Abstract
We examined whether estrogen negatively modulates cannabinoid-induced regulation of food intake, core body temperature and neurotransmission at proopiomelanocortin (POMC) synapses. Food intake was evaluated in ovariectomized female guinea pigs abdominally implanted with thermal DataLoggers and treated s.c. with the cannabinoid CB1/CB2 receptor agonist WIN 55,212-2, the CB1 receptor antagonist AM251 or their cremephor/ethanol/0.9% saline vehicle, and with estradiol benzoate (EB) or its sesame oil vehicle. Whole-cell patch clamp recordings were performed in slices through the arcuate nucleus. WIN 55,212-2 produced dose- and time-dependent increases in food intake. EB decreased food intake 8–24 h after administration, but rapidly and completely blocked the increase in consumption caused by WIN 55,212-2. EB also attenuated the WIN 55,212-2-induced decrease in core body temperature. The AM251-induced decrease in food intake was unaffected. The diminution of the WIN 55,212-2-induced increase in food intake caused by EB correlated with a marked attenuation of cannabinoid receptor-mediated decreases in glutamatergic miniature excitatory postsynaptic current frequency occurring within 10–15 minutes of steroid application. Furthermore, EB completely blocked the depolarizing shift in the inactivation curve for the A-type K+ current caused by WIN 55,212-2. The EB-mediated, physiologic antagonism of these presynaptic and postsynaptic actions elicited upon cannabinoid receptor activation was observed in arcuate neurons immunopositive for phenotypic markers of POMC neurons. These data reveal that estrogens negatively modulate cannabinoid-induced changes in appetite, body temperature and POMC neuronal activity. They also impart insight into the neuroanatomical substrates and effector systems upon which these counter-regulatory factors converge in the control of energy homeostasis.
Keywords: estrogen, POMC, appetite, cannabinoids, glutamate, K+ channels, electrophysiology
1. Introduction
Estrogen exerts far-reaching effects on homeostatic functions including, but not limited to, reproduction, food intake and body temperature regulation. For example, estrogen exerts negative and positive feedback on the reproductive axis, which regulates ovarian steroidogenesis, ovulation, endometrial thickness, breast development and sexual behavior (Yuen et al., 1973;Owen, 1975;Sinchak and Micevych, 2001). Estrogen also decreases appetite in several rodent species via peripheral (Palmer and Gray, 1986;McCaffrey and Czaja, 1989;Santollo et al., 2007), intracerebroventricular (Gao et al., 2007) and intrahypothalamic (Butera and Czaja, 1984;Palmer and Gray, 1986) routes of administration, and in human females energy intake is lowest during the periovulatory phase of the ovarian cycle, in which estrogen levels are at their highest (Johnson et al., 1994). In addition, core body temperature is lower during the estrogen-dominated follicular phase (Cagnacci et al., 1997;Stephenson and Kolka, 1999;Stachenfeld et al., 2000), and hot flashes represent the chief complaint among women during reproductive senescence (Deecher and Dorries, 2007). These estrogenic effects are largely related to the actions of the steroid on thermoregulatory centers in the preoptic area (Silva and Boulant, 1986;Tsai et al., 1992), and on proopiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus, where estrogen regulates synaptology (Gao and Horvath, 2008), POMC gene expression (Thornton et al., 1994;Cheung and Hammer, 1995;Roepke et al., 2007) peptide release (Ferin et al., 1984), and alters the coupling of metabotropic receptors to their effector systems (Kelly and Wagner, 1999;Nguyen and Wagner, 2006) to collectively affect the excitability of POMC neurons and neurotransmission at POMC synapses.
Cannabinoids also impact these same homeostatic processes. For example, Δ9-tetrahydrocannabinol decreases gonadotropin secretion, thereby depressing ovarian steroidogenesis and disrupting ovarian cyclicity and pregnancy (Asch et al., 1981;Asch and Smith, 1986). Endogenous cannabinoids have also recently been shown to tonically inhibit sexual behavior in steroid-primed female rats (López et al., 2009). By contrast, cannabinoid CB1 receptor agonists stimulate appetite in mice (Sinnayah et al., 2008), rats (Williams and Kirkham, 2002) and guinea pigs (Ho et al., 2007;Diaz et al., 2009), and in humans dronabinol and marijuana have been utilized as pharmacotherapeutic adjuncts to ameliorate body wasting in cancer (Walsh et al., 2005) and HIV/AIDS (Woolridge et al., 2005;Haney et al., 2005) patients. These hyperphagic effects are due, in least in part, to cannabinoid-induced alterations in both excitatory and inhibitory amino acid neurotransmission and thus the activity of cells comprising the hypothalamic feeding circuitry such as melanin-concentrating hormone (Jo et al., 2005;Huang et al., 2007) and POMC (Hentges et al., 2005;Nguyen and Wagner, 2006;Diaz et. al., 2009) neurons. In addition, hypothalamic levels of endogenous cannabinoids are influenced by feeding-relevant humoral factors such as leptin (Di Marzo et al., 2001) and ghrelin (Kola et al., 2008). Finally, cannabinoid receptor agonists elicit hypothermia due to activation of CB1 receptors in the preoptic anterior hypothalamus (Fitton and Pertwee, 1982;Rawls et al., 2002).
In light of these convergent lines of evidence, we tested the hypothesis that estrogen would pervasively modulate cannabinoid CB1 receptor agonist- and antagonist-induced changes in energy homeostasis in a physiologically-pertinent way as measured via changes in food intake, core body temperature and neurotransmission at POMC synapses. Since arcuate neurons, including POMC neurons, receive convergent glutamatergic and GABAergic synaptic input (Decavel and van den Pol, 1992;Hentges et. al., 2005;Nguyen and Wagner, 2006;Gao et. al., 2007;Diaz et. al., 2009), we evaluated cannabinoid modulation of both miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) in estrogen- and vehicle-treated hypothalamic slices. Given that POMC neurons also exhibit an A-type K+ current (IA) that is enhanced by cannabinoid CB1 receptor agonists (Ibrahim et al., 2003;Tang et al., 2005), and estrogen has been shown to attenuate the IA in gonadotropin-releasing hormone (GnRH) neurons (DeFazio and Moenter, 2002), we therefore examined the modulatory effect of estrogen on CB1 receptor-mediated augmentation of the IA.
2. Materials and Methods
2.1 Animals
All animal procedures described in this study are in accordance with institutional guidelines based on NIH standards. Female and male Topeka guinea pigs (350–500 g) were obtained from Elm Hill Breeding Labs (Chelmsford, MA, USA), kept under controlled temperature (69–73°F) and light (12 h on: 12 h off), and provided with food and water ad libitum. They were ovariectomized and implanted with a DataLogger (SubCue, Calgary, Alberta) into the abdominal cavity to monitor temporal fluctuations in core body temperature under ketamine/xylazine anesthesia (33 mg/kg & 6 mg/kg, respectively; s.c.) at least 4 days prior to experimentation. Following recovery, the animals underwent treatment with estradiol benzoate (EB; 4 or 10 μg; s.c.), or with its sesame oil vehicle (0.1 ml; s.c.), as will be described subsequently.
2.2 Drugs
For the feeding studies, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate (WIN 55,212-2) and N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) were dissolved in cremephor/ethanol/0.9% saline (1/1/18; v/v/v). For the electrophysiological experiments, tetrodotoxin (TTX) with citrate was dissolved in Ultrapure H2O to a stock concentration of 1 mM. Tetraethylammonium chloride (TEA) was dissolved in UltraPure H2O to a stock concentration of 500 mM. 4-Aminopyridine (4-AP) and nickel chloride hexahydrate (NiCl2) were dissolved in UltraPure H2O to a stock concentration of 100 mM. Arachidonyl-2′-chloroethylamide (ACEA) was dissolved in ethanol to a stock concentration of 1 mM. cis-4-[Phosphomethyl]-2-piperidinecarboxylic acid (CGS 19755; 10 mM) was dissolved in 0.1N NaOH and then diluted to the final volume with UltraPure H20. WIN-55,212-2, AM251 and 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) were dissolved in dimethyl sulfoxide to stock concentrations of 10 mM. Stock solutions of 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (SR 95531) and ω-conotoxin MVIIC (10 mM and 10 μM, respectively) were prepared with UltraPure H2O.
2.3 Feeding Behavior
The feeding studies were performed as previously described (Diaz et. al., 2009). Briefly, food intake was monitored around the clock for seven days using a Comprehensive Lab Animal Monitoring System (Columbus Instruments; Columbus, OH, USA). Each morning at 8:00 a.m. the animals were injected with either the mixed cannabinoid CB1/CB2 receptor agonist WIN 55,212-2 (0.1–1.0 mg/kg; s.c.), the CB1 receptor antagonist AM251 (3 mg/kg; s.c.) or their cremephor/ethanol saline (1/1/18; v/v/v) vehicle (1 ml/kg; s.c.). Every other morning they were also injected with EB (4–10 μg) or its sesame oil vehicle.
2.4 Electrophysiology
Electrophysiological recordings from arcuate neurons were performed using an in vitro hypothalamic slice preparation as previously described (Tang et. al., 2005;Nguyen and Wagner, 2006). Briefly, electrode resistances varied from 3 – 8 MΩ. Membrane currents were recorded in voltage clamp with access resistances ranging from 8–20 MΩ, and underwent analog-digital conversion via a Digidata 1322A interface coupled to pClamp 8.2 software (Axon Instruments). The access resistance, as well as the resting membrane potential and the input resistance, were monitored throughout the course of the recording. If the access resistance deviated greater than 20% of its original value, the recording was ended. To ascertain whether estrogen could rapidly modulate cannabinoid receptor agonist-induced decreases in glutamatergic mEPSCs or GABAergic mIPSCs, cells were perfused in artificial cerebrospinal fluid in the presence of 500 nM TTX and 10 μM SR 95531, or 3 μM NBQX and 10 μM CGS 19755, to block GABAA or ionotropic glutamate receptor-mediated synaptic input, respectively, and also with 100 nM EB or its ethanol vehicle (0.00376% by volume), for 10–15 minutes. Baseline recordings were performed from a holding potential of −75 mV (for mEPSCs) or −30 mV (for mIPSCs) for 3–4 minutes. Both EB-treated and vehicle-treated slices were then perfused with varying concentrations of the cannabinoid receptor agonist WIN 55,212-2 (30 nM – 10 μM) or the cannabinoid CB1 receptor antagonist AM251 (1 μM), and 3–4 more minutes of data were collected. Measurements were obtained from at least 100 contiguous mEPSCs or mIPSCs, and were analyzed to determine alterations in frequency and amplitude prior to, and in the presence of, these compounds. To determine whether estrogen could modulate the A-type K+ (IA) current prevalent in arcuate POMC neurons (Ibrahim et. al., 2003;Tang et. al., 2005), recordings were performed in slices perfused with EB or vehicle, or occasionally in slices obtained from animals treated 24 h prior with either EB or vehicle. Neurons that exhibited transient outward tail currents evoked immediately following a hyperpolarizing voltage command (≥ 20 mV) from rest were selected for further analysis. The cells were perfused for 6–7 min with 25 mM TEA, 100 μM 4-AP, 1 μM TTX, 10 μM SR 95531, 3 μM NBQX and 10 μM CGS 19755 to block other depolarization-activated K+ channels (except for the IA, which is resistant to TEA and to low concentrations of 4-AP (Storm, 1988), and to isolate the cells from synaptic input impinging upon it. Cells were then subjected to baseline inactivation protocols. The inactivation of the IA was evaluated by holding the membrane potential at −60 mV and giving 10 mV pre-pulses (500 msec) from −110 to −40 mV, with each pulse followed by a depolarizing test command to −10 mV. The resultant outward current elicited by the depolarizing test command was measured for each of the pre-pulse potentials. After collecting the baseline measurements, slices were perfused with either WIN 55,212-2 (1μM) or the anandamide analog ACEA (1μM) in the presence of TEA, 4-AP, TTX, SR 95531, NBQX and CGS 19755 for 4–6 min, and then the inactivation protocols were run again. The amplitude and voltage-dependence of the IA were analyzed using p-Clamp and SigmaPlot 8.0 software. We obtained estimates of the half-maximal voltage (V½) and maximal peak current (Imax) from the inactivation curves generated by fitting the data (peak current vs. membrane voltage) to the Boltzmann equation (Deadwyler et al., 1995). If we encountered confounding Ca2+ currents that were ≥10% of the Imax, then we added 300 μM NiCl2 and 100 nM ω-conotoxin MVIIC to block T-, N- and P/Q-type Ca2+ channels. After recording, some slices were processed for immunohistofluoresence as described previously (Ronnekleiv et al., 1990).
2.5 Statistics
Comparisons between treatment groups were performed using the one-way or two-way analysis of variance (ANOVA) followed by the Least Significant Difference (LSD) test. Differences were considered statistically significant if the probability of error was less than 5%.
3. Results
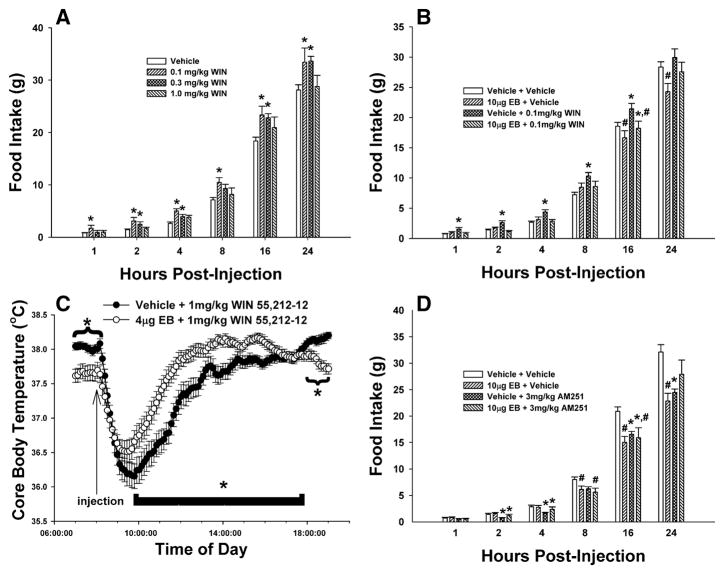
Cannabinoid effects on feeding behavior were assessed in ovariectomized female guinea pigs co-treated with EB (4 or 10 μg; s.c.) or its sesame oil vehicle. These doses of EB per se decreased daily food intake (Fig. 1A), caused uterine hypertrophy (Fig. 1B & 1C) and produced concentrations of the steroid (4 μg: 41.2±16.6 pg/ml; 10 μg: 91.2±17.5 pg/ml; n=4–11) commonly encountered over the follicular phase of the ovarian cycle (Owen, 1975). Fig. 2 depicts the effect of EB on the cannabinoid modulation of food intake and core body temperature. The cannabinoid receptor agonist WIN 55,212-2 produced a dose- and time-related increase in intake, with significant alterations initially observed as early as one h and continuing out to 24 h post-administration (Fig. 2A). The decrease in food intake caused by EB (10 μg) per se first manifested itself 8–16 h and persisted through 24 h after administration of the steroid (Fig. 1A & 2B). Most importantly, EB rapidly and completely blocked the rise in food intake produced by WIN 55,212-2 (Fig. 2B). In addition, it markedly attenuated the drop in core body temperature elicited by WIN 55,212-2; manifested by a ~0.4 °C reduction in the peak hypothermic response, and a ~6 h reduction in the duration of action (Fig. 2C). Conversely, the cannabinoid CB1 receptor antagonist AM251 (3 mg/kg) produced a significant and persistent reduction in food intake that was first observed two h post-injection, and EB co-treatment was without effect on this decrease in consumption (Fig. 2D).
Fig. 1.
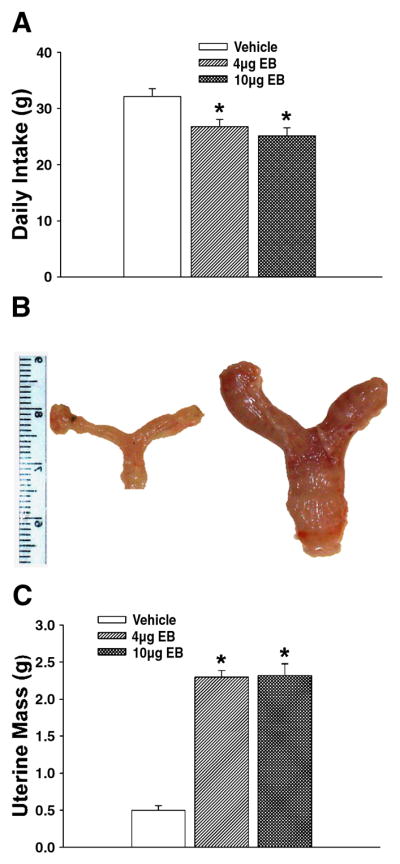
EB decreases food intake concomitantly with uterine hypertrophy. A, Composite bar graph showing the EB-induced decrease in cumulative food intake over 24 h. B, Composite photomicrograph of uteri and oviducts harvested from ovariectomized, vehicle- (left) and EB-treated (right) female guinea pigs. C, Composite bar graph illustrating the EB-induced hypertrophy of the female reproductive tract. Bars represent means and lines 1 S.E.M. of the daily intake and uterine masses under vehicle- and steroid-treated conditions. *, Values from EB-treated animals that are significantly different (one-way ANOVA/LSD; P<.05) than those from vehicle-treated controls.
Fig. 2.
EB rapidly modulates cannabinoid-induced changes in food intake and core body temperature. A, The dose-response effects of WIN 55,212-2. *, Values of food intake measured in animals treated with WIN 55,212-2 that are significantly different (multifactorial ANOVA/LSD; P<.05; n=4) than those measured in vehicle-treated controls. B, EB blocks the hyperphagic effect of WIN 55,212-2. *, Values of food intake measured in animals treated with WIN 55,212-2 that are significantly different (multi-factorial ANOVA/LSD; P<.05; n=4–7) than those measured in vehicle-treated controls. #, Values from EB-treated animals that are significantly different (multi-factorial ANOVA/LSD; P<.05; n=4–7) than those from their vehicle-treated counterparts. C, EB attenuates the hypothermic effect of WIN 55,212-2. *, Values from animals EB-treated animals that are significantly different (multi-factorial ANOVA/LSD; P<0.05; n=4–7) than those observed in vehicle-treated controls. D, Estrogen is without effect on the hypophagic effect of AM251. *, Values of food intake measured in AM251-treated animals that are significantly different (multi-factorial ANOVA/LSD; P<.05; n=4–7) than those measured in vehicle-treated controls. #, Values from EB-treated animals that are significantly different (multi-factorial ANOVA/LSD; P<.05; n=4–7) than those from their vehicle-treated counterparts. Columns represent the mean and vertical lines 1 S.E.M. of the food intake measured at 1, 2, 4, 8, 16 and 24 h for seven days in animals treated daily (8:00 a.m.) with WIN 55,212-2 (0.1–1 mg/kg; s.c.), AM251 (3 mg/kg; s.c.) or its cremephor/ethanol/saline vehicle, and every other day with EB (4–10 μg; s.c.) or its sesame oil vehicle. Symbols represent the means and vertical lines 2 S.E.M. of the core body temperature recorded every seven minutes by DataLoggers inserted into the abdominal cavity at the time of castration.
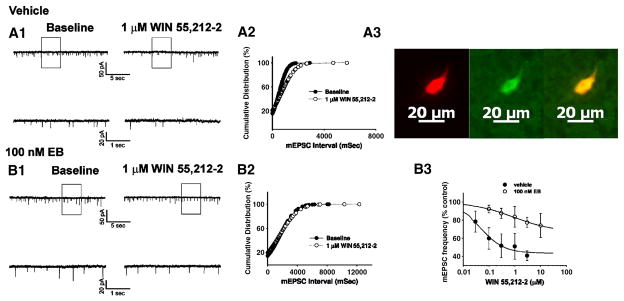
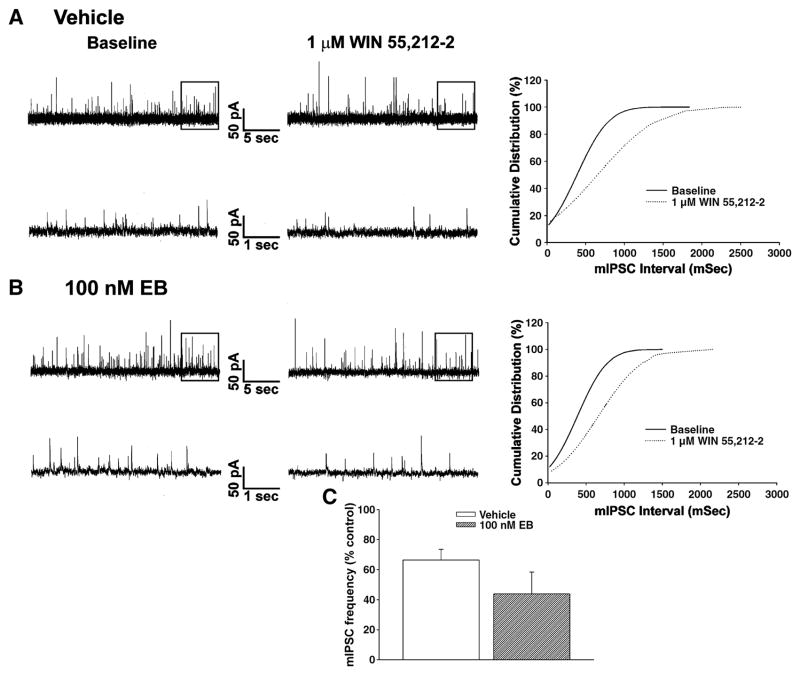
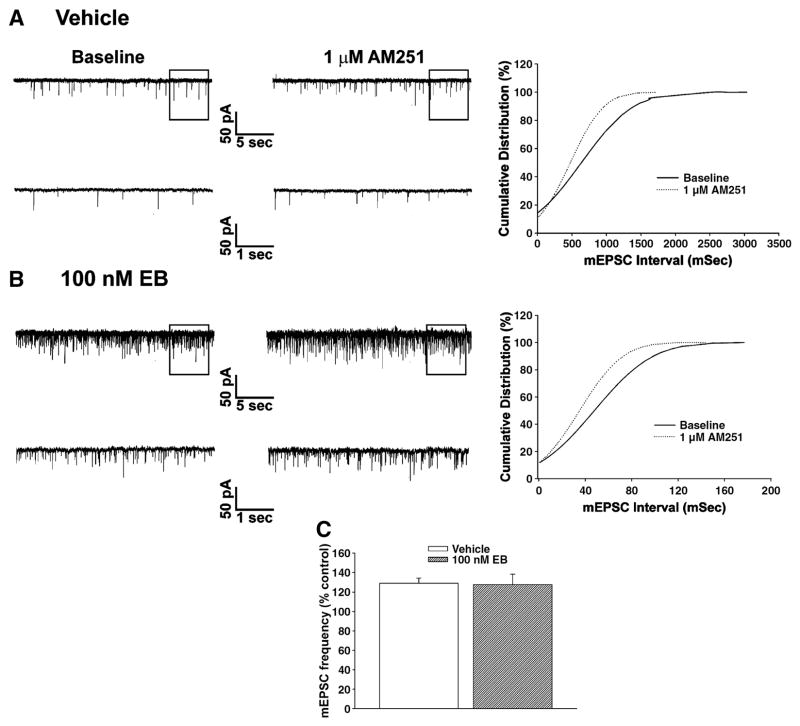
We have demonstrated previously that cannabinoid CB1 receptor agonists presynaptically inhibit amino acid neurotransmitter input onto anorexigenic POMC neurons in the guinea pigs as evidenced by: 1) a reduction in the evoked E/IPSC amplitude, 2) an increase in the S2:S1 ratio and 3) a decrease in mE/IPSC frequency (Nguyen and Wagner, 2006;Ho et. al., 2007). Therefore, we next sought to determine if the rapid estrogenic modulation of the hyperphagia produced by WIN 55,212-2 could be attributed to attenuation of this cannabinoid-induced presynaptic inhibition of transmitter release. Fig. 3A1 & 3B1 show glutamatergic mEPSCs observed prior to, and in the presence of, WIN 55,212-2 in slices obtained from castrated female guinea pigs that were perfused for 10–15 min with either EB (100 nM) or its ethanol vehicle. As can be seen from the traces of membrane current in Fig. 3A1, and from the cumulative probability plots in Fig. 3A2, bath application of WIN 55,212-2 (1 μM) produced a clearly discernable increase in the interval between contiguous mEPSCs in this arcuate neuron that was subsequently found to be immunopositive for cocaine- and amphetamine-regulated transcript (CART; Fig. 3A3), and was without effect on mEPSC amplitude (not shown). However, EB pre-treatment rapidly and markedly dampened the increase in mEPSC interval produced by WIN 55,212-2 (Fig. 3B1 & 3B2); ultimately resulting in a rightward shift in the dose-response curve for the cannabinoid receptor agonist to decrease mEPSC frequency (f; Fig. 3B3) that is manifested by a ~20-fold reduction in potency (vehicle: IC50 = 46.1 nM; EB: IC50 = 919.9 nM) and a ~2-fold reduction in efficacy (vehicle: Δfmax = 56.6%; EB: Δfmax = 33.4%). WIN 55,212-2 also decreased mIPSC frequency (Fig. 4A), but EB application did not affect this diminution (Fig. 4B & 4C). Likewise, as expected based on the results of Fig. 2D, EB did not alter the AM251-induced increase in mEPSC frequency (Fig. 5).
Fig. 3.
EB rapidly attenuates the cannabinoid-induced presynaptic inhibition of glutamatergic synaptic input onto arcuate POMC neurons. A1, Membrane current tracings showing the spontaneous mEPSCs recorded in a vehicle-treated arcuate neuron at baseline (left) and following exposure to 1 μM WIN 55,212-2 (right). The bottom traces represent excerpts from expanded portions of their respective upper traces that are contained within the bracket. A2, Cumulative probability plot illustrating the increase in interval (inverse of frequency) between contiguous mEPSCs in the cell in A1. WIN 55,212-2 elicited a ~30% decrease in mEPSC frequency (1.4 Hz vs. 2.0 Hz under basal conditions). A3, Double-labeling of the cell in A1 & A2 that is immunopositive for phenotypic markers characteristic of arcuate POMC neurons. Left, Color photomicrograph of the biocytin-streptavidin-AF546 labeling seen in this arcuate neuron. Middle, Color photomicrograph of the CART immunofluorescence in the perikarya on the left as visualized with AF488. Right, Composite overlay illustrating the double labeling in this arcuate neuron. B1, Membrane current tracings showing spontaneous mEPSCs in a cell perfused with 100 nM EB at baseline (left) and following exposure to 1 μM WIN 55,212-2 (right). B2, Cumulative probability plot illustrating the interval between contiguous mEPSCs that substantiates the lack of cannabinoid effect in the EB-treated slice containing the cell in B1. B3, Composite dose-response curves for the decrease in mEPSC frequency produced by WIN 55,212-2 in arcuate neurons from ethanol vehicle treated (●) and EB-treated (○) slices (n=4–5). The curves were fit via logistic equation to the data points. Symbols represent means and vertical lines 2 S.E.M. of the mEPSC frequency seen with varying concentrations of WIN 55,212-2 that were normalized to their respective control values.
Fig. 4.
EB does not affect the cannabinoid-induced presynaptic inhibition of GABAergic synaptic input onto POMC neurons. Membrane current tracings show the spontaneous mIPSCs recorded in a control cell (A) and EB-treated cell (B) at baseline (left) and following exposure to 1 μM WIN 55,212-2 (middle). On the right are the cumulative probability plots illustrating the comparable increase in interval between contiguous mIPSCs. C, Composite bar graph illustrating the decrease in mIPSC frequency produced by WIN 55,212-2 in arcuate neurons from ethanol vehicle-treated and EB-treated slices (n=4–5). Bars represent means and vertical lines 1 S.E.M. of the mIPSC frequency seen with 1 μM WIN 55,212-2 that were normalized to their respective control values.
Fig. 5.
EB is without effect on AM251-induced increase in glutamatergic synaptic input onto POMC neurons. Membrane current tracings show the spontaneous mEPSCs recorded in cells from vehicle-treated (A) and EB-treated (B) slices at baseline (left) and in the presence of 1 μM AM251 (middle). On the right are the cumulative probability plots illustrating the decrease in interval between contiguous mEPSCs. C, Composite bar graph illustrating the increase in mEPSC frequency produced by AM251 in arcuate neurons from ethanol vehicle-treated and EB-treated slices (n=4–5). Bars represent means and vertical lines 1 S.E.M. of the mIPSC frequency seen with 1 μM AM251 that were normalized to their respective control values.
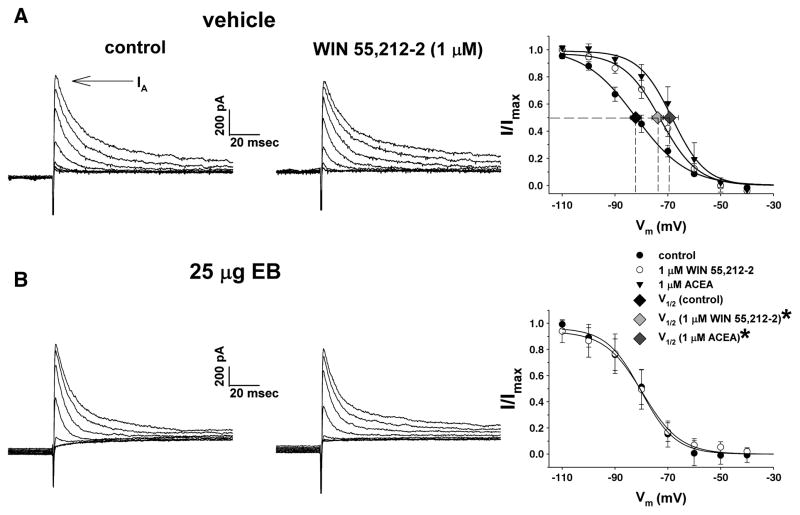
We have also shown that cannabinoid CB1 receptor agonists positively modulate the IA in POMC neurons of the female guinea pig by eliciting a depolarizing shift in the voltage dependence of its inactivation, with no effect on activation or peak current amplitude (Tang et. al., 2005). Therefore, to determine whether the negative estrogenic modulation of cannabinoid-induced hyperphagia may also involve an uncoupling of the CB1 receptor from the IA, we next examined the effects of WIN 55,212-2 and the anandamide analog ACEA on the inactivation properties of the IA in slices treated with EB or its ethanol vehicle. Traces of membrane current like those shown in Fig. 6A & 6B were used to generate inactivation curves from which the voltage dependence of IA inactivation was assessed. Both WIN 55,212-2 (1 μM) and ACEA (1 μM) right-shifted the inactivation curve for the IA in neurons from vehicle-treated slices and/or animals, which was associated with significant, ~10 mV depolarizations of the V½ (control: −82.2 ± 2.1 mV; WIN 55,212-2: −73.8 ± 2.2 mV; ACEA: −69.3 ± 3.3 mV; p<.05; Fig. 6A) with no effect on the Imax (not shown). Short-term bath application of EB (100 nM; 10–15 min) per se did not alter basal values for the V½ or the Imax (not shown), but completely reversed the rightward shift in the IA inactivation curve produced by WIN 55,212-2 (Fig. 6B), an effect that persisted at least 24 h in neurons from steroid-treated animals (not shown).
Fig. 6.
EB antagonized the CB1 receptor-mediated shift in the voltage dependence of the IA. This figure depicts the IA evoked in arcuate neurons from vehicle-treated (A) and EB-treated (B) slices during the inactivation protocol. The IA is observed as a transient outward current immediately following delivery of the test pulse (denoted by the arrow). On the left is the IA observed under baseline control conditions. In the middle is the IA evoked in the presence of WIN 55,212-2 (1 μM). On the right are the composite inactivation curves for the IA. The Boltzmann equation fits the curves to the corresponding data points. Symbols and accompanying vertical lines represent means ± 2 S.E.M. of peak currents normalized to the Imax that were observed at the test pulse following a given pre-pulse. Symbols and accompanying horizontal lines represent means ± 2 S.E.M. of the V½ derived from the inactivation curves prior to (vehicle: n=18; EB: n=6), and in the presence of, WIN 55,212-2 (vehicle: n=13; EB: n=6) or ACEA (1 μM; n=5). *, The estimated V½ derived from arcuate neurons in the presence of WIN 55,212-2 and ACEA that is significantly different (P<.05; one-way ANOVA/LSD) than that observed under baseline control conditions.
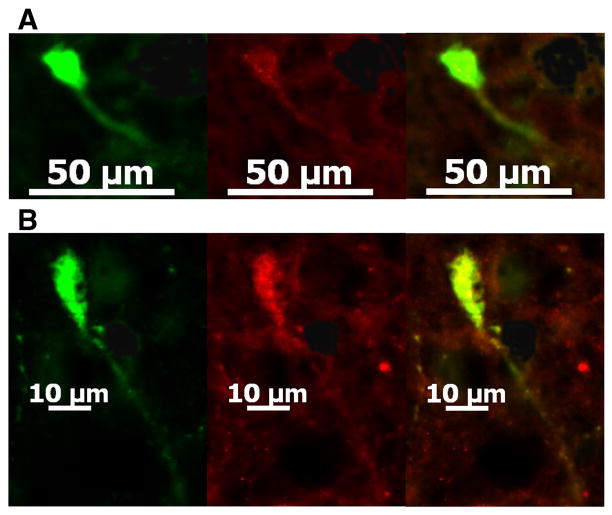
Of the 77 cells from we obtained electrophysiological recordings, nine were immunopositive for CART like the one shown in Fig. 3A3, eight were immunopositive for α-melanocyte-stimulating hormone (α-MSH) and six were immunopositive for β-endorphin. Ten of these cells had somas which were observed in two adjacent sections, which enabled us to test with an additional phenotypic marker. Of these, three neurons showed immunoreactivity for both CART and α-MSH like the one shown in Fig. 7, two cells exhibited immunoreactivity for both CART and β-endorphin and one other neuron displayed immunoreactivity for both α-MSH and β-endorphin.
Fig. 7.
POMC neurons express multiple phenotypic markers. A, Color photomicrographs of an arcuate neuron whose electrophysiological recording is shown in Fig. 4B that illustrate the biocytin-streptavidin-AF488 labeling (left), CART immunofluorescence as visualized with AF546 (middle), and the composite overlay of the double labeling in this neuron (right). B, Color photomicrographs of the same arcuate neuron seen from an adjacent section that illustrate the biocytin-streptavidin-AF488 labeling (left), α-MSH immunofluorescence as visualized with AF546 (middle), and the composite overlay illustrating the double labeling in this same cell (right).
4. Discussion
These data indicate that estrogen causes a rapid and pervasive attenuation of cannabinoid CB1 receptor-mediated changes in energy homeostasis via presynaptic and postsynaptic actions on POMC neurons. These conclusions are based on the following observations: 1) estrogen rapidly and completely abolished the cannabinoid-induced hyperphagia observed in ovariectomized female guinea pigs, 2) estrogen rapidly attenuated the cannabinoid-induced hypothermia, as evidenced by reductions in peak response and duration of action, 3) estrogen rapidly diminished the cannabinoid-induced presynaptic inhibition of glutamatergic input onto POMC neurons and 4) estrogen antagonized the cannabinoid-induced augmentation of the IA.
Cannabinoid CB1 receptor activation increases appetite in both rodents (Williams and Kirkham, 2002;Cota et al., 2003;Sinnayah et. al., 2008) and humans (Mattes et al., 1994;Budney et al., 2001). Presently, WIN 55,212-2 exhibited an inverted U-shaped dose-relationship concerning its ability to stimulate food intake in our guinea pig animal model, which is consistent with previous reports in rats (Koch, 2001;Miller et al., 2004). Stimulation of cannabinoid receptors also decreased mEPSC and mIPSC frequency in guinea pig POMC neurons, which is in agreement with prior studies in transgenic mice whose POMC neurons were genetically targeted with enhanced green fluorescent protein (eGFP; (Hentges et. al., 2005). However, in these mice GABAergic but not glutamatergic synaptic input is tonically inhibited by endogenous cannabinoids acting on the distal dendrites of POMC neurons, as evidenced by the ability of CB1 receptor antagonists to increase mIPSC frequency (Hentges et. al., 2005;Hentges, 2007;Sinnayah et. al., 2008). Exactly what impact this has on the appetite-modulating properties of cannabinoids in mice is unclear, as it has been reported that cannabinoid receptor agonists and antagonists respectively increase and decrease food intake in both Ay mice that over express agouti-related peptide (AgRP) and their wildtype controls (Sinnayah et. al., 2008). AgRP overexpression in Ay mice produces comparatively mild obesity consisting of hyperphagia, hyperinsulinemia and hyperleptinemia (Cone, 2006), and while Huszar and co-workers (1997) report increased daily intake in these mice, Sinnayah and colleagues (2008) did not describe any change in basal intake in either fed or fasted Ay mice relative to wildtype controls. On the other hand, melanocortin 4 (MC4) receptor-deficient and male β-endorphin knockout mice are hyperphagic, hyperglycemic, hyperinsulinemic, hyperleptinemic; with the former also being resistant to lipopolysaccharide-induced cachexia (Huszar et al., 1997;Appleyard et al., 2003;Marks and Cone, 2003). In addition, the double knockout of both the MC4 receptor and β-endorphin results in a significantly greater amount of food consumed than ablation of either one per se (Appleyard et. al., 2003). This raises the possibility that the MC4 receptor and/or β-endorphin knockouts may produce a more consistent and reliable disruption of POMC neuronal function. In any case, the AM251-induced decrease in food intake encountered in the present study was associated with a ~30% increase in mEPSC frequency at guinea pig POMC synapses. This is comparable to the increase in excitatory input observed in parvocellular neurons of the paraventricular nucleus (Malcher-Lopes et al., 2006), and suggests that the hypophagia produced by the cannabinoid CB1 receptor antagonist can be attributed, in part, to increased glutamatergic neurotransmission in hypothalamic anorexigenic nuclei. Guinea pigs, like humans, do not manufacture vitamin C (Horton et al., 1975;Odumosu, 1981), and have proven historically to be a very sensitive model than for the development of anorectic serotonergic drugs such as fenfluramine (Mennini et al., 1991) and fluoxetine (Anelli et al., 1992). Thus, in our guinea pig animal model of feeding, the appetite-modulating properties of cannabinoids are consistent with changes in amino acid neurotransmission – in particular glutamatergic neurotransmission – at POMC synapses.
The results of the present study clearly demonstrate that estrogen exerts powerful activational influences on cannabinoid-induced increases in food intake. Our findings also demonstrate that estrogen profoundly disrupts the pleiotropic actions of cannabinoids at POMC synapses via attenuation of the presynaptic inhibition of glutamate but not GABA release, and the enhancement of the postsynaptic IA. Arguably the principal target of estrogen involved in the hypothalamic control of homeostasis is the POMC neuron. Not only does estrogen increase POMC gene expression (Thornton et. al., 1994;Cheung and Hammer, 1995;Roepke et. al., 2007), but it also promotes asymmetric synapse formation (Gao et. al., 2007) and stimulates the release of posttranslational POMC byproducts such as β-endorphin (Ferin et. al., 1984). In addition, both the subcutaneous injection of estrogen and the intrascapular implantation of an estrogen-filled silastic capsule have been previously shown to increase excitatory synaptic input onto POMC neurons anywhere from 24–72 h following steroid administration (Nguyen and Wagner, 2006;Gao et. al., 2007). Moreover, estrogen alters the coupling of metabotropic receptors to their effector systems at POMC synapses to modulate cell excitability and neurotransmission (Kelly and Wagner, 1999;Nguyen and Wagner, 2006).
It is well-recognized that estrogen rapidly impedes postsynaptic Gi/o-coupled receptors from G protein-activated, inwardly-rectifying K+ (GIRK) channels in POMC neurons (Eckersell et al., 1998;Kelly and Wagner, 1999). This uncoupling is due to the membrane associated actions of estrogen, whereby the steroid activates a phospholipase C (PLC)/protein kinase C (PKC)/protein kinase A (PKA) pathway (Lagrange et al., 1997;Qiu et al., 2003;Qiu et al., 2006). This effect occurs with an EC50 of 7.5 nM and is blocked by the competitive estrogen receptor antagonist ICI 164,385 with a Ke of < 1 nM (Lagrange et. al., 1997). However, the diminishing of GIRK channel function does not occur in estrogen receptor α (ERα) or ERβ knockout mice (Qiu et. al., 2006), which suggests that this rapid uncoupling is mediated by a putative membrane estrogen receptor distinct from ERα or ERβ. On the other hand, estrogen has been reported to rapidly increase the amplitude of kainate-induced currents in dissociated hippocampal neurons in a PKA-dependent manner (Gu and Moss, 1996), and activate metabotropic glutamate receptors to modulate PLC-dependent intracellular signaling and sexual behavior via a membrane associated ERα (Boulware et al., 2005;Dewing et al., 2007). However, all of these estrogenic effects are targeted to the postsynaptic neuron. Thus, this is the first report to demonstrate that estrogen can thwart Gi/o-coupled receptors from presynaptically inhibiting glutamate neurotransmission at POMC synapses within minutes following its application. Such an effect would rapidly oppose the cannabinoid-induced inhibition, and lead to a net increase in the excitatory glutamatergic tone onto POMC neurons.
By contrast, systemic estrogen was without effect on the hypophagia caused by AM251, and transient bath application of the steroid failed to alter the cannabinoid CB1 receptor antagonist-induced increase in mEPSC frequency. This is inconsistent with our previous study demonstrating that EB administered 24 h prior can block the AM251-induced increase in glutamatergic synaptic input onto POMC neurons (Nguyen and Wagner, 2006), and suggesting that more prolonged estrogen exposure may decrease endogenous cannabinoid expression, as has been described for leptin (Di Marzo et. al., 2001). Indeed, estrogen stimulates leptin secretion (Lin et al., 2005), and both estrogen and leptin receptors converge upon several signal transduction elements such as STAT3, and phosphatidylinositol 3′-kinase (Roepke et. al., 2007;Gao and Horvath, 2008;Schwarz et al., 2008). AM251 is considered both an antagonist and inverse agonist at the cannabinoid CB1 receptor, and as such can elevate intracellular levels of cAMP and activate the PKA pathway (Pertwee, 2005). If the AM251-induced hypophagia and increase in mEPSC frequency were due to its ability to antagonize the actions of endogenous cannabinoid CB1 receptor agonists, then estrogen should have been able to rapidly mimic these actions due to its own ability to negatively modulate the coupling of the CB1 receptor to its effector systems at POMC synapses. However, if these effects of AM251 are due to its inverse agonism of the cannabinoid CB1 receptor associated with decreased guanine nucleotide binding and subsequent dissociation of the Gi/o heterotrimer, then as the data suggest the negative modulatory effects of estrogen on CB1 receptor/effector coupling would be occluded.
Our findings also provide the first description of an estrogen-induced disruption of a Gi/o-coupled receptor from A-type K+ channels in POMC neurons such as the Kv4.1 (Roepke et. al., 2007) or Kv4.2 (Tang et. al., 2005) subtypes. The IA is a depolarization-activated current that increases the interspike interval between action potentials, thereby reducing firing frequency (Rudy, 1988). Cannabinoid CB1 receptor agonists augment the IA in POMC and dissociated hippocampal neurons by altering the voltage dependence of its inactivation (Deadwyler et. al., 1995;Tang et. al., 2005). Hence, the estrogenic obstruction of the cannabinoid CB1 receptor-mediated depolarizing shift of the IA inactivation curve means that more of a hyperpolarizing stimulus would be required for deinactivation. It follows that at most membrane potentials fewer channels would be available to help offset subsequent depolarizing stimuli and slow neuronal firing. This is functionally similar to what happens in GnRH neurons, where estrogen per se diminishes the IA by decreasing peak current amplitude and density; thereby lowering threshold and decreasing latency to neuronal firing (DeFazio and Moenter, 2002). Thus, the collective estrogenic attenuation of the cannabinoid CB1 receptor-mediated presynaptic inhibition of glutamate release and postsynaptic activation of the IA would greatly impair the ability of CB1 receptor activation to decrease POMC neuronal activity, and result in increased release of the anorexic peptides α-MSH, β-endorphin and CART co-expressed in these cells. This would account, in part, for the overt, estrogen-induced physiologic antagonism of the hyperphagia presently observed following cannabinoid CB1 receptor activation.
These activational effects extend beyond appetite to include diminutions in the cannabinoid CB1 receptor-mediated hypothermia. While estrogen respectively decreases and increases the expression of α2-adrenergic and β3-adrenergic receptors in cultured brown adipocytes, it decreases mitochondrial cytochrome c oxidase activity and norepinephrine-induced thermogenesis, and does not affect lipolysis or uncoupling protein expression (Nava et al., 1994;Puerta et al., 1998;Rodríguez et al., 2002;Monjo et al., 2003). In addition, thermogenesis does not change over the estrous cycle (Puerta et. al., 1998). Alternatively, the silencing of ERα in the hypothalamic ventromedial nucleus reportedly reduces diet-induced thermogenesis (Musatov et al., 2007). Centrally, estrogen increases the firing of warm-sensitive neurons in the preoptic area (Silva and Boulant, 1986), thereby decreasing the threshold for heat dissipation and lowering body temperature (Stephenson and Kolka, 1999;Stachenfeld et. al., 2000). Conversely, cannabinoid CB1 receptor activation directly decreases thermogenesis (Perwitz et al., 2006), and also decreases the firing rate and increases the thermosensitivity of the primary preoptic thermodetector units (Schmeling and Hosko, 1980). Warm-sensitive neurons express an IA like the one enhanced by cannabinoid CB1 receptor agonists in this and other studies (Deadwyler et. al., 1995;Tang et. al., 2005), whose amplitude is inversely proportional to the ambient temperature (Boulant, 1998). Thus, it is certainly plausible that cannabinoid CB1 receptor activation augments an estrogen-sensitive IA to affect a decrease in the activity of warm-sensitive neurons that may be occluded by the steroid. Future studies will determine if this is, in fact, the case.
5. Conclusion
In conclusion, these results reveal that estrogen rapidly exerts multiple, negative modulatory influences on the cannabinoid regulation of food intake, core body temperature and neurotransmission at POMC synapses. The data also offer new insight into the interaction between estrogen and cannabinoids in the hypothalamic regulation of energy homeostasis. Finally, they have implications for the therapeutic role of cannabinoids in appetite stimulation in women.
Acknowledgments
Grant support: This study was supported by PHS Grant DA024314, and an intramural research grant from Western University of Health Sciences.
References
- Anelli M, Bizzi A, Caccia S, Codegoni AM, Fracasso C, Garattini S. Anorectic activity of fluoxetine and norfluoxetine in mice, rats and guinea-pigs. J Pharm Pharmacol. 1992;44:696–698. doi: 10.1111/j.2042-7158.1992.tb05500.x. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for endogenous β-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- Asch RH, Smith CG. Effects of delta9-THC, the principal psychoactive component of marijuana, during pregnancy in the rhesus monkey. J Reprod Med. 1986;31:1071–1081. [PubMed] [Google Scholar]
- Asch RH, Smith CG, Siler-Khodr TM, Pauerstein CJ. Effects of delta9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (Macaca mulatta) J Clin Endocrinol Metab. 1981;52:50–55. doi: 10.1210/jcem-52-1-50. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Hypothalamic neurons: Mechanisms of sensitivity to temperature. Ann NY Acad Sci. 1998;856:108–115. doi: 10.1111/j.1749-6632.1998.tb08319.x. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Volpe A, Paoletti AM, Melis GB. Regulation of the 24-hour rhythm of body temperature in menstrual cycles with spontaneous and gonadotroopin-induced ovulation. Fertil Steril. 1997;68:421–425. doi: 10.1016/s0015-0282(97)00242-2. [DOI] [PubMed] [Google Scholar]
- Cheung S, Hammer RP. Gonadal steroid hormone regulation of proopiomelanocortin gene expression in arcuate neurons that innervate the medial preoptic area of the rat. Neuroendocrinology. 1995;62:283–292. doi: 10.1159/000127015. [DOI] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschöp M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthhorst ACE, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE, Mu J, Whyte A, Childers S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp Ther. 1995;273:734–743. [PubMed] [Google Scholar]
- Decavel C, van den Pol AN. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J Comp Neurol. 1992;316:104–116. doi: 10.1002/cne.903160109. [DOI] [PubMed] [Google Scholar]
- Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flashes and night sweats) that occur in perimenopause, menopause and postmenopause life stages. Arch Womens Ment Health. 2007;10:247–257. doi: 10.1007/s00737-007-0209-5. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2255–2265. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych PE. Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparahu SK, Wang L, Liu J, Bátkai S, Járai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of μ-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferin M, Van Vugt D, Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent Prog Horm Res. 1984;40:441–485. doi: 10.1016/b978-0-12-571140-1.50015-3. [DOI] [PubMed] [Google Scholar]
- Fitton AG, Pertwee RG. Changes in body temperature and oxygen consumption rate of conscious mice produced by intrahypothalamic and intracerebroventricular injections of Δ9-tetrahydrocannabinol. Br J Pharmacol. 1982;75:409–414. doi: 10.1111/j.1476-5381.1982.tb08802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab. 2008;294:E817–E826. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao X-B, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Medicine. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17β-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV+ marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl) 2005;181:170–178. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]
- Hentges ST. Synaptic regulation of proopiomelanocortin neurons can occur distal to the arcuate nucleus. J Neurophysiol. 2007;97:3298–3304. doi: 10.1152/jn.00051.2007. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, Cox JM, Wagner EJ. Cannabinoid-induced hyperphagia: Correlation with inhibition of proopiomelanocortin neurons? Physiol Behav. 2007;92:507–519. doi: 10.1016/j.physbeh.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BJ, West CE, Turley SD. Diurnal variation in the feeding pattern of guinea pigs. Nutr Metabol. 1975;18:294–301. doi: 10.1159/000175607. [DOI] [PubMed] [Google Scholar]
- Huang H, Acuna-Goycolea C, Li Y, Cheng HM, Obrietan K, van den Pol AN. Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: implications for cannabinoid actions on food intake and cognitive arousal. J Neurosci. 2007;27:4870–4881. doi: 10.1523/JNEUROSCI.0732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Jo Y-H, Chen Y-JL, Chua SC, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WG, Corrigan SA, Lemmon CR, Bergeron KB, Crusco AH. Energy regulation over the menstrual cycle. Physiol Behav. 1994;56:523–527. doi: 10.1016/0031-9384(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ. Estrogen modulation of G-protein-coupled receptors. Trends Endocrinol Metab. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- Koch JE. Δ9-THC stimulates food intake in Lewis rats: Effects on chow, high-fat and sweet high-fat diets. Pharmacol Biochem Behav. 2001;68:539–543. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Lin K-C, Sagawa N, Yura S, Itoh H, Fujii S. Simultaneous increases of leptin and gonadotropin-releasing hormone following exogenous estrogen administration in women with normally menstrual cycle. Endocrine Journal. 2005;52:449–454. doi: 10.1507/endocrj.52.449. [DOI] [PubMed] [Google Scholar]
- López HH, Webb SA, Nash S. Cannabinoid receptor antagonism increases female sexual motivation. Pharmacol Biochem Behav. 2009;92:17–24. doi: 10.1016/j.pbb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng F-J, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DL, Cone RD. The role of the melanocortin-3 receptor in cachexia. Ann NY Acad Sci. 2003;994:258–266. doi: 10.1111/j.1749-6632.2003.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Engelman K, Shaw LM, Elsohly MA. Cannabinoids and appetite stimulation. Pharmacol Biochem Behav. 1994;49:187–195. doi: 10.1016/0091-3057(94)90475-8. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Czaja JA. Diverse effects of estradiol-17β: concurrent suppression of appetite, blood pressure and vascular reactivity in conscious, unrestrained animals. Physiol Behav. 1989;45:649–657. doi: 10.1016/0031-9384(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Mennini T, Bizzi A, Caccia S, Codegoni A, Fracasso C, Frittoli E, Guiso G, Padura IM, Taddei C, Uslenghi A, Garattini S. Comparative studies on the anorectic activity of d-fenfluramine in mice, rats and guinea pigs. Naunyn-Schmiedeberg’s Arch Pharmacol. 1991;343:483–490. doi: 10.1007/BF00169550. [DOI] [PubMed] [Google Scholar]
- Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav. 2004;80:611–616. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Monjo M, Rodríguez AM, Palou A, Roca P. Direct effects of testosterone, 17β-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144:4923–4930. doi: 10.1210/en.2003-0537. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor α in the ventromedial nucleus of the hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava MP, Fernández A, Abelenda M, Puerta M. Dissociation between brown adipose tissue thermogenesis and sympathetic activity in rats with high plasma levels of oestradiol. Pflugers Arch. 1994;426:40–43. doi: 10.1007/BF00374668. [DOI] [PubMed] [Google Scholar]
- Nguyen QH, Wagner EJ. Estrogen differentially modulates the cannabinoid-induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology. 2006;84:123–137. doi: 10.1159/000096996. [DOI] [PubMed] [Google Scholar]
- Odumosu A. Vitamin C and weight reducing drugs on brain ascorbic acid in guinea pigs. Acta Vitaminol Enzymol. 1981;3:96–102. [PubMed] [Google Scholar]
- Owen JA. Physiology of the menstrual cycle. Am J Clin Nutr. 1975;28:333–338. doi: 10.1093/ajcn/28.4.333. [DOI] [PubMed] [Google Scholar]
- Palmer K, Gray JM. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav. 1986;37:187–189. doi: 10.1016/0031-9384(86)90404-x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Perwitz N, Fasshauer M, Klein J. Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Horm Metab Res. 2006;38:356–358. doi: 10.1055/s-2006-925401. [DOI] [PubMed] [Google Scholar]
- Puerta M, Rocha M, González-Covaleda S, McBennett SM, Andrews JF. Changes in cytochrome oxidase activity in brown adipose tissue during oestrous cycle in the rat. Eur J Endocrinol. 1998;139:433–437. doi: 10.1530/eje.0.1390433. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A g-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Rodríguez AM, Monjo M, Roca P, Palou A. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cell Mol Life Sci. 2002;59:1714–1723. doi: 10.1007/PL00012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Loose MD, Erickson KR, Kelly MJ. A method for immunocytochemical identification of biocytin-labeled neurons following intracellular recording. BioTechniques. 1990;9:432–438. [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA. Acute activation of ERα decreases food intake, meal size and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- Schmeling WT, Hosko MJ. Effect of Δ9-tetrahydrocannabinol on hypothalamic thermosensitive units. Brain Res. 1980;187:431–443. doi: 10.1016/0006-8993(80)90213-9. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang S-L, Thompson SM, McCarthy MM. Estradiol induces dendritic spines by enhancing glutamate release independent of transcription: A mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NL, Boulant JA. Effects of testosterone, estradiol and temperature on neurons in preoptic tissue slices. Am J Physiol Regul Integr Comp Physiol. 1986;19:R625–R632. doi: 10.1152/ajpregu.1986.250.4.R625. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnayah P, Jobst EE, Rathner JA, Caldera-Siu AD, Tonelli-Lemos L, Eusterbrock AJ, Enriori PJ, Pothos EN, Grove KL, Cowley MA. Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLoS ONE. 2008;3:e2202. doi: 10.1371/journal.pone.0002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol. 2000;88:1643–1649. doi: 10.1152/jappl.2000.88.5.1643. [DOI] [PubMed] [Google Scholar]
- Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol. 1999;86:22–28. doi: 10.1152/jappl.1999.86.1.22. [DOI] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Tang SL, Tran V, Wagner EJ. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. J Neurophysiol. 2005;94:2983–2986. doi: 10.1152/jn.01187.2004. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Loose MD, Kelly MJ, Rönnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- Tsai CL, Kanosue K, Matsumura K. Effects of estradiol treatment on responses of rat preoptic warm sensitive neurons to progesterone in vitro. Neurosci Lett. 1992;136:23–26. doi: 10.1016/0304-3940(92)90638-n. [DOI] [PubMed] [Google Scholar]
- Walsh D, Kirkova J, Davis MP. The efficacy and tolerability of long-term use of dronabinol in cancer-related anorexia: A case series. J Pain Symptom Manage. 2005;30:493–495. doi: 10.1016/j.jpainsymman.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Yuen BH, Keye WR, Jaffe RB. Human prolactin: secretion, regulation and pathophysiology. Obstet Gynecol Survey. 1973;28:527–541. [PubMed] [Google Scholar]