Abstract
Rationale and Objectives
To compare two subjective methods for the identification of changes suggestive of early interstitial lung disease (ILD) on chest computed tomographic (CT) scan.
Materials and Methods
The CT scans of the first 100 subjects enrolled in the COPDGene Study from a single institution were examined using a sequential reader and a group consensus interpretation scheme. CTs were evaluated for the presence of parenchymal changes consistent with ILD using a scoring system of “0” or normal, “1” or equivocal for the presence of ILD, “2” highly suspicious for ILD, “3” classic ILD changes. A statistical comparison of early ILD cases with normal subjects was performed.
Results
There was a high degree of agreement between methods (Kappa 0.84 95% CI (0.73–0.94) P <0.0001 for the sequential and consensus methods). The sequential reading method had both high positive (1) and negative values (0.97) for predicting a consensus read despite a 58% reduction in the number of chest CT evaluations. Regardless of interpretation method, the prevalence of chest CT changes consistent with early ILD in this subset of smokers from COPDGene varies between 5–10%. Subjects with early ILD tended to have greater tobacco smoke exposure than subjects without early ILD (P=0.053).
Conclusions
A sequential CT interpretation scheme is efficient method for the visual interpretation of CT data. Further investigation is required to independently confirm our findings and further characterize early ILD in smokers.
Keywords: Early Interstitial Lung Disease, CT scan, Smoker
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common idiopathic interstitial lung disease (ILD) with an estimated 50% three year survival (1–3). IPF is typically diagnosed 3 to 4 years after the development of symptoms by which time most patients have advanced lung remodeling and fibrosis where experimental treatments have proven largely ineffective(4, 5). Our group has previously demonstrated both chest computed tomographic (CT) and pathologic evidence of early interstitial lung disease (early ILD) in asymptomatic members in 18 kindreds affected with familial IPF(6). This suggests that screening using high-risk populations may, in some cases, detect early stages of ILD.
While early disease detection in ILD may lead to a better understanding of the natural history of IPF, strategies for detection and screening in high-risk populations have not been formally investigated. To address this issue we compared an efficient sequential reading method (7) to a consensus reading method for identification early ILD in a population of smokers (both with and without COPD) enrolled in the COPDGene Study. We report a high degree of correlation between methods and present the comparison of the clinical characteristics of subjects identified as having early ILD in the COPDGene Study.
Methods
COPDGene
The COPDGene Study is a multicenter investigation focused on examining the genetic and epidemiologic basis of chronic obstructive pulmonary disease (COPD) and other smoking related lung diseases. Study participants complete a protocol that includes questionnaires, medical record review, physical examination, and spirometric measures of lung function before and after the administration of a short acting inhaled bronchodilator. Common metrics of lung function reported from this maneuver include the Forced Expiratory Volume in 1 second (FEV1) which is the volume of gas that a subject can forcibly exhale during the first second of effort, and the Forced Vital Capacity (FVC) which is to total volume of gas that can be forcibly exhaled from the lungs. Both measures are expressed as a percent of their predicted values based upon those found in a normal population. Additional testing performed included a 6 minute walk test (6-MWT), collection of a blood sample for genetic testing, and high resolution CT scanning of the chest at full inspiration and expiration. The first 100 COPDGene subjects enrolled at a single institution were included in this analysis. All subjects provided written informed consent for participation in COPDGene. The study was approved by the Institutional Review Boards of all participating COPDGene centers.
Computed Tomographic Scan (CT)
All subjects were scanned with a 16 or 64-detector CT scanner (Definition 16, Sensation 64, Siemens Medical Solutions). Imaging was performed during breath-hold at full inflation (total lung capacity) and expiration (functional residual capacity) with the patient in the supine position. Every patient was carefully instructed how to breathe before the scan. Only the inspiratory images were utilized in this investigation.
MSCT scan parameters were as follows: collimation, 0.5-mm; 120kV; 200mA; gantry rotation time, 0.5s, pitch, 1.1. The images reviewed for this analysis were reconstructed using a high resolution reconstruction algorithm with a slice thickness of 1 mm and a reconstruction interval of 10 mm. All images were reviewed on Picture Archiving Communication Systems (PACS) workstations (Centricity, GE Healthcare) using axial images with a window level of -700 HU and a window width of 1500 HU.
CT scoring
CT findings were scored by 3 readers (including 1 pulmonologists and 2 radiologists) as “0”, no evidence of ILD, “1”, equivocal for ILD, “2”, suspicious for ILD, and “3”, ILD. Equivocal for ILD (Score of 1) was defined as focal or unilateral ground glass attenuation, focal or unilateral reticulation, and patchy ground glass abnormality (less than 5% of the lung). Suspicious for ILD (Score of 2) was defined as follows: nondependent ground glass abnormality affecting more than 5% of any lung zone, non-dependent reticular abnormality, diffuse centrilobular nodularity with ground glass abnormality, honeycombing, traction bronchiectasis, non-emphysematous cysts, architectural distortion. ILD (Score of 3) was defined as bilateral fibrosis in multiple lobes associated to honeycombing and traction bronchiectasis in a sub-pleural distribution.
Sequential Reading Method
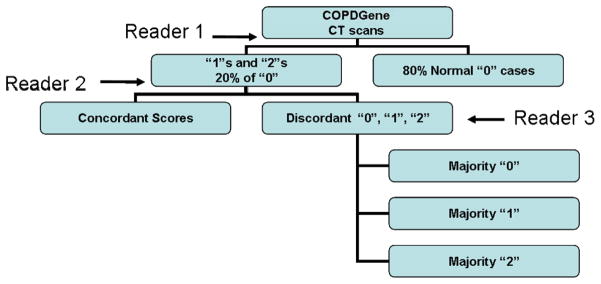
The sequential reading process is similar to a method similar to that proposed by Lynch and colleagues(7). In the sequential reading process (illustrated in Figure 1), the 100 CT scans were divided amongst 4 PACS workstations. Reader #1 would review the scans at their station and provide a score of 0, 1, 2, or 3. CT scans including those scans given a score of “1” or “2” and a random selection of approximately 20% of the normal scans (score “0”) would be provided to reader #2 who was blinded to the initial interpretation. Finally, reader #3, who was blinded to the interpretations of reader #1 and #2, provided majority opinion on those scans discordantly scored between Readers #1 and #2.
Figure 1.
Sequential Computed Tomographic Interpretation.
Consensus Reading Method
Once scored by the sequential method, consensus opinion for each CT scan was provided by the group after collectively reviewing the all of the CT scans.
Statistical Analysis
Kappa statistics were provided to evaluate the agreement between the 2 CT interpretation schemes. Positive and negative predictive values (identifying score “2” vs. scores “1” and “0”) are presented to compare the effectiveness of the sequential reading method in comparison to the consensus method.
A comparison of early ILD cases with normal subjects was performed with Fisher’s Exact tests (used to examine the significance of the association between two small sample sizes of binary variables)(8) and Wilcoxon Rank Sum tests for continuous variables (non-parametric method for the comparison of the medians of two populations) (9). Data analysis was performed using SAS, version 9.1 (Carey, NC). P values less than 0.05 were considered statistically significant.
Results
Cohort Demographics
The demographic and functional data of the study cohort are provided in Table 1. The median age of the cohort was 61 years and 44% of the cohort was male. Forty seven of the subjects were current smokers and the median pack year tobacco history (average number of packs per day multiplied by the number of years smoked) was 37.6. The median forced expiratory volume in 1 second expressed as a percent of predicted (FEV1% predicted) was 87 (73–100) and the median forced vital capacity expressed as a percent of predicted (FVC% predicted) was 94.5 (83–103.5). The median ratio of the FEV1 to FVC (FEV1/FVC) was 0.73 (0.63–0.81). Sixty one of the 100 subjects had normal spirometry, 11 subjects had GOLD stage 1 disease, 20 with GOLD stage 2 disease, and 8 subjects with GOLD stage 3 disease. None of the 100 subjects included in this analysis had very severe GOLD stage 4 disease.
Table 1.
Demographic and functional data of the study cohort (n=100).
| Characteristic | |
|---|---|
| Male Sex | 44 |
| Age (years) | 61.5 (56.6–67.1) |
| Age Started Smoking | 16 (14.5–18.5) |
| Current Smoker | 47 |
| Pack Years Tobacco | 37.6 (24.2–53.9) |
| FEV1 % Predicted | 87 (73–100) |
| FVC % Predicted | 94.5 (83–103.5) |
| FEV1/FVC | 0.73 (0.63–0.81) |
| Normal Spirometry (n) | 61 |
| GOLD Stage 1 (n) | 11 |
| GOLD Stage 2 (n) | 20 |
| GOLD Stage 3 (n) | 8 |
| GOLD Stage 4 (n) | 0 |
| 6 MWT (feet) | 1695 (1400–1860) |
Sequential Reading Method
Out of 100 cases, 69 cases were classified as normal (score “0”), 23 cases were classified as equivocal ILD (score “1”), and 8 cases were classified as suspected ILD (score “2”). No subjects were assigned a score of “3”. Forty four of those initial 100 cases (29 with score of “1” or “2”, and 15 cases with a score of “0”) were then evaluated by Reader #2. In 50% (n=22) of cases, the first and second readers agreed in the scoring while there was disagreement in the remaining 22 cases requiring a third review. Reader #3 provided majority opinion for the discordant readings. Out of 15 cases with a score of “0” by the first reader, 10 cases had a final score of “0” and the remaining 5 cases had a final score of “1”. Importantly, throughout the sequential analysis of the CT scans by readers #1, #2, and #3, no case initially given a score of “0” was upgraded to a score of “2”. The total number of interpretations performed by the first, and second, and third readers was 166 (100 plus 44 plus 22) (Table 2).
Table 2.
Comparison of the CT scan Evaluation Methods.*
| Sequential Analysis | Score of “0” | Score of “1” | Score of “2” | Number of Readings |
| 69 | 23 | 8 | 166 | |
| Consensus Analysis | Score of “0” | Score of “1” | Score of “2” | Number of Readings |
| 64 | 26 | 10 | 400 |
The methodologies are designated as “Sequential Analysis”, where each scan was reviewed in series, and “Consensus Analysis” where each score was agreed upon by 3 simultaneous readers. The number of readings was calculated as the total number of CT interpretations. The kappa statistic for the correlation between these two methods (Kappa 0.84 95% confidence interval [0.73–0.94] P <0.0001 for the sequential and consensus scheme).
Consensus Reading Method
Following the sequential and modified sequential visual scoring of the CT scans, the four readers simultaneously viewed and scored the CT scans on a single PACs workstation. Using this method, 64 cases were classified as normal (score “0”), 26 cases were classified as equivocal (score “1”), and 10 cases were classified as suspected (score “2”). A total of 400 interpretations (100 CT scans and 4 readers for each scan) were performed using this method (Table 2). A description of the CT findings for each of the 10 cases is provided in Table 3. These interpretations were provided by an independent reader (DL) who was not part of the sequential or consensus reading methods. Of the 10 CT scans described in Table 3, cases 1, 3, 7, 9, and 10 were felt to be most consistent with early ILD. The findings in the remaining cases were felt to be either too non-specific (cases 2, 5, 6, 8) or not representative of interstitial lung disease (case 4). Selected examples of these findings are shown in Figures 2A–D.
Table 3.
CT findings of the 10 cases assigned a score of “2” by consensus review.
| Case # | Description |
|---|---|
| 1* | Mild basal asymmetric reticular abnormality with septal thickening |
| 2 | Patchy upper lobe ground glass opacities |
| 3* | Upper lobe irregular cysts |
| 4 | Basal linear scarring or dependent ground glass |
| 5 | Mild basal peripheral ground glass |
| 6 | Basal bronchiectasis and centrilobular nodules, right basal ground glass |
| 7 | Upper lobe centrilobular nodules with mild ground glass |
| 8 | Mild basal asymmetric reticular and ground glass |
| 9 | Patchy bilateral basal ground glass and reticular abnormality and traction |
| Bronchiectasis | |
| 10 | Mild asymmetric bibasilar ground glass with traction bronchiectasis |
denotes cases that were given a score of “1” on sequential read and a “2” on consensus evaluation.
Figure 2.
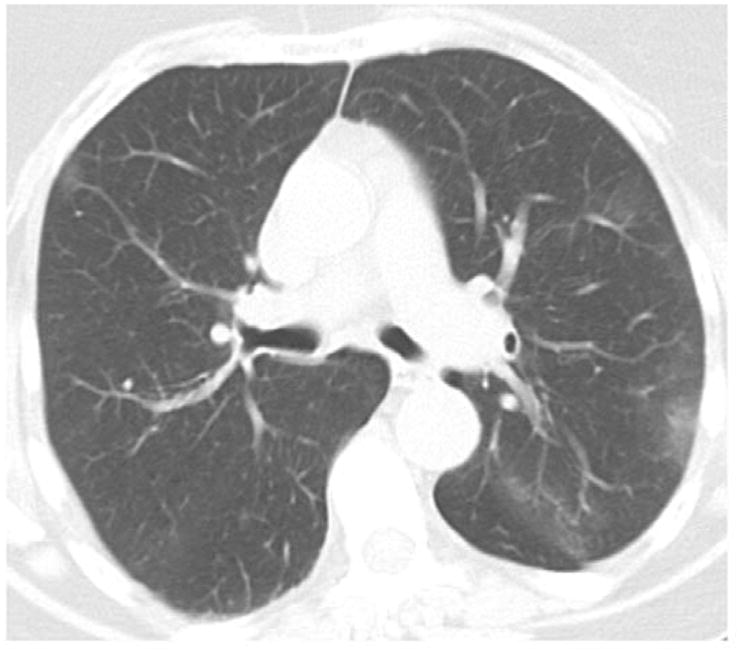
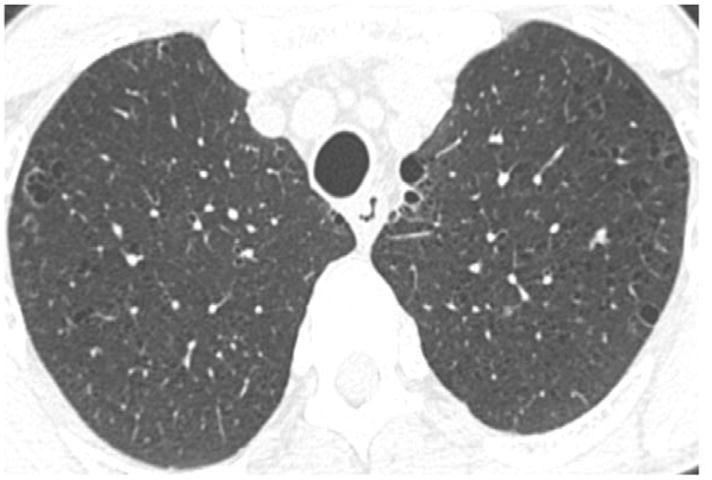
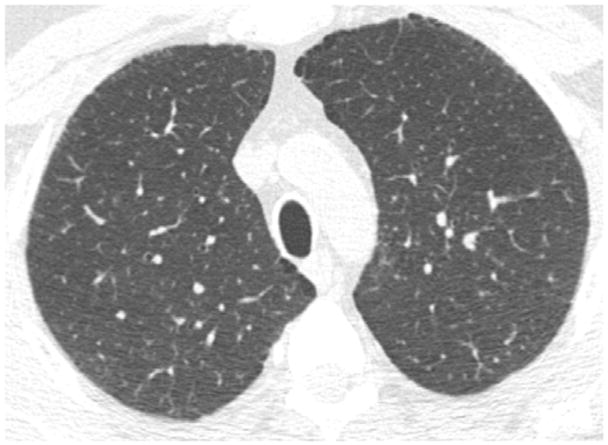
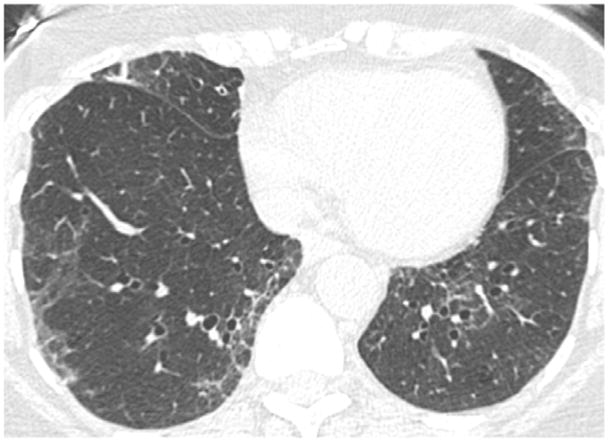
Figure 2A. Example of patchy upper lung ground glass opacities (Case #2 in Table 3).
Figure 2B. Example of upper lobe irregular cysts (Case #3 in Table 3).
Figure 2C. Example of upper lobe centrilobular nodules with mild ground glass (Case #7 in Table 3).
Figure 2D. Example of patchy bilateral basal ground glass and reticular abnormality and traction bronchiectasis (Case # 9 in Table 3).
Comparison of Reading Methods
There was a high degree of agreement between methods (Kappa 0.84 95% CI (0.73–0.94) P <0.0001 for the sequential and consensus methods). A score of “2” by the sequential method had a positive predictive value of 1 for being scored as a “2” by the consensus method. A combined score of “0” or “1” had a negative predictive value for 0.98 for being scored as either a “0” or “1” by the consensus method. A score of “0” had a negative predictive value for 0.97 for being scored as a “0” by the consensus method. The sequential reading method resulted in 59% fewer Chest CT evaluations per reader. Regardless of interpretation method, the prevalence of chest CT changes assigned a score of “2” in this subset of smokers from COPDGene varied between 8–10%.
Clinical Characteristics of early ILD by Consensus Reading
A comparison of the differences between early ILD subjects to normal subjects (identified by the consensus method) is presented in Table 4. There was a trend towards a statistically significant difference in the number of pack-years of tobacco smoke exposure between early ILD subjects and normal smokers (P=0.05). There a large percentage of subjects with early ILD were current smokers (70%) in contrast to those subjects without early ILD (42%) although this difference was not statistically significant (P=0.2).
Table 4.
Clinical Characteristics of COPDGene Subjects Stratified by Early Interstitial Lung Disease Status.*
| Characteristic | Score “0” (n=65) | Score “2” (n=10) | P Value† |
|---|---|---|---|
| Age (years) | 61 (56–65) | 64 (60–69) | 0.4 |
| Age Started Smoking | 17 (15–18) | 17 (14–18) | 0.8 |
| Gender (female) | 36 (55%) | 5 (50%) | 1.0 |
| Race (white) | 57 (88%) | 9 (90%) | 1.0 |
| COPD Diagnosis‡ | 16 (25%) | 3 (30%) | 0.7 |
| Current Smoker | 27 (42%) | 7 (70%) | 0.2 |
| Pack Years Tobacco | 33 (23–48) | 43 (33–90) | 0.05 |
| FEV1 % Predicted§ | 93 (77 – 108) | 82 (79 – 99) | 0.2 |
| FVC % Predicted§ | 101 (90 – 109) | 89 (86 – 102) | 0.2 |
| FEV1/FVC | 0.73 (0.61 – 0.82) | 0.69 (0.66 – 0.76) | 0.7 |
| 6 MWT (meters) | 1700 (1400–1861) | 1739 (1560–1780) | 0.7 |
A comparison of the differences in the clinical characteristics of subjects with CT findings suggestive of early ILD subjects and normal subjects from the COPDGene study identified by the consensus Chest CT reading method are presented. Median and interquartile ranges or numbers and percentages are presented where appropriate.
P values presented result from the comparison of early ILD status and normal subjects by Fisher Exact tests (for binary variables) and Wilcoxon Rank Sum tests (for continuous variables).
COPD was defined using modified Global Initiative for Chronic Obstructive Lung Disease (GOLD) Stage II criteria(24) (an FEV1/FVC ratio < 0.7 and an FEV1 < 80% of predicted).
Predicted values for FEV1 and FVC are derived from Hankinson et al.(25)
Discussion
In this manuscript we present the first comparison of chest CT reading methods for the identification of subjects with early ILD. To establish an efficient sequential CT evaluation method that objectively qualifies early ILD changes, we have undertaken review of a subset of chest CT scans (N=100) from the COPDGene study. Our data demonstrates a high degree of correlation between a sequential reading and a consensus reading method. The sequential reading method may be an effective and efficient methodology for screening large numbers of CT scans for early ILD as it involves a 58% reduction in the number of chest CT evaluations compared to a consensus reading.
The chest CT abnormalities including increased septal lines, peri-bronchovascular thickening, reticulation, and ground glass opacities observed in this study were suggestive of diverse histologic subtypes of ILD previously described in smokers (NSIP, RB-ILD, LCH and COP)(10). Our study suggests that an increased pack-year history of degree of tobacco smoke exposure may predispose subjects to early ILD. Previous findings from our research group have also identified tobacco smoke exposure as a risk factor the development of early ILD among both subjects with rheumatoid arthritis and asymptomatic family members of kindreds with familial pulmonary fibrosis (6, 11). These findings are intriguing as numerous epidemiologic studies have identified smoke exposure as a risk factor for IPF (12–16).
Of note 20–30% of patients affected with ILD do not have a history of smoking and most patients who smoke do not develop ILD, suggesting that gene-by-environment interactions may be important in the development of this disease. This concept is further supported by family studies which demonstrate clustering of ILD cases (17–22) and recent evidence that heterozygous mutations in telomerase reverse transcriptase (TERT) and telomere shortening is present in up to 8% of kindred’s affected with pulmonary fibrosis and may be more frequent in smokers (22, 23).
Our study has several limitations. First, the cases presented here as being suspicious for early ILD were not confirmed by open lung biopsies. Indeed, we describe a broad spectrum of radiographic abnormalities in this subset of subjects with a score of “2”, not all of which are typical of interstitial lung disease. Additional disease entities may be present in this cohort such as aspiration, pneumonia, drug reaction, or even pulmonary hemorrhage. While five of the 10 subjects displayed findings more suggestive of the presence of early ILD such as centrilobular nodularity with ground glass or patchy reticular abnormalities associated with traction bronchiectasis, the CT findings in the remaining subjects are too non-specific to make such a judgment. Further longitudinal investigation is required to determine if such radiographic changes persist and evolve into a more recognizable disease state. Second, small sample size limits some the conclusions that can be drawn from our study. Finally, our analysis was performed in a cohort of both former and current smokers with a relatively high prevalence of COPD. Caution should be exercised in extrapolating the prevalence of early ILD noted in our study to heterogeneous populations.
In summary we compared two chest CT evaluation methods for the identification early ILD in a population of smokers enrolled in the COPDGene Study. Our findings suggest that a sequential chest CT reading method may be an effective and efficient method for identifying subjects with early ILD in larger cohorts. We believe that further characterization of early ILD could positively impact future clinical care by allowing us to detect and treat early stages of pulmonary fibrosis in subjects at risk of developing IPF.
Acknowledgments
Grants: Funding/Support: COPDGene is supported by NIH Grant Numbers U01 HL089897 and U01 HL089856
Dr. Washko is supported by NIH Grant Number: K23 HL089353 and an award from the Parker B. Francis Foundation.
Dr. Hunninghake is supported by NIH grant: K08 HL092222.
Dr. Rosas is supported by NIH Grant Number HL087030
Dr. Hatabu is support by NIH Grant Number: 5R21CA116271-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King TEJ, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting Survival in Idiopathic Pulmonary Fibrosis. Scoring System and Survival Model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and Prevalence of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 3.Gross TJ, Hunninghake GW. Idiopathic Pulmonary Fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 4.Demedts M, Behr J, Buhl R, et al. High-Dose Acetylcysteine in Idiopathic Pulmonary Fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Jr, Behr J, Brown KK, et al. BUILD-1: A Randomized Placebo-controlled Trial of Bosentan in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 6.Rosas IO, Ren P, Avila NA, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch MCA, Sahin H, Garg K. Prevalence of Infiltrative Lung Disease Identified on CT in Participants in the National Lung Screening Trial. :609. Abstract RSNA 2008; SSQ04-04. [Google Scholar]
- 8.Fisher RA. On the interpretation of X2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85:87094. [Google Scholar]
- 9.Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- 10.Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Myers JL, Martinez FJ. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics. 2008;28:1383–1396. doi: 10.1148/rg.285075223. discussion 1396–1388. [DOI] [PubMed] [Google Scholar]
- 11.Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168:159–166. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 12.Caminati A, Harari S. Smoking-related Interstitial Pneumonias and Pulmonary Langerhans Cell Histiocytosis. Proc Am Thorac Soc. 2006;3:299–306. doi: 10.1513/pats.200512-135TK. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 14.MDKRF, Hunninghake GG. Smoking: An Injury with Many Lung Manifestations. Am J Respir Crit Care Med. 2005;172:1070–1071. doi: 10.1164/rccm.2508006. [DOI] [PubMed] [Google Scholar]
- 15.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 16.Baran CP, Opalek JM, McMaken S, et al. Important Roles for Macrophage Colony-stimulating Factor, CC Chemokine Ligand 2, and Mononuclear Phagocytes in the Pathogenesis of Pulmonary Fibrosis. 2007:78–89. doi: 10.1164/rccm.200609-1279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nogee LM, Dunbar AE, Wert SE, Askin F, Hamvas A, Whitsett JA. A Mutation in the Surfactant Protein C Gene Associated with Familial Interstitial Lung Disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax. 2002;57:338–342. doi: 10.1136/thorax.57.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steele MP, Speer MC, Loyd JE, et al. Clinical and Pathologic Features of Familial Interstitial Pneumonia. 2005:1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall RP, Puddicombe A, Cookson WO, Laurent GJ. Adult familial cryptogenic fibrosing alveolitis in the United Kingdom. Thorax. 2000;55:143–146. doi: 10.1136/thorax.55.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitterman PB, Rennard SI, Keogh BA, Wewers MD, Adelberg S, Crystal RG. Familial idiopathic pulmonary fibrosis. Evidence of lung inflammation in unaffected family members. N Engl J Med. 1986;314:1343–1347. doi: 10.1056/NEJM198605223142103. [DOI] [PubMed] [Google Scholar]
- 22.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proceedings of the National Academy of Sciences. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armanios MY, Chen JJL, Cogan JD, et al. Telomerase Mutations in Families with Idiopathic Pulmonary Fibrosis. 2007:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 24.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 25.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]