviad et al, 2008). This may explain why murine Lass2 is not able to rescue the schlankG0349 mutants to the pupal stage, but rather causes partial rescue to the second/third larval instar stages (Supplementary Figure 1C). On the other hand, the fact that we do obtain a rescue of schlank mutants with Lass2 further strengthens our conclusion that schlank encodes a ceramide synthase.
Interestingly, our analysis of schlank mutants and overexpression of schlank and murine Lass2 revealed an additional phenotype and function of Lass family members that has not been observed in the previous studies in mammalian tissue culture cells. We identified schlank as an important regulator of the balance between lipogenesis and lipolysis. We found that schlank-mutant larvae or larvae analysed upon downregulation of schlank by RNAi are largely devoid of storage fat (Figure 4 and Supplementary Figure S3C), which is deposited as TAGs in the fat body, whereas larvae overexpressing schlank showed increased TAG levels, as determined by TLC analysis (Figure 4A and B; Table I). Even more, overexpression of schlank in schlankG0349 mutants could restore the fat body development and lead also to increasing TAG, DAG and FA levels (Supplementary Figure S3D and E). The loss of storage fat in schlank mutants or upon downregulation of schlank by RNAi in Drosophila larvae is partly due to upregulation of mRNA levels of lipases (Figure 5A), including brummer encoding an ATGL lipase (Grönke et al, 2005; Haemmerle et al, 2006), and of lip3, which encodes a TAG lipase (Zinke et al, 2002; Fuss et al, 2006). When schlank or murine Lass2 were overexpressed, TAG, DAG and FA levels were increased and expression of lipolytic lipases was decreased, confirming the negative regulatory effect on lipases (Figure 5B).
Schlank regulates the balance between lipogenesis and lipolysis during growth
Our data suggest that the role of schlank in lipogenesis can be mediated, at least in part, through positive regulation of SREBP, a key regulator required for synthesis of cholesterol and unsaturated FAs in mammals and saturated FAs in Drosophila (Sato et al, 1994; Seegmiller et al, 2002). The decrease of SREBP mRNA levels in schlank mutants (Figure 6A) is in line with the reduced transcription of the SREBP-dependent enzymes of FA biosynthesis and with the reduced TAG levels in these animals (Table I). In particular, we found downregulation myristic acid (C14:0), myristoleic acid (C14:1) and palmitic acid (C16:1) in schlank-mutant larvae, as demonstrated by ESI-MS analysis and quantification of FA (Figure 5C). In contrast, we found an increase of the level SREBP mRNA and of SREBP-dependent target gene expression, including FAS, upon overexpression of schlank (Figure 6C). In line with these data, we found elevated levels of TAG in larvae overexpressing schlank (Figure 4 and Table I). Interestingly, ubiquitous expression of the murine Lass2 in larvae also lead to increase of TAG, DAG and FA levels. This suggests a conserved function of Lass proteins in TAG lipid homeostasis. It is of note that ceramide synthesis is dependent on the availability of long-chain saturated FAs, which participate in the initial rate-limiting reaction involving the condensation of a FA-CoA and serine (Adachi-Yamada et al 1999; Batheja et al, 2003), and in the conversion of (dihydro)sphingosine to (dihydro)ceramid, which involves amino acylation with a long-chain FA at carbon-2 of sphingosine. schlank may, thus, act as a metabolic sensor linking sphingolipid homeostasis with FA metabolism in cells. Together, these data identify schlank as a positive regulator of SREBP and are consistent with phenotypes of dSREBP and schlank mutants both showing similar growth and metabolic defects and the fact that both mutants can be rescued by supplementation of food with soy lipids (Figure 6D).
Cholesterol in mammals or unsaturated FAs in mammals and in Drosophila are known regulators of transcriptional and posttranscriptional processing of SREBP. Recent findings demonstrated that modification of ceramide synthesis can also contribute to SREBP regulation (Worgall et al, 2004). It has been shown that leptin-induced reduction of the expression of the ceramide synthases Lass2 and 4 resulted in downregulation of SREBP-1c mRNA and reduced lipogenesis in lean tissue (Gallardo et al, 2007), whereas proteolytic maturation of SREBP-1c was reduced in white adipose tissue (Bonzón-Kulichenko et al, 2009). These observations are in line with our data using schlank mutants showing reduced ceramide synthase activity and reduced lipogenesis, which may be exerted, at least in part, through downregulation of SREBP mRNA. How this occurs mechanistically is not yet known. It is of note that no rescue of schlank349 mutants was obtained when we expressed the mutated schlankH215D protein in which the ceramide synthase function is severely affected in the fat body of schlank349 mutants. This is consistent with the idea that ceramide biosynthesis is essential for tissue growth and survival of the animals. In contrast, overexpression of the schlankH215D variant did not alter ceramide levels; however, it caused an increase in TAG, DAG and FA levels comparable to that seen when overexpressing wild-type schlank or murine Lass2 (Figure 4 and Table I). This suggests the possibility that some effect of schlank on TAG metabolism may be independent of its ceramide synthase function. In this context, the N-terminally located homeobox (Hox) domain, which is contained within many of the Lass proteins of higher organisms including Lass 2, may be relevant. Future studies will have to reveal whether the homeodomain is involved in some of the regulatory effects exerted by schlank.
How schlank expression is regulated is not known. Lipid and carbohydrate metabolism are regulated by a variety of hormones, including insulin and glucagon in mammals or insulin-like peptides and the glucagon-like adipokinetic hormones in insects (Brogiolo et al, 2001; Van der Horst et al, 2001; Fuss et al, 2006; Hafner et al, 2006). However, mechanisms by which cellular lipid metabolism might be interlaced with hormone-dependent body fat regulation are unknown at present.
Together, our results identify schlank as a Drosophila member of the Lass family of ceramide synthases. Furthermore, our data provide evidence that schlank acts as a regulator of the balance between lipogenesis and lipolysis during larval growth, suggesting a novel role for ceramide synthases in regulating body fat metabolism.
Materials and methods
Fly strains, temperature shift and rescue experiments
Fly stocks were obtained from the Bloomington stock centre. schlank alleles schlankG0370, schlankG0349, schlankG0163 and schlankG0061 were isolated by screening the Göttingen X-chromosome collection of P-lines (Peter et al, 2002). schlankG0370, schlankG0349 and schlankG0163 are strong alleles and schlankG0061 is a weak allele. For phenotypic analysis, schlank alleles were balanced over FM7c, P{KrGAL4} and P{UAS-GFP}. For rescue experiments, flies of the genotype schlankG0349/FM7c, P{KrGAL4}, P{UAS-GFP}; +; UASschlankHA or UASlass2HA were crossed with the pplGAL4 transgene (gift from I Zinke) to express full-length schlank or the murine lass2 cDNA in a schlank-mutant background. Heterozygous progeny, males and females expressing Krüppel (Kr; Hoch et al, 1990, 1991) GFP can thereby be distinguished from the hemizygous males and heterozygous females carrying the schlankG0349 mutation.
Cloning and transgene production
schlankHA or lass2HA was generated by PCR reaction using specific schlank or lass2 primer pairs. The pP{UASschlankHA}and pP{UASlass2HA}were cloned by standard procedures and constructs were integrated into w1118 fly genomes by P-element-mediated transformation (Rubin and Spradling, 1982).
Site-directed mutagenesis
Site-directed mutagenesis for pP{UASschlankH215D} was performed with the GeneTailor site-directed mutagenesis system (Invitrogen Corp., Carlsbad, CA, USA) following the manufacturer's recommendation.
Real-time RT-PCR
Isolation of mRNA and quantification by real-time RT–PCR were performed as described by Fuss et al (2006). All template reactions were performed in parallel and repeated with independently isolated RNA samples from different larval or cell collections.
Antibody generation
Polyclonal antiserum was generated in guinea pig (BioGenes, Berlin, Germany) against a schlank-specific oligopeptide (SRAGARVATTERREE) (Supplementary Figure S4A).
Purification of schlankHA
For partial purification of schlankHA cell lysate of larvae expressing UASschlankHA were subjected to a 0.5 ml anti-HA affinity matrix column and schlankHA was purified using the anti-HA affinity matrix kit (Roche, Germany) according the manufacturer's instructions.
Immunoblotting and immunohistochemistry
Immunoblotting or immunohistochemistry experiments were performed as described earlier (Bauer et al, 2006). The following antibodies were used for immunoblotting: anti-HA (rat, 1:500; clone 3F10; Roche) or anti-schlank (guinea pig, 1:400). For immunohistochemical staining we used anti-schlank (1:40), anti-HA (rat, 1:100; clone 3F10; Roche) and anti-Spectrin (mouse, 1:40; Developmental Studies, Hybridoma Bank).
De novo ceramide synthase activity
Ceramide synthase activity was determined by measuring de novo ceramide generation after 12-h labelling of larvae with L-[3-14C]-serine. For metabolic labelling, larvae were fed with heat inactivated yeast paste containing L-[3-14C]-serine (54 mCi/mmol) as a precursor for sphingolipids (GE, Germany). Ceramides were separated by TLC as described above by loading the same amounts of radioactivity to each line on the TLC plates. Radioactive bands were visualized with a Bio Imaging Analyser 1000 (Fuji, Japan) and quantification was performed with the image analysis software Tina (Raytest, Staubenhardt, Germany). Ceramides were identified and quantified using commercially available reference standards (ceramide (C18:1/C18:0) (Sigma, Germany), phytoceramide (Cosmoferm, Delft, Netherlands), and stearic acid (Fluka)). In order to rule out that any yeast ceramides are included in our determination of Drosophila ceramides, yeast ceramides of a yeast control were analysed for every experiment in parallel. Ceramide synthase assay was performed as described previously (Wang and Merrill, 1999), with minor modifications.
Lipid analysis in larvae
Lipids were of larvae were extracted and separated as described by Reichelt et al (2004). Unpolar lipids (FA, TAG) were separated by TLC with n-hexane/diethylether/glacial acetic acid (70:30:1, vol/vol/vol) and ceramides with chloroform/methanol/glacial acetic acid (190:9:1, vol/vol/vol). Detail information is provided in the Supplementary data. For ESI-MS we used a ESI-Q-Tof 2 mass spectrometer equipped with a nanospray source (MicroMass, Manchester, UK) and FA quantification we used D3-stearic acid (Sigma, Germany). Details about methods and primers are provided in Supplementary data.
Supplementary Material
Supplementary data
Review Process File
Acknowledgments
We thank Mustapha Diallo for technical help with RNAi experiments and quantification of the lipids in SL-2 cells, Dr Guenter Schwarzmann for synthesis of the radiolabelled dihydrosphingosin and Heike Hupfer for technical assistance. We thank Ingo Zinke for the pplGAL4 line and for support in real-time PCR analysis and Monika Koehnke for cloning of the UASlass2HA construct. We also thank Robert Rawson for providing the IgG-3B2 SREBP antibody. This work was supported by the SFB 645 (grants to MH and RB, KS and US), GRK 804 (IH and MH) and by the seventh framework program of the EU-funded ‘LipidomicNet', proposal number 202272 (to KS).
Footnotes
The authors declare that they have no conflict of interest.
References
- Acharya U, Acharya JK (2005) Enzymes of sphingolipid metabolism in Drosophila melanogaster. Cell Mol Life Sci 62: 128–142 [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, Gotoh T, Sugimura I, Tateno M, Nishida Y, Onuki T, Date H (1999) De novo synthesis of sphingolipids is required for cell survival by down-regulating c-Jun N-terminal kinase in Drosophila imaginable discs. Mol Cell Biol 19: 7276–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguila JR, Suszko J, Gibbs AG, Hoshizaki DK (2007) The role of larval fat cells in adult Drosophila melanogaster. J Exp Biol 210: 956–963 [DOI] [PubMed] [Google Scholar]
- Arrese EL, Wells MA (1997) Adipokinetic hormone induced lipolysis in the fat body of an insect, Manduca sexta: synthesis of sn-1,2-diacylglycerols. J Lipid Res 38: 68–76 [PubMed] [Google Scholar]
- Batheja AD, Uhlinger DJ, Carton JM, Ho G, D'Andrea MR (2003) Characterization of serine palmitoyltransferase in normal human tissues. J Histochem Cytochem 51: 687–696 [DOI] [PubMed] [Google Scholar]
- Bauer R, Weimbs A, Hoch M (2006) DE-cadherin, a core component of the adherens junction complex modifies subcellular localization of the Drosophila gap junction protein innexin2. Cell Commun Adhes 13: 103–114 [DOI] [PubMed] [Google Scholar]
- Bonzón-Kulichenko E, Schwudke D, Gallardo N, Moltó E, Fernández-Agulló T, Shevchenko A, Andrés A (2009) Central leptin regulates total ceramide content and sterol regulatory element binding protein-1C proteolytic maturation in rat white adipose tissue. Endocrinology 150: 169–178 [DOI] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA (2002) Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell 2: 239–249 [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E (2001) An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol 11: 213–221 [DOI] [PubMed] [Google Scholar]
- Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. Annu Rev Nutr 21: 23–46 [DOI] [PubMed] [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carré C, Noselli S, Léopold P (2005) Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310: 667–670 [DOI] [PubMed] [Google Scholar]
- D'mello NP, Childress AM, Franklin DS, Kale SP, Pinswasdi C, Jazwinski SM (1994) Cloning and characterization of LAG1, a longevity-assurance gene in yeast. J Biol Chem 3: 15451–15459; erratum in J Biol Chem 1994; 269: 28522 [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB (2002) Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science 3: 879–883 [DOI] [PubMed] [Google Scholar]
- Felsenstein J (1989) PHYLIP—Phylogeny Inference Package (version 3.2). Cladistics 5: 164–166 [Google Scholar]
- Fuss B, Becker T, Zinke I, Hoch M (2006) The cytohesin Steppke is essential for insulin signalling in Drosophila. Nature 444: 945–948 [DOI] [PubMed] [Google Scholar]
- Gallardo N, Bonzón-Kulichenko E, Fernández-Agulló T, Moltó E, Gómez-Alonso S, Blanco P, Carrascosa JM, Ros M, Andrés A (2007) Tissue-specific effects of central leptin on the expression of genes involved in lipid metabolism in liver and white adipose tissue. Endocrinology 148: 5604–5610 [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Affolter M, Burglin T (1994) Homeodomain proteins. Annu Rev Biochem 63: 487–526 [DOI] [PubMed] [Google Scholar]
- Gibbons GF, Islam K, Pease RJ (2000) Mobilisation of triacylglycerol stores. Biochim Biophys Acta 1483: 37–57 [DOI] [PubMed] [Google Scholar]
- Grönke S, Mildner A, Fellert S, Tennagels N, Petry S, Müller G, Jäckle H, Kühnlein RP (2005) Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab 1: 323–330 [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspo M, Hoefler G, Zechner R (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312: 734–737 [DOI] [PubMed] [Google Scholar]
- Hafner M, Schmitz A, Grüne I, Srivatsan SG, Paul B, Kolanus W, Quast T, Kremmer E, Bauer I, Famulok M (2006) Inhibition of cytohesins by SecinH3 leads to hepatic insulin resistance. Nature 444: 941–944 [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C, Argraves KM (2001) Enzymes of sphingolipid metabolism: from modular to integrative signalling. Biochemistry 40: 4893–4903 [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18: 1926–1945 [DOI] [PubMed] [Google Scholar]
- Hojjati MR, Li Z, Jiang XC (2005) Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta 1737: 44–51 [DOI] [PubMed] [Google Scholar]
- Hoch M, Schröder C, Seifert E, Jackle H (1990) Cis-acting control elements for Krüppel expression in the Drosophila embryo. EMBO J 9: 2587–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch M, Seifert E, Jackle H (1991) Gene expression medicated by cis-regulatory elements of the Krüppel gene in response to the anterior morphogens bicoid and hunchback. EMBO J 10: 2267–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Kirchman PA, Zagulski M, Hunt J, Jazwinski SM (1998) Homologs of the yeast longevity gene LAG1 in Caenorhabditis elegans and human. Genome Res 8: 1259–1272 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846 [DOI] [PubMed] [Google Scholar]
- Kolter T, Sandhoff K (2006) Sphingolipid metabolism diseases. Biochim Biophys Acta 1758: 2057–2079 [DOI] [PubMed] [Google Scholar]
- Kunte AS, Matthews KA, Rawson RB (2006) Fatty acid auxotrophy in Drosophila larvae lacking SREBP. Cell Metab 3: 439–448 [DOI] [PubMed] [Google Scholar]
- Lahiri S, Futerman AH (2007) The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci 64: 2270–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH Jr, Futerman AH (2008) Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem 283: 5677–5684 [DOI] [PubMed] [Google Scholar]
- Li CM, Park JH, Simonaro CM, He X, Gordon RE, Friedman AH, Ehleiter D, Paris F, Manova K, Hepbildikler S, Fuks Z, Sandhoff K, Kolesnick R, Schuchman EH (2002) Insertional mutagenesis of the mouse acid ceramidase gene leads to early embryonic lethality in homozygotes and progressive lipid storage disease in heterozygotes. Genomics 79: 218–224 [DOI] [PubMed] [Google Scholar]
- Martin DE, Hall MN (2005) The expanding TOR signaling network. Curr Opin Cell Biol 17: 158–166 [DOI] [PubMed] [Google Scholar]
- Martin S, Parton RG (2006) Lipid droplets: a unified view of a dynamicorganelle. Nat Rev Mol Cell Biol 7: 373–378 [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller G F, Spiegel S, Proia RL (2005) Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani Y, Kihara A, Igarashi Y (2005) Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J 390: 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RT, Soulages JL, Hariharasundaram B, Arrese EL (2005) Activation of the lipid droplet controls the rate of lipolysis of triglycerides in the insect fat body. J Biol Chem 280: 22624–22631 [DOI] [PubMed] [Google Scholar]
- Peter A, Schöttler P, Werner M, Beinart N, Dowe G, Burkert P, Mourkioti F, Dentzer L, He Y, Deak P, Benos PV, Gatt MK, Murphy L, Harris D, Barrell B, Ferraz C, Vidal S, Brun C, Demaille J, Cadieu E et al. (2002) Mapping and identification of essential gene functions on the X chromosome of Drosophila. EMBO Rep 3: 34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Ben-Dor S, Futerman AH (2006) When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J Biol Chem 281: 25001–25005 [DOI] [PubMed] [Google Scholar]
- Reichelt J, Breiden B, Sandhoff K, Magin TM (2004) Loss of keratin 10 is accompanied by increased sebocyte proliferation and differentiation. Eur J Cell Biol 83: 747–759 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC (1982) Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353 [DOI] [PubMed] [Google Scholar]
- Sato R, Yang J, Wang X, Evans MJ, Ho YK, Goldstein JL, Brown MS (1994) Assignment of the membrane attachment, DNA binding, and transcriptional activation domains of sterol regulatory element-binding protein-1 (SREBP-1). J Biol Chem 24: 17267–17273 [PubMed] [Google Scholar]
- Seegmiller AC, Dobrosotskaya I, Goldstein JL, Ho YK, Brown MS, Rawson RB (2002) The SREBP pathway in Drosophila: regulation by palmitate, not sterols. Dev Cell 269: 229–238 [DOI] [PubMed] [Google Scholar]
- Simha V, Garg A (2006) Lipodystrophy: lessons in lipid and energy metabolis. Curr Opin Lipidol 17: 162–169 [DOI] [PubMed] [Google Scholar]
- Spassieva S, Seo JG, Jiang JC, Bielawski J, Alvarez-Vasquez F, Jazwinski SM, Hannun YA, Obeid LM (2006) Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J Biol Chem 281: 33931–33938 [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S (2000) Sphingosine-1-phosphate: signaling inside and out. FEBS Lett 30: 55–57 [DOI] [PubMed] [Google Scholar]
- Teufel A, Maass T, Galle PR, Malik N (2009) The longevity assurance homologue of yeast lag1 (Lass) gene family. Int J Mol Med 476: 135–140 [PubMed] [Google Scholar]
- Van der Horst DJ, Van Marrewijk WJ, Diederen JH (2001) Adipokinetic hormones of insect: release, signal transduction, and responses. Int Rev Cytol 211: 179–240 [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Futerman AH (2002) Do longevity assurance genes containing Hox domains regulate cell development via ceramide synthesis? FEBS Lett 528: 3–4 [DOI] [PubMed] [Google Scholar]
- Wang E, Merrill AH Jr (1999) Ceramide synthase. Methods Enzymol 311: 15–21 [DOI] [PubMed] [Google Scholar]
- Winter E, Ponting CP (2002) TRAM, LAG1 and CLN8: members of a novel family of lipid-sensing domains? Trends Biochem Sci 27: 381–383 [DOI] [PubMed] [Google Scholar]
- Worgall TS, Juliano RA, Seo T, Deckelbaum RJ (2004) Ceramide synthesis correlates with the posttranscriptional regulation of the sterol-regulatory element-binding protein. Arterioscler Thromb Vasc Biol 24: 943–948 [DOI] [PubMed] [Google Scholar]
- Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R (2005) Lipolysis: pathway under construction. Curr Opin Lipidol 16: 333–340 [DOI] [PubMed] [Google Scholar]
- Zinke I, Schutz CS, Katzenberger JD, Bauer M, Pankratz MJ (2002) Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J 21: 6162–6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Review Process File