Abstract
Glioblastoma multiforme is the most common primary intracranial tumor in humans. Despite continued advances in cancer therapy, the outcome for patients diagnosed with this disease remains bleak. Novel treatments involving the use of conditionally replicating adenoviruses (CRAds) to target malignant brain tumors have undergone extensive research and proven to be a promising mode of glioblastoma therapy. CRAds are genetically manipulated to replicate within tumor cells, exhibiting a high degree of infectivity, cytotoxicity, and transgene expression. While the use of various CRAds has been deemed safe for intracranial injection in preclinical trials, a significant therapeutic effect has yet to be seen in patients. This shortcoming stems from the distribution limitations involved with local delivery of virolytic agents. To enhance this modality of treatment, stem cells have been explored as cellular vehicles in virotherapy applications, given that they possess an intrinsic tropism for malignant brain tumors. Stem cell loaded CRAd delivery offers a more specific and effective method of targeting disseminated tumor cells and forms the basis for this review.
Keywords: Stem cells, mesenchymal stem cells, neural stem cells, virotherapy, adenovirus, glioma, glioblastoma, brain cancer
INTRODUCTION
Intracranial gliomas are the most prevalent form of primary brain cancer. The dynamic biology of these tumors compounded with their elusive location within the brain renders them very difficult to treat by means of conventional therapy. Despite continued advances in surgical and medical therapies, the outcome for patients diagnosed with this form of cancer remains dismal. Gliomas, like many other tumors, are subject to unpredictable genotypic and phenotypic alterations resulting in treatment resistant clones [1]. The selective permeability of the blood brain barrier limits the effectiveness of most chemotherapeutic agents, impeding their ability to reach distant tumor cells [2]. Surgical treatment also faces many obstacles, as the biological nature of these tumors causes them to infiltrate diffusely within surrounding brain parenchyma [1]. In light of these challenges, novel techniques and alternative approaches are being explored to treat this terminal condition.
Gene therapy is one method in particular that is being investigated to treat malignant gliomas. With the help of viral vectors, gene therapy allows for the introduction of genetic material into deficient cells to compensate for abnormal genes or to make a beneficial protein. Due to the fact that most brain tumors do not metastasize beyond the central nervous system, gene therapy and local delivery of vectors carrying therapeutic genes appear to be a promising frontier in experimental brain tumor treatment. While treating patients with radiation and chemotherapeutic agents such as temozolomide following tumor resection provides a survival benefit of several months, the effects are not long lasting [3]. The inability of chemotherapy, radiation and gross total resection to effectively treat patients diagnosed with this form of cancer beckons for an alternative pathway to combat this disease.
The use of viruses to specifically kill tumor cells while sparing normal cells is known as virotherapy [4]. Viruses have evolved to infiltrate host cells and usurp the cellular machinery in order to replicate and package their own genome. Virotherapy, in this sense, involves the targeting of malignant tumor cells in order to infect them with oncolytic viruses. Once internalized, these viruses gain control over the proliferating tumor and induce cell death. The most common viral vectors used in virotherapy include: herpes simplex virus, retrovirus, measles virus, reovirus, and adenovirus [5]. The delivery of oncolytic agents to targeted tumor cells can be accomplished using a mélange of different viruses as vessels; however, this article will focus on the delivery of an oncolytic adenovirus to target glioblastoma.
A key component for successful and effective gene therapy resides in the capacity of the gene delivery vectors. Adenoviruses possess many appealing characteristics that render them an excellent model for developing effective oncolytic therapies for brain tumors. These features, including their intrinsic ability to grow to high titers, large carrying capacity for transgenes, broad tropism, efficient transduction, and mild pathogenicity all reveal why using adenoviruses presents significant therapeutic potential [4, 6]. Additionally, the viral genome is large enough to incorporate foreign genes and it has been carefully identified and fully sequenced allowing it to be easily manipulated for therapeutic purposes.
ADENOVIRAL THERAPY
The use of conditionally replicating adenoviruses (CRAds) to treat malignant brain tumors has undergone extensive research and proves to be a promising mode of cancer therapy. Conditionally-replicating adenoviruses are genetically altered to replicate within tumor cells while exhibiting a high degree of infectivity, cytotoxicity and specificity [4, 6-8]. With cytotoxic replication as the presumed goal for oncolytic virotherapy, it is imperative that CRAds specifically target tumor cells and spare surrounding normal cells. The precise targeting ability of CRAds has been accomplished by manipulating specific elements residing in the early regions (E1A and E1B) of the adenovirus genome [8]. Vectors of this class that have gained recent attention include ONYX-015, Delta-24-RGD, and CRAd-Survivin-pk7.
The conditionally replicating adenoviral vector (CRAd) dl1520, known as ONYX-015, has been engineered to replicate efficiently in neoplastic cells characterized by disruptions in the p53 tumor suppressor pathway [9]. While other mechanisms of replicative selectivity may be operative, it is believed that ONYX-015 attains tumor specificity from an 800bp deletion in the E1B region encoding the 55kDa protein, which in infected cells, binds and inactivates cellular p53. Targeting the p53 pathway is important in glioma therapy because approximately 30-50% of gliomas harbor a p53 mutation [7]. The safety of delivering ONYX-015 to treat malignant gliomas lies in the fact that should the virus encounter a non-neoplastic cell bordering the tumor, replication would be aborted because ONYX-015 is incapable of countering the proficient p53 defense mechanism characteristic of non-tumor cells.
While the use of ONYX-015 to combat gliomas is relatively recent, clinical trials have revealed the safety and potential efficacy of using this vector in the treatment of intractable head and neck cancers in both Phase I and Phase II trials [10, 11]. In 2004, Chiocca et al. conducted the first Phase I trial consisting of dose-escalating intracerebral injections of ONYX-015 in 24 patients with recurrent malignant gliomas. Following tumor resection, ONYX-015 was injected (volume of 100μL per injection) at 10 different sites into the wall of the resection cavity at doses varying from 1×107 to 1×1010 p.f.u. The median time to progression after treatment was 46 days (range 13 to 452 +/− days) and the median survival time was 6.2 months (range 1.3 to 28.0 +/− months). None of the 24 patients experienced serious adverse affects related to ONYX-015; on the contrary, the study showed that injections of ONYX-015 into glioma cavities was well tolerated at doses up to 1010 p.f.u. While this study revealed the overall safety of injecting ONYX-015 into the brain, a significant therapeutic effect was not realized in patients. A limitation involved with local injection of CRAds is the lack of adequate viral distribution in tumor tissue. As such, tumor recurrence remains inevitable, and a new approach in the delivery of CRAds is required to improve the outcome of patients.
An alternative method to combat gliomas using the CRAd Delta-24-RGD is also being investigated for its potential to improve the prognosis of patients diagnosed with this form of cancer. Delta-24-RGD has been engineered by Fueyo et al. to selectively replicate in cells harboring dysfunctional retinoblastoma (Rb) protein or its regulatory pathway by virtue of a 24bp deletion in the E1A region. To overcome the paucity of CAR expressed on many tumor cells, Delta-24-RGD has been further enhanced to express the RGD motif in its fiber knob H1 loop to increase viral internalization and infectivity [12]. This modification allows for efficient viral internalization entirely independent of CAR.
Although Delta-24-RGD is currently awaiting clinical evaluation, its application has proven to be highly effective against tumors in mice bearing xenografts. A preclinical study conducted by Fueyo et al. [13] examining the therapeutic potential of Delta-24-RGD to target tumor stem cells reveals that this vector eliminates both brain tumor stem cells as well as non-stem cell populations. These findings are of great importance to brain tumor therapy because tumor stem cells are the driving force sustaining tumor growth and recurrence. Tumor stem cells are both chemo-resistant and radio-resistant and are therefore considered to be responsible for tumor progression and recurrence even after conventional methods of glioma therapy have been performed [14]. The results of the Fueyo's study indicate that intracranial injection of Delta-24-RGD induces autophagic cell death, and the treatment of xenografts derived from brain tumor stem cells with Delta-24-RGD significantly improved the survival of glioma-bearing mice (mean survival: 38.5 versus 66.3 days, difference=27.8 days, 95% confidence interval =19.5 to 35.9 days, P<0.001). Such impressive results have gained Delta-24-RGD much attention in the quest for a better modality of treatment, and this vector is about to undergo a Phase I clinical trial [Fueyo, 2008 personal correspondence].
Another approach to treating gliomas utilizes the tumor specific promoter survivin (S), which is a member of the inhibitor of apoptosis protein (IAP) family that acts as a suppressor of apoptosis and plays a vital role in cell division [15, 16]. Owing to its tremendous up-regulation in tumor cells, defective expression of survivin has been associated with playing a fundamental role in tumorigenesis, increased rates of tumor recurrence, and resistance to chemo- and radiotherapy [15, 17]. Transcriptional targeting by means of exploiting tumor specific promoters allows for adenoviral replication and oncolysis to occur exclusively within tumor tissue because viral replication is mediated by specific internal cellular cues emitted by host tumor cells [15, 16].
Since it has been demonstrated that incorporating a survivin promoter into the E1A region of an adenoviral genome enhances viral replication and oncolysis in malignant gliomas, a tremendous effort has been put forth to engineer an optimal CRAd possessing this feature [15-17]. The adenoviral vector, CRAd-S-pk7, belongs to the class of Ad-5 sero-types that have been carefully crafted with the survivin promoter in addition to having a polylysine residue (pk7) incorporated into its fiber knob enhancing its transduction capability. Ulasov et al. [15] showed that injecting CRAd-S-pk7 into U87MG glioma xenografts in mice induces strong cytotoxicity in target cells and does not harm surrounding normal cells. In the study, injection of CRAd-S-pk7 inhibited xenograft tumor growth by more than 300% (p<0.001), allowing long-term survivorship to the mice bearing intracranial gliomas (>110 days; p<0.005).
DELIVERY OF AN ONCOLYTIC ADENOVIRUS
While the use of various adenoviral vectors has proven safe for intracranial injection in several preclinical trials, a significant therapeutic effect is yet to be seen in patients. The reason behind this shortcoming is largely a result of the distribution limitations involved with local delivery of virolytic agents by themselves. Due to the complex biological nature of high grade gliomas, local injection of adenoviral vectors fails to reach scattered infiltrative tumor cells within the brain parenchyma [1, 9]. The therapeutic effect of intracereberal injections of CRAds is only seen in the vicinity of the injection site, however, a broader vector distribution is necessary to impact all tumor cells. To remedy this and prevent possible tumor recurrence, stem cells have been explored as vehicles for gene therapy in brain tumors given that they possess an intrinsic tropism for pathologies [18, 19].
The initiative behind exploiting stem cells as delivery vehicles for CRAds is based on the previous success in delivering other cancer toxic agents and transgenes to tumors in the brain. Aboody et al. [19] showed that NSCs can selectively deliver therapeutic genes to intracranial tumor sites including prodrug activating enzymes (cytosine deaminase, carboxylesterase, thymidine kinase), interleukins (IL-2, IL-4, IL-12, IL-23), interferon-β, apoptosis-promoting genes (tumor necrosis factor-related apoptosis-inducing ligand) and metalloproteinases (PEX). The well established delivery potential of stem cells for gene therapy in the brain has prompted researchers to investigate their application in virotherapy as well.
Given their ability to localize to tumor sites, stem cell mediated CRAd delivery offers a more specific and thorough therapeutic effect than local delivery of CRAds alone. A benefit to using a cell-based delivery approach of an adenovirus is that a stem cell is capable if responding to diverse pathological signals released by tumor tissue [19-21]. Stem cell specificity and tropism is likely mediated by several cell surface receptors as well as secreted cytokines and growth factors. Additionally, extracellular matrix proteins have been also been associated with the tumor-tropism of stem cells [19]. While the exact mechanism of their tumor affinity has yet to be fully elucidated, neural stem cells (NSC) and mesenchymal stem cells (MSC) are currently being examined as viable vehicles for targeting and delivering CRAds to disseminated tumor cells.
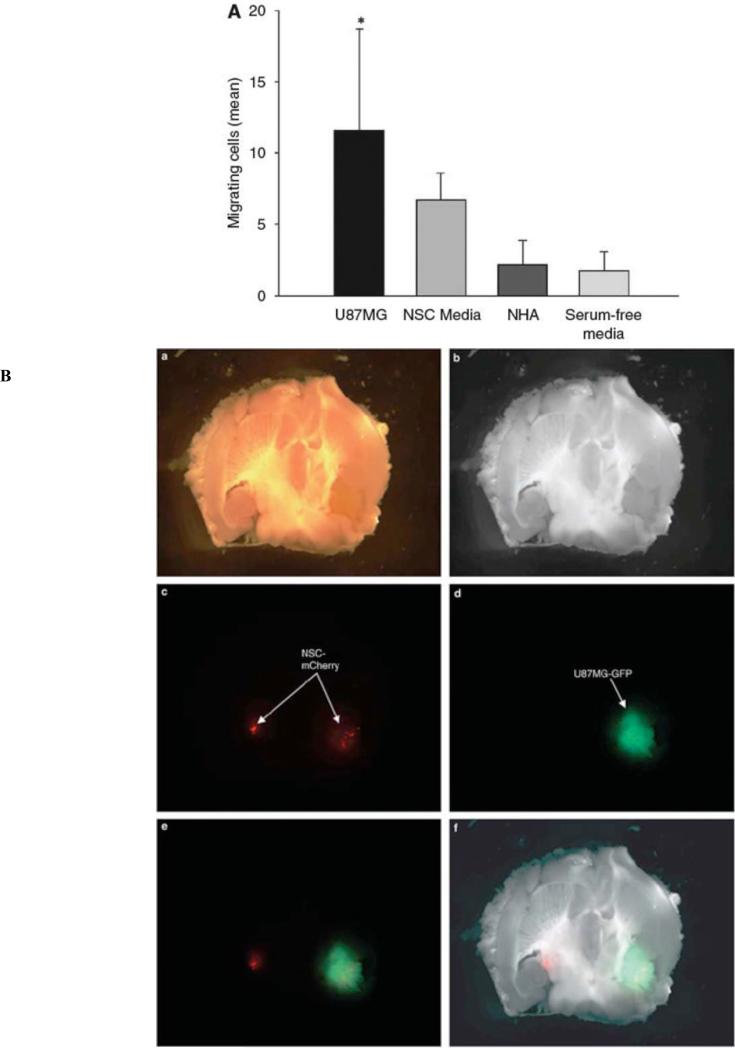
Aside from the ethical and technical difficulties associated with isolating NSC, their endogeneity to the CNS renders them a vastly explored vehicle for vector delivery in the brain. Aboody et al. [19] revealed that NSC have the ability to invade tumor foci and track single insidious tumor cells infiltrating into normal tissue away from the primary tumor mass. Experiments evaluating the delivery potential of NSC revealed that these cells possess an inherent tropism and unique capacity to target invading glioma stem cells in vitro as well as in vivo (Figs. 1A and B) [20-22].
Fig. (1).
NSCs migrate in response to glioma. (A) An in vitro migration assay was conducted using a migration chamber. As shown, NSCs migrate preferentially in response to media conditioned from U87MG cells and not normal human astrocytes (B) In vivo assessment of NSC migratory potential toward malignant glioma. A total of 5×105 U87MG-GFP cells were injected into the right hemisphere of male nude mice. Two weeks later, 5×104 NSCmCherry cells were injected directly contralateral to the site of U87MG-GFP injection. The data presented show a mouse brain that was extracted 12 days after NSC-mCherry implantation. (a) Bright phase image of mouse brain; (b) grayscale rendering; (c) live NSC-mCherry cells visualized using the Cy3 channel (red band-pass filter); (d) U87MGGFP cells are visualized using a GFP channel (green band-pass filter); (e) overlay of Cy3 and GFP channels and (f) overlay of grayscale, GFP and Cy3 captured images. Reprinted with permission. Reference [22].
In terms of delivering an oncolytic adenovirus, Tyler et al. [22] demonstrated that loading NSC cells with CRAds does not significantly compromise their homing abilities.NSC permissivity for Ad-vectors is due to the fact that NSC express many Ad- target surface receptors including: avb3 and avb5 integrins, adhesion proteins targeted by vectors possessing RGD motifs, CAR, CD46, and syndecan and perlecan, two heparan sulfate proteoglycans that bind to vectors containing a poly-L-lysine (pk7) modification. A luciferase assay analyzing the transduction of NSC with various recombinant Ad vectors revealed that the pk-7 modified vector, AdWT-pk7, showed the greatest transduction capacity followed by the AdWT.
In addition to providing a carrier function, it is also imperative that NSC allow for adequate CRAd genome amplification to achieve optimal infectivity and sustained tumor toxicity upon reaching distant glioma cells. Tyler et al. [22] proposed that tumor specific promoters (TSP), which control transcription and translation of the viral E1A replication gene, are vital to this process. Qualitative RT-PCR revealed that two tumor specific promoters, survivin and chemokine receptor CXCR4, allow for robust transcriptional activity in most glioma cell lines, while exhibiting relatively modest activity in normal cell lines. As such, the authors compared CRAd-S-pk7 and CRAd-CXCR4-5/3, two oncolytic vectors possessing these promoters, by evaluating their activity in NSC mediated delivery to gliomas. The results indicate relatively attenuated replicative cytotoxicity in NSC, but sufficient replicative cytotoxicity in U87MG tumor cells. In particular, CRAd-S-pk7 displayed limited toxicity to the NSC carrier, superb levels of NSC transduction, potent cytotoxicity to glioma cells, and could be delivered to U87MG cells in vitro.
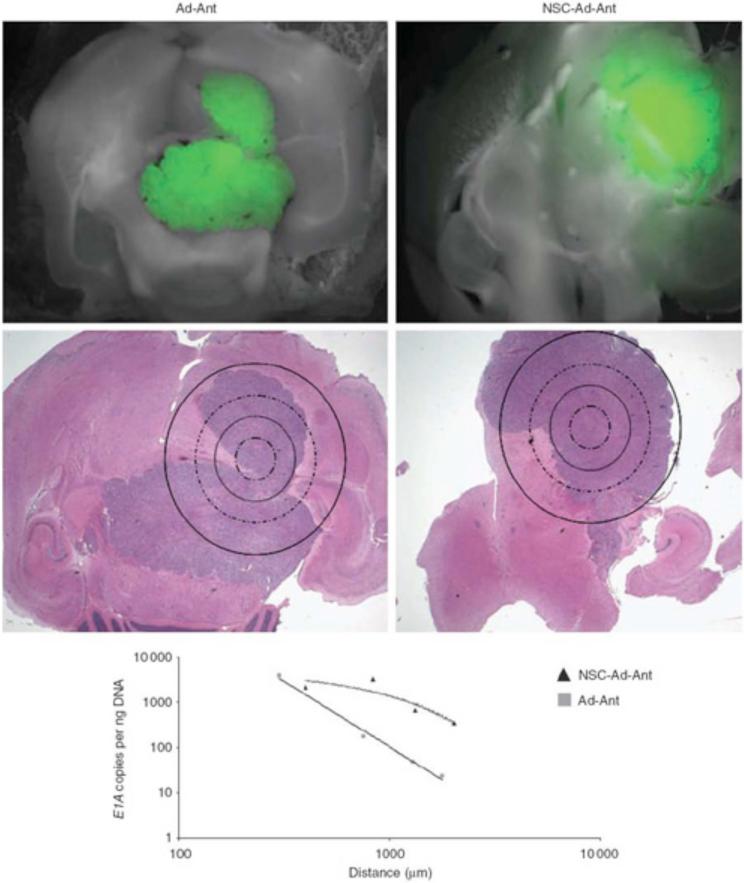
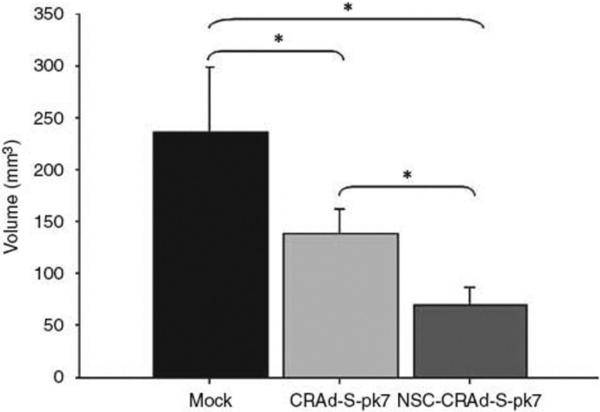
When comparing the effectiveness of delivering NSC loaded with CRAd-S-pk7 versus CRAd-S-pk7 alone, results show that vector distribution to distant tumor cells is drastically enhanced when mediated by a stem cell carrier (Fig. 2). qPCR of laser-captured brain tissue sections from mice receiving injections of NCS-loaded-CRAd-S-pk7 showed greater E1A gene distribution than those of mice injected with CRAd-S-pk7 alone [22]. In addition, an in vivo efficacy study investigating the ability of NCS-loaded-CRAd-S-pk7 to reduce U87 tumor growth in athymic mice revealed an overall reduction in tumor volume when compared to mice receiving intratumoral injections of CRAd-S-pk7 alone (Fig. 3). It should be noted that despite these appealing results utilizing NSC in glioma therapy, the clinical application of NSC may be hampered by the ethical and logistical problems surrounding their isolation and immunologic incompatibility requiring allogenic transplantation with harvesting. Finally, although our preliminary studies suggest that NSCs infected with an oncolytic vector can survive for up to one month, this issue requires further studies to determine clinical applicability.
Fig. (2).
Effectiveness of delivering NSC loaded with CRAd-S-pk7 versus CRAd-S-pk7 alone. NSCs delivering CRAd-S-pk7 enhance vector distribution in an intracranial glioma model. As shown, tissue was collected separately at increasing distances from the identified injection site by drawing concentric circles of increasing diameter around the injection site. Viral E1A copy numbers were quantified (y-axis) as a function of the distance from the injection site (x-axis). Data shown are representative of n¼4 mice from each group killed at day 12 after viral injection anterior to the tumor injection site. Reprinted with permission. Reference [22].
Fig. (3).
NSC-mediated delivery of a CRAd shows an enhanced antitumor effect. Male athymic (nude) mice received subcutaneous injections of tumor cells into the right hind leg (flank). Ten days after experimental treatment, tumor volumes were recorded for each treatment group. Data are presented as mean tumor volume for each experimental group. Reprinted with permission. Reference [22].
Mesenchymal stem cells appear to be a more likely candidate for clinical application for a number of reasons. First, MSCs can be derived easily from patients, expanded in culture, and genetically manipulated for therapeutic purposes. Additionally, if needed for the same patient, autologous transplantation ensures immunologic compatibility. Like NSC, MSC are bio-responsive and home towards tumor cells via secreted growth factors and cytokines [23]. In vitro studies quantifying the migration of MSC to gliomas confirm that exposure to conditioned media from normal human astrocytes (NHA) or fibroblasts induces no observable response, whereas exposure to conditioned media from U87 glioma cells elicits a migratory response [23].
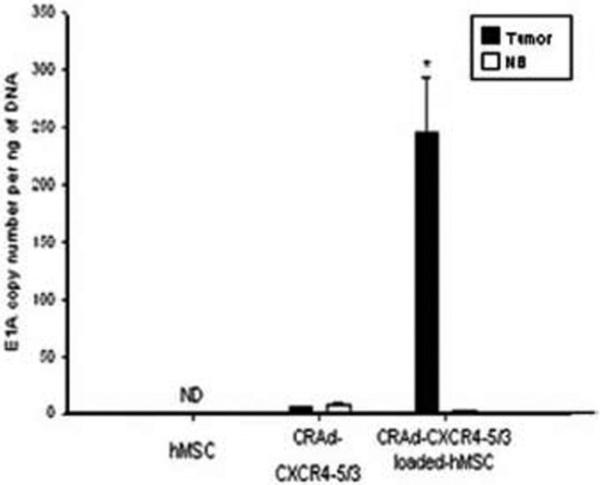
In terms of their viability as vessels in virotherapy, it has been shown in other cancer models that MSC can be loaded with CRAds, migrate towards tumors, and deliver CRAds to distant tumor cells [24]. In light of their success in the treatment of other cancers, Sonabend et al. [25] tested MSC for their application in a glioma model. When evaluating adenoviral transduction of MSC with several CRAds, it was found that Ad5/3 transduction is significantly greater than AdWT and AdWT-RGD, presumably because Ad5/3 is genetically modified for coxsackie and adenovirus receptor-independent infection. Given these results, Ad5/3 was favored for testing in vivo and further enhanced with the C-X-C chemokine receptor 4 (CXCR4) tumor specific promoter. The rational behind using CXCR4-modified vectors is based on the findings that MSC express CXCR4 and that this promoter is active in human gliomas, allowing robust transcriptional activity and replication to occur solely within these two cell types [25-27]. In vivo studies evaluating CRAd delivery in mice bearing intracranial U87MG xenografts revealed that injecting CRAd-CXCR4-5/3 loaded MCS near the tumor site (5mm away) delivered 46-fold more viral copies than injecting the virus alone without a carrier (Figs. 4 and 5).
Fig. (4).
Quantitative assessment of CRAd-CXCR4–5/3 delivery by hMSC into glioma cells was performed on tumor and brain parenchyma samples by laser microdissection, and subsequently, DNA was isolated and viral replication was analyzed by quantitative polymerase chain reaction (qPCR).Reprinted with permission. Reprinted with permission. Reference [25].
Fig. (5).
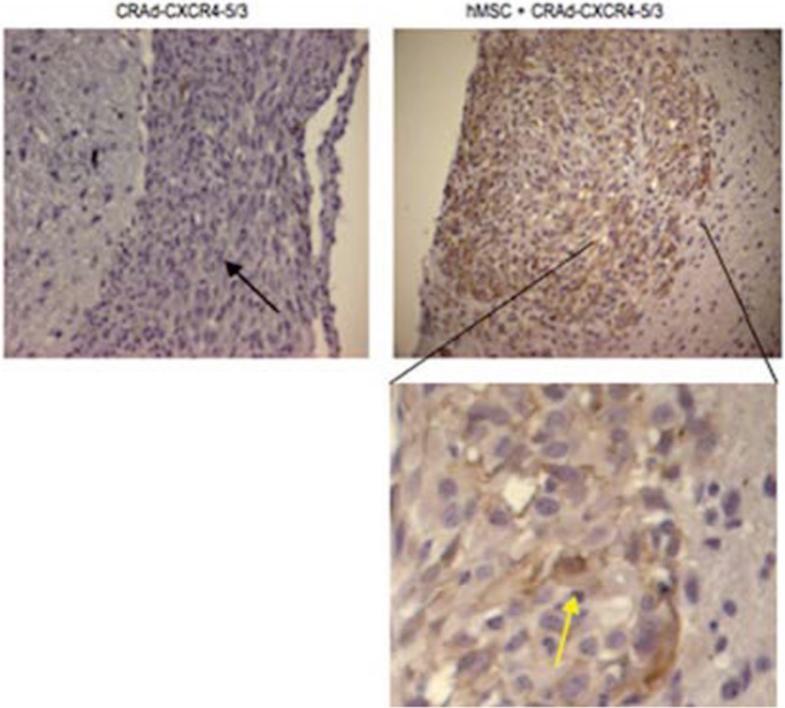
Delivery of CRAd-CXCR4–5/3 to glioma cells in murine brains with and without hMSC carrier. Immunohistochemical staining of the tumor slices was performed using an anti-human CD29 antibody, an integrin that is known to be expressed by hMSC. Tumors of mice (black arrow) treated with hMSC infected with CRAd-CXCR4–5/3 stained positive for CD29 (yellow arrow), whereas tumors of mice treated with CRAd-CXCR4–5/3 did not show any staining for this antigen. Reprinted with permission. Reference [25].
A complication encountered in this experiment involved elevated cytotoxicity within the stem cell carrier, as the presence of a transcriptional regulator such as CXCR4 does not limit the replicative cytotoxicity of CRAds in MSC [25]. While this study produced encouraging results, the delivery of CRAds by MSC still needs to be perfected. Specifically, MSC are susceptible to CRAd induced cytotoxicity, therefore if viral amplification is too affluent in the carrier vessel, MSC can be exposed to unwanted toxicity resulting in suboptimal delivery. Despite this, the established capacity of MSC to migrate through the brain parenchyma suggests that these cells can survive within the CNS for a prolonged period of time, a prerequisite for using MSC within a much larger brain [25].
Finally, an important concern of stem cell based gene therapy is the issue of safety. Potential adverse events could arise using these cells, though the current data suggests otherwise. For example, Dr. Aboody's work on immortalized NSCs (HB1.F3) has shown that these cells are non-immunogenic and non-tumorigenic [28]. Moreover, the appeal of using NSC in virotherapy relies on the ability of the virus to replicate within the cell and eventually destroy it in order for new progeny to be released. Ultimately, however, the safety of this treatment will have to be tested in a phase I clinical trial, whereby NSC loaded with an oncolytic agent will be delivered to the tumor bed, either in newly diagnosed tumors, recurrent tumors, or both.
CONCLUSIONS
The goal underlying effective and successful treatment of malignant gliomas with oncolytic adenoviruses is based on tumor selectivity by restricting viral replication and infectivity to targeted glial cells. As discussed, the current approaches used to modify adenoviruses for greater tumor specificity include: functional deletions in essential viral genes; tumor specific promoters used to control the expression of these viral genes; and tropism modifications to bestow a greater affinity for tumor cells. While several preclinical trials have yielded hopeful results, others have highlighted the limitations encountered thus far in this mode of therapy. With the overall safety and efficacy of utilizing CRAds being secure, improving their ability to target disseminated tumor cells is the next important step. Perhaps the combination of conventional forms of cancer treatment along with the delivery of oncolytic adenoviruses can successfully combat this resistant malignancy. In the future, continuous progress in elucidating mechanisms of tumor formation and viral replication must persist, and the search for an optimal stem cell carrier to deliver CRAds to scattered glioma cells must advance to improve the overall survival of patients.
FINANCIAL SUPPORT
This work was supported by the National Cancer Institute (R01-CA122930), the National Institute of Neurological Disorders and Stroke (K08-NS046430), The Alliance for Cancer Gene Therapy Young Investigator Award, and the American Cancer Society (RSG-07-276-01-MGO).
REFERENCES
- 1.Ehtesham M, Stevenson CB, Thompson RB. Stem cell therapies for malignant glioma. Neurosurg Focus. 2005;19:E5. doi: 10.3171/foc.2005.19.3.6. [DOI] [PubMed] [Google Scholar]
- 2.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3:499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;10:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Chu RL, Post DE, Khuri FR, Van Meir EG. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin Cancer Res. 2004;10:5299–312. doi: 10.1158/1078-0432.CCR-0349-03. [DOI] [PubMed] [Google Scholar]
- 5.Zheng S, Ulasov IV, Han Y, Tyler MA, Zhu ZB, Lesniak MS. Fiber knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–60. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- 6.Sonabend AM, Ulasov IV, Lesniak MS. Gene therapy trials for the treatment of high grade gliomas. Gene Ther Mol Biol. 2007;11(A):79–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Geoerger B, Grill J, Opolon P, et al. Oncolytic activity of the E1B-55kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62:764–72. [PubMed] [Google Scholar]
- 8.Ulasov IV, Tyler MA, Rivera AA, Nettlebeck DM, Douglas JT, Lesniak MS. Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy. J Med Virol. 2008;80:1595–603. doi: 10.1002/jmv.00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated Adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas in the adjuvant setting. Mol Ther. 2004;10:958–66. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Lemont JP, Nemunaitis J, Kuhn JA, Landers SA, McCarthy TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience.) Ann Surg Oncol. 2000;7:588–92. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- 11.Nemunaitis J, Khuri F, Ganly I, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–98. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 12.Vecil GG, Lang FF. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J Neuro Oncol. 2003;65:237–46. doi: 10.1023/b:neon.0000003653.45635.32. [DOI] [PubMed] [Google Scholar]
- 13.Fueyo J, Jiang H, Gomez-Monzano C, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99:1410–4. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 14.Altaner C. Glioblastoma and Stem Cells. Neoplasma. 2008;5:369–74. [PubMed] [Google Scholar]
- 15.Ulasov IV, Zhu ZB, Tyler MA, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 16.Van Houdt WJ, Haviv YS, Lu B, et al. The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg. 2006;104:583–92. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]
- 17.Das A, Tan WL, Teo J, Smith RD. Expression of survivin in primary glioblastomas. J Cancer Res Clin Oncol. 2002;128:302–6. doi: 10.1007/s00432-002-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–52. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 20.Zhenggang Z, Quan J, Feng J, et al. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004;23:281–7. doi: 10.1016/j.neuroimage.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Khalid S, Emilie B, Kim DE, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 22.Tyler MA, Ulasov IV, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16:262–78. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived medenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 24.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–66. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 25.Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–41. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 26.Ehtesham M, Winston JA, Kabos P, Thompson RC. CXCR4 expression mediates glioma cell invasiveness. Oncogene. 2006;25:2801–6. doi: 10.1038/sj.onc.1209302. [DOI] [PubMed] [Google Scholar]
- 27.Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–45. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 28.Kim SU, Nakagawa E, Hatori K, Nagai A, Lee MA, Bang JH. Production of immortalized human neural crest stem cells. Methods Mol Biol. 2002;198:55–65. doi: 10.1385/1-59259-186-8:055. [DOI] [PubMed] [Google Scholar]