SUMMARY
Mitochondrial dysfunction has long been associated with neurodegenerative disease. Therefore, mitochondrial protective agents represent a unique direction for the development of drug candidates that can modify the pathogenesis of neurodegeneration. This review discusses evidence showing that mitochondrial dysfunction has a central role in the pathogenesis of Alzheimer’s, Parkinson’s and Huntington’s diseases and amyotrophic lateral sclerosis. We also debate the potential therapeutic efficacy of metabolic antioxidants, mitochondria-directed antioxidants and Szeto-Schiller (SS) peptides. Since these compounds preferentially target mitochondria, a major source of oxidative damage, they are promising therapeutic candidates for neurodegenerative diseases. Furthermore, we will briefly discuss the novel action of the antihistamine drug Dimebon on mitochondria.
Keywords: metabolic antioxidants, mitochondria, mitochondria-directed antioxidants, neurodegeneration, SS peptides
INTRODUCTION
Although the brain represents only 2% of the body weight, it receives 15% of cardiac output and accounts for 20% of total body oxygen consumption. This energy requirement is largely driven by neuronal demand for energy to maintain ion gradients across the plasma membrane that is critical for the generation of action potentials. This intense energy requirement is continuous; even brief periods of oxygen or glucose deprivation result in neuronal death [1].
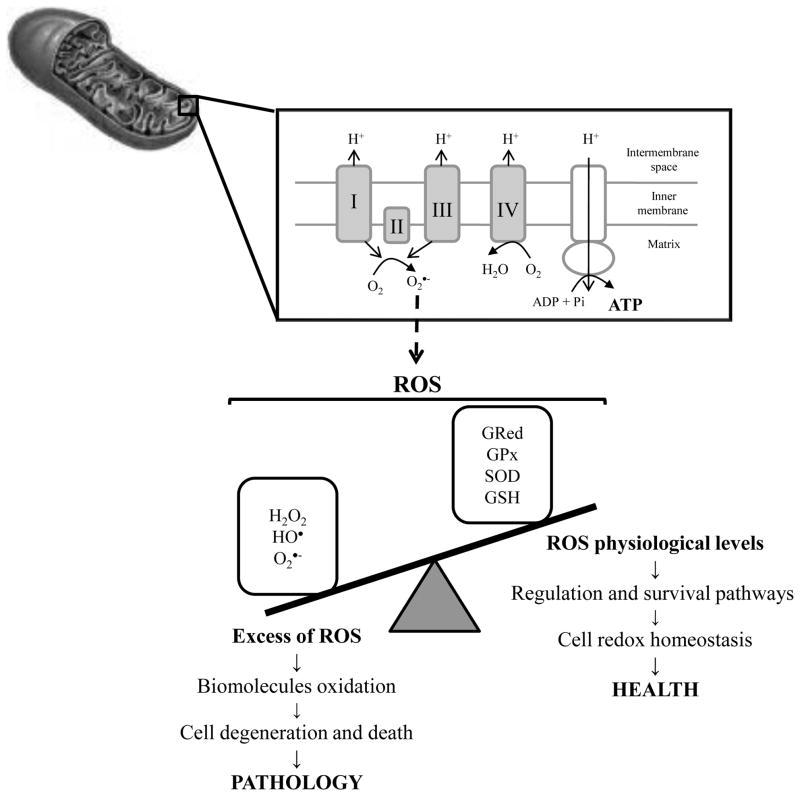
Mitochondria perform pivotal biochemical functions necessary for homeostasis and are arbiters of cell death and survival, in addition to being a source of ATP. They represent a convergence point for death signals triggered by both extracellular and intracellular cues. As such, the mitochondria sit at a strategic position in the hierarchy of cellular organelles to either promote the healthy life of the cell or to terminate it [2–4] (Fig. 1). Mitochondria are essential for neuronal function because the limited glycolytic capacity of neurons makes them highly dependent on aerobic oxidative phosphorylation (OXPHOS) for their energetic needs [1, 5, 6] (Fig. 1). However, OXPHOS is a major source of toxic endogenous free radicals, including hydrogen peroxide (H2O2), hydroxyl (•OH) and superoxide (O2− •) radicals that are products of normal cellular respiration. When the electron transport chain (ETC) is inhibited, electrons accumulate in complex I and coenzyme Q, where they can be donated directly to molecular oxygen to yield O2− • that can be further detoxified by the mitochondrial manganese superoxide dismutase (MnSOD) producing H2O2 that, in turn, can be converted to H2O by glutathione peroxidase (GPx). However, O2−• in the presence of nitric oxide (NO•), formed during the conversion of arginine to citrulline by nitric oxide synthase (NOS), can lead to the formation of peroxynitrite (ONOO−). Furthermore, H2O2 in the presence of reduced transition metals can be converted to the toxic product •OH via Fenton and/or Haber Weiss reactions.
Figure 1. The two faces of mitochondria.
Besides the fundamental role of mitochondria in the generation of energy (ATP), these organelles are also the main producers of reactive oxygen species (ROS). If ROS levels overwhelm the defense mechanisms of the cells, oxidative damage of proteins, lipids and DNA occurs. Besides other constituents of the cells, mitochondria are highly affected by oxidative damage leading to the impairment of ATP production. Other consequences of mitochondria impairment is the opening of the permeability transition pore and release of pro-apoptotic factors that ultimately contributes to cell degeneration and death. Consequently, it is unsurprising that mitochondrial oxidative damage is intimately involved in neurodegenerative diseases. Although ROS are traditionally viewed as toxic agents contributing to cellular pathology, emerging evidence suggests that low/moderate levels of ROS are involved in survival and regulation pathways being critical in cellular homeostasis. H2O2, hydrogen peroxide; hydroxyl radical, HO•, GPx, glutathione peroxidase; GRed, glutathione reductase; GSH, reduced glutathione; O2•−, superoxide anion radical; SOD, superoxide dismutase
It is well recognized that reactive oxygen species (ROS) and reactive nitrogen species (RNS) play a dual role since they can be either harmful or beneficial to living systems [7]. Beneficial effects of reactive species occur at low/moderate concentrations and involve physiological roles in cellular responses to noxia, as for example in defense against infectious agents and in the function of a number of cellular signaling systems. One further beneficial example of ROS at low/moderate concentrations is the induction of a mitogenic response [7]. However, oxidative stress occurs if the amount of free radical species produced overwhelms the cells’ capacity (enzymatic and non-enzymatic antioxidant defenses) to neutralize them, which is followed by mitochondrial dysfunction and neuronal damage. Reactive species generated by mitochondria have several cellular targets including mitochondrial components themselves (lipids, proteins and DNA). The lack of histones in mitochondrial DNA (mtDNA) and diminished capacity for DNA repair render mitochondria an especially vulnerable target of oxidative stress events [8] (Fig. 1).
Many lines of evidence suggest that mitochondria have a central role in age-related neurodegenerative diseases. The recent realization that mitochondria are at the intersection of the life and death of a cell, particularly through the involvement of mitochondrial damage in a range of diseases has made mitochondria a promising target for drug discovery and therapeutic interventions. In this review, we will discuss the close association between mitochondrial dysfunction and neurodegeneration. Furthermore, we will evaluate several mitochondria-directed therapies namely metabolic antioxidants, mitochondria-targeted antioxidants and Szeto-Schiller (SS) peptides and Dimebon.
MITOCHONDRIAL DYSFUNCTION AND NEURODEGENERATION
A major obstacle to the development of new therapies is our inadequate knowledge of the etiology of the neurodegenerative diseases. Although genetic mutations account for a small number of these disorders, the majority of cases appear to be sporadic in nature. Many lines of evidence suggest that mitochondria have a central role in aging-related neurodegenerative diseases. Indeed, mitochondrial dysfunction has been described in several neurodegenerative disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS).
AD is the most common cause of dementia among older people. This degenerative brain disease typically begins with a subtle decline in memory and progresses to global deterioration in cognitive and adaptive functioning. The neuropathological features associated with the disease include the presence of extracellular senile plaques, intracellular neurofibrillary tangles (NFT), and the loss of basal forebrain cholinergic neurons that innervate the hippocampus and the cortex. NFT are formed from paired helical filaments composed of neurofilaments and hyperphosphorylated tau protein. Senile plaques are formed mostly from the deposition of the amyloid-β (Aβ) peptide, a 39–43 amino acid peptide generated through the proteolytic cleavage of the amyloid-β precursor protein (AβPP) by the β- and γ-secretases. Accumulating evidence indicates that mitochondrial abnormalities and oxidative damage are early events in AD [9, 10]. Indeed, Manczak and colleagues [11] found an increase in H2O2 and a decrease in cytochrome oxidase activity in young Tg2576 mice, prior to the appearance of Aβ plaques suggesting that oxidative stress is an early event in AD pathophysiology.
Evidence for mitochondrial dysfunction in AD pathogenesis comes from impaired activity of three tricarboxylic acid cycle (TCA) complexes, pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase, observed in postmortem AD brain and fibroblasts [12, 13]. Similarly, reduced respiratory chain activities in complexes I, III and IV have been found in platelets and lymphocytes from AD patients and postmortem brain tissue [14–17]. Several in vitro studies corroborate the idea that mitochondria are key players in AD. First, Aβ requires functional mitochondria to induce toxicity [18]. Previous studies showed that Aβ1–40 induced a significant increase in H2O2 production in brain mitochondria isolated from diabetic rats [19, 20]. It was also reported that the continuous intracerebroventricular infusion of both Aβ25–35 and Aβ1–40 for up to 14 days stimulated H2O2 generation in isolated neocortex mitochondria [21].
In addition, an active γ-secretase complex in rat brain mitochondria was identified [22]. Being composed of nicastrin (NCT), anterior pharynx-defective-1 (APH-1), and presenilin enhancer protein 2 (PEN2), this γ-secretase complex cleaves, among other substrates, AβPP generating Aβ and AβPP-intracellular domain. Furthermore, AβPP was detected in mitochondrial membranes of PC12 cells bearing the Swedish double mutation in AβPP gene [23]. Both Aβ and A PP have strong copper (Cu)-reductase activity, generating Cu+ from Cu2+. This reaction produces H2O2 as a by-product [24]. In addition to Cu2+, A also binds zinc (Zn2+) and iron (Fe3+) potentiating oxidative stress [24]. Aβ also potentiates the opening of the mitochondrial permeability transition pore (PTP) induced by Ca2+ [25, 26]. It was reported that the interaction of cyclophilin D, an integral part of the PTP, with mitochondrial Aβ potentiates mitochondrial, neuronal, and synaptic stress [27]. It was also observed that cyclophilin D deficiency substantially improves learning and memory and synaptic function in an AD mouse model and alleviates Aβ-mediated reduction of long-term potentiation [27]. Recently we showed that sporadic AD fibroblasts present alterations in mitochondria morphology and distribution [28]. We demonstrated that these mitochondrial abnormalities are due to a decrease in dynamin-like protein 1 (DLP1), a regulator of mitochondrial fission and distribution [28]. Further, we reported that overproduction of Aβ causes abnormal mitochondrial dynamics including morphology, distribution, and function via differential modulation of mitochondrial fission/fusion proteins [29]. Similarly, Cho and collaborators [30] found that NO• produced in response to Aβ triggers mitochondrial fission, synaptic loss, and neuronal damage, in part via S-nitrosylation of DLP1. Studies have also shown increased autophagic degradation of mitochondria in AD brain [31, 32].
PD is the second most common neurodegenerative disease and affects individuals over the age of 65 years. This disease is characterized by degeneration and death of dopaminergic neurons in the pars compacta of the substantia nigra and by the presence of Lewy bodies. Dopaminergic neurons in the substantia nigra operate in a pathway that controls voluntary movement. Death of dopamine-containing neurons results in the inability to coordinate movement. It is well established that oxidative stress and mitochondrial dysfunction are associated with the degeneration of dopaminergic neurons in PD. Mitochondria was demonstrated to be one of the direct targets of α-synuclein during the pathogenesis of PD [33]. α-synuclein accumulates in the mitochondria of the striatum and substantia nigra of PD patients impairing mitochondrial complex I activity and causing oxidative stress. Moreover, the neurotoxin 1-methyl-4-phenylpyridium (MPP+), which is commonly used to induce parkinsonism in animal models, inhibits mitochondrial complex I resulting in the generation of free radicals [34], whereas the overexpression of Akt prevents ROS increase and apoptosis [35]. It has been previously shown that brain mitochondria isolated from rotenone-treated rats, a model of PD, presented an impaired respiratory chain associated with an increased O2•− production and probability of PTP opening [36]. It was also shown that organotypic striatal slice cultures exposed to 1 mM rotenone presented a significant increase in lactate dehydrogenase activity and O2•− and NO• levels [37]. Furthermore, Dahm and colleagues [38] reported that the S-nitrosation of mitochondrial complex I, a common situation in PD, was correlated with a significant loss of activity and an increased O2•− production from complex I. These findings point to a significant role for complex I S-nitrosation and consequent dysfunction during nitrosative/oxidative stress in disorders such as PD.
Domingues et al. [39] showed that mitochondrial-DNA-depleted cells (rho0) compromised at the mitochondrial and ubiquitin-proteasomal system (UPS) levels and presented were an alteration of the oxidative status. In parental cells (rho+), MPP+ induced a clear inhibition of complex I activity and an increase in the levels of ubiquitinylated proteins. Moreover, MPP+ induced a decrease in 20S chymotrypsin-like and peptidyl-glutamyl peptide hydrolytic-like proteolytic activities and an increase in ROS [39]. These results suggest that mitochondrial alterations lead to an imbalance in cellular oxidative status inducing proteasomal deregulation, which may exacerbate protein aggregation and, consequently, engender degenerative events. It was also shown that, under basal conditions, PD cybrid lines (cells in which endogenous mtDNA from PD was expressed within human teratocarcinoma cells) produce less ATP, more LDH release, depolarized mitochondria, less mitochondrial cytochrome c and higher caspase 3 activity [40]. Mitochondrial dysfunction can also induce α-synuclein oligomerization via ATP depletion-driven microtubule depolymerization and via ROS increase-driven protein oxidation [41]. Petit-Paitel and colleagues [42] demonstrated that both cytosolic and mitochondrial glycogen synthase kinase-3β (GSK-3β) were involved in mitochondrial dysfunction and neuronal cell death in MPP+-treated neurons. Further, it has been shown that defects in PTEN induced kinase 1 (PINK1), a mitochondrial Ser/Thr kinase, causes mitochondrial dysfunction, proteasomal deficit and α-synuclein aggregation in cell culture models of PD [43]. Recently, Moisoi and collaborators [44] demonstrated that the loss of HtrA2 resulted in transcriptional upregulation of nuclear genes characteristic of the integrated stress response, including the transcription factor CHOP, selectively in the brain. The same authors also showed that the loss of HtrA2 results in the accumulation of unfolded proteins in the mitochondria, defective mitochondrial respiration and enhanced production of ROS that contributed to the induction of CHOP expression and to neuronal cell death [44].
HD is an autosomal-dominant disease caused by an abnormal expanded polyglutamine repeat in the huntingtin (htt) protein, which leads to the degeneration of the neurons in the striatum and cortex. A number of studies indicate that mitochondrial dysfunction is central to the pathogenesis of HD. Reduction of the activities of complexes II–III and IV has been observed in the caudate and putamen of HD patients [45]. A recent study showed increased glucose utilization relative to oxygen utilization in the striatum of early HD patients [46]. Chen and collaborators [47] also observed mitochondrial abnormalities and oxidative damage in the peripheral blood of HD patients. Moreover, Lim and co-workers [48] reported that mutant htt expression induced PTP opening and disruption of mitochondrial Ca2+ homeostasis. Similar results were obtained in mitochondria isolated from cells expressing mutant htt [49], suggesting that mitochondrial dysfunction plays a central role in HD pathogenesis. Mitochondrial NAD+-linked state 3 respiration and complex I activity were found to be compromised in the cerebral cortex of the 3-nitropropionic acid (3-NP)-induced rat model of HD [50]. Kumar and Kumar [51] reported that 3-NP administration for 14 days significantly induced HD like symptoms in rats as indicated by change in body weight, locomotor activity, rotarod activity performance, oxidative damage (elevated levels of lipid peroxidation, nitrite concentration and depletion of antioxidant enzyme levels) and impairment of mitochondrial complexes-I, II, II and IV in striatum, cortex and hippocampus. It was also reported that PC12 cells treated with 3-NP present high levels of H2O2 and a decline in ATP levels these effects being prevented by Bcl2 overexpression [52]. However, Kim and Chan [53] report that O2•− is involved in excitotoxicity and DNA fragmentation in striatal vulnerability in mice after treatment with the mitochondrial toxin 3-NP. Recently, it was suggested that mtDNA damage is an early biomarker for HD-associated neurodegeneration supporting the hypothesis that mtDNA lesions might contribute to the pathogenesis observed in HD [54]. Orr and colleagues [55] reported that specific N-terminal mutant htt fragments, before they form aggregates, would impair mitochondrial function directly.
ALS is the most common adult-onset motor neuron disease resulting in weakness, paralysis and subsequent death. Some 90% of cases are sporadic, i.e., unknown cause, and 10% of cases are familial. In 20% of the familial cases of ALS, motor neuron loss is related to mutations of the Cu/Zn superoxide dismutase (SOD1) gene. Mutant SOD1 has been shown to exert deleterious effects through a gain of function rather than a loss of activity [56]. Additionally, the presence of mutant SOD1 within motor neuron causes alterations of the mitochondrial respiratory chain [57], specifically in mitochondrial complexes II and IV [56]. Indeed, Zimmerman and colleagues [58]) reported that mitochondrial-produced O2•− play a critical role in mutant SOD1-mediated neuronal toxicity. A recent study also shows that SOD1 increases the vulnerability of mitochondria and perturbs Ca2+ homeostasis in SOD1G93A mice, a mouse model of ALS [59]. Further, it was reported that mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration, this effect being prevented by mitochondrial-targeted antioxidants [60]. Kawamata and collaborators [61] reported that the mitochondrial lysyl-tRNA synthetase (mitoKARS) interacted with mutant SOD1 contributing to mitochondrial dysfunction in ALS. There is evidence of abnormal structure and number of mitochondria and compromised mitochondrial function in ALS motor neurons and skeletal muscle. This is corroborated by findings of altered respiratory chain enzyme activities and CNS energy hypometabolism in the spinal cord and motor cortex of ALS patients [62–64].
The evidence presented above clearly indicates that mitochondrial abnormalities are intimately involved in the pathophysiology of neurodegenerative diseases.
MITOCHONDRIAL-DIRECTED THERAPIES
The discovery that mitochondrial dysfunction underlies the pathogenesis of many neurodegenerative diseases has opened a window for new therapeutic strategies aimed at preserving/ameliorating mitochondrial function. In nearly all cases where mitochondrial dysfunction contributes to disease, a major cause of damage is overproduction of ROS by mitochondria, either directly or as a secondary consequence of other malfunctions [1, 3].
Metabolic antioxidants
Metabolic antioxidants are involved in cellular energy production and act as cofactors of several metabolic enzymes (Fig. 2). The creatine/phosphocreatine system, regulated by mitochondrial creatine kinase, plays an important role in maintaining energy balance in the brain. The presence of this energy buffer system keeps the ATP/ADP ratio high at subcellular sites where ATP is needed. This also minimizes the loss of adenosine nucleotides, which causes cellular dysfunction. Recently, it was shown that the progression of mild cognitive impairment (MCI) to dementia is associated with a decline of N-acetyl aspartate and creatine [65]. Furthermore, creatine supplementation was found to be neuroprotective in several in vitro and in vivo studies. Chronic administration of creatine was shown to significantly increase survival, tyrosine hydroxylase immunoreactive fiber density, and soma size of dopaminergic neurons in mesencephalic cultures by protecting against neurotoxic insults induced by serum and glucose deprivation, MPP+ and 6-hydroxydopamine [66, 67]. It has been shown that creatine protects against dopamine loss and attenuates neuron loss in the substantia nigra of mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [68]. The same group also showed that oral creatine administration reduces the size of ischemic brain infarctions in mice [69] and attenuates striatal excitotoxic lesions produced by N-methyl-D-aspartate (NMDA) [70]. In addition, creatine was shown to affect the morphogenesis of dendritic spines and synapses [71]. Oral administration of creatine produces a dose-dependent improvement in motor performance and extends survival in G93A transgenic ALS mice, where it protects against loss of both motor and substantia nigra neurons [72]. Creatine administration protects against glutamate and Aβ toxicity in rat hippocampal neurons [73]. Creatine is also beneficial in animal models of traumatic brain injury and cerebral ischemia [74, 75]. The success of creatine in experimental studies led to clinical trials in neurodegenerative diseases, including PD, HD, and ALS. In a small pilot trial, creatine improved patient mood, but had no effect on the overall Unified Parkinson’s Disease Rating Scale (UPDRS) or dopamine transporter single photon emission computed tomography (SPECT) [76]. A recent study shows that long-term creatine supplementation is safe in aged PD patients [77]. It has also been shown that creatine treatment of HD patients is safe, tolerable, biovailable in the brain and reduces serum 8-hydroxy-2′-deoxyguanosine levels, an indicator of oxidative injury to DNA [78]. However, Verbessem and collaborators [79] reported that one year of creatine treatment, at a dose that could improve functional muscle capacity in healthy subjects and patients with neuromuscular disease (5 g/day), did not improve functional, neuromuscular, and cognitive status in HD patients. Similarly, a previous clinical trial did not find a significant effect of creatine on ALS patients [80]. Despite the positive effects obtained in in vitro and animal studies, clinical trials do not support creatine supplementation for the treatment of neurodegenerative conditions.
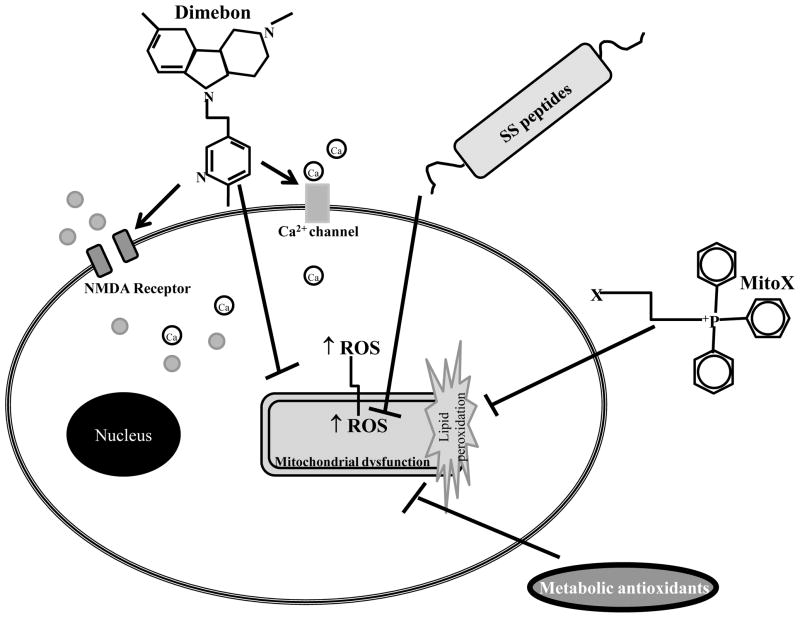
Figure 2. Mitochondrial-directed therapies.
Metabolic antioxidants (creatine, α-lipoic acid, N-acetyl-carnitine and coenzyme Q10) are involved in cellular energy production and act as cofactors of several metabolic enzymes. Additionally, they have also potent antioxidant actions avoiding damage of lipids, proteins and DNA. Mitochondria-targeted antioxidants and SS peptides selectively accumulated into mitochondria, a major source of reactive oxygen species (ROS) protecting against oxidative damage of mitochondrial and cellular components. Dimebon binds to NMDA receptors on the outside of the cell and decreases glutamate influx. It also binds Ca2+ channels and prevents an influx of Ca2+ from entering the cell. Recent evidence shows that Dimenbon stabilizes mitochondria by preventing the opening of the permeability transition pore.
LA is a coenzyme for mitochondrial pyruvate dehydrogenase and α-ketoglutarate dehydrogenase. Furthermore, it is a powerful antioxidant and can recycle other antioxidants, such as vitamins C and E and glutathione. Hager and collaborators [81, 82] reported that the administration of 600 mg LA/day to AD patients promoted stabilization of cognitive measures. We have recently shown that LA and/or N-acetyl cysteine (NAC; an antioxidant and glutathione precursor) decrease mitochondrial-related oxidative stress in fibroblasts isolated from AD patients [83]. Abdul and Butterfield [84] investigated whether pretreatment of cortical neurons with LA and acetyl-L-carnitine (ALCAR; a compound that acts as an intracellular carrier of acetyl groups across the inner mitochondrial membrane) protected cortical neuronal cells from 4-hydroxy-2-nonenal-mediated oxidative stress and neurotoxicity. This study showed crosstalk between phosphoinositol-3 kinase (PI3K), PKG, and ERK1/2 pathways in cortical neurons that contributed to ALCAR and LA-mediated pro-survival signaling mechanisms [84]. Suh and collaborators [85] reported that old rats injected with LA showed improvement in glutathione redox status of both cerebral and myocardial tissues when compared with control rats. They also showed that LA produced significantly increased survival in both R6/2 and N171-82Q transgenic mouse models of HD [86]. A recent study also showed that chronic dietary LA reduces deficits in hippocampal memory of aged Tg2576 mice [87]. Aliev and collaborators [88] reported that LA and ALCAR supplementation significantly reduced the number of severely damaged mitochondria and increased the number of intact mitochondria in the hippocampus of aged rats. A recent study also showed that ApoE4 mice fed ALCAR and LA have some improvement in cognitive performance [89]. Bianchetti and collaborators performed an open study to evaluate the effect of ALCAR (2g/day orally for 3 months) in association with donepezil or rivastigmine in 23 patients with mild AD who had not responded to treatment with a acetylcholinesterase inhibitor (AChEI) [90]. Clinical effects were evaluated by assessing cognitive functions, functional status and behavioral symptoms. The response rate, which was 38% after AChEI treatment, increased to 50% after the addition of ALCAR, indicating that the combination of these two drugs may be a useful therapeutic option in AD patients. However, this study does not identify the possible mechanism of action by which the two treatments synergize. Furthermore, the efficacy of ALCAR in MCI and mild AD was investigated with a meta-analysis of double-blind, placebo-controlled prospective, parallel group comparison studies of at least 3 months duration [91]. The authors reported beneficial effects on both the clinical scales and the psychometric tests. To establish whether ALCAR is clinically effective in the treatment of people with dementia, Hudson and Tabet analyzed and compared 11 double-blind randomized trials involving people with dementia, in which treatment with ALCAR was compared with a placebo group [92]. The analysis indicated a benefit of ALCAR on cognitive global impression, but there was no evidence of improvement using objective assessments in any other area of outcome. Furthermore, the authors emphasized that given the large number of comparisons the statistical significance may result from chance.
CoQ10 serves as an important cofactor of the ETC, where it accepts electrons from complexes I and II. CoQ10 acts as a potent antioxidant, blocks apoptosis by inhibiting the PTP and is a co-factor of mitochondrial uncoupling proteins [93]. In vitro studies showed that CoQ10 pre-treatment prevents a decrease in mitochondrial transmembrane potential and reduces mitochondrial ROS generation [94]. Furthermore, it was shown that CoQ10 treatment counteracts brain mitochondrial alterations induced by Aβ1–40 [19]. Previous studies also showed that CoQ10 protects against paraquat- and rotenone-induced mitochondrial dysfunction and neuronal death in human neuroblastoma cells (SHSY-5Y) and primary rat mesencephalic neurons, respectively [95, 96]. CoQ10 also protects against iron-induced apoptosis in dopaminergic neurons [97] and exerts anti-amyloidogenic effects by destabilizing preformed Aβ fibrils in vitro [98]. Further, CoQ10 protects SHSY-5Y cells against Aβ toxicity through inhibition of PTP opening [99]. Similarly, pretreatment of neuronal cells with CoQ10 maintains mitochondrial membrane potential during oxidative stress and reduces the amount of mitochondrial ROS generation [94]. Moreover, several studies have been conducted using CoQ10 in in vivo models. Recently, Yang and collaborators [100] tested the effect of CoQ10 on Aβ in aged transgenic mice overexpressing Alzheimer presenilin 1-L235P. The treatment, feeding the transgenic mice with CoQ10 for 60 days (1,200 mg/kg/day), partially attenuated Aβ overproduction and intracellular Aβ deposits in the cortex of the transgenic mice compared with the untreated age-matched transgenic mice. Meanwhile, an increased oxidative stress reaction was demonstrated by elevated levels of malondialdehyde (MDA) and decreased activity of SOD in the transgenic mice relative to the wild-type mice; supplementation with CoQ10 partially decreased the MDA level and upregulated the activity of SOD [100]. Sharma and collaborators [101] reported that the administration of CoQ10 increased complex I activity and partially improved motor performance in weaver mutant mice. The same authors reported that direct exposure to rotenone reduced CoQ10, complex I activity and mitochondrial membrane potential in SK-N-SH cells. Rotenone-induced down-regulation of complex I activity was attenuated by CoQ10 treatment, suggesting that complex I might be down-regulated due to depletion of CoQ10 in the brain [101]. Further, dietary administration of CoQ10 results in significant protection in a chronic MPTP model [102]. It was also shown that administration of CoQ10 exerted a therapeutic benefit in a dose dependent manner in R6/2 mice, a model of HD [103]. Stack and collaborators [104] evaluated the effects of combined minocycline and CoQ10 treatment in the R6/2 mouse. Combined minocycline and CoQ10 therapy provided an enhanced beneficial effect, ameliorating behavioral and neuropathological alterations; minocycline and CoQ10 treatment significantly extended survival and improved rotarod performance to a greater degree than either minocycline or CoQ10 alone. In addition, combined treatment attenuated gross brain atrophy, striatal neuron atrophy, and htt aggregation in the R6/2 mice [104], suggesting that this may offer therapeutic benefit to patients suffering from HD.
Altogether these results suggest that the delivery of antioxidants that protect mitochondria and reduce oxidative stress-related events represent a promising therapeutic approach for the treatment of neurodegenerative diseases. However, several clinical trials have been somewhat disappointing. A significant problem with the majority of clinical studies is that they have been conducted in patient populations who already have a neurodegenerative condition. This makes it difficult to assess the full potential of antioxidants to help prevent neurodegeneration. However, the broad occurrence of the disease, the limited regenerative nature of the CNS, and the fact that the diagnosis often does not occur until late in disease progression, suggest that the ideal antioxidant should be used as a prophylactic treatment in the population.
Mitochondria as a target for antioxidants and SS peptides
A major limitation to antioxidant therapy in treating age-related neurodegenerative diseases has been the inability to enhance the antioxidant levels within mitochondria. However, in the last several years, considerable progress has been made in developing mitochondria-targeted antioxidants (i.e., antioxidants that are selectively accumulated in mitochondria) (Fig. 2). Several have been developed by conjugating the lipophilic triphenylphosphonium (TPP+) cation to an antioxidant moiety, such as coenzyme Q (MitCoQ) and α-tocopherol (MitoVitE) [105]. This approach makes use of the potential gradient across the mitochondrial inner membrane. As a result of the proton gradient, a negative potential to 150 to 180mV is generated across the inner membrane. Lipophilic cations may therefore accumulate 100- to 1000-fold in mitochondria. In fact, MitoVitE is taken up by mitochondria ≈ 80-fold more than vitamin E [106]. The authors observed that MitoVitE was far more effective in protecting mitochondria against oxidative stress than vitamin E itself. Furthermore, it has been shown that MitoVitE is 800-fold more potent than idebenone, a CoQ10 analog, in protecting against GSH depletion in cultured fibroblasts from patients with Friedreich ataxia and is 350 fold more potent than trolox, a vitamin E analog [107]. Recently, it was shown that MitVitE mitigates ethanol-induced accumulation of intracellular oxidants and counteracts suppression of glutathione peroxidase/glutathione reductase functions, protein expression of γ-glutamylcysteine synthetase and total cellular glutathione levels in cerebellar granule cells [108].
MitoQ is a promising therapeutic antioxidant that has been successfully targeted to mitochondria. Coenzyme Q (or ubiquinone) is a respiratory chain component that accepts electrons from complexes I or II, to form the reduced product ubiquinol, which donates electrons to complex III. The ubiquinone pool in vivo exists largely in a reduced ubiquinol form, acting as an antioxidant and a mobile electron transfer. Ubiquinol has been reported to function as an antioxidant by donating a hydrogen atom from one of its hydroxyl groups to a lipid peroxyl radical, thereby decreasing lipid peroxidation within the mitochondrial inner membrane [109]. James and colleagues [110] reported that the favored orientation of MitoQ is with the TPP+ moiety near the membrane surface and the ubiquinone penetrating into the membrane core. This orientation enables the ubiquinone moiety to access the membrane core to act as a chain breaking antioxidant and allows recycling of MitoQ to its ubiquinol form via reduction by complex II. The ubiquinol derivative thus formed is an effective antioxidant that prevents lipid peroxidation and protects mitochondria from oxidative damage [111]. However, MitoQ cannot be oxidized by complex III explaining why it does not function as an electron carrier in mitochondrial respiration [112].
Recently, the effects of MitoQ on mitochondria in several in vitro cell models were tested [107, 113–115]. In cultured fibroblasts from Friedreich ataxia patients, MitoQ prevented cell death known to be caused by endogenous oxidative stress [107]. Low concentrations of MitoQ selectively inhibited serum deprivation-induced apoptosis in PC12 cells [114]. MitoQ reduces ROS formation and preserves mitochondrial function after glutathione depletion, even in cells lacking mtDNA [116]. These studies suggest that MitoQ may reduce free radicals, decrease oxidative damage, and maintain mitochondrial function. Since oxidative damage is intimately involved in the pathophysiology of AD, there is strong interest in determining whether mitochondria-targeted antioxidants can decrease oxidative damage in the neurons of AD patients [117]. In phase I trials, MitoQ showed good pharmacokinetic behavior with oral dosing at 80 mg (1 mg/kg), resulting in a plasma Cmax=33.15 ng/ml and Tmax=1 h. This formulation is now in phase II clinical trials for PD and Friedreich ataxia (Antipodean Pharmaceuticals Inc., San Francisco, CA).
There is a novel class of small cell permeable peptide antioxidants that target mitochondria in a potential-independent manner (Fig. 2). The structural motif of these Szeto Schiller (SS) peptides centers on alternating aromatic residues and basic amino acids [118]. SS-31 has a remarkable potency that can be explained by its extensive cellular uptake and selective partitioning into mitochondria. Intracellular concentrations of [3H]SS-31 were 6-fold higher than extracellular concentrations. Studies using isolated mitochondria revealed that [3H]SS-31 was concentrated 5000-fold in the mitochondrial fraction. By concentrating in the inner mitochondrial membrane, SS-31 became localized to the site of ROS production, and protected against mitochondrial oxidative damage and against further ROS production [119]. SS-31 protects neuronal cells against tert-butyl-hydroperoxide-induced mitochondrial depolarization and apoptotic cell death by reducing intracellular ROS, decreasing markers of apoptotic cell death and caspase activity [120]. It decreases mitochondrial ROS production and inhibits PTP and mitochondrial depolarization in isolated mitochondria [119]. Daily injections of SS-31 into G93A SOD1 mutants, an animal model of ALS, before onset of symptoms, lead to a significant increase in survival and improvement of motor performance [121]. Recently, Yang and collaborators [122] examined the ability of SS-31 and SS-20, to protect against MPTP neurotoxicity in mice. SS-31 produced complete dose-dependent protection against loss of dopamine and its metabolites in the striatum, as well as loss of tyrosine hydroxylase immunoreactive neurons in substantia nigra. SS-20, which does not possess the intrinsic ability to scavenge ROS, also demonstrated significant neuroprotective effects on dopaminergic neurons of MPTP-treated mice. Both SS-31 and SS-20 were very potent in preventing MPP+-induced cell death in cultured dopaminergic cells [122]. Studies with isolated mitochondria showed that both SS-31 and SS-20 prevented MPP+-induced inhibition of oxygen consumption and ATP production and mitochondrial swelling [122]. These findings provide strong evidence that these neuroprotective peptides, which target both mitochondrial dysfunction and oxidative damage, are promising for the treatment of neurodegenerative disorders.
Dimebon
The antihistamine drug Dimebon was first used to treat allergies in Russia in the early 1980s. Recently, Dimebon has been proposed to be useful for treating neurodegenerative disorders [123] (Fig. 2). Results of the first pivotal clinical trial of Dimebon in AD showed that this drug improved the clinical course of the disease [124]. In this randomized, double-blind, placebo-controlled trial of 183 patients with mild-to-moderate AD, patients treated with Dimebon experienced statistically significant improvements compared to placebo in all of the key aspects of the disease: memory and thinking, activities of daily living, behavior and overall function. After both 6 months and a full year of treatment, Dimebon-treated patients performed significantly better than placebo-treated patients in all key measures of the disease [124]. A phase III trial of Dimebon in AD treatment will soon be initiated. Dimebon also demonstrated efficacy in a phase II trial with HD patients conducted by Medivation and the Huntington Study Group (DIMOND). Despite extremely encouraging results in clinical trials, the mechanisms responsible for the beneficial actions of Dimebon remain poorly understood. Previous reports show that Dimebon is an inhibitor of NMDA receptors [125–127] and voltage-gated Ca2+ channels [127, 128]. A previous study also showed that Dimebon blocks opening of the mitochondrial PTP induced by Aβ25–35 and MPP+ [129]. Together these studies suggest that the clinical benefits exerted by Dimebon may be due to its ability to stabilize neuronal Ca2+ homeostasis and mitochondrial function. Currently, the scientific community is actively investigating the mode of action of Dimebon in neurodegenerative diseases.
Conclusions
Many lines of evidence show that mitochondrial abnormalities and oxidative damage play a central role in the pathogenesis of several neurodegenerative diseases. Therefore, the development of approaches to prevent or decrease mitochondrial dysfunction may provide therapeutic efficacy. The metabolic antioxidants and mitochondria-directed antioxidants and SS peptides have proved to be effective in pre-clinical and small clinical studies substantiating their promise. Furthermore, combinations of antioxidants may be more effective than the use of single agents. However, larger clinical trials with larger numbers of participants are needed to provide more definitive information on the therapeutic efficacy of these compounds. The novel therapeutic candidate Dimebon is effective in ameliorating the cognitive decline in AD and HD patients. However, its mechanism of action remains unclear highlighting the need for more research with this agent, particularly in animal models. In the future, mitochondrial-directed therapies will open new avenues for the manipulation of mitochondrial function providing protection from neurodegenerative diseases.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health (AG031852 to XWZ and AG026151 to MAS) and the Alzheimer’s Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moreira PI, Santos MS, Oliveira CR. Alzheimer’s disease: a lesson from mitochondrial dysfunction. Antioxid Redox Signal. 2007;9:1621–1630. doi: 10.1089/ars.2007.1703. [DOI] [PubMed] [Google Scholar]
- 2.Budd SL, Nicholls DG. Mitochondria in the life and death of neurons. Essays Biochem. 1998;33:43–52. doi: 10.1042/bse0330043. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–976. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- 4.Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma. 2000;17:843–855. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan PG, Keller JN, Mattson MP, Scheff SW. Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J Neurotrauma. 1998;15:789–798. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- 6.Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer’s disease. J Alzheimers Dis. 2009;16:741–761. doi: 10.3233/JAD-2009-0972. [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 10.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 12.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 13.Huang HM, Ou HC, Xu H, Chen HL, Fowler C, Gibson GE. Inhibition of alpha-ketoglutarate dehydrogenase complex promotes cytochrome c release from mitochondria, caspase-3 activation, and necrotic cell death. J Neurosci Res. 2003;74:309–317. doi: 10.1002/jnr.10756. [DOI] [PubMed] [Google Scholar]
- 14.Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN. Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- 15.Parker WD, Jr, Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, Cullum CM. Reduced platelet cytochrome c oxidase activity in Alzheimer’s disease. Neurology. 1994;44:1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- 16.Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol Aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 17.Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, Reiman EM. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6:323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J. 2001;15:1439–1441. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- 19.Moreira PI, Santos MS, Sena C, Nunes E, Seica R, Oliveira CR. CoQ10 therapy attenuates amyloid beta-peptide toxicity in brain mitochondria isolated from aged diabetic rats. Exp Neurol. 2005;196:112–119. doi: 10.1016/j.expneurol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Moreira PI, Santos MS, Sena C, Seica R, Oliveira CR. Insulin protects against amyloid beta-peptide toxicity in brain mitochondria of diabetic rats. Neurobiol Dis. 2005;18:628–637. doi: 10.1016/j.nbd.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Kaminsky YG, Kosenko EA. Effects of amyloid-beta peptides on hydrogen peroxide-metabolizing enzymes in rat brain in vivo. Free Radic Res. 2008;42:564–573. doi: 10.1080/10715760802159057. [DOI] [PubMed] [Google Scholar]
- 22.Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. J Biol Chem. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- 23.Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, Muller-Spahn F, Haass C, Czech C, Pradier L, Muller WE, Eckert A. Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem. 2004;279:50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- 24.Sayre LM, Moreira PI, Smith MA, Perry G. Metal ions and oxidative protein modification in neurological disease. Ann Ist Super Sanita. 2005;41:143–164. [PubMed] [Google Scholar]
- 25.Moreira PI, Santos MS, Moreno A, Oliveira C. Amyloid beta-peptide promotes permeability transition pore in brain mitochondria. Biosci Rep. 2001;21:789–800. doi: 10.1023/a:1015536808304. [DOI] [PubMed] [Google Scholar]
- 26.Moreira PI, Santos MS, Moreno A, Rego AC, Oliveira C. Effect of amyloid beta-peptide on permeability transition pore: a comparative study. J Neurosci Res. 2002;69:257–267. doi: 10.1002/jnr.10282. [DOI] [PubMed] [Google Scholar]
- 27.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008 doi: 10.2353/ajpath.2008.071208. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- 32.Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G. Increased autophagic degradation of mitochondria in Alzheimer disease. Autophagy. 2007;3:614–615. doi: 10.4161/auto.4872. [DOI] [PubMed] [Google Scholar]
- 33.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cleeter MW, Cooper JM, Schapira AH. Irreversible inhibition of mitochondrial complex I by 1-methyl-4-phenylpyridinium: evidence for free radical involvement. J Neurochem. 1992;58:786–789. doi: 10.1111/j.1471-4159.1992.tb09789.x. [DOI] [PubMed] [Google Scholar]
- 35.Salinas M, Martin D, Alvarez A, Cuadrado A. Akt1/PKBalpha protects PC12 cells against the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium and reduces the levels of oxygen-free radicals. Mol Cell Neurosci. 2001;17:67–77. doi: 10.1006/mcne.2000.0921. [DOI] [PubMed] [Google Scholar]
- 36.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005;280:42026–42035. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 37.Moldzio R, Piskernik C, Radad K, Rausch WD. Rotenone damages striatal organotypic slice culture. Ann N Y Acad Sci. 2008;1148:530–535. doi: 10.1196/annals.1410.009. [DOI] [PubMed] [Google Scholar]
- 38.Dahm CC, Moore K, Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J Biol Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 39.Domingues AF, Esteves AR, Swerdlow RH, Oliveira CR, Cardoso SM. Calpain-mediated MPP+ toxicity in mitochondrial DNA depleted cells. Neurotox Res. 2008;13:31–38. doi: 10.1007/BF03033365. [DOI] [PubMed] [Google Scholar]
- 40.Esteves AR, Domingues AF, Ferreira IL, Januario C, Swerdlow RH, Oliveira CR, Cardoso SM. Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8:219–228. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM. Oxidative stress involvement in alpha-synuclein oligomerization in Parkinsons disease cybrids. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2247. in press. [DOI] [PubMed] [Google Scholar]
- 42.Petit-Paitel A, Brau F, Cazareth J, Chabry J. Involvment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. PLoS ONE. 2009;4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W, Vives-Bauza C, Acin-Perez R, Yamamoto A, Tan Y, Li Y, Magrane J, Stavarache MA, Shaffer S, Chang S, Kaplitt MG, Huang XY, Beal MF, Manfredi G, Li C. PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and alpha-synuclein aggregation in cell culture models of Parkinson’s disease. PLoS ONE. 2009;4:e4597. doi: 10.1371/journal.pone.0004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moisoi N, Klupsch K, Fedele V, East P, Sharma S, Renton A, Plun-Favreau H, Edwards RE, Teismann P, Esposti MD, Morrison AD, Wood NW, Downward J, Martins LM. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ. 2009;16:449–464. doi: 10.1038/cdd.2008.166. [DOI] [PubMed] [Google Scholar]
- 45.Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 46.Powers WJ, Videen TO, Markham J, McGee-Minnich L, Antenor-Dorsey JV, Hershey T, Perlmutter JS. Selective defect of in vivo glycolysis in early Huntington’s disease striatum. Proc Natl Acad Sci U S A. 2007;104:2945–2949. doi: 10.1073/pnas.0609833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochem Biophys Res Commun. 2007;359:335–340. doi: 10.1016/j.bbrc.2007.05.093. [DOI] [PubMed] [Google Scholar]
- 48.Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 49.Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J Biol Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- 50.Pandey M, Varghese M, Sindhu KM, Sreetama S, Navneet AK, Mohanakumar KP, Usha R. Mitochondrial NAD+-linked State 3 respiration and complex-I activity are compromised in the cerebral cortex of 3-nitropropionic acid-induced rat model of Huntington’s disease. J Neurochem. 2008;104:420–434. doi: 10.1111/j.1471-4159.2007.04996.x. [DOI] [PubMed] [Google Scholar]
- 51.Kumar P, Kumar A. Possible role of sertraline against 3-nitropropionic acid induced behavioral, oxidative stress and mitochondrial dysfunctions in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:100–108. doi: 10.1016/j.pnpbp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 52.Mandavilli BS, Boldogh I, Van Houten B. 3-nitropropionic acid-induced hydrogen peroxide, mitochondrial DNA damage, and cell death are attenuated by Bcl-2 overexpression in PC12 cells. Brain Res Mol Brain Res. 2005;133:215–223. doi: 10.1016/j.molbrainres.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 53.Kim GW, Chan PH. Involvement of superoxide in excitotoxicity and DNA fragmentation in striatal vulnerability in mice after treatment with the mitochondrial toxin, 3-nitropropionic acid. J Cereb Blood Flow Metab. 2002;22:798–809. doi: 10.1097/00004647-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Acevedo-Torres K, Berrios L, Rosario N, Dufault V, Skatchkov S, Eaton MJ, Torres-Ramos CA, Ayala-Torres S. Mitochondrial DNA damage is a hallmark of chemically induced and the R6/2 transgenic model of Huntington’s disease. DNA Repair (Amst) 2009;8:126–136. doi: 10.1016/j.dnarep.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menzies FM, Grierson AJ, Cookson MR, Heath PR, Tomkins J, Figlewicz DA, Ince PG, Shaw PJ. Selective loss of neurofilament expression in Cu/Zn superoxide dismutase (SOD1) linked amyotrophic lateral sclerosis. J Neurochem. 2002;82:1118–1128. doi: 10.1046/j.1471-4159.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 57.Dupuis L, Gonzalez de Aguilar JL, Oudart H, de Tapia M, Barbeito L, Loeffler JP. Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neuro-degenerative diseases. 2004;1:245–254. doi: 10.1159/000085063. [DOI] [PubMed] [Google Scholar]
- 58.Zimmerman MC, Oberley LW, Flanagan SW. Mutant SOD1-induced neuronal toxicity is mediated by increased mitochondrial superoxide levels. J Neurochem. 2007;102:609–618. doi: 10.1111/j.1471-4159.2007.04502.x. [DOI] [PubMed] [Google Scholar]
- 59.Jaiswal MK, Keller BU. Cu/Zn superoxide dismutase typical for familial amyotrophic lateral sclerosis increases the vulnerability of mitochondria and perturbs Ca2+ homeostasis in SOD1G93A mice. Mol Pharmacol. 2009;75:478–489. doi: 10.1124/mol.108.050831. [DOI] [PubMed] [Google Scholar]
- 60.Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de Leon A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawamata H, Magrane J, Kunst C, King MP, Manfredi G. Lysyl-tRNA synthetase is a target for mutant SOD1 toxicity in mitochondria. J Biol Chem. 2008;283:28321–28328. doi: 10.1074/jbc.M805599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hatazawa J, Brooks RA, Dalakas MC, Mansi L, Di Chiro G. Cortical motor-sensory hypometabolism in amyotrophic lateral sclerosis: a PET study. J Comput Assist Tomogr. 1988;12:630–636. doi: 10.1097/00004728-198807000-00019. [DOI] [PubMed] [Google Scholar]
- 63.Borthwick GM, Johnson MA, Ince PG, Shaw PJ, Turnbull DM. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: implications for the role of mitochondria in neuronal cell death. Ann Neurol. 1999;46:787–790. doi: 10.1002/1531-8249(199911)46:5<787::aid-ana17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki S, Warita H, Murakami T, Shibata N, Komori T, Abe K, Kobayashi M, Iwata M. Ultrastructural study of aggregates in the spinal cord of transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathol. 2005;109:247–255. doi: 10.1007/s00401-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 65.Pilatus U, Lais C, Rochmont Adu M, Kratzsch T, Frolich L, Maurer K, Zanella FE, Lanfermann H, Pantel J. Conversion to dementia in mild cognitive impairment is associated with decline of N-actylaspartate and creatine as revealed by magnetic resonance spectroscopy. Psychiatry Res. 2009;173:1–7. doi: 10.1016/j.pscychresns.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 66.Andres RH, Ducray AD, Perez-Bouza A, Schlattner U, Huber AW, Krebs SH, Seiler RW, Wallimann T, Widmer HR. Creatine supplementation improves dopaminergic cell survival and protects against MPP+ toxicity in an organotypic tissue culture system. Cell Transplant. 2005;14:537–550. doi: 10.3727/000000005783982756. [DOI] [PubMed] [Google Scholar]
- 67.Andres RH, Huber AW, Schlattner U, Perez-Bouza A, Krebs SH, Seiler RW, Wallimann T, Widmer HR. Effects of creatine treatment on the survival of dopaminergic neurons in cultured fetal ventral mesencephalic tissue. Neuroscience. 2005;133:701–713. doi: 10.1016/j.neuroscience.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- 69.Zhu S, Li M, Figueroa BE, Liu A, Stavrovskaya IG, Pasinelli P, Beal MF, Brown RH, Jr, Kristal BS, Ferrante RJ, Friedlander RM. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J Neurosci. 2004;24:5909–5912. doi: 10.1523/JNEUROSCI.1278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malcon C, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine administration against NMDA and malonate toxicity. Brain Res. 2000;860:195–198. doi: 10.1016/s0006-8993(00)02038-2. [DOI] [PubMed] [Google Scholar]
- 71.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 73.Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem. 2000;74:1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- 74.Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–729. [PubMed] [Google Scholar]
- 75.Prass K, Royl G, Lindauer U, Freyer D, Megow D, Dirnagl U, Stockler-Ipsiroglu G, Wallimann T, Priller J. Improved reperfusion and neuroprotection by creatine in a mouse model of stroke. J Cereb Blood Flow Metab. 2007;27:452–459. doi: 10.1038/sj.jcbfm.9600351. [DOI] [PubMed] [Google Scholar]
- 76.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 77.Bender A, Samtleben W, Elstner M, Klopstock T. Long-term creatine supplementation is safe in aged patients with Parkinson disease. Nutrition research (New York, NY) 2008;28:172–178. doi: 10.1016/j.nutres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, Como P, Zimmerman C, Lin M, Zhang L, Ulug AM, Beal MF, Matson W, Bogdanov M, Ebbel E, Zaleta A, Kaneko Y, Jenkins B, Hevelone N, Zhang H, Yu H, Schoenfeld D, Ferrante R, Rosas HD. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology. 2006;66:250–252. doi: 10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- 79.Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Van Leemputte M, Hespel P, Dom R. Creatine supplementation in Huntington’s disease: a placebo-controlled pilot trial. Neurology. 2003;61:925–930. doi: 10.1212/01.wnl.0000090629.40891.4b. [DOI] [PubMed] [Google Scholar]
- 80.Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, Urbinelli L, Qureshi M, Zhang H, Pestronk A, Caress J, Donofrio P, Sorenson E, Bradley W, Lomen-Hoerth C, Pioro E, Rezania K, Ross M, Pascuzzi R, Heiman-Patterson T, Tandan R, Mitsumoto H, Rothstein J, Smith-Palmer T, MacDonald D, Burke D. A clinical trial of creatine in ALS. Neurology. 2004;63:1656–1661. doi: 10.1212/01.wnl.0000142992.81995.f0. [DOI] [PubMed] [Google Scholar]
- 81.Hager K, Marahrens A, Kenklies M, Riederer P, Munch G. Alpha-lipoic acid as a new treatment option for Azheimer type dementia. Arch Gerontol Geriatr. 2001;32:275–282. doi: 10.1016/s0167-4943(01)00104-2. [DOI] [PubMed] [Google Scholar]
- 82.Hager K, Kenklies M, McAfoose J, Engel J, Munch G. Alpha-lipoic acid as a new treatment option for Alzheimer’s disease--a 48 months follow-up analysis. J Neural Transm. 2007;(Suppl):189–193. doi: 10.1007/978-3-211-73574-9_24. [DOI] [PubMed] [Google Scholar]
- 83.Moreira PI, Harris PL, Zhu X, Santos MS, Oliveira CR, Smith MA, Perry G. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis. 2007;12:195–206. doi: 10.3233/jad-2007-12210. [DOI] [PubMed] [Google Scholar]
- 84.Abdul HM, Butterfield DA. Involvement of PI3K/PKG/ERK1/2 signaling pathways in cortical neurons to trigger protection by cotreatment of acetyl-L-carnitine and alpha-lipoic acid against HNE-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Free Radic Biol Med. 2007;42:371–384. doi: 10.1016/j.freeradbiomed.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suh JH, Wang H, Liu RM, Liu J, Hagen TM. (R)-alpha-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for GSH synthesis. Arch Biochem Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andreassen OA, Ferrante RJ, Dedeoglu A, Beal MF. Lipoic acid improves survival in transgenic mouse models of Huntington’s disease. Neuroreport. 2001;12:3371–3373. doi: 10.1097/00001756-200110290-00044. [DOI] [PubMed] [Google Scholar]
- 87.Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, Stackman RW., Jr Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28:213–225. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Aliev G, Liu J, Shenk JC, Fischbach K, Pacheco GJ, Chen SG, Obrenovich ME, Ward WF, Richardson AG, Smith MA, Gasimov E, Perry G, Ames BN. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. Journal of cellular and molecular medicine. 2009;13:320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shenk JC, Liu J, Fischbach K, Xu K, Puchowicz M, Obrenovich ME, Gasimov E, Alvarez LM, Ames BN, Lamanna JC, Aliev G. The effect of acetyl-L-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer’s disease. J Neurol Sci. 2009 doi: 10.1016/j.jns.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bianchetti A, Rozzini R, Trabucchi M. Effects of acetyl-L-carnitine in Alzheimer’s disease patients unresponsive to acetylcholinesterase inhibitors. Curr Med Res Opin. 2003;19:350–353. doi: 10.1185/030079903125001776. [DOI] [PubMed] [Google Scholar]
- 91.Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer’s disease. Int Clin Psychopharmacol. 2003;18:61–71. doi: 10.1097/00004850-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 92.Hudson S, Tabet N. Acetyl-L-carnitine for dementia, Cochrane database of systematic reviews (Online) 2003:CD003158. doi: 10.1002/14651858.CD003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36:381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- 94.Somayajulu M, McCarthy S, Hung M, Sikorska M, Borowy-Borowski H, Pandey S. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q10. Neurobiol Dis. 2005;18:618–627. doi: 10.1016/j.nbd.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 95.Moon Y, Lee KH, Park JH, Geum D, Kim K. Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone: protective effect of coenzyme Q10. J Neurochem. 2005;93:1199–1208. doi: 10.1111/j.1471-4159.2005.03112.x. [DOI] [PubMed] [Google Scholar]
- 96.McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 97.Kooncumchoo P, Sharma S, Porter J, Govitrapong P, Ebadi M. Coenzyme Q(10) provides neuroprotection in iron-induced apoptosis in dopaminergic neurons. J Mol Neurosci. 2006;28:125–141. doi: 10.1385/JMN:28:2:125. [DOI] [PubMed] [Google Scholar]
- 98.Ono K, Hasegawa K, Naiki H, Yamada M. Preformed beta-amyloid fibrils are destabilized by coenzyme Q10 in vitro. Biochem Biophys Res Commun. 2005;330:111–116. doi: 10.1016/j.bbrc.2005.02.132. [DOI] [PubMed] [Google Scholar]
- 99.Li G, Zou LY, Cao CM, Yang ES. Coenzyme Q10 protects SHSY5Y neuronal cells from beta amyloid toxicity and oxygen-glucose deprivation by inhibiting the opening of the mitochondrial permeability transition pore. Biofactors. 2005;25:97–107. doi: 10.1002/biof.5520250111. [DOI] [PubMed] [Google Scholar]
- 100.Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008;34:165–171. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- 101.Sharma SK, El Refaey H, Ebadi M. Complex-1 activity and 18F-DOPA uptake in genetically engineered mouse model of Parkinson’s disease and the neuroprotective role of coenzyme Q10. Brain Res Bull. 2006;70:22–32. doi: 10.1016/j.brainresbull.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 102.Cleren C, Yang L, Lorenzo B, Calingasan NY, Schomer A, Sireci A, Wille EJ, Beal MF. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104:1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- 103.Smith KM, Matson S, Matson WR, Cormier K, Del Signore SJ, Hagerty SW, Stack EC, Ryu H, Ferrante RJ. Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington’s disease mice. Biochim Biophys Acta. 2006;1762:616–626. doi: 10.1016/j.bbadis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 104.Stack EC, Smith KM, Ryu H, Cormier K, Chen M, Hagerty SW, Del Signore SJ, Cudkowicz ME, Friedlander RM, Ferrante RJ. Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington’s disease mice. Biochim Biophys Acta. 2006;1762:373–380. doi: 10.1016/j.bbadis.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 105.Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Advanced drug delivery reviews. 2000;41:235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 106.Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 107.Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 108.Siler-Marsiglio KI, Pan Q, Paiva M, Madorsky I, Khurana NC, Heaton MB. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Res. 2005;1052:202–211. doi: 10.1016/j.brainres.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 109.Ernster L, Forsmark P, Nordenbrand K. The mode of action of lipid-soluble antioxidants in biological membranes: relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. Biofactors. 1992;3:241–248. [PubMed] [Google Scholar]
- 110.James AM, Sharpley MS, Manas AR, Frerman FE, Hirst J, Smith RA, Murphy MP. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282:14708–14718. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- 111.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 112.James AM, Cocheme HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 113.Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med. 2001;7:1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bedogni B, Pani G, Colavitti R, Riccio A, Borrello S, Murphy M, Smith R, Eboli ML, Galeotti T. Redox regulation of cAMP-responsive element-binding protein and induction of manganous superoxide dismutase in nerve growth factor-dependent cell survival. J Biol Chem. 2003;278:16510–16519. doi: 10.1074/jbc.M301089200. [DOI] [PubMed] [Google Scholar]
- 115.Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J, Kalyanaraman B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem. 2004;279:37575–37587. doi: 10.1074/jbc.M404003200. [DOI] [PubMed] [Google Scholar]
- 116.Lu C, Zhang D, Whiteman M, Armstrong JS. Is antioxidant potential of the mitochondrial targeted ubiquinone derivative MitoQ conserved in cells lacking mtDNA? Antioxid Redox Signal. 2008;10:651–660. doi: 10.1089/ars.2007.1865. [DOI] [PubMed] [Google Scholar]
- 117.Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol. 2006;2006:31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 120.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 121.Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;98:1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- 122.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal F. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2445. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, Grigorieva I, Ivanov Y, Sablin S, Zefirov N. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 124.Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 125.Grigorev VV, Dranyi OA, Bachurin SO. Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bull Exp Biol Med. 2003;136:474–477. doi: 10.1023/b:bebm.0000017097.75818.14. [DOI] [PubMed] [Google Scholar]
- 126.Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, Kristal BS, Hayden MR, Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu J, Li Q, Bezprozvanny I. Evaluation of Dimebon in cellular model of Huntington’s disease. Mol Neurodegener. 2008;3:15. doi: 10.1186/1750-1326-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lermontova NN, Redkozubov AE, Shevtsova EF, Serkova TP, Kireeva EG, Bachurin SO. Dimebon and tacrine inhibit neurotoxic action of beta-amyloid in culture and block L-type Ca(2+) channels. Bull Exp Biol Med. 2001;132:1079–1083. doi: 10.1023/a:1017972709652. [DOI] [PubMed] [Google Scholar]
- 129.Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. discussion 345–339. [DOI] [PubMed] [Google Scholar]