Summary
Mitochondrial dysfunction is an important intracellular lesion associated with a wide variety of diseases including neurodegenerative disorders. In addition to aging, oxidative stress and mitochondrial DNA mutations, recent studies have implicated a role for the mitochondrial accumulation of proteins such as plasma membrane associated amyloid precursor protein (APP) and cytosolic alpha synuclein in the pathogenesis of mitochondrial dysfunction in Alzheimer’s disease (AD) and Parkinson’s disease (PD), respectively. Both of these proteins contain cryptic mitochondrial targeting signals, which drive their transport across mitochondria. In general, mitochondrial entry of nuclear coded proteins is assisted by import receptors situated in both outer and inner mitochondrial membranes. A growing number of evidence suggests that APP and alpha synclein interact with import receptors to gain entry into mitochondrial compartment. Additionally, carboxy terminal cleaved product of APP, ∼ 4kDa Abeta, is also transported into mitochondria with the help of mitochondrial outer membrane import receptors. This review focuses on the mitochondrial targeting and accumulation of these two structurally different proteins and the mode of mechanism by which they affect the physiological functions of mitochondria
Keywords: Mitochondrial import; Outer membrane translocases; Amyloid precursor protein; alpha synuclein, mitochondrial dysfunction, Alzheimer’s disease, Parkinson’ disease
(1) Introduction
Nature of signal sequences required for directing a protein molecule to a specific cellular compartment have been defined (1, 2 ). Studies for the past several decades on the protein targeting have remarkably contributed to our understanding of mechanisms underlying the transport of protein molecule to a specific cellular compartment (as reviewed in ref# 1-11). Recent studies have demonstrated that several physiologically important protein molecules belonging to animal and plant kingdoms also target to more than one compartment suggesting the presence of multiple hidden signals in these proteins (12-26). Studies have also suggested the need for post translational modifications to activate these hidden signals (13, 19, 21-25). Nevertheless, we are still beginning to understand the mechanisms involved in the activation of hidden signals during the targeting of these proteins to multiple compartments and cellular consequences of multiple organelle localization. Mitochondria are vital organelles for various neuronal functions. The mitochondrion, a double-membrane structure organelle, contains machinery for transcription, translation, and five protein complexes involved in the oxidative phosphorylation to generate adenosine triphosphate (ATP). Each mitochondrion contains multiple copies of 16.5 kb DNA that codes for the 13 proteins. Among 13 proteins, seven are part of complex I, one of complex III, three of complex IV and two of complex V. To carry out the cellular commitments, mitochondria need to import a large number of proteins that are coded by nuclear DNA. Recent proteomic studies suggest that over 1500 nuclear encoded proteins are reported to be imported into mammalian mitochondria under physiological conditions (27). Furthermore, dysfunction of these mitochondrial complexes is well documented during the pathogenesis of neurodegenerative disorders (28-48). However, the precise cause for dysfunction of these complexes in the neurodegenerative disorders is not well understood. A large body of literature has suggested an important role for a number of factors including oxidative stress, mitochondrial DNA mutations, imbalance in calcium homeostasis and aging in the dysfunction of mitochondrial complexes (28-34, 40, 46, 47). In addition, recent studies have also implicated a role for targeting and accumulation of plasma membrane APP and cytosolic alpha synuclein to mitochondria in the pathogenesis of mitochondrial dysfunction in Alzheimer’s and Parkinson’s diseases, respectively. (49-66). It is not clear how APP and alpha synuclein accumulate in the mitochondrial compartment during the pathogenesis of AD and PD respectively. Mitochondrial targeting of alpha synuclein and APP is a challenging and newly emerging field, which may be an important contributor in understanding the mitochondrial dysfunction in neurodegenerative disorders. This review focuses on the role of players involved in the mitochondrial targeting of APP and alpha synuclein and the inhibitory effects of mitochondrial accumulated APP and alpha synuclein on wide varieties of mitochondrial physiological functions resulting in the mitochondrial dysfunction as seen in AD and PD, respectively.
(2) Alpha synuclein and mitochondrial dysfunction in PD models
PD is the second most common progressive neurodegenerative disorder in humans, which is associated with loss of dopaminergic neurons in substantia nigra (67-69). Clinically, PD is characterized by severe motor dysfunction including uncontrollable resting tremor, muscular rigidity, impaired postural reflexes, and bradykinesia. One of the pathological hallmarks of PD and related synucleinopathies is intracellular inclusions called lewy bodies that consist of aggregated alpha synuclein (67-72). Although, the physiological functions of alpha synuclein are not clear but several lines of evidence suggest that it may act as a chaperone that plays a role in regulating membrane stability, neuronal plasticity and enzymatic activities (67, 68, 71-73). Moreover, constitutive levels of alpha synuclein may be important for maintaining the functional integrity of mitochondria inner membrane complexes I and III (57, 74).
Alpha Synuclein exhibits dynamic structural changes based on the local cellular conditions. Various triggering factors, either environmental or genetic, can lead to a cascade of events involving misfolding or loss of normal function of alpha synuclein (67-69,71-73,75). Importantly, two autosomal dominant mutations (A53T), and (A30P) and triplication of the alpha synuclein gene resulting in the increased study state levels of synuclein were linked to familial early onset PD (76-78). It is thought that mutant alpha synuclein proteins tend to aggregate more rapidly than the wild type human alpha synuclein, to form lewy body-like intraneuronal inclusions (67-69, 71, 72).
Several groups have shown mitochondrial dysfunction, oxidative stress and impairment of complex I in pathogenesis of PD (34-39). Complex I is the largest and first of five electron transport linked oxidative phosphorylation complexes of mitochondria and catalyzes the oxidation of NADH, reduction of ubiquinone to generate proton gradient across the membrane. Defect in the function of complex I results in the production of reactive oxygen free radicals. Mammalian complex I is an L shaped structure consisting of 45 subunits. Functionally, the complex I can be subdivided into three distinct fragments. The first part is flavo mononucleotide containing NADH dehydrogenase segment, which is exposed to matrix side of mitochondria. The second part is iron sulfur clusters containing membrane buried portion, which is involved in electron transfer to the electron transporter ubiquinone. The membrane bound transporter segment is the third part of complex I, which is involved in proton translocation. Evidence for impaired complex I mediated mitochondrial dysfunction in PD comes from studies using cybrids that contained mitochondria from PD patients, which showed reduced complex I activity (79, 80). Moreover, chronic administration of rotenone, an inhibitor of mitochondrial complex I, to rat induced the degeneration of tyrosine hydroxylase positive neurons in nigrostriatal region indicating that the perturbation of mitochondrial functions may trigger PD like symptoms (81). Furthermore, overexpression of either wild type or mutant alpha synuclein forms in cell culture systems as well as in transgenic animal models is associated with mitochondrial abnormalities, oxidative stress, and cell loss (57,59,62-64,66). These studies clearly demonstrate the possible relationships among increased alpha synuclein levels, mitochondrial defects and PD pathology in human and rodent models
(3) Mitochondrial abnormalities and APP in AD models
AD is the fourth leading cause of death in the developed world. Besides dementia, the most prominent clinicopathological features of this disease are extracellular deposition of amyloid plaques, intracellular neurofibrillary tangles, synaptic and progressive neuronal degeneration/loss (82, 83). Amyloid plaques consist of deposits of ∼4 kDa peptide called beta amyloid (Abeta), which are derived through proteolytic processing of APP. APP occurs as 3 major isoforms due to alternative splicing of the gene. The shortest form, APP695, lacks the serine protease inhibitor domain and occurs predominantly in neurons while longer non-neuronal forms such as APP770 and APP751 contain the serine protease inhibitor domain (82, 53). However, normal physiological functions of endogenous APP are not thoroughly understood but are thought to be involved in the stabilizing contact points between synapses and maintaining mitochondrial functions (51, 82, 84).
Decreased energy metabolism, decreased mitochondrial fluidity and decreased activity of mitochondrial cytochrome c oxidase, a 13 subunit terminal oxidase in the respiratory chain, leading to mitochondrial dysfunction have been reported in various AD models (33, 40-45). A growing number of studies have reported a possible interconnection among accumulation of full length APP and it’s cleaved product, especially Abeta, oxidative stress and mitochondrial dysfunction in the cellular, transgenic and human AD models including Down’s syndrome patients (85-98). Upregulation of APP, which is influenced by aging, stress and depletion of tropic factors, is also considered to play an important role in the cellular abnormalities including mitochondrial dysfunction in AD (99-102). However, the amount of APP expression needed to bring about cellular abnormalities various from model to model and the presence of familial mutations in and around abeta domain of APP (49, 52, 85-90, 103). Higher levels of mutations bearing neuronal and non-neuronal forms of APP are reported to bring about mitochondrial abnormalities faster than their wild type counterparts (103). Using biochemical and electron microscopy techniques, studies have observed that over expression of non neuronal form APP751 in cultured human muscle-fiber cells and mouse embryonal carcinoma (P19) cells was associated with mitochondrial structural abnormalities and altered mitochondrial membrane potentials (88,89). Collectively, these studies indirectly suggest the involvement of APP in the mitochondrial dysfunction
(4) Mitochondrial Import machinery
The investigations of nature of targeting signals and the interaction with mitochondrial receptors of APP and alpha synuclein are of great importance to understand their direct role in the mitochondrial dysfunction. Mitochondrial targeting signals are rich in basic amino acids, which can form amphipathic α-helices. Majority of mitochondrial proteins have N-terminal mitochondrial targeting signals but in some proteins these signals can either be found at C-terminus or in the internal part of the protein molecule (104-106). In addition, mitochondrial targeted proteins are required to maintain import competent unfolded confirmation to be recognized by translocases of outer membrane (TOM). The functions of these mitochondrial import receptors are well conserved in prokaryotic and eukaryotic organisms (106). Mitochondrial import signals are first recognized by a group of major translocases of outer membrane namely TOM 70, TOM 20 and TOM 22 in a sequential manner. Recent study demonstrates that TOM70 can act like a chaperone to keep proteins in import competent confirmation (107,108). Following the recognition of signals by these surface receptors, proteins are transported through TOM 40, which is a general import pore (GIP) forming protein (109). Barrel forming outer membrane proteins are further recognized by another group of receptors called SAM (sorting and assembly machinery) complex, which assists the insertion of these proteins in to outer membrane ( 104, 105, 106, 110). Recent study has suggested that mitochondrial translocation of some proteins including the ones with N-terminal chimeric signals may involve by passing of outer membrane receptors such as TOM70, 20 and 22 but not TOM40 (111, 112). Proteins that are passed through TOM40 are further recognized by inner membrane translocases (TIM) namely TIM22 and TIM23. TOM40 channel is thought to be larger than TIM 22 and 23 channels. Importantly, targeting to inner membrane receptors requires ATP as well as mitochondrial membrane potential (104-112). Polytypic inner membrane proteins are recognized by the TIM22 complex consists of channel forming TIM22 and peripheral Tim12, Tim8 and Tim13. Matrix and inner membrane anchored mitochondrial proteins are recognized by TIM 23 complex consists of channel forming Tim23 and peripheral (104-106, 110). Though many of matrix targeted proteins contain N-terminal cleavable signals, but there are a large number of mitochondrial proteins do not contain cleavable signals (104, 105, 112).
(5) Mitochondrial targeting signals of alpha synuclein and APP
(a) Mitochondrial signals of Alpha synuclein
Alpha synuclein, a 140 amino acid presynaptic soluble protein. Prediction analyses suggest the possible presence of mitochondrial targeting like signals (57). In addition, the alignment of N-terminal sequence of alpha synuclein with cleavable N-terminal mitochondrial targeting signals of bonafied mitochondrial cytochrome P450Scc (CYP11A1) and cytochrome P450 sterol 27-hydroxylase (CYP27A1) suggests that first 32 amino acids of N terminus of alpha synuclein contains 6 positively charged amino acids (Lysine) and are capable of forming helical structure. These properties of N-terminus of alpha synuclein resemble the physico-chemical properties of mitochondrial targeting sequences (Fig.1A). However, it is unclear whether mitochondrial targeting signals of alpha synuclein are cleaved by matrix proteases following the intramitochondrial entry. Recent reports including our laboratory have observed constitutive presence of synuclein in the mitochondria from the brains of rodents and humans (57-61). Using biochemical and cell biological approaches including deletion constructs of alpha synuclein, Devi et al (57) have shown that N-terminal 32 amino acid region of synuclein may be critical for mitochondrial targeting in dopaminergic DAN neurons and isolated mitochondria.
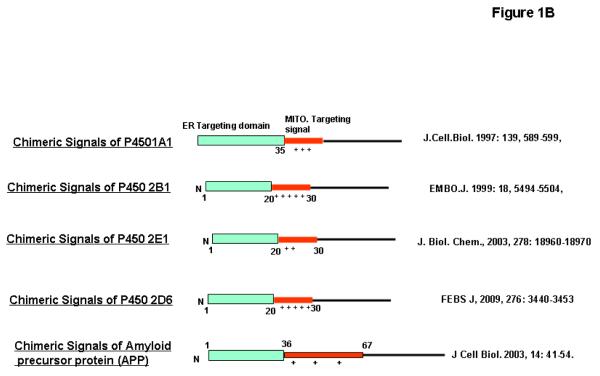
Figure 1. Mitochondrial targeting signals in alpha synuclein and APP proteins.
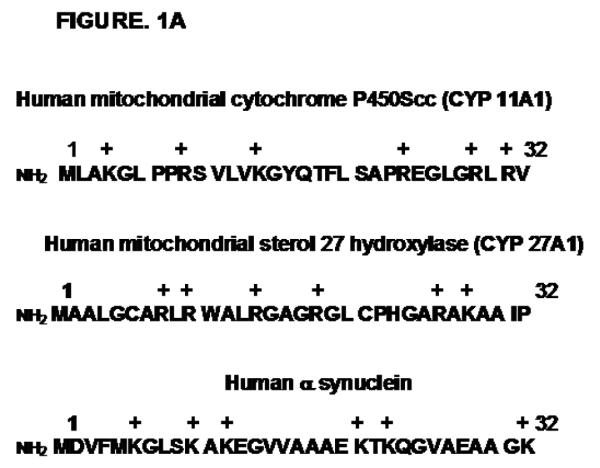
A, Physico-chemical similarities of N-terminal 32 aminoacid sequence of human alpha synuclein with mitochondrial targeting signals of human P40Scc and P450 c27. (B) Comparison of N-terminal chimeric signals of P450 1A1, 2B1, 2E1 and 2D6 with N-terminus of APP comprising of hydrophobic ER-targeting domain followed by the positively charged mitochondrial-targeting domain.
(b) Mitochondrial signal of APP
Unlike alpha synuclein, mitochondrial targeting signals of APP, which are conserved in all three major forms of APP, seem to be different and show similarities with non-cannonical chimeric signals that are discovered and characterized by Avadhani and colleagues (22-26). Chimeric signals are defined as the combination of endoplasmic reticulum (ER) and mitochondrial targeting signals arranged in tandem. These signals can localize a protein translated from a single gene to ER and mitochondria. By virtue of these chimeric signals at the N-terminus, xenobiotic-inducible cytochrome P4501A1, 2B1, 2E1 and 2D6 are targeted to both endoplasmic reticulum and mitochondria (22-26). The first 35 amino acid residues of APP resemble ER targeting signal, while 35-67 aminoacid sequence resemble the mitochondrial targeting signal of cytochrome P4501A1, 2B1 and 2E1 (Fig.1B). Studies for the past decade showed that the activation of mitochondrial signals hidden in the chimeric signals of proteins is under the control of several physiological factors such as proteases and phosphorylation. These factors likely vary from protein to protein. For example, mitochondrial targeting signals of P4501A1 are activated by soluble serine protease, which cleaves the ER targeting domain of this protein (22, 25). In contrast, PKA-mediated phosphorylation at ser 128 and ser 129 activates mitochondrial signals of cytochrome P4502B1 and 2E1, respectively (23, 24).
Using in vitro mitochondrial import, in vivo transient transfection and confocal immunofluoroscence our laboratory showed that the endogenous as well as ectopically expressed Alzheimer’s full length wild type and Swedish APP695 in HCN-1A neuronal cells are localized to both plasma membrane and mitochondria (49). Furthermore, the positively charged residues at 40, 44, and 51 of APP are seemed to be important for targeting to mitochondria (49). Consistent with these results, using biochemical and immunofluorescence techniques several reports have demonstrated that endogenous as well as ectopically expressed APP forms are localized to mitochondria in wide variety of cell lines such as PC12, COS, HEK 293, MEF cells (49-52,54,55). However, the mechanism by which hidden mitochondrial targeting signals are activated in APP protein is unclear.
(6) Interactions of alpha synuclein and APP with mitochondrial import receptors
(a) Interaction of Alpha synuclein with import receptors
Mitochondrial localization of alpha synuclein seems to vary with physiological conditions, cell lines and species (57-64). Very limited number of studies has focused on the involvement of mitochondrial outer and inner membrane receptors in the import of alpha synuclein (57-65). However, recent study suggests that the interaction of alpha synuclein with mitochondria is very selective and instant (113). Furthermore, intra mitochondrial localization of α synuclein is dependent on energy and membrane potential (57). Using in vitro import, it has been shown that the intra mitochondrial entry of alpha synuclein is blocked by antibodies to outer membrane TOM40 protein suggesting the requirement of general import pore forming TOM 40 protein (57). In support of this, an interesting study using in vitro pull down of mitochondrial proteins by synthetic C-terminal peptide of alpha synuclein suggests the interaction of alpha synuclein with TOM40 (65). In addition, alpha synuclein is also reported to interact with SAM 50, an outer membrane protein involved in the insertion of beta barrel protein (65).
(b) Interaction of APP with import receptors
Topology of mitochondrial full length APP as judged by limited trypsin digestion is such that its NH2-terminus is located inside the mitochondria while the COOH-terminal of the protein facing the cytoplasmic side (49). Nin-Cout orientation of mitochondrial APP was further supported by using multiple biochemical and proteomic approaches (54). Furthermore, mitochondrially associated APP is a non-glycosylated protein as opposed to plasma membrane associated APP, which is glycosylated. Combination of chemical cross-linking and immunoelectron microscopy study suggest that mitochondrial associated APP is in contact with mitochondrial outer membrane (TOM20, 22 and 40) and inner membrane (TIM23) translocase proteins (49). This is in contrast to the import mode of putative mitochondrial proteins whose interactions with translocases are transient during their entry in to mitochondria (97). Additionally, acidic domain spanning 220–290 amino acids of APP may be responsible for the Nmito- Ccyto topology and the incomplete intra mitochondrial entry resulting in the contact with import receptors (49). This phenomenon was also observed in the mitochondria of post mortem AD brains by Blue-native gel electrophoresis coupled western blotting with antibodies specific to import receptors, which showed outer membrane TOM40 associated ∼ 480 and ∼620 kDa complexes and inner membrane translocase TIM 23 associated ∼620 kDa complex (56). These results suggest the possibility that mitochondrial APP may form at least two different steady state import intermediates in AD brain mitochondria.
(7) Sub mitochondrial localization of alpha synuclein and APP
(a) Localization of Alpha synuclein
Alpha synuclein is reported to be localized to outer membrane, intermembrane space and inner membrane of mitochondria depending on species, cell lines and variations in the intracellular pH (57-62, 64). Interestingly, under acidic conditions, ectopically expressed alpha synuclein has been shown to localize exclusively to outer membrane (62). Studies have shown the constitutive presence of alpha synuclein in mitochondria of rodents and humans (57, 59-61). Electron microscopy analysis suggest that constitutively expressed alpha synuclein is exclusively localized to outer membrane in mouse mitochondria (57, 63), while outer and inner membranes of mitochondria from rat and human brain show the immunoreactivity for alpha synuclein (57,59-61). In vitro imported alpha synuclein in the presence of energy predominantly localizes to inner membrane (57). In agreement with these studies, using sub mitochondrial fractionation technique a recent study demonstrated that the in vitro transported alpha synuclein was mainly accumulated in the inner membrane of mitochondria (61). However, the topology of mitochondrial alpha synuclein and the reason for it’s versatile sub mitochondrial localization is not known.
(b) Localization of APP
In vitro, neuronal cultures and human AD models show that full length APP resides in mitochondria in Nin mito-Cout cyto orientation as well as in close association with outer membrane channel forming TOM40 protein (49, 56). However, ∼4 kDa abeta, a derivative of full length APP is predominantly localized to inner membrane of mitochondria (56, 90, 93, 95, 97,114).
(8) Regional and cellular distribution of mitochondrial alpha synuclein and APP under normal and disease status
The brain is a complex organ, with cellular, regional and functional heterogeneity. Pathology of PD and AD that includes mitochondrial dysfunction is regional and cellular specific. However, the cause for not regional and cellular specificity of these pathologies is known. Clearly, alpha synuclein and APP are implicated in the pathogenesis of PD and AD, respectively. Furthermore, mitochondrial accumulation of these proteins is associated with mitochondrial dysfunction in the culture systems (49, 52, 53, 57, 59, 62). Thus, investigation of mitochondrial association of alpha synuclein and APP in different regions and neurons in normal and disease brains may help in understanding the regional and cellular specificity of these proteins in causing mitochondrial dysfunction during the pathogenesis of PD and AD.
(a) Distribution of mitochondrial Alpha synuclein
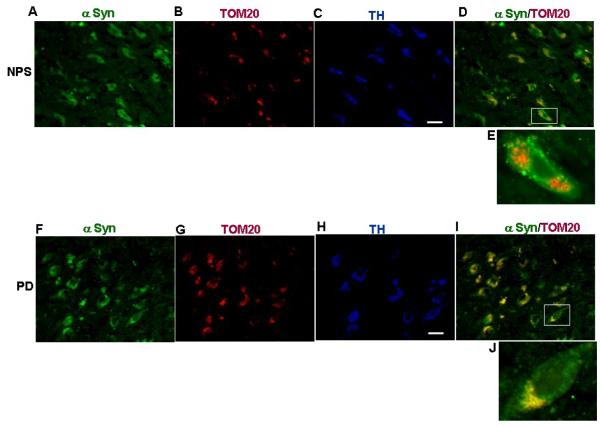
Constitutive presence of alpha synuclein in the mitochondria of brain differs from region to region in rat and humans (57, 60, 61). Mitochondria from cerebellum and cortex have relatively lower levels of alpha synuclein than hippocampus, striatum and substantia nigra (57, 60, 61). However, alpha synuclein seems to be accumulating in the striatum, substantia nigra and cortex of PD brains and these levels varies from 10-100 ng/mg mitochondrial protein (57). Dopaminergic neurons are the most affected neuronal type in the PD. Ectopically expressed alpha synuclein in dopaminergic DAN neurons accumulates in the mitochondria compartment (57). To test the presence of α synuclein in mitochondria of dopaminergic neurons of PD brains, we carried out using triple immuno staining on deparaffinised tissue sections from postmortem substantia nigra (SN) of normal and PD subjects (obtained from NDRI, Philadelphia according to institutional IRB approved protocols and were characterized as described ref # 57) with antibodies against alpha synuclein and TOM20. Following this, sections were also stained with tyrosine hydroxylase (TH) antibodies to identify dopaminergic neurons. In the SN of normal, a robust intracellular staining of alpha synuclein antibodies (Fig 2A.) and TOM 20 (Fig 2B.)-specific staining of particulate structures reminiscent of mitochondria was observed. However, very little staining of α synuclein overlapped with mitochondrial TOM 20 staining in normal of SN (Fig. 2D) and some of these neurons stained positively for TH antibody (Fig.2C). However, in the SN of PD, the α synuclein antibody stained appreciable amount of extranuclear punctate structures (Fig.2F) while TOM20 antibody showed specific mitochondrial staining (Fig.2G). Contrary to control, extranuclear punctate structures of α synuclein staining in the SN of PD overlapped with a significant number of mitochondrial TOM 20 staining (Fig.2I) and some of these neurons also stained for TH antibody (Fig.2H). These results suggest the presence of low levels of alpha synuclein in the mitochondria of dopaminergic neurons of normal brain while in the dopaminergic neurons of PD brain contains higher levels of alpha synuclein.
Figure 2. Immunofluorescence microscopy analysis of mitochondrial α synuclein in dopaminergic neurons of substantia nigra of non-PD and PD subjects.
Figures A-E: Triple labeling of dopaminergic neurons of substantia nigra of post mortem non-PD subject (NPS # 11) with anti rabbit α synuclein (A), anti goatTOM20 (B), and anti mouse TH (C). (D) Merged image of A and B. E= enlarged neuron. Figures F-J: Triple labeling of dopaminergic neurons of substantia nigra of post mortem PD subject (#11) with anti rabbit α synuclein (F), anti goatTOM20 (G), and anti mouse TH (H). (I) Merged image of F and G. J= enlarged neuron. Immunostaining was carried out as described in ref# 56. Bar= 100 μm.
(b) Distribution of mitochondrial APP
APP immunoreactivity in the mitochondria from post mortem AD brains (n=20) was higher than that of non-AD brains (n=20) (56). Furthermore, accumulation of mitochondrial APP in AD brains is dependent on the severity of the disease, which varied from region to region. Using quantitative ELISA, it is estimated that mitochondrial APP levels were in the range of 0.1 to 2.5 μg APP antibody reactive protein/mg mitochondrial protein (56). Interestingly, AD vulnerable brain regions such as frontal cortex, hippocampus and amygdale showed higher amounts of mitochondrial APP in AD brains. However, mitochondrial APP levels seem to be below detectable in non-AD brains (56). Mitochondrial accumulation of APP in different neurons in AD brain also show variability with respect to the severity of the disease. AD brains of all stages showed the accumulation of APP in the mitochondria of cholinergic neurons while mitochondrial APP accumulation was observed in dopaminergic, GABAergic and glutamatergic of AD brains belong to severe category (56). Collectively, these results suggest that mitochondrial accumulation of APP in various neuronal systems in different brain regions may have far reaching influence on mitochondrial dysfunction that might influence the neuronal survival in the pathogenesis of AD.
. It is known that the presence of ApoE4 allele is considered to be one of the risk factors in the pathogenesis of sporadic AD (115,116). Studies have reported that apolipoprotein E (ApoE) genotype may also influence mitochondrial dysfunction (117). Interestingly, majority of AD subjects showed one or two ApoE4 alle and also possessed highest amounts of mitochondrial APP (56). Nevertheless, the precise relationship between mitochondrial accumulation of APP and ApoE genotyping in the pathogenesis of AD subjects is not known. However, it is noteworthy to mention that recent studies have shown mutations on TOM40 gene, which is located on the chromosome (19q) in close proximity to upstream of ApoE, as a possible risk factor in the genesis of AD (118,119). Based on this, one can speculate that mutations on TOM40 gene may result in the dysfunction of general import pore TOM40 protein, which may in turn accentuate the mitochondrial translocational arrest of APP and associated mitochondrial dysfunction. However, it remains to be seen whether mutations render impairment of TOM40 functions during the pathogenesis of AD.
(9) Mitochondrial accumulation of synuclein and mitochondrial dysfunction in cellular and human PD models
The involvement of alpha synuclein in bringing about the complex I dysfunction in alpha synuclein linked PD or sporadic PD has been a subject of intense investigation. Recent evidences suggest that alpha synuclein and mitochondria interact with each other (57-65, 113). Lee et al (120) have also shown that an impaired mitochondrial function can induce increased alpha synuclein expression and formation of alpha synuclein inclusions. Alpha synuclein overexpression is also associated with the release of cytochrome c, increase of mitochondrial calcium and nitric oxide, and oxidative modification of mitochondrial components (121,122). Since increased expression of alpha synuclein and mitochondrial complex I deficiency are both implicated in PD pathogenesis, alpha synuclein localization in mitochondria may indirectly suggest a functional link between alpha synuclein and complex I.
Biochemical and immunoelectron microscopy data suggest that mitochondria-localized alpha synuclein is predominantly associated with inner membrane in the human and rat systems (57, 61). In DAN cell culture system and under in vitro import conditions alpha synuclein was shown to accumulate with time predominantly in the inner mitochondrial membrane (57). Interestingly, accumulation of α-synuclein in the mitochondria of human dopaminergic neurons caused reduced mitochondrial complex I activity and increased production of reactive oxygen species (ROS), whereas α-synuclein lacking the mitochondrial targeting signal failed to associate with the mitochondria and was not able to induce mitochondrial dysfunction (57). These results show a direct link between mitochondrial accumulation of alpha synuclein and mitochondrial dysfunction. Mitochondria from SN, striatum, and cerebellum of postmortem PD patients and controls showed the constitutive presence of α-synuclein in the mitochondria of all three brain regions from normal subjects. Interestingly, mitochondria isolated from SN and striatum but not cerebellum from PD subjects showed significant accumulation of α-synuclein and decreased complex I activity (57). Studies on the incubation of alpha synuclein with isolated cerebellar and striatal mitochondria show the inhibition of complex I activity (57, 61). Furthermore, this inhibition by alpha synuclein was dose-dependent, with the minimal effective concentration being as low as 1 pM (61). Blue native gel electrophoresis and immunocapture analysis further revealed the mitochondrial accumulated synuclein in both PD brains and neuronal cultures were associated with holo complex I (57). In addition, these experiments also revealed the presence of sub complexes of complex I. In support of these results a study using proteomic approach also showed the interaction between alpha synuclein and complex I subunits (65). Recently, it has been shown that the intricate assembly of complex I comprises the assembly of small sub complexes to a holo complex I in a sequential manner (123). However, it is not clear how alpha synuclein brings about the complex I dysfunction either by preventing the assembly or by disrupting the holo complex I. Nevertheless, these results for the first time show a direct connection between higher levels of mitochondrial synuclein and complex I mediated mitochondrial dysfunction in the etiology of PD. Alpha synuclein has been shown to interact with other mitochondrial complexes. Using yeast two-hybrid study, wild type alpha synuclein was found to interact with the mitochondrial cytochrome c oxidase (124). It was also reported that mutant (A53T) alpha synuclein over expressing transgenic mice developed mitochondrial degeneration as well as reduced complex IV activity (64).Taken together, the above literature suggests that the background levels of mitochondrial alpha synuclein may be an important factor influencing mitochondrial functions
(10) Implications of mitochondrial accumulation of full length APP and its C-terminal product abeta in AD models
(a) Mitochondrial accumulation of APP and mitochondrial dysfunction
Results from ours and other’s laboratories have observed decreased mitochondrial functions such as defects in oxidative phosphorylation, decreased ATP, decreased membrane potential, and increased production of ROS, perturbation in mitochondrial fusion and fission following the mitochondrial association of full length APP in various cellular, transgenic and human AD models (49-56). Accumulation of full-length APP in the mitochondrial compartment of cortex and hippocampus was observed in familial APP over-expressing Tg2576 mouse model (12 months old), which is accompanied by decreased impaired cytochrome c oxidase activity and decreased ATP level. (49). Decreased cytochrome c oxidase activity, decreased ATP levels and increased nitric oxide levels were also accompanied by mitochondrial accumulation of APP in PC12 and HEK cells (49,52). These abnormalities took place at a faster rate in models expressing APP with familial mutations than in wild type expressing models. Furthermore, mitochondrial accumulation of APP and the associated mitochondrial abnormalities were progressive.
Consistent with animal and cellular models, mitochondria isolated from these AD brains showed increased H2O2 levels, indicating impaired cytochrome c oxidase activity, which was associated with translocationally arrested mitochondrial APP (56). These studies indicate that mitochondrial function may be a direct target for APP. However, the expression of mitochondrial targeting mutant was accompanied by reduced mitochondrial dysfunction suggesting the direct involvement of mitochondrial accumulated APP in bringing about the some of the mitochondrial anomalies. In addition, accumulation of APP lacking the acidic domain was also accompanied by decreased mitochondrial dysfunction implying the involvement of incomplete mitochondrial translocation of APP mediated by the acidic domain spanning 220-290 amino acids of the protein in causing mitochondrial dysfunction. (49) Translocational arrest by acidic domain may result in the inhibition of the import of proteins essential for normal mitochondrial functions. In vitro mitochondrial import using freshly isolated mitochondria from AD brains showed the inhibition of cytochrome c oxidase subunits IV and Vb (56). The consequences of such inhibition under in vivo environment may result in oxidative stress from perturbation of cytochrome c oxidase. In support of the negative role associated with the acidic domain, a study showed that intracellular accumulation of acidic domain is capable of inducing cell death. However, the precise mechanism for acidic domain mediated mitochondrial translocational arrest in the pathogenesis of AD is unclear.
In contrast, constitutive low levels of APP in mitochondria are important for maintaining mitochondrial functions (51). Furthermore, mitochondrial APP is reported to be interacting with complex V in primary neuronal cultures (50). Collectively, these studies suggest that the like mitochondrial alpha synuclein, background concentration of APP associated with mitochondria may play a critical role in influencing mitochondrial functions.
(b) Mitochondrial accumulation of Abeta and mitochondrial dysfunction
Using immunoelectron microscopy, Yamaguchi et al (125) for the first time reported the presence of immunoreactive Abeta in the mitochondria of AD brains. A large number of studies using multiple approaches have found ∼4kDa Abeta in the mitochondria of transgenic mouse, cellular and human AD models (56, 90-97,114). Very recently, Abeta is reported to be imported from outside into mitochondria (94, 114). Using antibody inhibition technique, Hanson, et al (114) found that mitochondrial import of Abeta requires the interaction with all major TOM proteins such as TOM20, TOM70 and channel forming protein TOM40. Though in vitro imported Abeta is localized to inner membrane of mitochondria interestingly, it’s import is not dependent on mitochondrial membrane potential (114). However, the presence of mitochondrial targeting signals in Abeta is yet to be identified. Several reports have reported the role of mitochondrial Abeta in affecting a wide variety of mitochondrial functions (90-97). In collaboration with Cu2+, Abeta was shown to inhibit the cytochrome c oxidase activity. in transgenic mouse models (92). Reddy and colleagues using various biochemical and cell biological approaches have shown that Abeta can induce hydrogen peroxide production, decreased cytochrome oxidase activity, synaptic dysfunction and increased formation of carbonyl proteins in transgenic and cellular models of AD (48,87,90,126). In addition, Yan and colleagues showed that mitochondrial localized Aβ has been directly shown to (a) inhibit Aβ binding alcohol dehydrogenase (b) perturb mitochondrial permeability transition pore functions and (c) impair enzymatic activities of respiratory chain complex III and IV resulting in the reduced rate of oxygen consumption in transgenic mouse and human AD models (93,95,97). These studies collectively suggest that Aβ localized to mitochondria may render mitochondria vulnerable to oxidative damage by interacting with mitochondrial proteins.
(11) Concluding remarks
Physico-chemical properties of mitochondrial targeting signals of alpha synuclein and APP share similarity with that of putative mitochondrial signals. Though, these signals interact with mitochondrial import receptors, the mechanisms by which these signals become fully active are not clear. Studies show that accumulation of these proteins in the mitochondrial compartment may bring about mitochondrial dysfunction. The available literature suggests that the mitochondrial targets for these proteins seem to be different. Mitochondrially accumulated alpha synuclein may prefer to target complex I while APP may prefer to target complex IV and import machinery. It is important to further investigate the mechanisms underlying sub-mitochondrial localization of these proteins in order to understand their influence on mitochondrial functions. Another note worthy point is that mitochondrial accumulation of these proteins in certain brain regions and neurons may be an important factor in inducing mitochondrial abnormalities in the pathogenesis of AD and PD. In conclusion, investigation of factors responsible for the regional and cellular variations in the mitochondrial targeting and accumulation of these proteins may be an important future direction to unravel the mitochondrial dysfunction in AD and PD.
Acknowledgements
We thank Dr. N.G. Avadhani ((University of Pennsylvania) for his valuable suggestions. This work was supported by Alzheimer’s Association grant IIRG-08-89896 and NIH grant R01 AG 021920
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Schatz G, Dobberstein B. Common Principles of Protein Translocation Across Membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- [2].Wickner W, Schekman R. Protein Translocation Across Biological Membranes. Science. 2005;310:1452–1456. doi: 10.1126/science.1113752. [DOI] [PubMed] [Google Scholar]
- [3].Dolezal P, Likic V, Tachezy J, Lithgow JT. Evolution of the Molecular Machines for Protein Import into Mitochondria. Science. 2006;313:314–318. doi: 10.1126/science.1127895. [DOI] [PubMed] [Google Scholar]
- [4].Subramani S. Components Involved in Peroxisome Import, Biogenesis, Proliferation, Turnover, and Movement. Physiol. Rev. 1998;78:171–188. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- [5].von Heinj G. Signal sequences. The limits of variation. J. Mol. Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- [6].Wilkinson BM, Regnacq M, Stirling CJ. Protein trans- location across the membrane of the endoplasmic reticulum. J Membr. Biol. 1997;155:189–197. doi: 10.1007/s002329900171. [DOI] [PubMed] [Google Scholar]
- [7].Gilmore R, Walter P, Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J. Cell Biol. 1982;95:470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wickner WT, Lodish HF. Multiple mechanisms of insertion into and across membranes. Science. 1985;230:400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- [9].Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- [10].Andres DA, Dickerson IM, Dixon JE. Variants of the carboxyl-terminal KDEL sequence direct intracellular retention. J. Biol. Chem. 1990;265:5952–5955. [PubMed] [Google Scholar]
- [11].Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- [12].Pino P, Foth BJ, Kwok LY, Sheiner L L, Schepers R, Soldati T, Soldati-Favre D. Dual targeting of antioxidant and metabolic enzymes to the mitochondrion and the apicoplast of Toxoplasma gondii. PLos Pathogens. 2007;3:1092–1108. doi: 10.1371/journal.ppat.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Regev-Rudzki N, Pines O. Eclipsed distribution: A phenomenon of dual targeting of protein and its significance. Bioessays. 2007;29:772–782. doi: 10.1002/bies.20609. [DOI] [PubMed] [Google Scholar]
- [14].Ratnayaka A, Paraoan L, Spiller DG, Hiscott P, Nelson G, White MRH, Grierson I. A dual Golgi- and mitochondria-localised Ala25Ser precursor cystatin C: An additional tool for characterising intracellular mis-localisation leading to increased AMD susceptibility. Exptl Eye Res. 2007;84:1135–1139. doi: 10.1016/j.exer.2006.01.030. [DOI] [PubMed] [Google Scholar]
- [15].Lu ZH, Chakraborty G, Ledeen RW, Yahya D, Wu G. N-acetylaspartate synthase is bimodally expressed in microsomes and mitochondria of brain. Mol. Brain Res. 2004;122:71–78. doi: 10.1016/j.molbrainres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- [16].Sass E, Blachinsky E, Karniely S, Pines O. Mitochondrial and cytosolic isoforms of yeast fumarase are derivatives of a single translation product and have identical amino termini. J. Biol.Chem. 2001;276:46111–46117. doi: 10.1074/jbc.M106061200. [DOI] [PubMed] [Google Scholar]
- [17].Chatre L, Matheson LA, Jack AS, Hanton SL, et al. Efficient mitochondrial targeting relies on co-operation of multiple protein signals in plants. J. Exptl Botony. 2009;60:741–749. doi: 10.1093/jxb/ern319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chabregas SM, Luche DD, Farias LP, Ribeiro AF, et al. Dual targeting properties of the N-terminal signal sequence of Arabidopsis thaliana THI1 protein to mitochondria and chloroplasts. Plant Mol Biol. 2001;46:639–650. doi: 10.1023/a:1011628510711. [DOI] [PubMed] [Google Scholar]
- [19].Karniely S, Pines O. Single translation to dual destination:mechanisms of dual protein targeting in eukaryotes. EMBO Rep. 2005;6:420–425. doi: 10.1038/sj.embor.7400394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Regev-Rudzki N, Pines O. Eclipsed distribution: A phenomenon of dual targeting of protein and its significance. Bioessays. 2007;29:772–782. doi: 10.1002/bies.20609. [DOI] [PubMed] [Google Scholar]
- [21].Aguiar M, Masse R, Gibbs BF. Regulation of cytochrome P450 by posttranslational modification. Drug Metab Rev. 2005;37:379–404. doi: 10.1081/dmr-46136. [DOI] [PubMed] [Google Scholar]
- [22].Addya S, Anandatheerthavarada HK, Biswas G, Bhagwat SV, Mullick J, Avadhani NG. Targeting of NH2-terminal-processed microsomal protein to mitochondria: a novel pathway for the biogenesis of hepatic mitochondrial P450MT2. J Cell Biol. 1997;139:589–599. doi: 10.1083/jcb.139.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Anandatheerthavarada HK, Biswas G, Mullick J, Sepuri NB, Otvos L, Pain D, Avadhani DNG. Dual targeting of cytochrome P4502B1 to endoplasmic reticulum and mitochondria involves a novel signal activation by cyclic AMP-dependent phosphorylation at ser128. EMBO J. 1999;18:5494–5504. doi: 10.1093/emboj/18.20.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Robin MA, Anandatheerthavarada HK, Biswas G, Sepuri NB, Gordon DM, Pain D, Avadhani NG. Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation. J Biol Chem. 2002;277:40583–40593. doi: 10.1074/jbc.M203292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boopathi E, Srinivasan S, Fang JK, Avadhani NG. Bimodal protein targeting through activation of cryptic mitochondrial targeting signals by an inducible cytosolic endoprotease. Mol Cell. 2008;32:32–42. doi: 10.1016/j.molcel.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sangar MC, Anandatheerthavarada HK, Tang W, Prabu SK, Martin MV, Dostalek M, Guengerich FP, Avadhani NG. Human liver mitochondrial cytochrome P450 2D6 — individual variations and implications in drug metabolism. 2009;276:3440–3453. doi: 10.1111/j.1742-4658.2009.07067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated Analysis of Protein Composition, Tissue Diversity, and Gene Regulation in Mouse Mitochondria. Cell. 2003;115:629–640. doi: 10.1016/s0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- [28].Manfredi G, Beal MF. The role of mitochondria in the pathogenesis of neurodegenerative diseases. Brain Pathol. 2000;10:462–472. doi: 10.1111/j.1750-3639.2000.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta. 1998;1366:211–223. doi: 10.1016/s0005-2728(98)00114-5. [DOI] [PubMed] [Google Scholar]
- [30].Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A Dawn for Evolutionary Medicine. Ann Rev Gen. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Ann Rev Neuroscience. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- [32].Lin Michael T., Flint M. Beal Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006 October 19;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- [33].Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res. Brain Res. Rev. 2005;49:618. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [34].Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson’s disease. IUBMB Life. 2001;52:135. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- [35].Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- [36].Keeney PM, Xie J, Capaldi RA, Bennett JP. J.P, Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- [38].Gu M, Gash MT, Cooper JM, Wenning GK, Daniel SE, Quinn NP, Marsden CD, Schapira AH. Mitochondrial respiratory chain function in multiple system atrophy. Mov Disord. 1997;12:418–422. doi: 10.1002/mds.870120323. [DOI] [PubMed] [Google Scholar]
- [39].Swerdlow RH, Parks JK, Davis JN, Cassarino DS, Trimmer PA, Currie LJ, Dougherty J, Bridges WS, Bennett JP, P J, Wooten GF, Parker WD. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson’s disease family. Ann.Neurol. 1998;44:873–881. doi: 10.1002/ana.410440605. [DOI] [PubMed] [Google Scholar]
- [40].Sims NR. Energy metabolism, oxidative stress and neuronal degeneration in Alzheimer’s disease. Neurodegeneration. 1996;5:435–440. doi: 10.1006/neur.1996.0059. [DOI] [PubMed] [Google Scholar]
- [41].Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Parker WD. Cytochrome oxidase deficiency in Alzheimer’s disease. Ann. N Y. Acad. Sci. 1991;640:59–64. doi: 10.1111/j.1749-6632.1991.tb00191.x. [DOI] [PubMed] [Google Scholar]
- [43].Chandrasekaran K, Hatanpaa K, Brady DR, Stoll J, Rapoport SI. Downregulation of oxidative phosphorylation in Alzheimer Disease: loss of cytochrome oxidase subunit mRNA in the hippocampus and entorhinal cortex. Brain Res. 1998;796:13–19. doi: 10.1016/s0006-8993(98)00248-0. [DOI] [PubMed] [Google Scholar]
- [44].Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol. Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- [45].Parker WD, Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, Cullum CM. Reduced platelet cytochrome c oxidase activity in Alzheimer’s disease. Neurology. 1994;44:1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- [46].LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- [47].Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- [48].Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- [49].Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J. Cell Biol. 2003;161:41. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schmidt C, Lepsverdize E, Chi SL, Das AM, Pizzo SV, Dityatev A, Schachner M. Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Mol Psychiatry. 2008;13:953–969. doi: 10.1038/sj.mp.4002077. [DOI] [PubMed] [Google Scholar]
- [51].Sheng BY, Niu Y, Zhou H, Yan JX, Zhao NM, Zhang XF, Gong YD. The mitochondrial function was impaired in APP knockout mouse embryo fibroblast cells. Chinese Science Bulletin. 2009;54:1725–1731. [Google Scholar]
- [52].Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, Muller-Spahn F, Haass C, Czech C, Pradier L, Muller WE, Eckert A. Amyloid betainduced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J. Biol. Chem. 2004;279:50310. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- [53].Anandatheerthavarada HK, Devi L. Mitochondrial translocation of amyloid precursor protein and its cleaved products: Relevance to mitochondrial dysfunction in Alzheimer’s disease. Rev in the Neurosciences. 2007;18:343–354. doi: 10.1515/revneuro.2007.18.5.343. [DOI] [PubMed] [Google Scholar]
- [54].Park HJ, Kim SS, Seong YM, Kim KH, Goo HG, Yoon EJ, Min d.S., Kang S, Rhim H. Beta-amyloid precursor protein is a direct cleavage target of HtrA2 serine protease. Implications for the physiological function of HtrA2 in the mitochondria. J. Biol. Chem. 2006;281:34277. doi: 10.1074/jbc.M603443200. [DOI] [PubMed] [Google Scholar]
- [55].Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain isassociated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li WW, Yang R, Guo JC, Ren HM, Zha XL, Cheng JS, Cai DF. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007;18:1543–1546. doi: 10.1097/WNR.0b013e3282f03db4. [DOI] [PubMed] [Google Scholar]
- [59].Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol. Life Sci. 2008;65:1272–1284. doi: 10.1007/s00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Uéda K, Yu S, Yang H. Semiquantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody. Brain Res. 2008;1244:40–52. doi: 10.1016/j.brainres.2008.08.067. [DOI] [PubMed] [Google Scholar]
- [61].Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Uéda K, Chan P, Yu S. alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neuroscience Lett. 2009;454:187–192. doi: 10.1016/j.neulet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- [62].Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exptl Cell Res. 2008;314:2076–2089. doi: 10.1016/j.yexcr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shavali S, Brown-Borg HM, Ebadi M, Porter J. Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett. 2008;439:125–128. doi: 10.1016/j.neulet.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neuroscience. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McFarland MA, Ellis CE, Markey SP, Nussbaum RL. Proteomics analysis identifies phosphorylation-dependent alpha-synuclein protein interactions. Mol. Cell Proteomics. 2008;7:2123–2137. doi: 10.1074/mcp.M800116-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory A,M, Wong J, Takenouchi T, Hashimoto M, Masliah E. Alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cookson MR. The biochemistry of Parkinson’s disease. Annu.Rev.Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- [68].Shults CW. Lewy bodies. Proc.Natl.Acad.Sci.U.S.A. 2006;103:1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- [70].Schapira AH. Etiology of Parkinson’s disease. Neurology. 2006;66:S10–S23. doi: 10.1212/wnl.66.10_suppl_4.s10. [DOI] [PubMed] [Google Scholar]
- [71].Bennett MC. The role of alpha-synuclein in neurodegenerative diseases. Pharmacol. Ther. 2005;105:311–331. doi: 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- [72].Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P. Alpha-synuclein and Parkinson’s disease. FASEB J. 2004;18:617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- [73].Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alphasynuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol.Cell. Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Liu Y, Yang H. Environmental toxins and alpha-synuclein in Parkinson’s disease. Mol Neurobiol. 2005;31:273–282. doi: 10.1385/MN:31:1-3:273. [DOI] [PubMed] [Google Scholar]
- [76].Gasser T. Genetics of Parkinson’s disease. J Neurol. 2001;248:833–840. doi: 10.1007/s004150170066. [DOI] [PubMed] [Google Scholar]
- [77].Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di IG, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- [78].Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- [79].Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Miller SW, Davis RE, D W. Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Exp Neurol. 2000;162:37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- [80].Borland MK, Mohanakumar KP, Rubinstein JD, Keeney PM, Xie J, Capaldi R, Dunham LD, Trimmer PA, Bennett JP. Relationships among molecular genetic and respiratory properties of Parkinson’s disease cybrid cells show similarities to Parkinson’s brain tissues. Biochim Biophys Acta. 2009;1792:68–74. doi: 10.1016/j.bbadis.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- [82].Price DL, Sisodia SS. Mutant genes in familial Alzheimer’s disease and transgenic models. Annu. Rev. Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- [83].Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:23–31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- [84].Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- [85].Janus C, Westaway D. Transgenic mouse models of Alzheimer’s disease. Physiol. Behav. 2001;73:873–886. doi: 10.1016/s0031-9384(01)00524-8. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto K, Akao Y, Yi H, Shamoto-Nagai M, Maruyama W, Naoi M. Overexpression of amyloid precursor protein induces susceptibility to oxidative stress in human neuroblastoma SH-SY5Y cells. J Neural Transm. 2006 Feb;113:125–135. doi: 10.1007/s00702-005-0318-0. 2006. [DOI] [PubMed] [Google Scholar]
- [87].Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Askanas V, McFerrin J, Baque S, Alvarez RB, Sarkozi E, Engel WK. Transfer of beta-amyloid Precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1314–1319. doi: 10.1073/pnas.93.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Grant SM, Shankar SL, Chalmers-Redman RM, Tatton WG, Szyf M, Cuello AC. Mitochondrial abnormalities in neuroectodermal cells stably expressing human amyloid precursor protein (hAPP751) Neuroreport. 1999;18:41–46. doi: 10.1097/00001756-199901180-00008. [DOI] [PubMed] [Google Scholar]
- [90].Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- [91].Casley CS, Land JM, Sharpe MA, Clark JB, Duchen MR, Canevari L. Betaamyloid fragment 25–35 causes mitochondrial dysfunction in primary cortical neurons. Neurobiol. Dis. 2002;10:258. doi: 10.1006/nbdi.2002.0516. [DOI] [PubMed] [Google Scholar]
- [92].Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloidbeta1-42. J. Neurosci. 2005;25:672. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Du H, Guo L, Fang F, Chen D, Sosunov A, McKhann M, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14:1097. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sirk D, Zhu Z, Wadia JS, Shulyakova N, Phan N, Fong J, Mills LR. Chronic exposure to sub-lethal beta-amyloid (Abeta) inhibits the import of nuclearencoded proteins to mitochondria in differentiated PC12 cells. J. Neurochem. 2007;103:1989. doi: 10.1111/j.1471-4159.2007.04907.x. [DOI] [PubMed] [Google Scholar]
- [95].Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’sdisease. FASEB J. 2005;19:2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- [96].Eckert A, Hauptmann S, Scherping I, Rhein V, Muller-Spahn F, Götz J, Muller WE. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5:157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- [97].Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- [98].Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down’s syndrome. Neuron. 2002;33:677–688. doi: 10.1016/s0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- [99].Stephenson DT, Rash K, Clemens JA. Amyloid precursor protein accumulates in regions of neurodegeneration following focal cerebral ischemia in the rat. Brain Res. 1992;593:128–135. doi: 10.1016/0006-8993(92)91274-i. [DOI] [PubMed] [Google Scholar]
- [100].Shepherd CE, Bowes S, Parkinson D, Cambray-Deakin M, Pearson RC. Expression of amyloid precursor protein in human astrocytes in vitro: isoform-specific increases following heat shock. Neuroscience. 2000;99:317–325. doi: 10.1016/s0306-4522(00)00197-4. [DOI] [PubMed] [Google Scholar]
- [101].Jeong SJ, Kim K, Suh YH. Age-related changes in the expression of Alzheimer’s beta APP in the brain of senescence accelerated mouse (SAM)-P/10. Neuroreport. 1997;8:1733–1737. doi: 10.1097/00001756-199705060-00033. [DOI] [PubMed] [Google Scholar]
- [102].Bahmanyar S, Higgins GA, Goldgaber D, Lewis DA, Morrison JH, Wilson MC, Shankar SKSK, Gajdusek DC. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer’s disease. Science. 1987;237:77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- [103].Hasimoto Y, Niikura T, Ito Y, Nishimoto I. Multiple mechanisms underlie neurotoxicity by different types of Alzheimer’s disease mutations of amyloid precursor protein. J.Biol.Chem. 2000;275:34541–34551. doi: 10.1074/jbc.M005332200. [DOI] [PubMed] [Google Scholar]
- [104].Neupert W, Herrmann JM. Translocation of Proteins into Mitochondria. Annu. Rev. Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- [105].Becker T, Gebert M, Pfanner N, Laan M. Biogenesis of mitochondrial membrane proteins. Curr Opin Cell Biol. 2009;21:1–10. doi: 10.1016/j.ceb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- [106].Dolezal P, Likic V, Tachezy J, Lithgow T. Evolution of the molecular machines for protein import into mitochondria. Science. 2006;313:314–318. doi: 10.1126/science.1127895. [DOI] [PubMed] [Google Scholar]
- [107].Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- [108].Fan AC, Bhangoo MK, Young JC. Hsp90 functions in the targeting and outer membrane translocation steps of Tom70-mediated mitochondrial import. J.Biol.Chem. 2006;281:33313–33324. doi: 10.1074/jbc.M605250200. [DOI] [PubMed] [Google Scholar]
- [109].Hill K, Model K, Ryan MT, Dietmeier K, Martin F, Wagner R, Pfanner N. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature. 1998;395:516–521. doi: 10.1038/26780. [DOI] [PubMed] [Google Scholar]
- [110].Endo T, Yamamoto H, Esaki M. Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J.Cell. Sci. 2003;116:3259–3267. doi: 10.1242/jcs.00667. [DOI] [PubMed] [Google Scholar]
- [111].Brix J, Dietmeier K, Pfanner N. Differential recognition of preproteins by the purified cytosolic domains of the mitochondrial import receptors Tom20, Tom22, and Tom70. J Biol Chem. 1997;272:20730–20735. doi: 10.1074/jbc.272.33.20730. [DOI] [PubMed] [Google Scholar]
- [112].Anandatheerthavarada HK, Sepuri NBV, Avadhani NG. Mitochondrial Targeting of Cytochrome P450 Proteins Containing NH2-terminal Chimeric Signals Involves an Unusual TOM20/TOM22 Bypass Mechanism. J.Biol.Chem. 2009;284:17352–17363. doi: 10.1074/jbc.M109.007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH. Optical Reporters for the Conformation of alpha-Synuclein Reveal a Specific Interaction with Mitochondria. J Neuroscience. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Petersen CA. Hansson, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kamboh MI. Molecular genetics of late-onset Alzheimer’s disease. Ann Hum Genet. 2004;68:381–404. doi: 10.1046/j.1529-8817.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- [116].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- [117].Gibson GE, Haroutunian V, Zhang H, Park LC, Shi Q, Lesser M, Mohs RC, Sheu RK, Blass JP. Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol. 2000;48:297–303. [PubMed] [Google Scholar]
- [118].Takei N, Miyashita A, Tsukie T, Arai H, Asada T, Imagawa M, et al. Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese. Genomics. 2009;93:441–448. doi: 10.1016/j.ygeno.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [119].Bekris LM, Millard SP, Galloway NM, Vuletic S, Albers JJ, et al. Multiple SNPs within and surrounding the apolipoprotein E gene influence cerebrospinal fluid apolipoprotein E protein levels. J Alzheimers disease. 2008;13:255–266. doi: 10.3233/jad-2008-13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lee HJ, Shin SY, Choi C, Lee YH, Lee SJ. Formation and removal of alphasynuclein aggregates in cells exposed to mitochondrial inhibitors. J. Biol. Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- [121].Zhou W, Schaack J, Zawada WM, Freed CR. Overexpression of human alpha-synuclein causes dopamine neuron death in primary human mese. Brain Res. 2002;926:42–50. doi: 10.1016/s0006-8993(01)03292-9. [DOI] [PubMed] [Google Scholar]
- [122].Lee MH, Hyun DH, Halliwell B, Jenner P. Effect of the overexpression of wild-type or mutant [alpha]-synuclein on cell susceptibility to insult. J. Neurochem. 2001;76:998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- [123].Antonicka H, Ogilvie I, Taivassalo T, Anitori RP, Haller RG, Vissing J, Kennaway NG, Shoubridge EA. Identification and characterization of a common set of complex I assembly intermediates in mitochondria from patients with complex I deficiency. J Biol Chem. 2003;278:43081–43088. doi: 10.1074/jbc.M304998200. [DOI] [PubMed] [Google Scholar]
- [124].Elkon H, Don J, Melamed E, Ziv I, Shirvan A, Offen D. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. J. Mol. Neurosci. 2002;18:229–238. doi: 10.1385/JMN:18:3:229. [DOI] [PubMed] [Google Scholar]
- [125].Yamaguchi H, Yamazaki T, Ishiguro K, Shoji M, Nakazato Y, Hirai S. Ultrastructural localization of Alzheimer amyloid beta/A4 protein precursor in the cytoplasm of neurons and senile plaque-associated astrocytes. Acta Neuropathol. 1992;85:15–22. doi: 10.1007/BF00304629. [DOI] [PubMed] [Google Scholar]
- [126].Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;2:103–117. doi: 10.3233/jad-2005-7203. [DOI] [PubMed] [Google Scholar]