Abstract
Background
Ubiquitous environmental chemicals, including endocrine-disrupting chemicals (EDCs), are associated with declining human reproductive health, as well as an increasing incidence of cancers of the reproductive system. Verifying such links requires animal models exposed to “real-life,” environmentally relevant concentrations/mixtures of EDC, particularly in utero, when sensitivity to EDC exposure is maximal.
Objectives
We evaluated the effects of maternal exposure to a pollutant cocktail (sewage sludge) on the ovine fetal reproductive neuroendocrine axes, particularly the kisspeptin (KiSS-1)/GPR54 (G-protein–coupled receptor 54) system.
Methods
KiSS-1, GPR54, and ERα (estrogen receptor α) mRNA expression was quantified in control (C) and treated (T) maternal and fetal (110-day) hypothalami and pituitary glands using semiquantitative reverse transcription polymerase chain reaction, and colocalization of kisspeptin with LHβ (luteinizing hormone β) and ERα in C and T fetal pituitary glands quantified using dual-labeling immunohistochemistry.
Results
Fetuses exposed in utero to the EDC mixture showed reduced KiSS-1 mRNA expression across three hypothalamic regions examined (rostral, mid, and caudal) and had fewer kisspetin immunopositive cells colocalized with both LHβ and ERα in the pituitary gland. In contrast, treatment had no effect on parameters measured in the adult ewe hypothalamus or pituitary.
Conclusions
This study demonstrates that the developing fetus is sensitive to real-world mixtures of environmental chemicals, which cause significant neuroendocrine alterations. The important role of kisspeptin/GPR54 in regulating puberty and adult reproduction means that in utero disruption of this system is likely to have long-term consequences in adulthood and represents a novel, additional pathway through which environmental chemicals perturb human reproduction.
Keywords: environmental chemicals, GPR54, hypothalamus, kisspeptin, pituitary, prenatal exposure, sheep
Exposure to ubiquitous environmental chemicals can have detrimental effects on human and animal health, including immune (Rier et al. 1995; Vine et al. 2000), thyroid (Langer et al. 1998), and cognitive and motor (Colborn et al. 1993) functions. Exposure to such chemicals has also been associated with increased incidences of reproductive disorders (Dickerson and Gore 2007; Fernandez et al. 2007; Toppari et al. 1996), such as precocious puberty, early menopause, and breast cancer in females (Buck Louis et al. 2008; Kortenkamp 2006) and an increased incidence of testicular dysgenesis syndrome in males (Olesen et al. 2007; Swan et al. 2000). The chemicals present within the environment include both inorganic (e.g., heavy metals such as cadmium and lead) and organic (e.g., alkylphenols, phthalates, polychlorinated biphenyls and organochlorine pesticides) compounds, and many are termed endocrine-disrupting compounds (EDCs) due to their effects on physiologic systems. These EDCs are normally present in the environment as complex mixtures and exert their physiologic effects via a variety of mechanisms (Safe 1994; Safe et al. 2002; Wilson et al. 2008); thus, the effects of each compound can be additive, synergistic, or antagonistic (Rajapakse et al. 2002) with others present within the mixture. Investigation of the complex mechanistic interactions that might occur after exposure to such mixtures is a major emerging issue for research and risk assessment (Kortenkamp 2008). The prenatal and early life stages of development, when the fetus and young are exposed to pollutants through maternal blood and/or milk, are times when the body is particularly vulnerable to the effects of EDCs (Rhind 2005), because during this time EDCs may permanently reprogram physiologic processes influencing later health and/or reproductive function (Rhind et al. 2003).
Sewage sludge, a by-product of treatment of waste water from domestic, agricultural, and industrial sources, contains numerous organic and inorganic pollutants (Giger et al. 1984; Lind et al. 2009; Smith 1995; Stevens et al. 2003; Webber and Lesage 1989), which broadly reflect the EDC mixture to which humans are typically exposed. Sewage sludge is widely used for land remediation and as an agricultural fertilizer, including on land used for grazing by farm animals. When applied to pastures, it results in modest increases in EDC concentrations in soil and herbage (Rhind et al. 2002) and provides an ideal model to investigate the effects of “real-life” exposure to complex mixtures of environmental concentrations of chemicals/EDCs (Rajapakse et al. 2002; Rhind 2002; Smith 1995; Welshons et al. 2003). Although the observed concentrations of the individual chemicals in this model would not be predicted to pose a health risk, sheep exposed to this cocktail of pollutants exhibit altered fetal testis (Paul et al. 2005) and ovary (Fowler et al. 2008) development, altered bone density and morphology (Lind et al. 2009), and altered adult behavior (Erhard et al. 2004).
Normal reproductive function depends on hypothalamic regulation of gonadal function via secretion of gonadotropin-releasing hormone (GnRH) and the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which then act on the ovaries and testes to stimulate gonadal maturation, gametogenesis, and steroidogenesis. The hypothalamo–pituitary–gonadal axis is regulated by a plethora of excitatory and inhibitory hypothalamic inputs and is highly sensitive to the organizational and activational effects of endogenous steroids during fetal and perinatal life (MacLusky and Naftolin 1981; Robinson et al. 2002). Therefore, alterations in reproductive function that manifest later in life, such as the earlier timing of puberty (Euling et al. 2008; Massart et al. 2006) or reduced fertility (Andersen et al. 2006; Carlsen et al. 1992; Skakkebaek et al. 2006), could be the result of fetal exposure to sex steroid mimetics, such as EDCs, during development of this regulatory axis.
Recent work has shown that the neuropeptide kisspeptin and its receptor G-protein–coupled receptor 54 (GPR54) are vital for the central regulation of GnRH neurosecretory activity and timing of puberty (Messager 2005; Roa et al. 2008). Kisspeptins are natural ligands for GPR54 (Kotani et al. 2001; Muir et al. 2001; Ohtaki et al. 2001). Thus, it is possible that the kisspeptin/GPR54 neurosecretory system is a target through which EDCs could perturb normal reproductive function (Navarro and Tena-Sempere 2008). This possibility is highlighted by findings showing that expression of KiSS-1 mRNA is regulated by estradiol (Navarro et al. 2004; Smith et al. 2005a, 2005b) and that kisspeptin can act as a transsynaptic modulator for conveying estradiol feedback to GnRH neurons (Navarro and Tena-Sempere 2008).
The objective of the present study was to determine whether exposure of pregnant sheep to environmental concentrations of a mixture of EDCs, via sewage sludge used as a fertilizer, disrupts the kisspeptin/GPR54 system, as determined by examination of KiSS-1, GPR54, and ERα (estrogen receptor-α) mRNA expression in the adult and fetal hypothalamus and pituitary and the proportions of kisspeptin positive LH-secretory gonadotropes and estradiol-receptive gonadotropes within the fetal pituitary gland.
Materials and Methods
Animals
Control (C) and treated (T) pregnant ewes were maintained on pasture treated with conventional inorganic fertilizers or sewage sludge, respectively. Sewage treatment has been applied to pastures since 1997, as described in detail elsewhere (Paul et al. 2005). Animals were treated humanely and with regard for alleviation of suffering. At 110 days of gestation, maternal and fetal animals were euthanized [Schedule 1, U.K. Animals (Scientific Procedures) Act of 1986]. Hypothalami and pituitary glands were collected from mothers (seven C and eight T) and their fetuses (12 C and 17 T) immediately after euthanasia and halved. For semi-quantitative reverse transcription polymerase chain reaction studies, tissues were frozen on dry ice and stored at −80°C before later mRNA extraction and analysis. For immunohistochemical studies, hemipituitaries from fetuses (seven C and eight T) were fixed in 4% paraformaldehyde for 24 hr and then stored in 30% sucrose at 4°C until processed.
Tissue preparation
While still frozen, maternal and fetal hypothalamic blocks were cut into three coronal slices (4 mm thick in adult tissue, 2 mm thick in fetal tissue) using external landmarks on the base of the brain as previously described (Whitelaw et al. 2009). The most rostral slice (Ros) encompassed the preoptic area (POA), the mid slice (Mid) encompassed the paraventricular nucleus, and the caudal slice (Cau) contained the arcuate nucleus (ARC). From each slice, approximately 100–200 mg of tissue was harvested for RNA extraction for each animal. Approximately 100–200 mg of tissue was also harvested from the midsagittal face of maternal and fetal pituitary glands for RNA extraction. Total RNA was extracted from each tissue using TRIzol (Invitrogen, Paisley, UK) as recommended by the manufacturers, and mRNA (200–300 ng) was then reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Invitrogen), random hexamers (Promega, Southampton, UK), and RNasin (Promega) as described previously (O’Shaughnessy and Murphy 1993). Purity and quantity of mRNA and cDNA were assessed using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Semiquantitative reverse transcription-polymerase chain reaction
We designed oligo-nucleotide primers and Taqman probes for the KiSS-1, ERα, and β-actin genes using published ovine GenBank [National Center for Biotechnology Information (NCBI) 2009] sequences DQ059506, AY033393 and U39357, respectively, and Primer Express Software (Applied Biosystems, Warrington, UK). The GPR54 primer sequences were obtained from previously published work conducted in the rat (Seminara et al. 2003). The GPR54 primer sequences used were 100% homologous to the NCBI ovine GPR54 (KiSS-1 receptor) sequence (EU 272411). The Taqman probes for KiSS-1, ERα, GPR54, and β-actin were synthesized by Eurogentec (Southampton, UK) and contained FAM (6-carboxyfluorescein) as the 5′-reporter and Blackhole Quencher 1 as the 3′-quencher (sequences shown in Table 1). The qPCR reactions were performed in duplicate, and a reagent blank was included within each plate to detect contamination by genomic DNA. To validate the primers for KiSS-1 and GPR54, before their use in qPCR, we initially used them in a reverse transcription PCR reaction and amplified the fragments, and then we separated the fragments by 1.5% agarose gel electrophoresis, and visualized them by ethidium bromide staining. We obtained and sequenced the PCR products of the predicted size for both genes for verification.
Table 1.
qPCR probes and primer sequences.
| Target | mRNA primer sequence 5′–3′ | Product (bp) | Reference |
|---|---|---|---|
| β-Actin | Forward: TCCTTCCTGGGCATGGAATC | 199 | U39357; NCBI 2009 |
| Reverse: GGGCGCGATGATCTTGATCT | |||
| Probe (FAM labeled): CCTTCCTTCCTGGGCATGGAATCC | |||
| KiSS-1 | Forward: CTGGTGCAGCGGGAGAAG | 57 | DQ059506; |
| Reverse: GCGCAGGCCGAAGGA | NCBI 2009 | ||
| Probe (FAM labeled): ACGTGTCCGCCTACA | |||
| ERα | Forward: CCCGGAAGACGTGAATCAGA | 66 | AY033393; |
| Reverse: GTTTGCAAGGAATGCGATGA | NCBI 2009 | ||
| Probe (FAM labeled): CAGCTGGCCACCACTGGCTGC | |||
| GPR54 | Forward: TACATCCAGCAGGTCTCGGTG | 71 | Seminara et al. 2003 |
| Reverse: ACGTACCAGCGGTCCACACT | |||
| Probe (FAM labeled): CACGTGTGCCACTCTGACCGCC |
The qPCRs for ovine KiSS-1, GPR54, and the endogenous reference gene β-actin were conducted using an Mx3000P real-time PCR system (Agilent Technologies UK Ltd, Stockport, UK)) according to the manufacturer’s instructions. Each qPCR reaction (25 μL) contained PCR Buffer II [Applied Biosystems; 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.001% (vol/vol) gelatin], 0.2 mM of each deoxynucleotide triphosphate, 0.625 U AmpliTaq Gold DNA polymerase (Applied Biosystems), template cDNA (2 μL), and optimized quantities of the respective probe and primers for the genes of interest and molecular biology grade H2O (VWR, Lutterworth, UK). Thermal cycling conditions were 10 min at 95°C (initial denaturation step), 45 cycles of 30 sec at 95°C (denaturation step), and 1 min at 60°C (primer annealing and elongation).
We quantified KiSS-1, ERα, and GPR54 mRNA expression using the comparative CT (cycle threshold) method (Schmittgen and Livak 2008), and we calculated gene expression relative to the reference gene (β-actin). Validation studies were conducted to ensure that the amplification efficiencies of the reference gene, KiSS-1, ERα, and GPR54 were equivalent. This involved generation of relative standard curves for each gene using serial dilutions of cDNA. We plotted the resultant ΔCT values between the housekeeping gene and KiSS-1, ERα, and GPR54 (y) versus log (dilution) (x), and calculated the slope of the line (by linear regression analysis). Slopes for KiSS-1, ERα, and GPR54 were all < 0.1, indicating equivalent amplification efficiencies (data not shown).
Immunohistochemistry
Frozen sections (5 μm) of fetal pituitaries were cut on a cryostat and thaw mounted onto Polysine-coated slides (VWR). We washed the cut sections from each animal (n = 8 C, n = 8 T) briefly in phosphate-buffered saline (PBS)and the sections underwent microwave antigen retrieval to unmask epitopic sites (Leong and Milios 1986; Shi et al. 1991). Briefly, we immersed the slides in 0.1 M sodium citrate buffer and heated them in a conventional 750-W microwave oven for 2 × 5 min as previously described for pituitary tissue (Franceschini et al. 2006). Slides were then left to cool to room temperature for 20 min before being washed in PBS. We blocked sections with PBS containing 10% normal goat serum (NGS) for 1 hr at room temperature and then rinsed them in PBS before addition of primary antibody. For kisspeptin and LHβ double immunolabeling, sections were incubated in a mixture of polyclonal kisspeptin antibody that was raised against bioactive residues 43–52 (kp10) of mouse metastin [1:1,000, IFR 145; Institut National de la Recherche Agronomique (INRA), Nouzilly, France] (Richard et al. 2008) and validated in ovine tissue (Franceschini et al. 2006) and a mono-clonal anti-LHβ antibody (1:10,000, generous gift from Alan McNeilly) in PBS containing 2% NGS, for 72 hr at 4°C. For KiSS-1 and ERα double immunolabeling, slides were incubated for 72 hr at 4°C with a mixture of polyclonal anti-KiSS-1 (as above, 1:1,000) and monoclonal anti-human ERα antibody (Dako UK Ltd, Ely, UK; 1:200) in PBS containing 2% NGS. After incubation with the appropriate mixture of primary antibodies, sections were then washed (3 × 5 min) in PBS and incubated for 1 hr at room temperature in a mixture of Alexafluor 488-conjugated goat anti-rabbit immunoglobulin G (IgG) and Alexafluor 594-conjugated goat anti-mouse IgG (Invitrogen, both 1:1,000 in 2% NGS). Sections were washed (3 × 5 min) in PBS before being mounted using Fluorsave mounting medium [Calbiochem (Merck), Beeston, UK] and coverslipped.
Immunohistochemical analysis of kisspeptin concentrated on expression within the pituitary. The study design did not allow collection of tissue in a manner that would allow visualization of kisspeptin immunopositive cell bodies in the hypothalamus as described by Franceschini et al. (2006).
For LHβ and ERα double immunolabeling, we sequentially immunostained sections using nickel-intensified diaminobenzidine (NiDAB) and diaminobenzidine (DAB). Briefly, sections were washed in PBS, and microwave antigen retrieval was carried out as detailed above. Sections were then incubated with 3% H2O2 in methanol for 10 min to block endogenous peroxidase activity, washed, blocked in 10% NGS in PBS (1 hr at room temperature), and incubated overnight at 4°C with monoclonal anti-human ERα antibody (Dako, 1:200 dilution) in PBS containing 2% NGS. Sections were then washed and incubated with an anti-mouse biotinylated secondary antibody (1:1,000, Dako) for 1 hr at room temperature, washed again, and positive immunostaining visualized using a Vecstatin ABC kit (Vector Laboratories, Peterborough, UK). Immunolocalization of ERα was visualized using NiDAB to produce a blue-black color. Slides were then washed and incubated with polyclonal anti-ovine LHβ (AFP 69707P, 1:10,000; Crawford et al. 2000) for 1 hr at room temperature before processing as detailed above, except that LHβ immunolocalization was visualized using DAB, to produce a brown product. Slides were then rapidly dehydrated in increasing concentrations of alcohol, mounted using DPX (Raymond Lamb, Eastbourne, UK) and coverslipped. Pituitary sections from all C and T animals were run for each antibody at the same time and under the same conditions to ensure comparability. Negative controls for all immunohistochemical procedures were included and indicated specific binding of all antibodies used.
The number of LHβ immunoreactive cells (brown cytoplasmic staining) with or without ERα nuclear immunolabeling (blue-black nucleus) and similarly the number of kisspeptin immunoreactive cells (green) that coexpressed LHβ or ERα (red) immunofluorescence were counted using a DM4000B microscope (Leica, Milton Keynes, UK). Images were captured at 40× magnification using a Leica DC480 digital camera and Leica Qwin software. Slides were evaluated subjectively but systematically by counting the number of immunodetectable cells or double-labeled cells from the same three areas for each pituitary gland (top, middle, and bottom). Because there was no difference in cell number between areas, the mean number of cells from the three areas was calculated for each pituitary and compared between C and T groups.
Statistical analyses
All data are presented as mean ± SE. For real-time data analysis, all data were log transformed and analyzed using two-way analysis of variance. For colocalization studies, cell numbers in C and T animals were compared using Student’s t-tests. A p-value of less than 0.05 was considered statistically significant.
Results
Fetal KiSS-1 and GPR54 mRNA expression
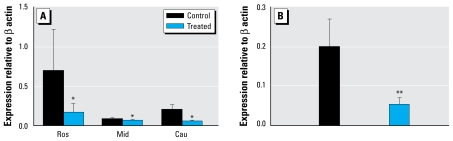
There was no effect of sex on the level of mRNA expression for KiSS-1 or GPR54 in either C or T fetuses, so data were pooled. Figure 1 depicts the relative levels of KiSS-1 mRNA expression in the hypothalamus and pituitary gland of the C and T fetuses. Hypothalamic KiSS-1 and GPR54 mRNA expression did not differ significantly between the areas of the hypothalamus studied. However, T fetuses expressed significantly (p < 0.05) less hypothalamic KiSS-1 mRNA than did C fetuses, particularly in the Ros and Cau regions (Figure 1A). KiSS-1 mRNA expression was also significantly lower (p < 0.05) in the pituitary glands of T than of C fetuses (Figure 1B). Despite the significant effects of treatment on KiSS-1 mRNA expression, no differences were seen in either hypothalamic or pituitary gland GPR54 mRNA expression (Ros: C, 0.14 ± 0.03; T, 0.14 ± 0.02; Mid: C, 0.60 ± 0.51; T, 0.23 ± 0.05; Cau: C, 0.14 ± 0.07; T, 0.13 ± 0.04; pituitary: C, 0.28 ± 0.086; T, 0.26 ± 0.06).
Figure 1.
KiSS-1 mRNA expression (mean ± SE) in fetal hypothalamus (A) and pituitary (B) after in utero exposure to sewage sludge chemicals (treated).
*p < 0.05, and **p < 0.02 compared with respective control value.
Maternal KiSS-1 and GPR54 mRNA expression
There were no significant differences in maternal hypothalamic KiSS-1 mRNA expression with treatment (Ros: C, 0.77 ± 0.37; T, 1.46 ± 0.79; Mid: C, 1.64 ± 0.45; T, 1.23 ± 0.76; Cau: C, 0.82 ± 0.39; T, 0.91 ± 0.91). In the maternal pituitary, KiSS-1 mRNA expression exhibited a nonsignificant trend (p = 0.15) toward lower expression in T (0.42 ± 0.61) relative to relative to C (1.76 ± 0.58) animals. No significant effects of treatment were seen on GPR54 mRNA expression in either tissue from adult ewes (Ros: C, 0.33 ± 0.2; T, 0.39 ± 0.17; Mid, C, 0.66 ± 0.54; T, 0.27 ± 0.14; Cau, C, 0.17 ± 0.03; T, 0.38 ± 0.25, pituitary: C, 0.99 ± 0.75; T, 0.70 ± 0.34).
ERα mRNA expression
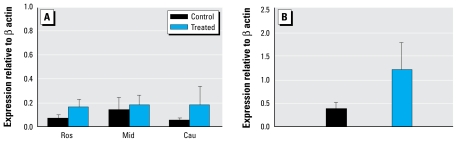
Figure 2 depicts relative levels of ERα mRNA expression in the hypothalamus and pituitary gland of the C and T dams. T dams exhibited a trend toward higher ERα mRNA expression than did C dams in all areas of the hypothalamus and in the pituitary glands (Figure 2A,B). In the fetal animals, exposure to treated pastures had no effect on ERα mRNA expression in either the hypothalamus or the pituitary gland (Ros: C, 0.041 ± 0.025; T, 0.017 ± 0.003; Mid, C, 0.011 ± 0.003; T, 0.039 ± 0.014; Cau, C, 0.11 ± 0.10; T, 0.009 ± 0.002, pituitary: C, 0.299 ± 0.138; T, 0.246 ± 0.076).
Figure 2.
Maternal ERα mRNA expression (mean ± SE) in the hypothalamus (A) and the pituitary (B) of T sheep were not significantly affected by exposure to sewage sludge relative to control sheep.
Kisspeptin and LHβ colocalization in the fetal pituitary
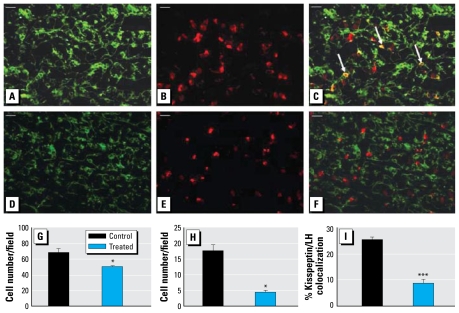
Given the decrease in KiSS-1 mRNA expression in the fetal animals, we used dual immunohistochemistry to examine whether exposure to sewage-sludge–treated pastures had any effect on the expression of kisspeptin within the fetal pituitary glands and, if so, in which cells. Figure 3 shows representative examples of the staining for LHβ and kisspeptin in the pituitary glands of C and T fetuses and the mean number of immunopositive cells observed. Exposure to sewage sludge was associated with a significant (p < 0.05) reduction in the number of LHβ immunopositive cells in the fetal pituitary gland (Figure 3G) and a significant reduction in the number of cells double labeled for LHβ and kisspeptin (expressed as the number of LHβ positive gonadotropes per field; p < 0.01; Figure 3H) or as a percentage of the total number of LHβ positive cells per field (p< 0.001; Figure 3I).
Figure 3.
Representative photomicrographs of staining for kisspeptin (A, D) and LHβ (B, E), and merged images (C, F), in fetal pituitaries from control (top) and treated (bottom) animals. Cells showing colocalization are stained yellow (indicated by arrows). Bar = 20 μm. (G–I) Quantification per field of view of the mean LHβ-immunopositive cell number (G), the mean number of cells immunopositive for both LHβ and kisspeptin cells (H), and percentage of kisspeptin/LHβ immunopositive cells as a proportion of the total number of LHβ-immunopositive cells (I) in fetal pituitary tissue sections from control and treated animals.
*p < 0.05, and ***p < 0.001, compared with respective control value.
Kisspeptin and ERα colocalization
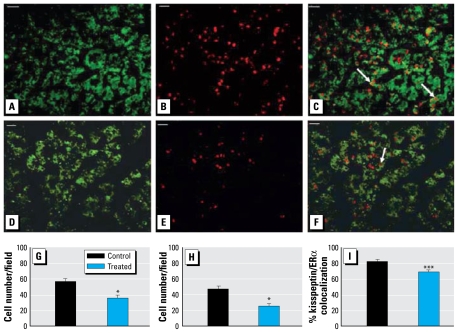
Kisspeptin expression was also analyzed relative to ERα expression in the fetal pituitary glands. Figure 4 shows representative images of kisspeptin, ERα, and double-labeled immunofluorescence and the mean number of cells expressing kisspeptin and ERα in the C and T fetuses. T fetuses exhibited significantly fewer (p < 0.01) ERα positive cells within the fetal pituitary glands compared with C fetuses (Figure 4G). Significantly (p < 0.01) fewer ERα positive cells were also present within the pituitary gland that coexpressed kisspeptin in the T relative to the C animals, expressed as both the number of cells (Figure 4H) and the percentage of colocalized cells (p < 0.001; Figure 4I).
Figure 4.
Representative photomicrographs of staining for kisspeptin (A, D) and ERα (B, E), and merged images (C, F), in fetal pituitaries from control (top) and treated (bottom) animals. Examples of cells showing colocalization are indicated by arrows. Bar = 20 μm. (G–I) Quantification per field of view of the mean ERα-immunopositive cell number (G), the mean kisspeptin/ERα cells immunopositive for both ERα and kisspeptin (H), and percentage of kisspeptin/ERα immunopositive cells as a proportion of the total number of ERα immunopositive cells (I) in fetal pituitary tissue sections from control and treated animals.
*p < 0.01, and ***p < 0.001, compared with respective control value.
LHβ and ERα colocalization
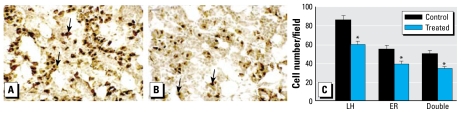
Figure 5, A and B, depicts representative images of pituitary tissue from C and T fetuses immunostained for LHβ and ERα, and the mean number of cells expressing LHβ or ERα or coexpressing both antigens is shown in Figure 5C. T fetuses had significantly fewer ERα positive cells (p < 0.01) and LHβ/ERα positive double-labeled cells (p < 0.005) per field (Figure 5C). However, the number of colocalized cells per field, expressed as a proportion of the total number of ERα immunopositive cells, was similar for C and T fetuses (C, 92 ± 1.7%; T, 88.7 ± 2.8%). As in the study of LHβ and kisspeptin coexpression, we observed significantly fewer (p < 0.01) LHβ immunopositive cells per field in T fetuses.
Figure 5.
Representative photomicrographs of ERα (blue-black) and LHβ (brown) double immunohistochemistry in fetal pituitaries from control (A) and treated (B) animals. Colocalized cells are indicated by arrows; bar = 20 μm. (C) Quantification per field of view of the mean of LHβ, ERα, and LHβ/ERα immunopositive cells in fetal pituitary tissue sections from control and treated animals.
*p < 0.05 compared with respective control value.
Discussion
We have shown for the first time that maternal exposure to a complex mixture of chemicals/EDCs, at environmental concentrations, negatively affects the fetal kisspeptin/GPR54 neuroendocrine system. This exposure reduced the expression of both KiSS-1 mRNA in the hypothalamus and pituitary of exposed fetuses and the proportion of kisspeptin immunopositive pituitary cells that expressed LHβ and ERα. Therefore, our results provide evidence of a mechanism through which low-level, long-term exposure to environmental EDCs may alter subsequent adult reproductive function (Navarro and Tena-Sempere 2008; Navarro et al. 2009).
This study showed that fetuses exposed to a mixture of EDCs through maternal contact with chemicals present in sewage sludge have reduced hypothalamic KiSS-1 mRNA expression; this effect was most pronounced in the regions of the hypothalamus that encompass the POA and ARC. In both areas, kisspeptin cells coexpress ERα (Smith et al. 2005b) and in the ARC, kisspeptin immunopositive cells have been implicated in mediating estrogen negative feedback onto GnRH neurons (Franceschini et al. 2006). Therefore, EDC exposure may disturb estrogen feedback systems within the hypothalamus, which may have consequences in later life (e.g., the timing of puberty). Although this has not yet been examined in this experimental model, kisspeptin signaling is fundamental to the initiation of puberty in rodents (Clarkson and Herbison 2006; Han et al. 2005) and reproductive activity in sheep (Clarke et al. 2009; Smith et al. 2008a), and hypothalamic KiSS-1 gene expression during puberty is significantly reduced if rats are neonatally exposed to estrogenic compounds (Navarro et al. 2009). Interestingly, despite a significant decrease in KiSS-1 expression in our exposed fetuses, GPR54 mRNA expression was not affected in either the hypothalamus or the pituitary gland. Although regulation of KiSS-1 mRNA expression by estradiol is widely accepted, hormonal regulation of GPR54 mRNA expression is not as well documented and appears to be less sensitive to steroid feedback, at least in the pituitary gland (Richard et al. 2008). Therefore, regulation of the kisspeptin/GPR54 system by estrogenic compounds is likely to be mediated primarily via the ligand rather than its receptor. Although it is not possible to determine which specific compounds were responsible for the effects noted in the T fetuses, the findings of Navarro et al. (2009) suggest that it is likely due to the activity of estrogenic EDCs known to be present in sewage sludge. Given the critical organizational events occurring in utero, alterations in the kisspeptin/GPR54 system could have long-term detrimental effects on adult reproductive function, and further studies will be required to investigate this possibility.
The results of the present study also indicate that maternal EDC exposure reduces fetal pituitary KiSS-1 mRNA expression as well as lowering the proportion of gonadotropes that coexpressed kisspeptin and LHβ and the number of LHβ positive gonadotropes. Although the present experimental design did not allow us to differentiate between direct effects of EDCs at the level of the pituitary and indirect central actions, the results clearly demonstrate that maternal EDC exposure could affect fetal intrapituitary modulation of LH release. However, not all kisspeptin positive cells within the pituitary were also LHβ positive; therefore, the observed reduction in KiSS-1 mRNA expression may also affect other pituitary cell types, including somatotropes, lactotropes, and gonadotropes that specifically synthesize FSH. Kisspeptin function within the pituitary gland, particularly during development, may not be restricted to LH release because gonadotropes are also a critical source of mitogenic and/or differentiation (Tilemans et al. 1992) and antiapoptotic factors (Melamed et al. 1999).
As with the hypothalamic kisspeptin/GPR54 system, the pituitary kisspeptin system is also sensitive to endogenous estradiol (Smith et al. 2008b). The results of this study show, again for the first time, that kisspeptin is expressed in pituitary cells that also express ERα, demonstrating that there may be important interactions between kisspeptin and estrogens in the fetal ovine pituitary. Moreover, the number of ERα positive cells, as well as the proportion of cells coexpressing ERα and kisspeptin, was significantly reduced in T fetuses. Because 92% of ERα-positive cells are also LHβ-positive gonadotropes, this reflects a significant disturbance of the interactions among kisspeptin, estrogens, and LH by chemical/EDC exposure in utero.
Our results extend previous work that reported the presence of kisspeptin and GPR54 in the adult pituitary gland of the rat (Gutierrez-Pascual et al. 2007; Richard et al 2008) and demonstrates for the first time that KiSS-1 and GPR54 mRNA and kisspeptin are expressed in the fetal ovine pituitary. The exact role played by pituitary kisspeptin and its relevance in the regulation of LH secretion (Smith et al 2008b) are presently subject to discussion. Our results indicate that kisspeptin could have paracrine/autocrine actions within the pituitary gland, and these have not yet been characterized. However, based on studies that have shown kisspeptin to be involved in LH release in the presence of GnRH (Gutierrez-Pascual et al. 2007; Navarro et al. 2005), it has been suggested that kisspeptin might act in the pituitary, in synergy with GnRH (and estradiol), as an endocrine/autocrine/paracrine signal to modulate hormone secretions from the anterior pituitary (Richard et al. 2009).
The late prenatal and early postnatal developmental stages are particularly sensitive periods of brain development with initiation of sexually dimorphic characteristics (Rhees et al. 1990). It is therefore perhaps not surprising that exposure to exogenous chemicals/EDCs in sewage sludge (Cespedes et al. 2008; Lind et al. 2009; Petrovic et al. 2002; Reddy et al. 2005) during this period has the potential to alter the kisspeptin/GPR54 systems and thus affect adult reproductive function. Under the present study conditions, the tissue levels of individual EDCs are modest (Rhind et al. 2002), so the effects seen must be due to combinations of small amounts of many different environmental chemicals (Willingham 2004). Interestingly, the adult female sheep, through which the chemical exposure occurred, were resilient to the effects of sewage sludge exposure, which supports the proposal that the effects of EDCs are more pronounced during the critical, early windows of development than in adulthood. Although the effects shown in fetuses in this study are very likely to be due to exposure of their mothers to a mixture of chemicals present in sewage sludge, identifying which particular chemicals or combination of chemicals are responsible is extremely difficult. Interpretation and, more important, extrapolation of the findings of this study to human health should be attempted with caution because it is impossible to know the exact level of human exposure and whether this is comparable to the levels of chemicals to which sheep are exposed after sewage sludge application to pasture. Although there are metabolic and physiologic differences between humans and sheep, human waste is an important contributor to sewage sludge. We therefore propose that humans themselves are exposed to many of the constituent chemicals/EDCs present in sewage sludge and in fact that the levels of some constituents in this model are probably lower than those to which humans are exposed.
We conclude that low-level exposure of fetuses to a mixture of environmental chemicals via maternal ingestion, throughout development, disrupts both the normal pattern of fetal hypothalamic and pituitary KiSS-1 mRNA expression and also the number and proportion of kisspeptin positive cells that coexpress LHβ and ERα in the fetal pituitary. This indicates that the regulation of gonadotropes by endogenous estradiol could be disrupted. Because kisspeptin plays a major role in many aspects of reproductive axis regulation, including puberty onset, alterations to this system during development of the sexually dimorphic neuroendocrine axis are likely to affect long-term reproductive function and fertility and this will be examined in future studies of adult animals exposed to EDCs in this experimental paradigm. Humans are also exposed to a cocktail of environmental chemicals on a daily basis throughout their lives, including during development, so the kiss-peptin/GPR54 system is a potential target for environmental EDCs to alter puberty timing and reproductive function in our own species.
Footnotes
We are grateful to C. Kyle (Macaulay Institute) for excellent technical assistance and to the staff of the Hartwood Research Station for their assistance in the management of experimental animals.
The Wellcome Trust supported this work (080388/Z/06/Z).
References
- Andersen AN, Gianaroli L, Felberbaum R, de Mouzon J, Nygren KG. Assisted reproductive technology in Europe, 2002. Results generated from European registers by ESHRE. Hum Reprod. 2006;21(7):1680–1697. doi: 10.1093/humrep/del075. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Gray LE, Jr, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics. 2008;121(suppl 3):S192–S207. doi: 10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespedes R, Lacorte S, Ginebreda A, Barcelo D. Occurrence and fate of alkylphenols and alkylphenol ethoxylates in sewage treatment plants and impact on receiving waters along the Ter River (Catalonia, NE Spain) Environ Pollut. 2008;153(2):384–392. doi: 10.1016/j.envpol.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN. Kisspeptin and seasonality in sheep. Peptides. 2009;30(1):154–163. doi: 10.1016/j.peptides.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JL, Currie RJ, McNeilly AS. Replenishment of LH stores of gonadotrophs in relation to gene expression, synthesis and secretion of LH after the preovulatory phase of the sheep oestrous cycle. J Endocrinol. 2000;167(3):453–463. doi: 10.1677/joe.0.1670453. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8(2):143–159. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- Erhard HW, Boissy A, Rae MT, Rhind SM. Effects of pre-natal undernutrition on emotional reactivity and cognitive flexibility in adult sheep. Behav Brain Res. 2004;151(1–2):25–35. doi: 10.1016/j.bbr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics. 2008;121(suppl 3):S167–S171. doi: 10.1542/peds.2007-1813C. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, Olmos B, Granada A, Lopez-Espinosa MJ, Molina-Molina JM, Fernandez JM, et al. Human exposure to endocrine-disrupting chemicals and prenatal risk factors for cryptorchidism and hypospadias: a nested case-control study. Environ Health Perspect. 2007;115(suppl 1):8–14. doi: 10.1289/ehp.9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler PA, Dora NJ, McFerran H, Amezaga MR, Miller DW, Lea RG, et al. In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol Hum Reprod. 2008;14(5):269–280. doi: 10.1093/molehr/gan020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Giger W, Brunner PH, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984;225(4662):623–625. doi: 10.1126/science.6740328. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Pascual E, Martinez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagon MM, Castano JP. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;19(7):521–530. doi: 10.1111/j.1365-2826.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A. Breast cancer, oestrogens and environmental pollutants: a re-evaluation from a mixture perspective. Int J Androl. 2006;29(1):193–198. doi: 10.1111/j.1365-2605.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int J Androl. 2008;31(2):233–240. doi: 10.1111/j.1365-2605.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276(37):34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Langer P, Tajtakova M, Fodor G, Kocan A, Bohov P, Michalek J, et al. Increased thyroid volume and prevalence of thyroid disorders in an area heavily polluted by polychlorinated biphenyls. Eur J Endocrinol. 1998;139(4):402–409. doi: 10.1530/eje.0.1390402. [DOI] [PubMed] [Google Scholar]
- Leong AS, Milios J. Rapid immunoperoxidase staining of lymphocyte antigens using microwave irradiation. J Pathol. 1986;148(2):183–187. doi: 10.1002/path.1711480209. [DOI] [PubMed] [Google Scholar]
- Lind PM, Gustafsson M, Hermsen SA, Larsson S, Kyle CE, Orberg J, et al. Exposure to pastures fertilised with sewage sludge disrupts bone tissue homeostasis in sheep. Sci Total Environ. 2009;407(7):2200–2208. doi: 10.1016/j.scitotenv.2008.12.035. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211(4488):1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Massart F, Parrino R, Seppia P, Federico G, Saggese G. How do environmental estrogen disruptors induce precocious puberty? Minerva Pediatr. 2006;58(3):247–254. [PubMed] [Google Scholar]
- Melamed P, Gur G, Rosenfeld H, Elizur A, Yaron Z. Possible interactions between gonadotrophs and somatotrophs in the pituitary of tilapia: apparent roles for insulin-like growth factor I and estradiol. Endocrinology. 1999;140(3):1183–1191. doi: 10.1210/endo.140.3.6571. [DOI] [PubMed] [Google Scholar]
- Messager S. Kisspeptin and its receptor: new gatekeepers of puberty. J Neuroendocrinol. 2005;17(10):687–688. doi: 10.1111/j.1365-2826.2005.01357.x. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276(31):28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145(10):4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146(4):1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Sanchez-Garrido MA, Castellano JM, Roa J, Garcia-Galiano D, Pineda R, et al. Persistent impairment of hypothalamic KiSS-1 system after exposures to estrogenic compounds at critical periods of brain sex differentiation. Endocrinology. 2009;150(5):2359–2367. doi: 10.1210/en.2008-0580. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Tena-Sempere M. The KiSS-1/GPR54 system: putative target for endocrine disruption of reproduction at hypothalamic-pituitary unit? Int J Androl. 2008;31(2):224–232. doi: 10.1111/j.1365-2605.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- NCBI (National Center for Biotechnology Information) Genbank Overview. 2009. [[accessed 30 July 2009]]. Available: http://www.ncbi.nlm.nih.gov/Genbank/
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Olesen IA, Sonne SB, Hoei-Hansen CE, Rajpert-DeMeyts E, Skakkebaek NE. Environment, testicular dysgenesis and carcinoma in situ testis. Best Pract Res Clin Endocrinol Metab. 2007;21(3):462–478. doi: 10.1016/j.beem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Murphy L. Cytochrome P-450 17 alpha-hydroxylase protein and mRNA in the testis of the testicular feminized (Tfm) mouse. J Mol Endocrinol. 1993;11(1):77–82. doi: 10.1677/jme.0.0110077. [DOI] [PubMed] [Google Scholar]
- Paul C, Rhind SM, Kyle CE, Scott H, McKinnell C, Sharpe RM. Cellular and hormonal disruption of fetal testis development in sheep reared on pasture treated with sewage sludge. Environ Health Perspect. 2005;113:1580–1587. doi: 10.1289/ehp.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic M, Sole M, Lopez de Alda MJ, Barcelo D. Endocrine disruptors in sewage treatment plants, receiving river waters, and sediments: integration of chemical analysis and biological effects on feral carp. Environ Toxicol Chem. 2002;21(10):2146–2156. [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xeno-estrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Iden CR, Brownawell BJ. Analysis of steroid conjugates in sewage influent and effluent by liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77(21):7032–7038. doi: 10.1021/ac050699x. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res Dev Brain Res. 1990;52(1–2):17–23. doi: 10.1016/0165-3806(90)90217-m. [DOI] [PubMed] [Google Scholar]
- Rhind SM. Endocrine disrupting compounds and farm animals: their properties, actions and routes of exposure. Domest Anim Endocrinol. 2002;23(1–2):179–187. doi: 10.1016/s0739-7240(02)00155-8. [DOI] [PubMed] [Google Scholar]
- Rhind SM. Are endocrine disrupting compounds a threat to farm animal health, welfare and productivity? Reprod Domest Anim. 2005;40(4):282–290. doi: 10.1111/j.1439-0531.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- Rhind SM, Rae MT, Brooks AN. Environmental influences on the fetus and neonate—timing, mechanisms of action and effects on subsequent adult function. Domest Anim Endocrinol. 2003;25(1):3–11. doi: 10.1016/s0739-7240(03)00041-9. [DOI] [PubMed] [Google Scholar]
- Rhind SM, Smith A, Kyle CE, Telfer G, Martin G, Duff E, et al. Phthalate and alkyl phenol concentrations in soil following applications of inorganic fertiliser or sewage sludge to pasture and potential rates of ingestion by grazing ruminants. J Environ Monit. 2002;4(1):142–148. doi: 10.1039/b107539j. [DOI] [PubMed] [Google Scholar]
- Richard N, Corvaisier S, Camacho E, Kottler ML. KiSS-1 and GPR54 at the pituitary level: overview and recent insights. Peptides. 2009;30(1):123–129. doi: 10.1016/j.peptides.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20(3):381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- Rier SE, Martin DC, Bowman RE, Becker JL. Immunoresponsiveness in endometriosis: implications of estrogenic toxicants. Environ Health Perspect. 1995;103(suppl 7):151–156. doi: 10.1289/ehp.95103s7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29(1):48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Taylor JA, Foster DL, Padmanabhan V. In utero programming of sexually differentiated gonadotrophin releasing hormone (GnRH) secretion. Domest Anim Endocrinol. 2002;23(1–2):43–52. doi: 10.1016/s0739-7240(02)00144-3. [DOI] [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24(2):87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Safe SH, Pallaroni L, Yoon K, Gaido K, Ross S, McDonnell D. Problems for risk assessment of endocrine-active estrogenic compounds. Environ Health Perspect. 2002;110(suppl 6):925–929. doi: 10.1289/ehp.02110s6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Jorgensen N, Main KM, Rajpert-De Meyts E, Leffers H, Andersson AM, et al. Is human fecundity declining? Int J Androl. 2006;29(1):2–11. doi: 10.1111/j.1365-2605.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008a;149(11):5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146(9):3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146(7):2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008b;149(4):1951–1959. doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- Smith S. Agricultural Recycling of Sewage Sludge and the Environment. Wallingford, UK: CAB International; 1995. pp. 207–236. [Google Scholar]
- Stevens JL, Northcott GL, Stern GA, Tomy GT, Jones KC. PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks, and polychlorinated n-alkanes in U.K. sewage sludge: survey results and implications. Environ Sci Technol. 2003;37(3):462–467. doi: 10.1021/es020161y. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilemans D, Andries M, Denef C. Luteinizing hormone-releasing hormone and neuropeptide Y influence deoxyribonucleic acid replication in three anterior pituitary cell types. Evidence for mediation by growth factors released from gonadotrophs. Endocrinology. 1992;130(2):882–894. doi: 10.1210/endo.130.2.1310281. [DOI] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Animals (Scientific Procedures) Act. 1986. [[accessed 3 August 2009]]. Available: http://scienceandresearch.homeoffice.gov.uk/animal-research/legislation/
- Vine MF, Stein L, Weigle K, Schroeder J, Degnan D, Tse CK, et al. Effects on the immune system associated with living near a pesticide dump site. Environ Health Perspect. 2000;108:1113–1124. doi: 10.1289/ehp.001081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber MD, Lesage S. Organic Contaminants in Canadian Municipal Sludges. Waste Manag Res. 1989;7:63–82. [Google Scholar]
- Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw CM, Robinson JE, Chambers GB, Hastie P, Padmanabhan V, Thompson RC, et al. Expression of mRNA for galanin, galanin-like peptide and galanin receptors 1–3 in the ovine hypothalamus and pituitary gland: effects of age and gender. Reproduction. 2009;137(1):141–150. doi: 10.1530/REP-08-0266. [DOI] [PubMed] [Google Scholar]
- Willingham E. Endocrine-disrupting compounds and mixtures: unexpected dose-response. Arch Environ Contam Toxicol. 2004;46(2):265–269. doi: 10.1007/s00244-003-2110-1. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Blystone CR, Hotchkiss AK, Rider CV, Gray LE., Jr Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int J Androl. 2008;31(2):178–187. doi: 10.1111/j.1365-2605.2007.00861.x. [DOI] [PubMed] [Google Scholar]