SUMMARY
Dysregulation of mitochondrial structure and function has emerged as a central factor in the pathogenesis of Parkinson's disease and related parkinsonian disorders (PD). Toxic and environmental injuries and risk factors perturb mitochondrial complex I function, and gene products linked to familial PD often affect mitochondrial biology. Autosomal recessive mutations in PTEN-induced kinase 1 (PINK1) cause an L-DOPA responsive parkinsonian syndrome, stimulating extensive interest in the normal neuroprotective and mitoprotective functions of PINK1. Recent data from mammalian and invertebrate model systems converge upon interactions between PINK1 and parkin, as well as DJ-1, α-synuclein and leucine rich repeat kinase 2 (LRRK2). While all studies to date support a neuroprotective role for wild type, but not mutant PINK1, there is less agreement on subcellular compartmentalization of PINK1 kinase function and whether PINK1 promotes mitochondrial fission or fusion. These controversies are reviewed in the context of the dynamic mitochondrial lifecycle, in which mitochondrial structure and function are continuously modulated not only by the fission-fusion machinery, but also by regulation of biogenesis, axonal/dendritic transport and autophagy. A working model is proposed, in which PINK1 loss of function results in mitochondrial reactive oxygen species (ROS), cristae/respiratory dysfunction and destabilization of calcium homeostasis, which trigger compensatory fission, autophagy and biosynthetic repair pathways that dramatically alter mitochondrial structure. Concurrent strategies to identify pathways that mediate normal PINK1 function and to identify factors that facilitate appropriate compensatory responses are both needed to halt the aging-related penetrance and incidence of familial and sporadic PD.
Keywords: PINK1, parkin, autophagy, kinases, mitochondria, neurodegeneration, oxidative stress, Parkinson’s disease, mitochondrial fission, calcium dysregulation, electron transport chain, cristae
1. Mitochondrial pathobiology is centrally implicated in Parkinson's disease
Parkinson's disease (PD) is a debilitating movement disorder that affects about a million people in North America, and which is expected to increase as the population grows older. Studies employing Mendelian models of parkinsonian neurodegeneration implicate an exciting convergence with mechanisms identified from studies of Parkinson's disease patient tissues and intoxication related to drug or environmental exposures [1, 2]. In short, these three major approaches to studying PD converge upon perturbations in mitochondrial structure, function and distribution [3]. There are currently no therapies to slow disease progression, although presumed loss-of-function mutations in autosomal recessive families result in accelerated disease presenting at younger ages. Thus, studying the regulation and function of the wild type gene products may yield important therapeutic insights to prevent or delay onset and progression of PD in general [4–7].
The proteins involved in autosomal recessive PD include parkin, PTEN-induced kinase 1 (PINK1), DJ-1 and ATP13A2 [8–11]. Parkin is an E3 ubiquitin ligase, and impaired proteasome function has been described in sporadic PD [5, 12]. Mitochondria are central to the actions of parkinsonian toxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydroxypyridine (MPTP) [13], rotenone [7] and 6-hydroxydopamine (6-OHDA) [14, 15], and PINK1 represents the only kinase known to exhibit a canonical N-terminal mitochondrial localization signal [10]. ATP13A2 is a lysosomal ATPase, and dysregulation of macroautophagy [16–18] and chaperone-mediated autophagy [19, 20] have been shown in mutiple models of dopaminergic neuronal injury. Finally, oxidative stress has long been considered a central factor in PD mechanisms based upon human tissue studies and PD toxin models [21, 22]. Data showing that DJ-1 localizes to mitochondria during oxidative stress [6], where it exhibits peroxiredoxin-like activity [23], lend further support to the concept of common pathways of dopaminergic (DA) neurodegeneration that are potentially amenable to therapeutic intervention [123].
2. Mitochondrial dynamics and neuronal differentiation and function
Due to their polarized nature, proper distribution of mitochondria within cellular subcompartments is particularly important in neurons. Mitochondrial content in neuronal processes (axons, synapses and dendrites, known collectively as neurites) plays a critical role in regulating outgrowth and synaptic remodeling into adulthood [24, 25]. Disruption of neurite remodeling and plasticity due to old age and disease likely contributes to memory loss and neurodegeneration. Interestingly, the dynamic remodeling of pre-existing mitochondria may play a more prominent role than biogenesis in regulating mitochondrial content in neurites [26, 27].
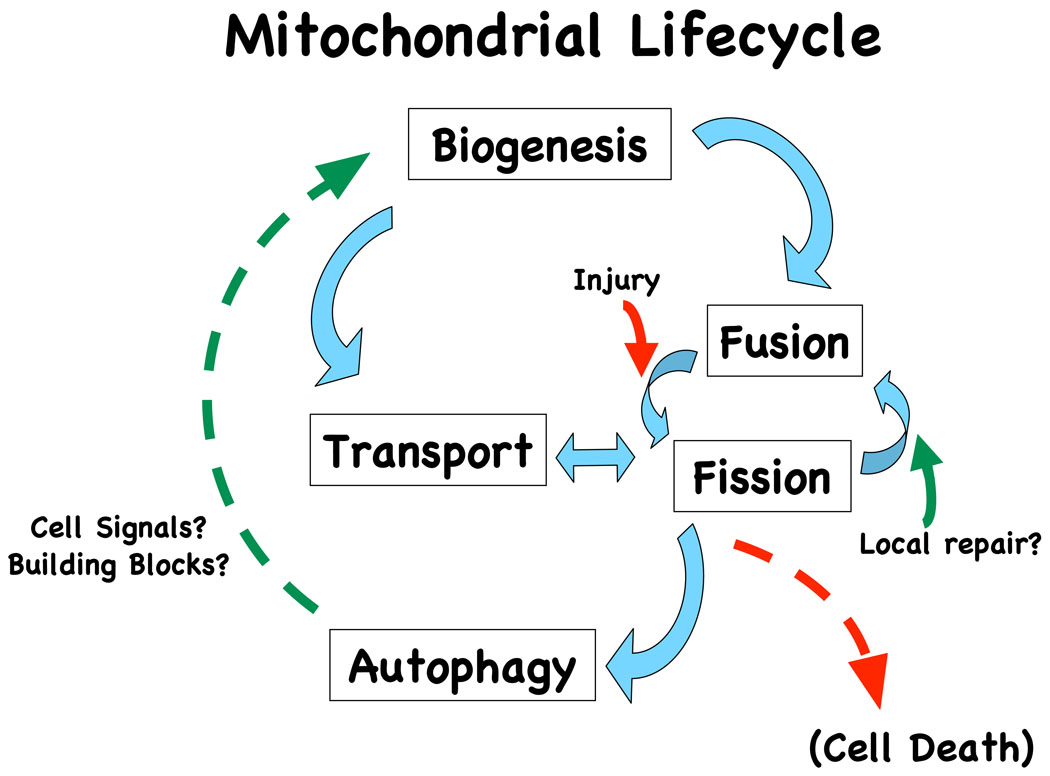
The classic view of mitochondrial dynamics focuses upon regulation of mitochondrial fission and fusion, although several other processes also regulate the steady state morphology and distribution of mitochondria within neurons (Figure 1). Mitochondrial fusion is mediated by the inner membrane protein optic atrophy 1 (Opa1) and two outer membrane mitofusins. In general, enhanced mitochondrial fusion and connectivity play critical roles in conferring resistance to many forms of cellular injury [28, 29], potentially through enhanced respiration, enhanced calcium buffering capacity or stabilization of the mitochondrial genome [30]. Mutations in the fusion machinery cause several forms of hereditary neurodegeneration, attesting to the importance of proper fission-fusion dynamics in neurons [31].
Figure 1. The lifecycle of a mitochondrion.
There are multiple factors that regulate the steady state distribution and morphology of the mitochondrial network in a cell. Dynamic processes that regulate the normal mitochondrial lifecycle are linked by blue arrows. Mitochondrial biogenesis is regulated by both nuclear and mitochondrial transcription factors, targeted translational and protein trafficking mechanisms and, in neurons, activity-dependent transport of the entire organelle to meet functional demands. A balance of fission and fusion events occur continuously, and is tied into axonal trafficking in neurons and cell cycle in non-neuronal cells. A portion of mitochondria isolated by fission undergoes basal autophagic turnover, releasing new building blocks and unknown signals related to biogenesis (green dashed arrow). Most injury states that have been studied promote mitochondrial fission (red arrow). While fission involving bax-recruitment is necessary for cell death in some systems [35], the role of fission in other injury contexts is less clear. We propose that fission serves predominantly as an injury response to isolate damaged segments of the mitochondrial network. Segments that can be repaired may refuse with other undamaged portions (green arrow). Fission protects the remainder of the network against harmful calcium fluxes [32], and enhances retrograde transport and mitochondrial autophagy, which serves to further sequester damaged mitochondria and reduce leakage of pro-death mediators. If neither repair nor autophagy is sufficient, or if degraded mitochondria are ineffectively replaced [39], the balance tips to favor cell death (red dashed arrow).
Mitochondrial fission is mediated by cytosolic and outer mitochondrial membrane proteins including dynamin-related protein (Drp1) and hFis1, which mediate mechanical constriction powered by GTP hydrolysis. While Drp1-dependent mitochondrial fission can limit injury associated with calcium waves [32], fission is often associated with cell death. Fission is elicited by the PD neurotoxins 6-OHDA [33] and rotenone [34], playing an important role in the execution of apoptosis when occurring in conjunction with Bax recruitment [35, 36]. On the other hand, a basal level of mitochondrial fission is important for cell division and mitochondrial trafficking, and dominant negative Drp1 homologues reduce both mitochondrial content and functional plasticity of synapses [25, 26]. Little is known about the posttranslational regulation of mitochondrial dynamics, although sumoylation and phosphorylation of Drp1 have been recently reported [28, 37].
In addition to dynamic changes in mitochondrial fission/fusion and trafficking, there is growing evidence that autophagic degradation plays a major role in regulating neurite morphology and mitochondrial content. Macroautophagy involves the regulated, membranous engulfment of cytoplasmic cargo destined for lysosomal degradation [38, 39], and represents the only major degradative pathway for organelles and insoluble proteins [40]. The autophagy machinery includes conjugating enzymes required for covalent attachment of ubiquitin-fold proteins Atg12 and Atg8/microtubule-associated light chain protein 3 (LC3) to nascent autophagic membranes [38]. RNAi knockdown of Atg conjugation components are effective at inhibiting induction of autophagy and mitophagy [16, 18].
Mitochondrial autophagy (mitophagy) comprises an adaptive response to hypoxia, mitochondrial damage or limiting amounts of aerobic substrates in many cell types [41]. However, in cardiac muscle and neuronal cells, which are dependent upon aerobic respiration, robust mitophagy may also prove harmful [18, 42, 43]. Autophagy induced by cerebral hypoxia exacerbates neuronal apoptosis in vivo [44, 45]. Since substantia nigra DA neurons exhibit decreased basal mitochondrial content compared to other midbrain neurons, it has been proposed that diminished reserves may render them more susceptible to compromise of mitochondrial homeostasis during PD pathogenesis [46]. We have postulated that the outcome of mitophagy induction may depend upon the ability of neurons to replace sequestered/degraded mitochondrial components with newly synthesized replacements [39], given data suggesting deficits in nuclear import and transcription in 6-OHDA treated neuronal cells and post-mortem PD tissues [47, 48]. Interestingly, impaired mtDNA replication has been implicated in PINK1 deficient cells [49], although evidence of compensatory mitochondrial and antioxidant transcription have also been reported [50, 51].
3. PINK1 is a novel neuroprotective kinase
PINK1 is a 581 amino acid protein with an N-terminal mitochondrial targeting signal, a putative transmembrane segment, a Ser/Thr kinase domain, and a C-terminal regulatory domain [Reviewed in [52]]. PINK1 was originally identified as a PTEN regulated message in cancer cells, but showed no effects on cell proliferation [53]. A homozygous G309D mutation and a truncating W437X mutation were first identified in consanguineous European PD families [10]. Subsequently, additional autosomal recessive PINK1 mutations, including heterozygote compound mutations at E240K and L489P, were identified in other PD families [54–57]. The possibility of PINK1 haploinsufficiency as a risk factor in sporadic PD has also been raised [58–60]. Although meta-analysis reveals a nonsignificant odds ratio for PINK1 heterozygotes, these patients may show accelerated disease progression [61].
Mutations in familial PINK1 involve several regions of the protein and specific effects are likely to vary depending upon the mutation (Reviewed in [52]). PD-associated mutations in PINK1 can affect PINK1 stability and expression levels [62], its association with heat shock protein 90 chaperones [63, 64], or its ability to phosphorylate pan-kinase substrates such as histone H1 and casein [65, 66], although neither mitochondrial import [62] nor ability to dimerize [67] appear to be affected by the point mutations. C-terminal truncations of PINK1 are likely to affect function, as in vitro kinase activity of PINK1 is regulated in different directions by portions of the C-terminal domain [65, 66].
Overexpression of wild type PINK1 confers protection from proteasome inhibitor-mediated cell death [10], a property abolished by PD-associated mutations [68], and PINK1 is found in mitochondria involved in aggresomes [69]. Wild type, but not mutant, PINK1 protects from staurosporine-induced apoptosis [70], although this is not seen in all systems [34]. PINK1 protects against MPTP toxicity in vivo and MPP+ toxicity in vitro [71], and siRNA knockdown of PINK1 expression enhances susceptibility to MPP+ and rotenone [72]. Despite these striking effects on survival, few target pathways for PINK1 have been identified. PINK1 phosphorylates the mitochondrial heat shock protein 75 (also known as TNF receptor-associated protein 1) [73] and interacts with p38 MAPK in phosphorylating the mitochondrial protease HtrA2 [74].
In addition to neuroprotection against pharmacologic injuries, PINK1 can reduce injury associated with dominant genetic PD models. Sporadic PD and autosomal dominant parkinsonism is typically characterized by Lewy bodies containing aggregated α-synuclein. In recent years, α-synuclein has also been implicated in mitochondrial degeneration, both in vitro [75] and in vivo [76]. PINK1 protects against α-synuclein-mediated retinal degeneration in Drosophila [77]. In cultured cells, a truncated mutant PINK1 exacerbates A53T α-synuclein toxicity [78] and PINK1 siRNA elicits proteasomal impairment and α-synuclein aggregation [67]. The dominant G2019S leucine rich repeat kinase 2 causes neurite degeneration through an autophagic mechanism [17]. PINK1 overexpression protects against G2019S LRRK2-mediated neurite injury (SJ Cherra & CT Chu, unpublished data), while nematode lrk-1 is necessary for expression of pink-1 null phenotypes [79]. Given the broad neuroprotective properties of PINK1 in both toxic and genetic models of parkinsonian injury, elucidating the normal function(s) of PINK1 holds much promise towards development of future therapies that will be effective in multiple forms of PD.
4. PINK1 is a unique kinase directly targeted to mitochondria (sometimes)
Following identification of PINK1 as a PD-associated gene, it was found to be broadly expressed in the cortex, striatum and brainstem [80], with regulation by post-transcriptional mechanisms [81]. PINK1 represents the first large canonical kinase to exhibit a canonical mitochondrial targeting sequence. This consists of an N-terminal amphipathic helix that is recognized by the translocase of the outer mitochondrial membrane (TOM) [82]. PINK1 colocalizes with mitochondria in cells [65] and in human brain tissues [83], but is also present in cytosolic fractions [62, 63]. In Drosophila, the rhomboid-7 mitochondrial proteinase may be involved inprocessing both PINK1 and HtrA2/Omi [84].
Recent studies show that the N-terminal mitochondrial localization signal is necessary for some [68], but not all effects of PINK1 [71]. This suggests that PINK1 neuroprotection could involve cytosolic targets even in models involving mitochondrially targeted injury. The ability to form cytosolic complexes with parkin and DJ-1, with effects on E3 ligase activity, has been recently reported [85]. On the other hand, recombinant variants of PINK1 lacking the N-terminal domain are still present in mitochondrial fractions due to binding interactions with other proteins [86], so these studies do not negate the probability of key mitochondrial targets for PINK1.
Similarly, PINK1 localization with respect to mitochondrial subcompartments is still unclear. While most studies indicate localization predominantly near the inner mitochondrial membrane [65, 73, 78, 83], PINK1 may also associate with outer membrane fractions to a lesser extent [78]. Moreover, one study suggests that the kinase domain faces outwards from the mitochondrion through an arrested import mechanism [87]. Like other kinases [88], it is likely that PINK1 has multiple functions in the cell involving dynamic trafficking between subcellular compartments and scaffold complexes, depending upon post-translational modifications and proteolytic processing.
One of the challenges with studying PINK1 has been difficulty detecting endogenous levels of the PINK1 protein. Thus, localization and even degree of RNAi knockdown has been estimated using overexpressed tagged recombinant PINK1. As the degree of protein overexpression cannot be estimated without a reliable measure of endogenous PINK1, it is possible that high levels of overexpression saturate transport or processing mechanisms. Using an antibody raised to the kinase domain, the major band detected in SH-SY5Y cells is a processed form that lacks both the N- and C-terminals, whereas recombinant C-terminal tagged PINK1 migrates as a full-length species that nevertheless associates with mitochondrial fractions [89]. While detection of tagged species can be markedly enhanced by proteasome inhibition in agreement with other studies [64], such treatments have little effect on endogenous bands. Conversely, other studies have shown that processed PINK1 is not limited to mitochondrial fractions, suggesting either efflux from mitochondria [62] or other sites of proteolytic processing. Other considerations include non-linearity of antibody responses, observations that one antibody may preferentially recognize one processed form over another even if equivalent amounts are present, and effects of as yet uncharacterized post-translational modifications on antibody avidity. It is clear that much work remains to be done to illuminate the distribution of PINK1 under different physiologic and injury states in pertinent neuronal populations.
5. PINK1 loss of function has major effects on mitochondrial morphology
Regardless of the major site(s) of PINK1 function, modulating PINK1 expression shows major effects on mitochondrial morphology in Drosophila (see below) and several mammalian studies (see Table I). Interestingly, silencing or knockout of PINK1 expression in mice does not cause neuronal cell death [90, 91], resulting instead in decreased evoked striatal dopamine release [91] and reduced state 3 maximal respiration in the presence of ADP [92]. Cortical regions are less susceptible to these changes, but these differences can be overcome by H2O2 or heat shock, suggesting that the mice are well compensated for developmental absence of PINK1.
Table I.
Summary of mitochondrial results in mammalian PINK1 loss of function studies
| Cell type | human fibroblast |
human HeLa; fibroblast |
mouse cortical neurons; human NSC |
human SH-SY5Y | human SH-SY5Y | human M17 | rat PC12 | rat B103 neuroblastoma |
mouse brain |
|---|---|---|---|---|---|---|---|---|---|
| Loss of function | Homozygous G309D | Transient siRNA; primary fibroblast (Q126P and G309D) | Stable shRNA NSC lines, DA differentiated | Stable shRNA lines, A and D series. Stable & transient WT PINK1. *Mouse cortical neurons expressing KD, E240K, L489P | Transient siRNA, Dharmacon (#1) & Qiagen (#2), passaged and retransfected q3 d | lentiviral shRNA | stable shRNA, transient overexpression of mutants | lentiviral co-expression of A53T α-syn and W437X PINK1 | germ-line deletion of exons 4–7. |
| Degree of knockdown | 96% decrease in mRNA | 90% decrease in mRNA using #1029 shRNA | 70–80% decrease in mRNA; 60–80% decrease of endogenous PINK1 | 80% decrease in mRNA; 52% decrease of overexpressed PINK1 | >80% decrease in mRNA | >80% decrease in mRNA | High level expression of HA tag in >85% of singly transfected cells | ||
| Cell death/viability | No change at 15 d, but increased cytotoxicity at 30–52 d after differentiation in NSC and 21–28 d after plating in primary cortical neurons | 20–40% decrease in basal viability over 5 d after passage. *30% decrease in cortical neurons for KD and L489P, but not E240K | No basal effect at 6 or 12 d; hypersensitive to paraquat at 12 d. (Deng et al 2005 [72] found 20% drop in basal viability; hypersensitive to MPP+ and rotenone) | No basal change; Hypersensitive to rotenone | no Δ in TH+ SNc neuron # at 2–3 yrs; no changes in striatal TH. (Zhou et al 2007 [90] also found no Δ in SNc neuron# in conditional PINK1 RNAi Tg with >90% decrease in brain mRNA) | ||||
| Membrane potential/ATP | Decreased (TMRM) | Decreased (TMRM), sensitive to oligomycin (maintained by complex V not by respiration) | Decreased (JC-1); restored by antioxidants | Decreased (JC-1) at 12 d; ATP also decreased at 12 d (with #2 siRNA only) | Decreasd (TMRM) | Decreased ATP synthesis | Reduced MTR fluorescence and decreased ATP | No change in ATP levels | |
| ETC | Decreased complex I, no change in complex IV | Oxygen consumption decreased, and no longer oligomycin sensitive; reversed by pyruvate. | No effect at 6 d; decreased complex IV at 12 d. | Decreased oxygen consumption | 4 mo striatum: reduced state 3 (maximum) for I and II; reduced state 4 (proton leak) for I; 4 mo cortex: no Δ 24 mo, reduced state 3 for 1, II, III/IV; increased state 4 for I | ||||
| ROS | Increased MDA, but not carbonyls or 8-oxoG; increased glutathione with no Δ in ratio. No Δ of O2- by EPR in lymphoblasts | Increased superoxide (MitoSOX, Het); decreased GSH at dd5 in differentiated NSC; role of NADPH oxidase | Increased superoxide (MitoSOX) | Increased carbonyls by 6 d; decreased total glutathione only at 12 d with no change in ratio | Reduced aconitase activity, but no change in TBARS, carbonyls, 4-HNE. No sign of astrogliosis. | ||||
| Morphology | Increased % cells with fragmentation; rescued by RNAi resistant PINK1 and parkin, but not by DJ-1 | Drp1-dependent fragmentation in PINK1 deficient lines by 3D-reconstruction, indices of interconnectivity and elongation; opposite effect with transient and stable overexpression. *WT, KD, L489P showed similar effects in cortical neurons | No change at 12 d | Increased % cells with fragmentation, additive with rotenone; reversed with stable and transient PINK1 overexpression. Decreased mobile fraction & mito fusion rates | Irregular profiles, increased diameter, abnormal cristae, inclusions. Rescued by WT α-syn, exacerbated by A53T | No change in average size of mitochondrial profiles; increase in 0.8–1 micron size bin at 4 mo, and in both 0.8–1 and >1 size bins at 24 mo. | |||
| Ultrastructure | Decreased cristae | Enlarged with disorganized cristae in surviving NSC | Decreased cristae density; mixture of pale swollen profiles and small fragments | Swollen cristae | No gross structural defects at 4 and 24 mo. | ||||
| Autophagy, Lysosomes | Increased lysotracker in differentiated NSC and mouse cortical neurons | Increased autophagy/mitophagy (LC3, EM, lysostracker); reversed by RNAi resistant PINK1, enhanced by parkin. *Increased autophagy/mitophagy in mutant PINK1 expressing 1° neurons | Possible association of mitochondria with lysosomes | Enlarged lysosomal structures. LC3 aggregates in coinfected cells | |||||
| Mitochondrial content | Induction of MnSOD | Evidence of mitochondrial biogenesis: increased complex I, III, V at dd43 (not at dd5) | Decreased mito-GFP, reversed by inhibiting lysosomal degradation; decreased steady state matrix and membrane protein expression | Decreased mtDNA in #1 and #2 by 9–12 d; no Δ in mtDNA encoded protein; No Δ in enzyme activity, porin, MTG | No change in Tim23 | Normal numbers in striata, no change in citrate synthase, porin, AIF, CxV, isocitrate dehydrogenase in young −/−. No change in MnSOD and other antioxidant enzymes at 24 mo. | |||
| Other findings | Patient fibroblasts and lymphoblasts appear to be compensated oxidative stress | Deficient Na+/Ca2+ antiporter causes elevated basal mitochondrial Ca2+ and decreased buffering capacity. ROS inhibits glucose uptake | Sequestration of damaged mitos by fission & parkin-enhanced autophagy is neuroprotective; ROS upstream of other effects | Stable parkin overexpression did not reverse mtDNA reduction or decreased mtDNA synthesis | Calcineurin dephosphorylates (and activates) Drp1 downstream of membrane potential | Wild type PINK1 dimerizes; property is preserved with L347P, E417G, but not Del-245. Buildup of CFP-degron and insoluble α-syn | Calcium uptake and mPTP opening implicated. | Evoked release of DA/catecholamines reduced in striatal slices and adrenal chromaffin cellsl; deficient corticostriatal LTP/LTD. | |
| References | Hoepken et al, 2006 [50] | Exner et al 2007 [96] | Wood-Kaczmar 2008 [51]; Gandhi et al 2009 [110] | Dagda et al 2009 [89]; *unpublished data | Gegg et al 2009 [49] | Sandebring et al 2009 [34] | Liu et al 2009 [67] | Marongiu et al 2009 [78] | Kitada et al 2007 [91]; Gautier 2008 [92] |
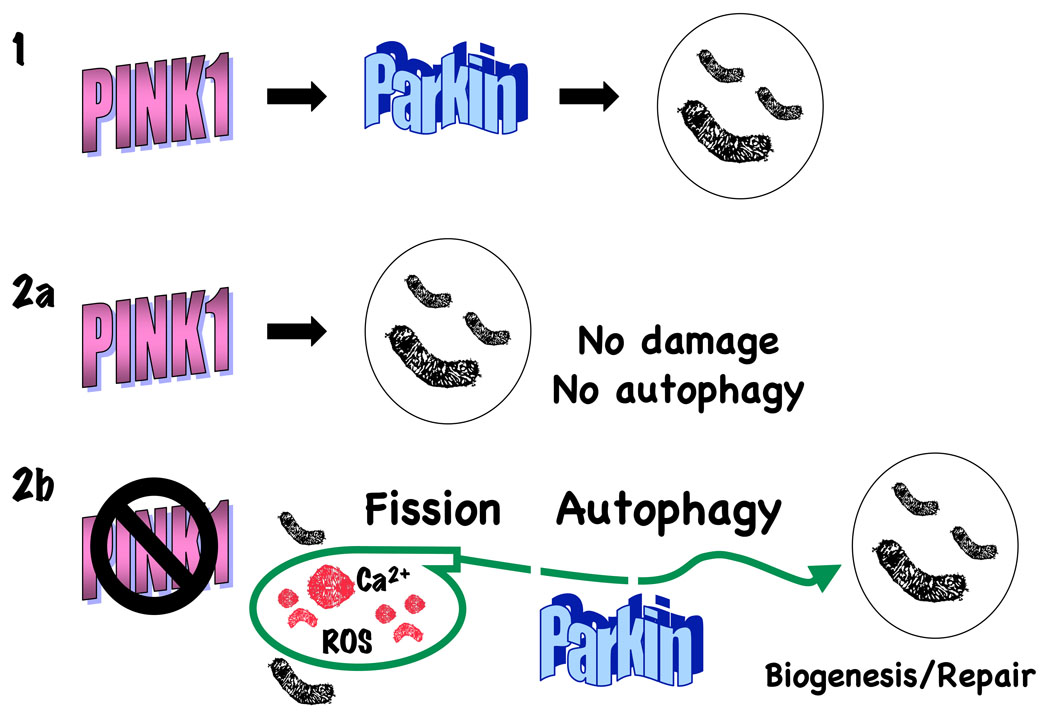
Drosophila studies show flight muscle degeneration accompanied by markedly swollen and enlarged mitochondria. Notably, PINK1 and parkin flies show similar phenotypes. While parkin overexpression reverses effects of PINK1 loss-of-function on mitochondrial morphology, the converse was not observed, suggesting that parkin functions downstream of PINK1 [93–95]. The ability of parkin overexpression to correct morphologic effects of PINK1 deficiency has also been observed in mammalian cells [89, 96]. Assuming that parkin overexpression acts to directly reverse the deficit left by reduced PINK1 function, the simplest model places parkin as a mediator of PINK1 mitoprotection (Fig. 2, top), although other potential mechanisms are also compatible with the data (Fig. 2, bottom and legend).
Figure 2. Hypothetical models for PINK1-Parkin interactions.
Several effects of PINK1 deficiency can be complemented by overexpression of Parkin in both mammalian and invertebrate systems.
1) The simplest model places Parkin as a downstream mediator of a common PINK1-Parkin pathway that affects mitochondrial dynamics, although there is disagreement in the literature over whether the fission-fusion balance is positively (Drosophila) or negatively (mammalian cells, C. elegans) associated with PINK1 activity.
2) An alternative model is suggested by data showing opposite effects of PINK1 and parkin activities on autophagy/mitophagy [89]. PINK1 maintains healthy mitochondrial networks regulated by a steady state fission-fusion balance, and in the absence of damage, there is no stimulus to induce autophagy. In PINK1 deficient cells, fission and autophagy are coordinately upregulated to isolate damaged and often swollen segments of mitochondria, preventing propagation of calcium waves and release of oxidants and other death mediators. Parkin overexpression augments selective clearance of depolarized mitochondria [105]. Thus, PINK1 and Parkin promote a similar steady state phenotype of healthy mitochondrial networks by acting at different ends of the process, with PINK1 maintaining mitochondrial health and Parkin eliminating damaged mitochondria. Experimentally increasing Drp1 indirectly protects against cell injury by further enhancing the compensatory fission-autophagy process (rather than by restoring a normal PINK1 function), while knockdown of Drp1 function and increased Opa serve to exacerbate injury.
These two models are not mutually exclusive, and there is at least one other model in which both proteins may affect a similar process through different mechanisms. For example, both Parkin and PINK1 may function to enhance mitochondrial biogenesis [49, 109], but Parkin does not rescue the effects of PINK1 deficiency in this context.
Additional studies supporting a direct relationship between PINK1 and parkin include observations that a parkin peptide can be phosphorylated by ΔN-PINK1 in vitro [97] and demonstrating physical interaction between overexpressed PINK1 and parkin in the cytosol [85, 98, 99]. It remains to be discovered whether or not the mitochondrial effects of altered PINK1 are modulated by parkin E3 ligase activity, nor is it known whether or not PINK1 kinase activity is important for this effect. With some exceptions (e.g. MAPKs), activated kinases typically do not stably dock with their substrates, but can form stable complexes with inhibitors, anchoring proteins and scaffolds.
Both Drosophila and mammalian cell studies show ultrastructural changes for PINK1 loss of function involving mitochondrial pallor with decreased or disorganized cristae [89, 100, 101], which resemble mitochondria in sporadic PD cybrid cell lines [102]. While some profiles appear enlarged, it is unclear whether this reflects swelling or fusion. The apparent size of ultrastructural profiles is influenced by the angle between the plane of section and the longitudinal mitochondrial axis, as well as by degree of branching and tortuosity. In addition, fluorescence and molecular genetic data in Drosophila [100, 101, 103] and mammalian culture systems [34, 89, 96] appear to support opposite conclusions in the fission-fusion balance.
In PINK1 deficient Drosophila, GFP-tagged mitochondria in longitudinal flight muscle sections show a dishomogeneous distribution with significant clumping that is distinct from the clearly fissioned appearance elicited by knockdown of Marf (Mfn homolog) [103]. Surprisingly, immunostaining of GFP-tagged mitochondria in DA neuron whole mount sections show similar mitochondrial aggregation in both dPINK1 deficient and dPINK1 overexpressing flies [100]. However, the mechanisms may be different as analysis of cultured Drosophila neurons, S2R cells and COS cells reveal that more PINK1 deficient cells are scored as cells with elongated profiles compared to dPINK1 overexpressing cells [100]. In Drosophila systems, molecular manipulations to increase fission or decrease fusion protected against muscle degeneration, mitochondrial swelling and loss of brain DA levels [100, 101, 103]. These include providing an extra copy of Drp1 or decreasing levels or function of Opa1 or Mfn. On the hand, decreased Drp1 suppresses the effects of PINK1 overexpression in the eye [101], and exacerbated injury in PINK1 deficient flies. Assuming that the effects of manipulating mitochondrial fission-fusion machinery directly reverses the effects of insufficient PINK1, these data suggest that the normal function of PINK1 may be to promote fission [100, 101]. Alternatively, data in Drosophila muscle and testes suggest that the effects of PINK1 are not mediated by a direct linear relationship with Drp1, as the mitochondrial phenotypes are distinct and a modest deficit in Drp1 causes lethality in PINK1 null flies [103]. As discussed below, the effects of Drp1 manipulation may also be compatible with modulation of a compensatory mechanism that protects from the effects of PINK1 deficiency.
In mammalian cells, three studies have shown the opposite phenotype, namely that PINK1 deficient cells exhibit fragmented rather than fused mitochondria [34, 89, 96]. The changes observed in stable deficient states may be time dependent, as a transient siRNA study showed no morphology changes at 12 d [49]. In mutant PINK1 patient fibroblasts, human HeLa cells and human M17 neuronal cells, significantly increased percentages of PINK1 deficient cells are scored as showing fragmented rather than interconnected mitochondria [34, 96]. Using a different technique involving computerized analysis of individual mitochondria within cells, average cellular indices of mitochondrial interconnectivity and elongation are decreased with PINK1 deficiency or Drp1 overexpression (as a positive control for fission) and increased with either transient or stable overexpression of wild type PINK1 and with a dominant negative Drp1 (as a positive control for fusion) [89]. Ultrastructural analysis of several independent PINK1 knockdown cell lines showed a mixture of small fragmented profiles and large rounded, pale profiles, both with diminished cristae density. The appearance of larger diameter profiles has also been described in aging PINK1 KO mice [92] and in cells co-expressing mutant α-synuclein and truncated PINK1 [78]. Confocal microscopy with 3-D reconstruction show that irregardless of diameter, mitochondrial fragments are isolated from each other, consistent with a fission-dependent process [89]. In contrast, overexpression of PINK1 results in a more interconnected network. Finally, live cell imaging was used to demonstrate that PINK1 deficient cells show reductions in the mobile fraction of mito-YFP, consistent with reduced interconnectivity [34]. In light of recent reports of PINK1 associating with mitochondrial adapters for microtubule dependent transport [86], this data could also be consistent with reduced overall mitochondrial mobility. Irregardless, rates of mitochondrial fusion were decreased in PINK1 deficient cells when assayed using photoactivated mito-GFP [34]. Taken together, these studies demonstrate a shift in the fission-fusion balance to favor Drp1-dependent fission in PINK1 deficient cells.
In human neuronal cells, the mitochondrial fragmentation phenotype is suppressed by dominant negative Drp1 [89], Drp1 knockdown, or Opa1/Mfn2 overexpression [34]. Overexpression of Drp1 promotes further fragmentation. Furthermore, PINK1 knockdown cells exhibit decreased phosphorylation of Drp1 at S637 through activation of calcineurin phosphatase activity [34], while PINK1 overexpressing cells exhibit Drp1 2D-gel mobility consistent with higher phosphorylation states (SJ Cherra & CT Chu, unpublished data). Because Drp1 phosphorylation at S637 inhibits its ability to mediate fission [104], these data are consistent with a role for PINK1 in suppressing fission. Interestingly, suppressing Drp1 activity exacerbated cellular injury in the setting of PINK1 deficiency [89], in agreement with Drosophila studies, despite reversing the mitochondrial morphology changes. Again, these data implicate fission as a response to injury elicited by PINK1 deficiency.
6. Alternative models for PINK1-parkin interactions and mitochondrial regulation
While direct studies of mitochondrial fission and fusion kinetics may help resolve some issues, it is important to recognize that fission and fusion are not the only modulators of the steady state appearance of the mitochondrial network (Fig. 1). Selective clearance of smaller somatic mitochondrial fragments through either axonal transport or autophagic degradation, or alterations in delivery of newly synthesized mitochondrial components, can each affect the distribution of elongated or punctate mitochondria. A recent pair of studies that together suggest an alternative model for PINK1-parkin interactions (Fig. 2 bottom), may also partially resolve some of the controversies associated with effects of Drp1 modulation between Drosophila and human neuronal lines.
It has been elegantly demonstrated that parkin regulates not only proteasomal degradation, but is recruited to chemically depolarized mitochondria where it promotes their degradation through autophagy [105]. While autophagy has been traditionally viewed as a nonselective bulk housekeeping process, recent work in injury and disease models support the concept that selective mechanisms of targeted degradation must also exist. For mitochondrial autophagy, eat-me signals may include decreased membrane potential [41, 105, 106], targets of mitogen activated protein kinase/extracellular signal regulated protein kinase activity [18], or lipid oxidation [107]. Interestingly, given the E3 ligase function of parkin, certain forms of ubiquitination may also be involved in autophagy regulation [108].
If parkin rescues from PINK1 deficiency by reconstituting a downstream function of PINK1, it would be logical to surmise that PINK1 may also function to promote clearance of damaged mitochondria. Instead, we found the opposite: wild type PINK1 suppresses injury-induced autophagy while PINK1 deficient lines showed induction of autophagy and mitochondrial degradation [89]. Further investigation revealed that parkin overexpression complements the effects of PINK1 knockdown in human neuronal cells by further enhancing autophagic clearance of mitochondria isolated by fission [89]. Also, an intact autophagy machinery is essential for parkin-mediated protection against cell death in PINK1 deficient cells (RK Dagda & CT Chu, unpublished data). These studies indicate that PINK1 and Parkin can promote a similar steady state phenotype of healthy, elongated mitochondrial networks by acting at different ends of the process, with PINK1 maintaining mitochondrial health and Parkin eliminating damaged mitochondria. Similarly, the ability of fission mediators to rescue against injury in Drosophila PINK1 mutants, may relate to enhancing the isolation and clearance of damaged mitochondria (Fig. 2, bottom). Undoubtedly both fission and fusion are activated as an injury response (Fig. 1), with the steady state outcome on mitochondrial morphology dependent upon cell type- and context-dependent differences in compensatory reserve and susceptibility to injury.
A third model must also be considered, as it is possible that PINK1 and parkin regulate a common process or outcome through distinct mechanisms. For example, PINK1 [49] and parkin [109] may be implicated in mitochondrial biogenesis through different pathways. Furthermore, although PINK1 does not rescue parkin null phenotypes in Drosophila, parkin can still function upstream of PINK1 by modulating its processing and subcellular distribution [63]. These models are not mutually exclusive, and it is likely that multiple levels of interaction exist depending upon the particular process regulated by PINK1.
7. Towards a unifying model of PINK1 deficiency studies
If the primary role of PINK1 is to maintain healthy mitochondrial networks, with perturbations in mitochondrial dynamics occurring secondary to injury, what then contributes to mitochondrial or cellular injury? The final portion of this review will focus upon data implicating 1) abnormal cristae structure and function, 2) calcium dysregulation, and 3) oxidative stress, each of which may provide signals to trigger mitochondrial remodeling and autophagy.
Abnormal cristae structure and membrane depolarization have been consistently described in all mammalian culture studies of PINK1 deficiency (Table I). Given that cristae are important for expanding the surface area of the inner mitochondrial membrane available for mitochondrial respiration, it is likely that these structural and functional alterations are linked. PINK1 may play a primary role in modulating the functional morphology of cristae, given its recently reported association with mitofilin [86]. These changes are unlikely to occur purely as a nonselective consequence of PINK1 deficient injury, as overexpression of PINK1 causes markedly increased cristae density without eliciting an autophagic injury response [89]. The mechanisms for abnormal electron transport chain function are still being elucidated, and may include substrate limitation [110] or direct effects on mitochondrial complex I function [111], although no consistent change in expression of mitochondrial electron transport complex components has been reported (Table I). Given that changes in mitochondrial membrane potential play important normal roles in regulating axonal transport [112, 113], mitophagy or apoptosis [114], additional work on the role of PINK1 in modulating membrane potential may yield new therapeutic directions.
Mitochondrial calcium dysregulation may play a key role in PINK1 deficient cells [110] and in a combined mutant α-synuclein and mutant PINK1 model [78]. Under normal circumstances, mitochondria play an important role in rapid buffering of cytosolic calcium, taking up calcium into the matrix through a membrane potential-dependent uniporter [115]. An unidentified sodium-calcium antiporter than releases calcium back into the cell, preventing saturation of this buffering system. In PINK1 deficient human and mouse neurons, defective antiporter function results in elevated basal mitochondrial calcium levels and impaired buffering capacity [110]; inhibiting calcium uptake into mitochondria protects against effects of overexpressed W437X PINK1 and A53T α-synuclein in rat neuroblastoma cells [78]. Among effects of elevated cytosolic calcium are activation of NADPH oxidase [110] and calcineurin, which promotes fission in PINK1 knockdown cells [34]. Antioxidants reverse glucose uptake deficits and restore function of the electron transport chain [34]. Interestingly, chronic lysosomal dysfunction results in accumulation of mitochondria with calcium buffering deficits and enhanced sensitivity to calcium mobilizing injuries [116]. Likewise, mitochondrial fission, which exacerbates cell death due to Drp1-dependent recruitment of Bax, protects against injuries involving propagating mitochondrial calcium waves [32]. Given that fission plays a protective role in PINK1 deficient cells [89, 100, 101, 103], these observations provide additional indirect support to the calcium dysregulation model for PINK1 deficiency.
Oxidative stress has been implicated in eye degeneration caused by dPINK1 inactivation in fruit flies [117] and in several mammalian neuronal systems [49, 51, 89]. While PINK1 has been implicated in a FOXO3a-mediated ROS defense pathway [118], increased electron leakage from dysfunctional respiration or calcium-mediated activation of NADPH oxidases could contribute to ROS generation in PINK1 deficient cells. ROS-mediated damage to nearby respiratory components and/or mtDNA could in turn set up a damaging positive feedback loop to further amplify injury. Whether ROS is a primary or secondary event, these species clearly contribute to injury. Although there is little evidence of oxidative injury in PINK1 knockout mice other than aconitase inactivation [113], antioxidant enzymes and pharmaceutical agents confer significant protection against several alterations observed in PINK1 deficient Drosophila [117] and human cells, including diminished membrane potential [89, 110].
It may be worth separating potential signaling functions of reactive oxygen species from pathologic levels of oxidative stress. For instance, reversible redox inactivation of autophagy proteins that remove LC3 from membranes may function as part of a physiologic regulatory system that allows initiation of starvation-induced autophagy [119]. Much higher levels of mitochondrial ROS, however, may result in overactivation of autophagy through robust mitochondrial ERK activation [15, 18] or loss of physiologic feedback mechanisms involving beclin 1 [120]. While autophagy is cytoprotective in the context of PINK1 deficiency, beclin 1 independent autophagy contributes to neurite retraction in the MPP+ and G2019S LRRK2 models [16, 17]. As with fission/fusion and other potential therapeutic targets, either insufficient or excess stimulation may prove harmful unless properly balanced [39].
7. Conclusions
Mitochondrial fragmentation, cristae loss and mitophagy have been observed in human neurodegenerative diseases [121, 122], suggesting that perturbations in fission-fusion homeostasis and autophagy could contribute to neuritic/synaptic dysfunction and neurodegeneration in PD. Current data in models of autosomal recessive PARK6 neurodegeneration suggest that aberrant cristae/inner membrane function, calcium dysregulation and oxidative stress trigger a series of pathogenic as well as compensatory pathways. Coordinated activation of mitochondrial fission and autophagy function to isolate dysfunctional mitochondria, limiting cell death, while fusion may also be activated to facilitate repair and mtDNA stabilization [30].
Complementation of PINK1 deficient states may be mediated by 1) restoring pathways dependent upon PINK1 function, 2) reversing pathologic pathways activated in its absence, or 3) facilitating success of compensatory mechanisms. Given that stimulating fission or autophagy can be beneficial in some contexts and harmful in others, additional studies are warranted to distinguish cause from correlation, and factors that determine success of a given compensatory or protective response. Both direct and indirect effects of altered PINK1 kinase activity and/or localization could form valid targets for therapeutic modulation. Understanding pathways of injury and compensation are important therapeutically whether or not they represent direct kinase substrates, as there are multiple mechanisms to achieve a common phenotype of a healthy neuronal mitochondrial network. Given the broad neuroprotective properties of PINK1 in both toxic and genetic models of parkinsonian injury, elucidating the normal function(s) of PINK1 offers exciting promise for therapies effective in multiple forms of PD.
ACKNOWLEDGMENTS
The authors' research is supported by the National Institutes of Health (AG026389-03S1, NS053777, DC009120).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nature clinical practice. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 2.Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson's disease: proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Laar VS, Berman SB. Mitochondrial dynamics in Parkinson's disease. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vila M, Przedborski S. Genetic clues to the pathogenesis of Parkinson's disease. Nat Med. 2004;10(suppl):S58–S62. doi: 10.1038/nm1068. [DOI] [PubMed] [Google Scholar]
- 5.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 6.Dekker MC, Bonifati V, van Duijn CM. Parkinson's disease: piecing together a genetic jigsaw. Brain. 2003;126:1722–1733. doi: 10.1093/brain/awg172. [DOI] [PubMed] [Google Scholar]
- 7.Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT. Mechanistic approaches to Parkinson's disease pathogenesis. Brain Pathol. 2002;12:499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitada T, Asakawa S, Matsumine H, Hattori N, Minoshima S, Shimizu N, Mizuno Y. Positional cloning of the autosomal recessive juvenile parkinsonism (AR-JP) gene and its diversity in deletion mutations. Parkinsonism Relat Disord. 1999;5:163–168. doi: 10.1016/s1353-8020(99)00032-2. [DOI] [PubMed] [Google Scholar]
- 9.Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, van Swieten JC, Brice A, van Duijn CM, Oostra B, Meco G, Heutink P. DJ-1(PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci. 2003;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- 10.Valente EM, P.M. Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 12.McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P. Failure of the ubiquitin-proteasome system in Parkinson's disease. Nat Rev Neurosci. 2001;2:589–594. doi: 10.1038/35086067. [DOI] [PubMed] [Google Scholar]
- 13.Przedborski S, Jackson-Lewis V. Mechanisms of MPTP toxicity. Mov Disord. 1998;13 Suppl 1:35–38. [PubMed] [Google Scholar]
- 14.Callio J, Oury TD, Chu CT. Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J. Biol. Chem. 2005;280:18536–18542. doi: 10.1074/jbc.M413224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulich S, Horbinski C, Patel M, Chu C. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Rad Biol Med. 2007;43:372–383. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plowey ED, Cherra SJ, 3rd, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress. Autophagy. 2008;4:770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann Neurol. 1998;44 Suppl 1:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- 22.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 23.Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung EC, McBride HM, Slack RS. Mitochondrial dynamics in the regulation of neuronal cell death. Apoptosis. 2007;12:979–992. doi: 10.1007/s10495-007-0745-5. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Westermann B. Molecular machinery of mitochondrial fusion and fission. J Biol Chem. 2008;283:13501–13505. doi: 10.1074/jbc.R800011200. [DOI] [PubMed] [Google Scholar]
- 32.Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordan J, Schrader M. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med. 2008;44:1960–1969. doi: 10.1016/j.freeradbiomed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Sandebring A, Thomas KJ, Beilina A, Brug Mvd, Cleland MM, Ahmad R, Miller DW, Zambrano I, Cowburn RF, Behbahani H, Cedazo-Mínguez A, Cookson MR. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurindependent dephosphorylation of Dynamin-related protein 1. PLoS ONE. 2009 doi: 10.1371/journal.pone.0005701. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 36.Yuan H, Gerencser AA, Liot G, Lipton SA, Ellisman M, Perkins GA, Bossy-Wetzel E. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death Differ. 2007;14:462–471. doi: 10.1038/sj.cdd.4402046. [DOI] [PubMed] [Google Scholar]
- 37.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 39.Cherra SJ, 3rd, Chu CT. Autophagy in neuroprotection and neurodegeneration: A question of balance. Future Neurol. 2008;3:309–323. doi: 10.2217/14796708.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin Z-H, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 41.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007 doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue L, Fletcher GC, Tolkovsky AM. Mitochondria are selectively eliminated from eukaryotic cells after blockade of caspases during apoptosis. Curr Biol. 2001;11:361–365. doi: 10.1016/s0960-9822(01)00100-2. [DOI] [PubMed] [Google Scholar]
- 43.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- 46.Liang CL, Wang TT, Luby-Phelps K, German DC. Mitochondria mass is low in mouse substantia nigra dopamine neurons: Implications for Parkinson's disease. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Chalovich EM, Zhu JH, Caltagarone J, Bowser R, Chu CT. Functional repression of cAMP response element in 6-hydroxydopamine-treated neuronal cells. J Biol Chem. 2006;281:17870–17881. doi: 10.1074/jbc.M602632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu CT, Plowey ED, Wang Y, Patel V, Jordan-Sciutto KL. Location, location, location: altered transcription factor trafficking in neurodegeneration. J Neuropathol Exp Neurol. 2007;66:873–883. doi: 10.1097/nen.0b013e318156a3d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gegg ME, Cooper JM, Schapira AH, Taanman JW. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS ONE. 2009;4:e4756. doi: 10.1371/journal.pone.0004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoepken HH, Gispert S, Morales B, Wingerter O, Del Turco D, Mulsch A, Nussbaum RL, Muller K, Drose S, Brandt U, Deller T, Wirth B, Kudin AP, Kunz WS, Auburger G. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis. 2007;25:401–411. doi: 10.1016/j.nbd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Wood-Kaczmar A, Gandhi S, Yao Z, Abramov AS, Miljan EA, Keen G, Stanyer L, Hargreaves I, Klupsch K, Deas E, Downward J, Mansfield L, Jat P, Taylor J, Heales S, Duchen MR, Latchman D, Tabrizi SJ, Wood NW. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills RD, Sim CH, Mok SS, Mulhern TD, Culvenor JG, Cheng HC. Biochemical aspects of the neuroprotective mechanism of PTEN-induced kinase-1 (PINK1) J Neurochem. 2008;105:18–33. doi: 10.1111/j.1471-4159.2008.05249.x. [DOI] [PubMed] [Google Scholar]
- 53.Unoki M, Nakamura Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene. 2001;20:4457–4465. doi: 10.1038/sj.onc.1204608. [DOI] [PubMed] [Google Scholar]
- 54.Rohe CF, Montagna P, Breedveld G, Cortelli P, Oostra BA, Bonifati V. Homozygous PINK1 C-terminus mutation causing early-onset parkinsonism. Ann Neurol. 2004;56:427–431. doi: 10.1002/ana.20247. [DOI] [PubMed] [Google Scholar]
- 55.Hatano Y, Li Y, Sato K, Asakawa S, Yamamura Y, Tomiyama H, Yoshino H, Asahina M, Kobayashi S, Hassin-Baer S, Lu CS, Ng AR, Rosales RL, Shimizu N, Toda T, Mizuno Y, Hattori N. Novel PINK1 mutations in early-onset parkinsonism. Ann Neurol. 2004;56:424–427. doi: 10.1002/ana.20251. [DOI] [PubMed] [Google Scholar]
- 56.Healy DG, Abou-Sleiman PM, Gibson JM, Ross OA, Jain S, Gandhi S, Gosal D, Muqit MM, Wood NW, Lynch T. PINK1 (PARK6) associated Parkinson disease in Ireland. Neurology. 2004;63:1486–1488. doi: 10.1212/01.wnl.0000142089.38301.8e. [DOI] [PubMed] [Google Scholar]
- 57.Rogaeva E, Johnson J, Lang AE, Gulick C, Gwinn-Hardy K, Kawarai T, Sato C, Morgan A, Werner J, Nussbaum R, Petit A, Okun MS, McInerney A, Mandel R, Groen JL, Fernandez HH, Postuma R, Foote KD, Salehi-Rad S, Liang Y, Reimsnider S, Tandon A, Hardy J, St George-Hyslop P, Singleton AB. Analysis of the PINK1 gene in a large cohort of cases with Parkinson disease. Arch Neurol. 2004;61:1898–1904. doi: 10.1001/archneur.61.12.1898. [DOI] [PubMed] [Google Scholar]
- 58.Valente EM, Salvi S, Ialongo T, Marongiu R, Elia AE, Caputo V, Romito L, Albanese A, Dallapiccola B, Bentivoglio AR. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- 59.Bonifati V, Rohe CF, Breedveld GJ, Fabrizio E, De Mari M, Tassorelli C, Tavella A, Marconi R, Nicholl DJ, Chien HF, Fincati E, Abbruzzese G, Marini P, De Gaetano A, Horstink MW, Maat-Kievit JA, Sampaio C, Antonini A, Stocchi F, Montagna P, Toni V, Guidi M, Dalla Libera A, Tinazzi M, De Pandis F, Fabbrini G, Goldwurm S, de Klein A, Barbosa E, Lopiano L, Martignoni E, Lamberti P, Vanacore N, Meco G, Oostra BA. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology. 2005;65:87–95. doi: 10.1212/01.wnl.0000167546.39375.82. [DOI] [PubMed] [Google Scholar]
- 60.Kumazawa R, Tomiyama H, Li Y, Imamichi Y, Funayama M, Yoshino H, Yokochi F, Fukusako T, Takehisa Y, Kashihara K, Kondo T, Elibol B, Bostantjopoulou S, Toda T, Takahashi H, Yoshii F, Mizuno Y, Hattori N. Mutation analysis of the PINK1 gene in 391 patients with Parkinson disease. Arch Neurol. 2008;65:802–808. doi: 10.1001/archneur.65.6.802. [DOI] [PubMed] [Google Scholar]
- 61.Marongiu R, Ferraris A, Ialongo T, Michiorri S, Soleti F, Ferrari F, Elia AE, Ghezzi D, Albanese A, Altavista MC, Antonini A, Barone P, Brusa L, Cortelli P, Martinelli P, Pellecchia MT, Pezzoli G, Scaglione C, Stanzione P, Tinazzi M, Zecchinelli A, Zeviani M, Cassetta E, Garavaglia B, Dallapiccola B, Bentivoglio AR, Valente EM. PINK1 heterozygous rare variants: prevalence, significance and phenotypic spectrum. Human mutation. 2008;29:565. doi: 10.1002/humu.20719. [DOI] [PubMed] [Google Scholar]
- 62.Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci U S A. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weihofen A, Ostaszewski B, Minami Y, Selkoe DJ. Pink1 Parkinson mutations, the Cdc37/Hsp90 chaperones and Parkin all influence the maturation or subcellular distribution of Pink1. Hum Mol Genet. 2007 doi: 10.1093/hmg/ddm334. [DOI] [PubMed] [Google Scholar]
- 64.Moriwaki Y, Kim YJ, Ido Y, Misawa H, Kawashima K, Endo S, Takahashi R. L347P PINK1 mutant that fails to bind to Hsp90/Cdc37 chaperones is rapidly degraded in a proteasome-dependent manner. Neurosci Res. 2008;61:43–48. doi: 10.1016/j.neures.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- 66.Sim CH, Lio DS, Mok SS, Masters CL, Hill AF, Culvenor JG, Cheng HC. C-terminal truncation and Parkinson's disease-associated mutations down-regulate the protein serine/threonine kinase activity of PTEN-induced kinase-1. Hum Mol Genet. 2006;15:3251–3262. doi: 10.1093/hmg/ddl398. [DOI] [PubMed] [Google Scholar]
- 67.Liu W, Vives-Bauza C, Acin-Perez R, Yamamoto A, Tan Y, Li Y, Magrane J, Stavarache MA, Shaffer S, Chang S, Kaplitt MG, Huang XY, Beal MF, Manfredi G, Li C. PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and alpha-synuclein aggregation in cell culture models of Parkinson's disease. PLoS ONE. 2009;4:e4597. doi: 10.1371/journal.pone.0004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang HL, Chou AH, Yeh TH, Li AH, Chen YL, Kuo YL, Tsai SR, Yu ST. PINK1 mutants associated with recessive Parkinson's disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol Dis. 2007;28:216–226. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Muqit MM, Abou-Sleiman PM, Saurin AT, Harvey K, Gandhi S, Deas E, Eaton S, Payne Smith MD, Venner K, Matilla A, Healy DG, Gilks WP, Lees AJ, Holton J, Revesz T, Parker PJ, Harvey RJ, Wood NW, Latchman DS. Altered cleavage and localization of PINK1 to aggresomes in the presence of proteasomal stress. J Neurochem. 2006;98:156–169. doi: 10.1111/j.1471-4159.2006.03845.x. [DOI] [PubMed] [Google Scholar]
- 70.Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- 71.Haque ME, Thomas KJ, D'Souza C, Callaghan S, Kitada T, Slack RS, Fraser P, Cookson MR, Tandon A, Park DS. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0705363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 2005;337:1133–1138. doi: 10.1016/j.bbrc.2005.09.178. [DOI] [PubMed] [Google Scholar]
- 73.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS biology. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- 75.Shavali S, Brown-Borg HM, Ebadi M, Porter J. Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett. 2008;439:125–128. doi: 10.1016/j.neulet.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Todd AM, Staveley BE. Pink1 suppresses alpha-synuclein-induced phenotypes in a Drosophila model of Parkinson's disease. Genome / National Research Council Canada = Genome / Conseil national de recherches Canada. 2008;51:1040–1046. doi: 10.1139/G08-085. [DOI] [PubMed] [Google Scholar]
- 78.Marongiu R, Spencer B, Crews L, Adame A, Patrick C, Trejo M, Dallapiccola B, Valente EM, Masliah E. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson's disease by disturbing calcium flux. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samann J, Hegermann J, Gromoff EV, Eimer S, Baumeister R, Schmidt E. Caenorhabditis elegans LRK-1 and PINK-1 act antagonistically in stress response and neurite outgrowth. J Biol Chem. 2009 doi: 10.1074/jbc.M808255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taymans JM, Van den Haute C, Baekelandt V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem. 2006;98:951–961. doi: 10.1111/j.1471-4159.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- 81.Scheele C, Petrovic N, Faghihi MA, Lassmann T, Fredriksson K, Rooyackers O, Wahlestedt C, Good L, Timmons JA. The human PINK1 locus is regulated in vivo by a non-coding natural antisense RNA during modulation of mitochondrial function. BMC genomics. 2007;8:74. doi: 10.1186/1471-2164-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abe Y, Shodai T, Muto T, Mihara K, Torii H, Nishikawa S, Endo T, Kohda D. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell. 2000;100:551–560. doi: 10.1016/s0092-8674(00)80691-1. [DOI] [PubMed] [Google Scholar]
- 83.Gandhi S, Muqit MM, Stanyer L, Healy DG, Abou-Sleiman PM, Hargreaves I, Heales S, Ganguly M, Parsons L, Lees AJ, Latchman DS, Holton JL, Wood NW, Revesz T. PINK1 protein in normal human brain and Parkinson's disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- 84.Whitworth AJ, Lee JR, Ho VM, Flick R, Chowdhury R, McQuibban GA. Rhomboid-7 and HtrA2/Omi act in a common pathway with the Parkinson's disease factors Pink1 and Parkin. Disease models & mechanisms. 2008;1:168–174. doi: 10.1242/dmm.000109. discussion 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, Xia K, Jiang W, Ronai Z, Zhuang X, Zhang Z. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest. 2009;119:650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 Forms a Multiprotein Complex with Miro and Milton, Linking Pink1 Function to Mitochondrial Trafficking (dagger) Biochemistry. 2009 doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou C, Huang Y, Shao Y, May J, Prou D, Perier C, Dauer W, Schon EA, Przedborski S. The kinase domain of mitochondrial PINK1 faces the cytoplasm. Proc Natl Acad Sci U S A. 2008;105:12022–12027. doi: 10.1073/pnas.0802814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 89.Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of pink1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009 doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou H, Falkenburger BH, Schulz JB, Tieu K, Xu Z, Xia XG. Silencing of the Pink1 gene expression by conditional RNAi does not induce dopaminergic neuron death in mice. International journal of biological sciences. 2007;3:242–250. doi: 10.7150/ijbs.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 94.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 95.Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim Y, Park J, Kim S, Song S, Kwon SK, Lee SH, Kitada T, Kim JM, Chung J. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 98.Shiba K, Arai T, Sato S, Kubo SI, Ohba Y, Mizuno Y, Hattori N. Parkin stabilizes PINK1 through direct interaction. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Um JW, Stichel-Gunkel C, Lubbert H, Lee G, Chung KC. Molecular interaction between parkin and PINK1 in mammalian neuronal cells. Mol Cell Neurosci. 2009;40:421–432. doi: 10.1016/j.mcn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trimmer PA, Borland MK, Keeney PM, Bennett JP, Jr, Parker WD., Jr Parkinson's disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. J Neurochem. 2004;88:800–812. doi: 10.1046/j.1471-4159.2003.02168.x. [DOI] [PubMed] [Google Scholar]
- 103.Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 105.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K(+)/H(+) exchange activity, osmotic swelling and mitophagy. Cell Death Differ. 2007 doi: 10.1038/sj.cdd.4402167. [DOI] [PubMed] [Google Scholar]
- 107.Kissova I, Deffieu M, Samokhvalov V, Velours G, Bessoule JJ, Manon S, Camougrand N. Lipid oxidation and autophagy in yeast. Free Radic Biol Med. 2006;41:1655–1661. doi: 10.1016/j.freeradbiomed.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 108.Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci U S A. 2008;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, Matsumoto T. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet. 2006;15:883–895. doi: 10.1093/hmg/ddl006. [DOI] [PubMed] [Google Scholar]
- 110.Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morais V, Verstreken P, Huang H, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandermakers W, Lees AJ, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gerencser AA, Nicholls DG. Measurement of instantaneous velocity vectors of organelle transport: mitochondrial transport and bioenergetics in hippocampal neurons. Biophysical journal. 2008;95:3079–3099. doi: 10.1529/biophysj.108.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 114.Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002;4:769–781. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 115.Walsh C, Barrow S, Voronina S, Chvanov M, Petersen OH, Tepikin A. Modulation of calcium signalling by mitochondria. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbabio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 116.Jennings JJ, Jr, Zhu JH, Rbaibi Y, Luo X, Chu CT, Kiselyov K. Mitochondrial Aberrations in Mucolipidosis Type IV. J Biol Chem. 2006;281:39041–39050. doi: 10.1074/jbc.M607982200. [DOI] [PubMed] [Google Scholar]
- 117.Wang D, Qian L, Xiong H, Liu J, Neckameyer WS, Oldham S, Xia K, Wang J, Bodmer R, Zhang Z. Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proc Natl Acad Sci U S A. 2006;103:13520–13525. doi: 10.1073/pnas.0604661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mei Y, Zhang Y, Yamamoto K, Xie W, Mak TW, You H. FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proc Natl Acad Sci U S A. 2009;106:5153–5158. doi: 10.1073/pnas.0901104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Embo J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chu CT, Zhu J, Dagda R. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy. 2007;3:663–666. doi: 10.4161/auto.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu J-H, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13:473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dagda RK, Zhu J, Chu CT. Mitochondrial kinases in Parkinson’s disease: converging insights from neurotoxin and genetic models. Mitochondrion. 2009;9:289–298. doi: 10.1016/j.mito.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]