Abstract
Background
Genital ulcer disease (GUD), including syphilis, is an important cause of morbidity in low and middle income (LMI) countries and syphilis transmission is associated with HIV transmission.
Methods
We conducted a literature review to evaluate syphilis infection among drug users in LMI countries for the period 1995–2007. Countries were categorized using the World Bank Atlas method (The World Bank, 2007) according to 2006 gross national income per capita.
Results
Thirty-two studies were included (N=13,848 subjects), mostly from Southeast Asia with some from Latin America, Eastern Europe, Central and East Asia, North Africa and the Middle East but none from regions such as Sub-Saharan Africa. The median prevalence of overall lifetime syphilis (N=32 studies) was 11.1% (interquartile range: 6.3% to 15.3%) and of HIV (N=31 studies) was 1.1% (interquartile range: 0.22% to 5.50%). There was a modest relation (r=0.27) between HIV and syphilis prevalence. Median syphilis prevalence by gender was 4.0% (interquartile range: 3.4% to 6.6%) among males (N=11 studies) and 19.9% (interquartile range: 11.4% to 36.0%) among females (N=6 studies). There was a strong relation (r= 0.68) between syphilis prevalence and female gender that may be related to female sex work.
Conclusion
Drug users in LMI countries have a high prevalence of syphilis but data are limited and, in some regions, entirely lacking. Further data are needed, including studies targeting the risks of women. Interventions to promote safer sex, testing, counseling and education, as well as health care worker awareness, should be integrated in harm reduction programs and health care settings to prevent new syphilis infections and reduce HIV transmission among drug users and their partners in LMI countries.
Keywords: syphilis, genital ulcer disease, drug user, sexual risk behaviors, HIV, developing countries
Introduction
There is a long-standing relationship between sexually transmitted infections (STIs) and illicit drug use. Up to 60% of injection drug users (IDUs) report histories of STIs (Nelson et al., 1991) and high rates are also seen among non-injection drug users (NIDUs), such as users of crack cocaine (Ross et al., 2002). STIs, including syphilis, have been shown to be independent risk factors for the sexual transmission of human immunodeficiency virus (HIV) (Lyles et al., 2007; Buchacz et al., 2005; Phipps et al., 2005; Browne et al., 2003; Harrell et al., 2003; Kalichman et al., 2000; Greenblatt et al., 1988). Additionally, untreated STIs can cause infertility, debilitating illness and death. Unlike HIV, many STIs can be easily treated and cured if diagnosed.
There are substantial gaps in our understanding of global syphilis epidemiology. Worldwide, there were 11.76 million reported new cases of syphilis in 1999 (World Health Organization, 2001) as shown in Table 1, the most recent year in which aggregate data are available. Drug users are an important target group for the prevention of both syphilis and HIV due primarily to frequent high-risk sexual behaviors, including commercial sex work to obtain money for drugs. In addition, drug users that have non-drug using sex partners often constitute a “bridge group”, through which they may facilitate the spread of STIs to non-drug using populations (Strathdee et al., 2008; Steinbrook et al., 2007; Liu et al., 2006; Ruan et al, 2006; Hahn et al., 1989). Despite having a high prevalence and incidence of STIs internationally, drug users can be a difficult-to-reach population who may be incompletely or inconsistently engaged in longitudinal or preventive health care, and for whom adherence to therapeutic interventions can be problematic.
Table 1.
Estimated new cases of syphilis (in million) among adults, 1995 and 1999 (Adapted from World Health Organization, 2001)
| Region | 1995 | 1999 | ||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
|
North America |
0.07 | 0.07 | 0.14 | 0.054 | 0.053 | 0.107 |
|
Western Europe |
0.10 | 0.10 | 0.20 | 0.069 | 0.066 | 0.136 |
|
North Africa & Middle East |
0.28 | 0.33 | 0.62 | 0.167 | 0.197 | 0.364 |
|
Eastern Europe & Central Asia |
0.05 | 0.05 | 0.10 | 0.053 | 0.052 | 0.105 |
|
Sub Saharan Africa |
1.56 | 1.97 | 3.53 | 1.683 | 2.144 | 3.828 |
|
South and South East Asia |
2.66 | 3.13 | 5.79 | 1.851 | 2.187 | 4.038 |
|
East Asia & Pacific |
0.26 | 0.30 | 0.56 | 0.112 | 0.132 | 0.244 |
|
Australia & New Zealand |
0.01 | 0.01 | 0.01 | 0.004 | 0.004 | 0.008 |
|
Latin America & Caribbean |
0.56 | 0.70 | 1.26 | 1.294 | 1.634 | 2.928 |
| Total | 5.55 | 6.67 | 12.22 | 5.29 | 6.47 | 11.76 |
We conducted a review of literature summarizing syphilis prevalence and associated factors among drug users in LMI countries to document syphilis prevalence and to explore related risk factors. Knowledge of these risk factors can be used to facilitate the development of targeted and effective interventions to reduce risky sexual and drug related behaviors and encourage early and effective treatment.
Methods
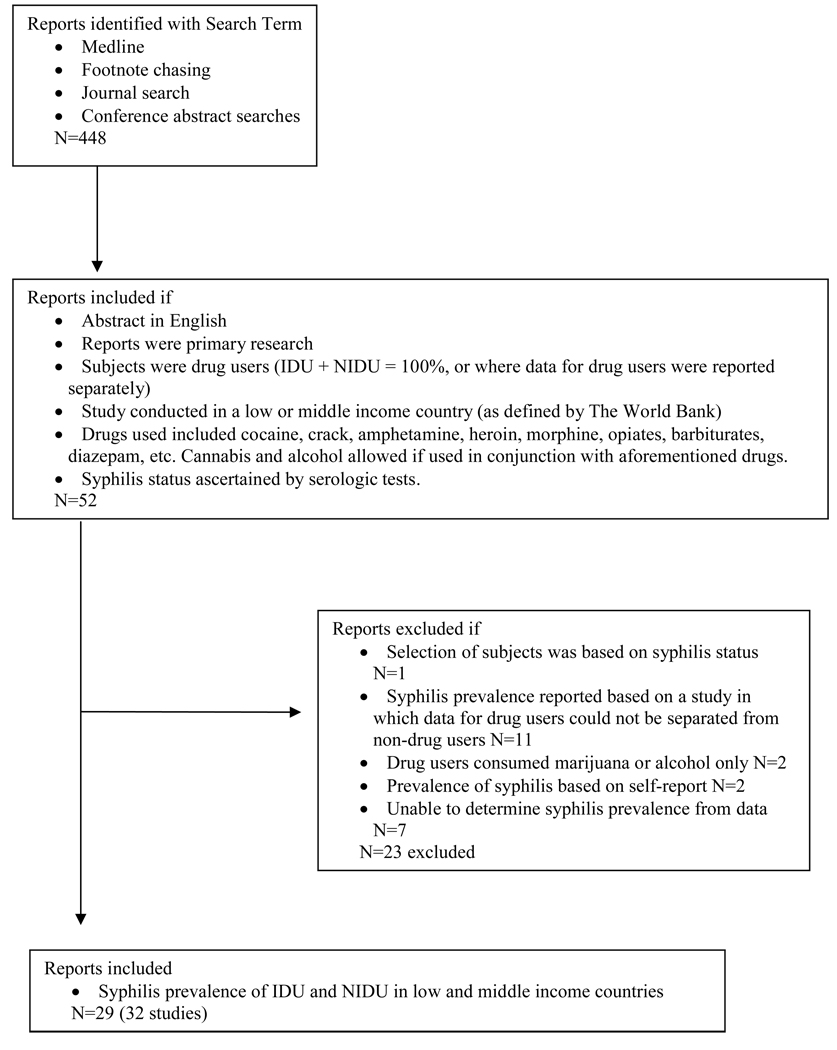
Searches of literature published from January 1995 to May 2007 were conducted via a PubMed portal on NCBI Entrez Databases. The following search terms were used: (Syphilis OR Treponema pallidum) AND (intravenous drug use OR intravenous drug abuse OR drug misuse OR drug addict OR injecting drug use OR drug abuse OR IDU). We supplemented the search with manual footnote chasing and review of relevant journals and supplements. The search revealed 448 papers. Figure 1 illustrates the process used to retrieve, screen and select studies for this review.
Figure 1.
Decision Tree Used to Retrieve and Select Papers
We included all primary research papers written in English or containing an English abstract. Only one paper was not published in English and the relevant data was extracted from the English abstract. All papers and abstracts that were included contained primary data on syphilis infection in people living in LMI countries who reported cocaine, crack, amphetamine, heroin, morphine, opiates, barbiturates, diazepam, sedatives and other illicit drug use via injecting, snorting, smoking or ingesting.
Countries were categorized by income groups according to 2006 gross national income (GNI) per capita. The World Bank Atlas method (The World Bank, 2007) groups countries as low income ($905 or less), lower-middle income ($906–3,595), upper-middle income ($3,596–11,115), and high income ($11,116 or more). Low, lower-middle, and upper-middle income countries (referred to as LMI) were included in this review.
To increase precision and reduce bias in estimating syphilis prevalence, we excluded papers in which selection of subjects was based on syphilis status, as this was our outcome of interest, and those that included non-drug users when this population could not be separated from the entire sample. Papers including alcohol or marijuana as participants’ sole drug use were excluded. Finally, to be certain that the diagnosis of syphilis was accurate in the papers under review, we excluded those that based syphilis status on self-report, including only those that used serologic testing for syphilis.
Data were transcribed onto a coding form developed by reviewing those used in other reviews, such as the HCV Synthesis Project (Scheinmann et al., 2007). The coding form included type of study (cohort vs. cross sectional); diagnostic tests and prevalence; demographics and other characteristics of the subjects such as age, gender, type of drug used and route of administration; sexual history and current sexual practices including condom usage, if working as a sex worker and if had sex with a sex worker. The principal outcomes were cases of syphilis organized into three categories: active syphilis, unspecified syphilis, and the total lifetime prevalence of syphilis. Total lifetime syphilis is the sum of active and unspecified. Subjects were considered to have active syphilis if they had a positive RPR or VDRL with a titer ≥ 1:8 and a positive confirmatory TPHA, FTA-ABS test, or TPPA test or if the authors explicitly stated that they were reporting laboratory-confirmed active cases (Mandell et al., 2005). Patients were considered to have unspecified syphilis if they had positive treponemal tests and or RPR or VDRL tests with a titer < 1:8. The list of diagnostic tests used in each study is located in Table 2.
Table 2.
Characteristics of drug users studies in our systematic review (n=32)
| Citation | Location | Enrollment Date |
Recruitment Setting |
Sample (Drug users) |
Female % (n) |
Active Syphilis % (n) |
Unspecified Syphilis % (n) |
Total Syphilis % (n) |
Diagnostic Test |
HIV % (n) |
Syphilis/ HIV co-infection % (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdala et al (2003) | St. Petersburg, Russia |
June 2000 – Aug 2000 |
SEP | 101 | 38.6 (39) | - | 6.9 (7) | 6.9 (7) | TREP- CHEK anti-Trep EIA (detects IgG antibodies) |
10.9 (11) |
0.0 (0 of 11) |
| Altaf et al (2007) | Karachi, Pakistan |
Oct 2003 – Nov 2003 |
SEP | 161 | 0.0 (0) | 13.0 (21) | - | 13.0 (21) | RPR, confirmed with TPHA |
0.6 (1) |
- |
| Azim et al (2000) | Bangladesh (4 regions) |
June 1998 – March 1999 |
Drug treatment |
402 | 0.3 (1) | 4.5 (18) | 12.9 (52) | 17.4 (70) | RPR, TPHA |
2.5 (10) |
- |
| Azim et al (2002) | Bangladesh (4 regions) |
June 1999 – June 2000 |
SEP, Drug treatment |
1236 | 0.4 (5) | 5.8 (72) | 15.4 (190) | 21.2 (262) | RPR, TPHA |
0.6 (7) |
- |
| Azim et al (2004) | Bangladesh (4 regions) |
2001 | SEP | 1015 | - | 2.2 (22) | 13.1 (133) | 15.3 (155) | RPR, TPHA |
0.7 (7) |
- |
| Azim et al (2004) | Bangladesh (4 regions) |
2002 | SEP | 1008 | - | 2.5 (25) | 13.7 (138) | 16.2 (163) | RPR+ & TPHA+ (represents non- active), TPHA & RPR titer of >=8 (represents reactive) |
1.6 (16) |
- |
| Azim et al (2006) | Dhaka, Bangladesh |
Dec 2004 – May 2005 |
Street outreach |
121 | 100 (121) | 9.1 (11) | 51.2 (62) | 60.3 (73) | RPR, TPPA (lifetime RPR titer <1:8), active RPR titer >= 1:8) |
0.0 (0) |
0.0 (0 of 0) |
| Baqi et al (1998) | Karachi, Pakistan |
Apr 1994 – July 1994 |
Drug treatment |
474 (272 tested for syphilis) |
0.0 (0) | 6.6 (18 of 272) |
- | 6.6 (18 of 272) |
VDRL, confirmed with FTA- ABS |
0.0 (0 of 316) |
0.0 (0 of 0) |
| Beyrer et al (2004) | Mae Rim, Thailand |
Feb 1999 – Jan 2000 |
Drug treatment |
1865 | 10.7 (200) | - | 2.2 (41) | 2.2 (41) | RPR, followed by Serodia- TP-PA for antibody confirmation |
10.3 (192) |
- |
| Carey et al (2006) | Bangalore, India |
Apr 2001 – Oct 2001 |
Drug ftreatment |
356 | 2.0 (7) | - | 12.9 (46) | 12.9 (46) | T. pallidum antibodies |
1.1 (4) |
- |
| Chen et al (2005) | Yunnan, China |
Nov 1999 – May 2000 |
Drug treatment, Medical setting |
292 | 100 (292) | 11.3 (33) | - | 11.3 (33) | RPR, confirmed with TPHA |
17.8 (52) |
- |
| De Carvalho et al (1996) | Santos, Sao Paulo, Brazil |
Oct 1990 – Dec 1992 |
Drug Treatment Street outreach |
220 (197 tested for syphilis) |
41.6 (89) | - | 34.0 (67 of 197) | 34.0 (67 of 197) |
FTA-abs | 57.0 (122 of 214) |
- |
| Dowe et al (2001) | Jamaica, West Indies |
1994 – 1999 | Drug treatment |
301 | 9.0 (27) | - | 6.3 (19) | 6.3 (19) | VDRL, FTA-ab |
2.7 (8) |
- |
| El Ghazzawi et al (1995) | Alexandria, Egypt |
June 1989 – Nov 1991 |
Drug treatment |
100 | 0.0 (0) | - | 3.0 (3) | 3.0 (3) | VDRL, MHA-TP |
0.0 (0) |
0.0 (0 of 0) |
| Frost et al (2007) | Tijuana and Ciudad Juarez, Mexico |
Feb 2005 – April 2005 |
Community setting/ street outreach |
428 (413 tested for syphilis) |
8.2 (35) | - | 9.2 (38 of 413) | 9.2 (38 of 413) |
RPR | 3.3 (14) |
- |
| Go et al (2006) | Northern Vietnam |
Aug 2003 – Sept 2003 |
Street outreach |
309 | 3.6 (11) | - | 1.0 (3) | 1.0 (3) | RPR, TPHA |
- | - |
| Karapetyan et al (2002) | St. Petersbur g, Russia |
Apr 1998 – Dec 1998 |
Storefront/ street outreach (Mobile van) |
910 | 31.3 (285) | - | 11.5 (105) | 11.5 (105) | microprecipitation reaction w/ cardiolipid antigen, confirmation w/IFA (indirect fluorescent antibodies) |
0.0 (0) |
0.0 (0 of 0) |
| Kurbanov et al (2003) | Uzbekistan | April 1999 – March 2000 |
Medical Settings |
18 | 11.1 (2) | - | 22.2 (4) | 22.2 (4) | TP Ab test |
100 (18) |
22.2 (4 of 18) |
| Liu et al (2006) | Anhui Province, China |
2003 | Drug treatment |
312 | 38.8 (121) | - | 11.2 (35) | 11.2 (35) | TRUST followed by TP-PA for confirmation |
0.3 (1) |
- |
| Ostrovskii et al (1999) | St. Petersburg, Russia |
- | Storefront/ street outreach (Mobile van) |
900 | - | - | 11.0 (99) | 11.0 (99) | - | 0.1 (1) |
- |
| Panda et al (1997) | Calcutta, India |
Nov 1994 – Feb 1995 |
drug treatment, jails |
111 | 0.0 (0) | - | 6.3 (7) | 6.3 (7) | VDRL | 0.9 (1) |
- |
| Panda et al (1998) | Calcutta, India |
July 1996 – Sept 1996 |
Street outreach |
103 (91 tested for syphilis) |
0.0 (0) | - | 4.4 (4 of 91) | 4.4 (4 of 91) |
VDRL (1/8 dilution) |
1.1 (1 of 91) |
- |
| Panda et al (2002) | Kolkata, India |
Dec 1999 – June 2000 |
Street outreach |
249 | 0.0 (0) | 0.0 (0) | 6.8 (17) | 6.8 (17) | VDRL (active), TPHA (lifetime) |
1.2 (3) |
- |
| Panda et al (2007) | Chennai, India |
Apr 2003 – July 2003 |
Street outreach |
211 | 0.0 (0) | 0.9 (2) | - | 0.9 (2) | RPR, TPHA (said active) |
0.0 (0) |
- |
| Peak et al (1995) | Kathmandu, Nepal |
1991 | Storefront/ Street outreach |
127 | 10.2 (13) | - | 14.2 (18) | 14.2 (18) | VDRL | 1.6 (2) |
- |
| Peak et al (1995) | Kathmandu, Nepal |
1992 | Storefront/ Street outreach |
39 | 7.7 (3) | - | 10.3 (4) | 10.3 (4) | VDRL | 2.6 (1) |
- |
| Peak et al (1995) | Kathmandu, Nepal |
1993 | Storefront/ Street outreach |
141 | 5.0 (7) | - | 14.2 (20) | 14.2 (20) | VDRL | 0.0 (0) |
- |
| Peak et al (1995) | Kathmandu, Nepal |
1994 | Storefront/ Street outreach |
117 | 3.4 (4) | - | 5.1 (6) | 5.1 (6) | VDRL | 0.0 (0) |
- |
|
Rhodes et al (2006)/ Platt et al (2007) |
Moscow, Volgogradand Barnaul, Russia |
Sept 2003 – Nov 2003 |
Community setting/ Street outreach |
1473 | 29.4 (433) | - | 10.7 (157) | 10.7 (157) | TP Ab test (ICE Syphilis, anenzyme immunoas say (EIA) modified for use with oral fluid) |
7.6 (112) |
14.3 (16 of 112) |
| Ruan et al (2004) | Sichuan province, China |
Nov 2002 – Nov 2004 |
Community setting |
379 | 17.4 (66) | 15.3 (58) | - | 15.3 (58) | ELISA Ab, confirmed with TPPA |
11.3 (43) |
30.2 (13 of 43) |
| Rumi et al (2000) | Bangladesh | Aug 1994 – May 1996 |
Medical setting |
198 | 0.0 (0) | - | 5.1 (10) | 5.1 (10) | PRP, TPHA |
1.0 (2) |
- |
| Wai et al (1996) | Kelantan, Malaysia |
- | Drug treatment |
171 | 100 (171) | - | 38.6 (66) | 38.6 (66) | VDRL, TPHA |
8.2 (14) |
- |
The meta package (Schwarzer, 2007) of the freely-available, open-source R program (R Development Core Team, 2008) was used to test prevalences for heterogeneity. Testing revealed significant heterogeneity among the identified studies precluding pooling of syphilis or HIV prevalence. Testing for heterogeneity revealed χ2 = 1085.76, degrees of freedom = 31, p< 0.0001 for overall syphilis data and χ2 = 18573.1, degrees of freedom = 30, p< 0.0001 for HIV data. We therefore report these data in terms of median and unweighted interquartile ranges.
Results
Twenty-nine published papers were included (Abdala et al., 2003; Altaf et al., 2007; Azim et al., 2000; Azim et al., 2001; Azim et al., 2004; Azim et al., 2006; Baqi et al., 1998; Beyrer et al., 2004; Carey et al., 2006; Chen et al., 2005; de Carvalho et al., 1996; Dowe et al., 2001; El Ghazzawi et al., 1995; Frost et al., 2007; Go et al., 2006; Karapetyan et al., 2002; Kurbanov et al., 2003; Liu et al., 2006; Ostrovskii et al., 1999; Panda et al., 2002; Panda et al., 2007; Panda et al., 1997; Panda et al., 1998; Peak et al., 1995; Rhodes et al., 2006; Platt et al., 2007; Ruan et al., 2004; Rumi et al., 2000; Wai et al., 1996). Two papers (Azim et al., 2004 and Peak et al., 1995) were serial cross-sectional studies involving data from multiple years; these papers were thus listed as two and four separate studies, respectively. Two papers by different authors clearly describe the same study and thus were considered one study (Rhodes et al., 2006 and Platt et al., 2007). Thus, our sample of 29 papers included 32 studies described in Table 2.
The total sample (13,848 subjects, 252 of whom were not tested for syphilis due to study methodology) ranged from a study of 18 HIV positive subjects presenting at an HIV clinic in Uzbekistan (Kurbanov et al., 2003) to a study of 1,865 subjects entering a heroin or methamphetamine drug treatment facility in Thailand (Beyrer et al., 2004), with a mean of 433 subjects and a median of 271 subjects per study.
Study methodology and available details varied. Investigators of 12 studies did at least part of their recruitment from drug treatment programs, while another 14 recruited in part from street outreach. Other recruitment sites included storefront (N=6), syringe exchange programs (N=5), community setting (N=4), medical setting (N=3) and jails (N=1). Some investigators recruited from more than one site, thus the sum of the locations exceeds the total number of studies. Most studies were cross-sectional (n=30); the remaining two were a retrospective case-control and baseline data from a cohort study.
We recorded all types of drugs used regardless of method of administration. Twenty two studies included only IDU: nine included subjects who injected heroin or buprenorphine only; eight reported injection of heroin, benzodiazepines and other pharmaceutical agents, in addition to the use of opium, buprenorphine, cocaine, methamphetamine, ephedrine, cannabis, crack, barbiturates and methadone. The remaining five IDU-only studies did not specify type of drugs injected. One study reported 100% NIDU using alcohol, cannabis, and cocaine. The remaining nine studies consisted of a mix of IDU and NIDU using amphetamines, opiates, cocaine, crack, pheniramine, meclizine/promethazine, diazepam/lorazepam, buprenorphine, cannabis, barbiturates, alcohol, nitrazepan, pentazocine, pethidine, chloropheniramine and other tranquilizer tablets.
Among the 29 studies reporting gender, 81.1% of subjects were men and 18.9% were women. Eight studies included only men and three included only women, while the remaining 18 studies were primarily men (range 56.8 – 99.6%). The three women-only studies (Azim et al., 2006; Chen et al., 2005; Wai et al., 1996) included a high proportion of sex workers (48%, 63% and 100%). Among the 21 studies reporting age, the mean age was 30.5 years.
The prevalence of total syphilis (active and unspecified) in all studies, ranged from 1.0% in a mostly male IDU study in Vietnam (Go et al., 2006) to 60.3% in an IDU study among female sex workers in Bangladesh (Azim et al., 2006), with a median of 11.1% (interquartile range: 6.3% to 15.3%). The prevalence of active syphilis, in the 11 studies reporting active cases, ranged from 0.0% in an IDU/NIDU study in India (Panda et al., 2002) to 15.3% in an IDU study in China (Ruan et al., 2004), with a median of 6.23% (interquartile range: 2.98% to 10.8%). Prevalence of unspecified syphilis, in the 27 studies that did not distinguish between active and lifetime syphilis, ranged from 1.0 % in an IDU study in Vietnam (Go et al., 2006) to 51.2% in a study of IDU sex workers from Bangladesh (Azim et al., 2006), with a median of 11.0% (interquartile range: 6.3% to 13.9%). There was no association between World Bank country income groups (low, lower-middle, and upper-middle) and observed syphilis rates. No studies distinguished among primary, secondary, and tertiary syphilis. Table 3 summarizes the overall, active and unspecified syphilis.
Table 3.
Syphilis and HIV prevalence among drug users
| Number of Studies |
Number of Subjects Tested for Syphilis |
Range | Median | Unweighted Interquartile Range of Proportions (1st to 3rd Quartiles) |
||
|---|---|---|---|---|---|---|
|
Overall Syphilis Prevalence |
all | 32 | 13,596 | 1.0% to 60.3% |
11.1% | 6.3% –15.3% |
| men | 11 | 3570 | 0.7% to 13.0% |
4.0% | 3.4% – 6.6% | |
| women | 6 | 1313 | 9.1% to 60.3% |
19.9% | 11.4% – 36.0% | |
|
Active Syphilis Prevalence |
11 | 5054 | 0.0% to 15.3% |
6.2% | 3.0% – 10.8% | |
|
Unspecified Syphilis Prevalence |
27 | 12,281 | 1.0% to 51.2% |
11.0% | 6.3% – 13.9% | |
|
HIV Prevalence |
31 | 13,358 | 0.0% to 100% |
1.1% | 0.2% – 5.5% | |
HIV prevalence, in the thirty-one studies that reported HIV prevalence, ranged from 0.0% to 100%, with a median of 1.1% (interquartile range: 0.2% to 5.5%; see Table 3). Kurbanov et al. included only HIV-positive subjects (Kurbanov et al., 2003). There was a modest correlation (r=0.27) between HIV and overall syphilis prevalence. Eight studies reported rates of syphilis/HIV co-infection. There was, however, significant variability due to five studies having no co-infected subjects. Three studies, from China (Ruan et al., 2004), Russia (Rhodes et al., 2006; Platt et al., 2007), and Uzbekistan (Kurbanov et al., 2003), (173 total subjects) found co-infection rates of 30.2%, 14.3%, and 22.4%, respectively (see Table 2).
Among the studies reporting syphilis rates by gender, prevalence among men (N=11 studies, 3,570 subjects) ranged from 0.7% to 13.0%, with a median of 4.0% (interquartile range: 3.4% to 6.6%); prevalence among women (N=6 studies, 1313 subjects) ranged from 9.1% to 60.3%, with a median of 19.9% (interquartile range: 11.4% to 36.0%; see Table 3). Of the mixed gender studies, most included only a small proportion of women (Azim et al., 2006; de Carvalho et al., 1996; Dowe et al., 2001; Frost et al., 2007; Karapetyan et al 2002; Panda et al., 2002; Panda et al., 2007). A study from Brazil reported a two-fold higher syphilis seroprevalence for women (OR: 2.44, 95% CI: 1.28–4.76; de Carvalho et al., 1996) and a study from Russia reported a 9-fold higher seroprevalence (OR: 9.4, 95% CI: 5.7–15.5; Karapetyan et al., 2002). Additionally, studies from Bangladesh and Russia found a significantly higher rate of syphilis among women sex workers compared to women non-sex workers (Azim et al., 2006; Karapetyan et al., 2002). There was a strong relation (r= 0.68) between overall syphilis prevalence and the proportion of women.
Twenty-three studies reported one or more sexual-risk factors. 84.6% of subjects in 15 studies reported ever having sex. Among 11 studies (N=5,355 subjects), 59.2% reported multiple sexual partners over various time frames (‘currently’ to ‘past 10 years’), and in 5 studies (N=1,016 subjects) the mean age of sexual debut was 16.1 years (range 14.0–19.0). The frequency of men who have sex with men was reported in six studies, ranging from 1.7% in a mixed IDU/NIDU study in India (Carey et al., 2006) to 51.2% in an IDU study in Brazil (de Carvalho et al., 1996).
Use of condoms was reported in 21 studies, using variable time frames from ‘last sex’ to the ‘last six months’ and variable categories including steady partners and paid partners. One Chinese study (Ruan et al., 2004) found that the majority of subjects reported never using a condom with a primary sex partner (88.2%) or with non-primary sex partners (62.9%) in the last month. The frequency of “some” condom use (N=15 studies) ranged from 9.3% in Pakistani IDUs to 100% in Nepalese IDUs. Reports of “always” using condoms (N=8 studies) ranged from 1.0% in Nepalese IDUs to 32.7% in Russian IDU. Five studies presented syphilis rates by frequency of condom use: prevalence among subjects “always” using condoms ranged from 0% to 4.2%, among those reporting “some” condom use ranged from 4.5% to 27.3%, and among those who “never” used condoms was 11.8%.
One Russian study (Karapetyan et al., 2002) found that 22% of syphilis positive respondents reported that they would have unprotected sex even if they knew they had syphilis. Further, 86% of those subjects who reported having sex without a condom while knowing they had syphilis were sex workers. One Pakistani study found that syphilis positive IDUs were more likely to have paid for sex and had a younger age of first sexual intercourse (Altaf et al., 2007).
Thirteen studies reported if subjects had ever worked as a sex worker. Reports of sex work ranged from 1.3% in a mixed gender study in Thailand to 100% in a study of women in China. Three studies reported overall syphilis prevalence among sex workers: 15.0% (Azim et al., 2006), 15.5% (Carey et al., 2006), and 64.7% (Rhodes et al., 2006/Platt et al., 2007). Seventeen studies reported if subjects had ever had sex with a sex worker, using variable time frames from ‘last month’ to ‘ever’. Reports of sex with sex workers ranged from 71.8% in study of IDU men in India to 14.7% in a mixed gender IDU study in Russia. Two studies reported syphilis prevalence among subjects who had sex with sex workers: 10.8% (Carey et al. 2006) and 19.1% (Altaf et al., 2007).
Discussion
Our review identified 32 published studies from LMI countries reporting syphilis prevalence and sexual risk factors in drug users. The prevalence of overall lifetime syphilis ranged from 0.3% to 60.3% in studies from 14 LMI countries. High-risk sexual behaviors are prevalent among drug users and awareness of transmission risks is low (Altaf et al., 2007; Platt et al., 2007; Azim et al., 2006; Liu et al., 2006; Rhodes et al., 2006; Panda et al., 1998; Wai et al., 1996). High rates of sex work, sex with sex workers and MSM coupled with variable but generally low rates of condom use among illicit drug users could contribute to the high syphilis prevalence in LMI countries.
Women surveyed were more likely both to have syphilis and to trade sex for money. The higher syphilis estimate among women is consistent with other studies illustrating a greater likelihood of syphilis infection among women drug users. This is especially true among women drug users exchanging sex for money and/or drugs (Watters et al., 1994; Platt et al., 2007) who may not perceive themselves to be at high risk of STI infection and transmission (Liu et al, 2006). Lacking awareness of infection status, transmission routes and/or prevention methods, women may continue to practice risky behaviors with their sex and drug partners.
The high-risk group of women sex workers using drugs may “bridge” the population of drug users to non-injecting populations that use the services of sex workers (Strathdee et al., 2008; Liu et al., 2006; Ruan et al., 2006; Karapetyan et al., 2002). One study from Mexico (Strathdee et al., 2008) found that women IDUs often used drugs in a sexual relationship with a drug using male partner, suggesting greater overlap in sexual and drug use networks for women relative to men. This study further demonstrated risky patterns of sexual behavior among women sex workers who injected drugs compared to non-IDUs; IDUs were seven times more likely to use drugs before sex than non-IDUs, a behavior known to lead to lower rates of condom use and reduced condom negotiation skills. Continued studies targeting the risks of women, including larger samples of women drug users, are needed.
Syphilis facilitates the transmission of HIV infection (Lyles et al., 2007; Buchacz et al., 2005; Phipps et al., 2005; Browne et al., 2003; Harrell et al., 2003; Kalichman et al., 2000; Greenblatt et al., 1988). Additionally, syphilis in HIV infected patients is associated with a significant decrease in CD4 cell counts and an increase in HIV viral load (Palacios et al., 2007). The elevated HIV viral load does not consistently decrease after syphilis treatment, likely due to persistent immune activation (Palacios et al., 2007). This may increase both the risk of disease progression in the individual and of disease transmission in the community, illustrating the importance of preventing new syphilis infections in the setting of HIV.
Our results are subject to a number of limitations. Studies reviewed varied substantially in target population, methodology and data coding. Studies defined drug users with varying criteria, which may have affected the estimated prevalence rates. Studies recruited from a variety of settings (prisons, rehabilitation facilities, job seekers, and syringe exchange programs) making it difficult to generalize prevalence rates. Syphilis prevalences were never reported by specific drug type and infrequently by sex-and drug-related risk factors, thus limiting summary results. Many studies focused on HIV or other illnesses, with syphilis as a secondary research objective, often requiring calculations to estimate syphilis prevalence or limiting the details of active versus lifetime rates. The variation between studies and the frequent availability of a single study in certain countries make regional prevalence estimates imperfect. Finally, we accessed only studies with English-language text or abstracts.
The observed rates of syphilis, although highly variable, were higher than those of general population rates in all regions. The variation between studies and the frequent availability of only one study per country limits the reliability of prevalence estimates. Our analysis demonstrates an association between drug use and syphilis, but confirms neither an independent association nor a direct causal relationship between drug use and syphilis. The association of syphilis and drug use is likely due to a convergence of independent, individual and environmental level risk factors. Nonetheless, the increased prevalence of syphilis and the fact that syphilis facilitates the transmission of HIV infection reinforces the need for integrated health care models addressing both issues.
Interventions for HIV prevention among drug users have historically centered on efforts to reduce syringe mediated transmission through reducing behaviors such as front and back loading and syringe sharing through education, syringe exchange and other harm reduction techniques, such as syringe bleaching. Other interventions for STI prevention thus far have included condom promotion and improved diagnosis and treatment with clinic services directed at communities such as fishermen, migrant laborers, truck drivers and street-based sex workers (Abdala, et al 2003; Panda et al., 2002; Karapetyan et al., 2002; Panda et al., 1997; Wai et al., 1996). As prevention efforts for drug users have had an impact in certain areas, it has become clear that drug users retain significant ongoing sexual risk for STI transmission (Des Jarlais et al, 2007a; Des Jarlais 2007b; Kral et al., 2001). The overlap between HIV and TB epidemics have highlighted the limitations of relying entirely on vertical health care programs, which address these related infections separately, highlighting the need for integrated models of care. Similarly, the often high prevalence of syphilis and other STIs found among drug using populations in LMI countries, as well as high income countries, further highlights the need for HIV prevention efforts to address issues both of drug related risks, and of sexual health, among drug using populations in these settings. This reinforces the importance of integrated (or horizontal) health care models and points towards the need for global models of primary health care.
Acknowledgements
Supported by grants R01-DA020841, P30 DA 011041 and NIDAR01 DA-03574 from NIDA.
The authors would like to thank colleagues at The Baron Edmond de Rothschild Chemical Dependency Institute, the Department of Medicine at Beth Israel Medical Center, and the Center for Drug Use and HIV Research at NDRI, Inc. We would also like to acknowledge the following individuals, Anna Wald, Courtney McKnight, Judith Milliken, Naomi Braine, Theresa Perlis, Phillip Coffin, and Chris Newberry, for their input and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdala N, Carney JM, Durante AJ, Klimov N, Ostrovski D, Somalí AM, Kozlov A, Heimer R. Estimating the prevalence of syringe-borne and sexually transmitted disease among injection drug users in St Petersburg, Russia. International Journal of STD & AIDS. 2003;14(10):697–703. doi: 10.1258/095646203322387965. [DOI] [PubMed] [Google Scholar]
- Altaf A, Shah SA, Zaidi NA, Memon A, Nadeemur-Rehman, Wray N. High risk behaviors of injection drug users registered with harm reduction programme in Karachi, Pakistan. Harm Reduction Journal. 2007;4(1):7. doi: 10.1186/1477-7517-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim T, Alam MS, Rahman M, Sarker MS, Ahmed G, Khan MR, Rahman S, Rahman ASMM, Sack DA. Impending concentrated HIV epidemic among injecting drug users in Central Bangladesh. International Journal of STD & AIDS. 2004;15(4):280–282. doi: 10.1258/095646204773557875. [DOI] [PubMed] [Google Scholar]
- Azim T, Bogarts J, Yirrell DL, Banerjea AC, Sarker MS, Ahmed G, Amin MMM, Rahman ASMM, Hussain AMZ. Injecting drug users in Bangladesh: Prevalence of syphilis. Hepatitis, HIV and HIV subtypes. AIDS. 2002;16(1):121–123. doi: 10.1097/00002030-200201040-00015. [DOI] [PubMed] [Google Scholar]
- Azim T, Chowdhury EI, Reza M, Ahmed M, Uddin MT, Khan R, Ahmed G, Rahman M, Khandakar I, Khan SI, Sack DA, Strathdee SA. Vulnerability to HIV infection among sex workers and non-sex worker female injecting drug users in Dhaka, Bangladesh: Evidence from the baseline survey of a cohort study. Harm Reduction Journal. 2006;3:33. doi: 10.1186/1477-7517-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim T, Islam MN, Bogaerts J, Mian MAH, Sarker MS, Fattah KR, Simmonds P, Jenkins C, Choudhury MR, Mathan VI. Prevalence of HIV and syphilis among high-risk groups in Bangladesh. AIDS. 2000;14(2):210–211. doi: 10.1097/00002030-200001280-00022. [DOI] [PubMed] [Google Scholar]
- Baqi S, Nabi N, Hasan SN, Khan AJ, Pasha O, Kayani N, Haque RA, Haq I, Khurshid M, Fisher-Hoch S, Luby SP, McCormick JB. HIV antibody seroprevalence and associated risk factors in sex workers, drug users, and prisoners in SINDO, Pakistan. Journal of Acquired Immune Deficiency Síndromes and Human Retrovirology. 1998;18(1):73–79. doi: 10.1097/00042560-199805010-00011. [DOI] [PubMed] [Google Scholar]
- Beyrer C, Razak MH, Jittiwutikarn J, Suriyanon V, Vongchak T, Srirak N, Kawichai S, Tovanabutra S, Rungruengthanakit K, Sawanpanyalert P, Sripaipan T, Celentano DD. Methamphetamine users in Northern Thailand: changing demographics and risks for HIV and STD among treatment-seeking substance abusers. International Journal of STD & AIDS. 2004;15(10):697–704. doi: 10.1177/095646240401501012. [DOI] [PubMed] [Google Scholar]
- Browne R, Nwokolo N, Boag F. Effect of treatment for sexually transmitted disease on HIV transmission. Journal of HIV Therapy. 2003;8(3):67–71. [PubMed] [Google Scholar]
- Buchacz K, Greenberg A, Onorato I, Janssen R. Syphilis epidemics and human immunodeficiency virus (HIV) incidence among men who have sex with men in the United States: implications for HIV prevention. Sexually Transmitted Diseases. 2005;32(10 Suppl):S73–S79. doi: 10.1097/01.olq.0000180466.62579.4b. [DOI] [PubMed] [Google Scholar]
- Carey MP, Ravi V, Chandra PS, Desai A, Neal DJ. Screening for sexually transmitted infections at a DeAddictions service in south India. Drug and Alcohol Dependence. 2006;82(2):127–134. doi: 10.1016/j.drugalcdep.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS, Yin YP, Liang GJ, Gong XD, Li HS, Poumerol G, Tuy N, Shi MQ, Yu YH. Sexually transmitted infections among female sex workers in Yunnan, China. AIDS Patient Care and STDs. 2005;19(12):853–860. doi: 10.1089/apc.2005.19.853. [DOI] [PubMed] [Google Scholar]
- de Carvalho HB, Mesquita F, Massad E, Bueno RC, Lopes GT, Ruiz MA, Burattini MN. HIV and infections of similar transmission patterns in a drug injectors community of Santos, Brazil. Journal of Acquired Immune Deficiency Síndromes and Human Retrovirology. 1996;12(1):84–92. doi: 10.1097/00042560-199605010-00012. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Hagan H, Arasteh K, McKnight C, Perlman D, Friedman SR. Herpes simples virus-2 and HIV among noninjection drug users in New York city. Sexually Transmitted Diseases. 2007;34(11):923–927. doi: 10.1097/OLQ.0b013e3180ca9647. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, Perlis T, Hagan H, Abdul-Quader A, Heckathorn DD, McKnight C, Bramson H, Nemeth C, Torian LV, Friedman SR. Convergente of HIV seroprevalence among injecting and non-injecting drug users in New York city. AIDS. 2007;21(2):231–235. doi: 10.1097/QAD.0b013e3280114a15. [DOI] [PubMed] [Google Scholar]
- Dowe G, Smikle MF, Thesiger C, Williams E. Bloodborne sexually transmitted infections in patients presenting for substance abuse treatment in Jamaica. Sexually Transmitted Diseases. 2001;28(5):266–269. doi: 10.1097/00007435-200105000-00004. [DOI] [PubMed] [Google Scholar]
- El Ghazzawi E, Drew L, Hamdy L, El Sherbini E, El Din Aly Sedek S, Saleh E. Intravenous drug addicts: A high risk group for infection with human immunodeficiency virus, hepatitis virus, cytomegalovirus and bacterial infections in Alexandria, Egypt. Journal of the Egyptian Public Health Association. 1995;70(1–2):127–150. [PubMed] [Google Scholar]
- Frost SDW, Brouwer KC, Firestone Cruz MA, Ramos R, Ramos ME, Lozada RM, Magis-Rodriguez C, Strathdee SA. Respondent-driven sampling of injection drug users in two U.S.-Mexico border cities: Recruitment dynamics and impact on estimates of HIV and syphilis prevalence. Journal of Urban Health. 2007;83(6 suppl):i83–i97. doi: 10.1007/s11524-006-9104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselquist D, Potterat J. Uncontrolled herpes simplex virus-2 as a cofactor in HIV transmission. Journal of Acquired Immune Deficiency Syndromes. 2003;33(1):119–120. doi: 10.1097/00126334-200305010-00020. [DOI] [PubMed] [Google Scholar]
- Go VF, Frangakis C, Nam LV, Bergenstrom A, Sripaipan T, Zenilman JM, Celentano DD, Quan VM. High HIV sexual behaviors and sexually transmitted disease prevalence among injection drug users in Northern Vietnam: Implications for a generalized HIV epidemic. Journal of Acquired Immune Deficiency Syndromes. 2006;42(1):108–115. doi: 10.1097/01.qai.0000199354.88607.2f. [DOI] [PubMed] [Google Scholar]
- Greenblatt RM, Lukehart SA, Plumier FA, Quinn TC, Critchlow CW, Ashley RL, D’Costa LJ, Ndinya-Achola JO, Corey L, Ronald AR, et al. Genital ulceration as a risk factor for Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS. 1988;2(1):47–50. doi: 10.1097/00002030-198802000-00008. [DOI] [PubMed] [Google Scholar]
- Hahn RA, Onorato IM, Jones TS, Dougherty J. Prevalence of HIV infection among intravenous drug users in the United States. Journal of the American Medical Association. 1989;261(18):2677–2684. [PubMed] [Google Scholar]
- Chesson HW, Pinkerton SD, Voigt R, Counts GW. HIV infections and associated costs attributable to syphilis coinfection among African Americans. American Journal of Public Health. 2003;93(6):943–948. doi: 10.2105/ajph.93.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Nelly JA, Sikkema KJ, Koslov AP, Shaboltas A, Granskaya J. The emerging AIDS crisis in Russia: review of enabling factors and prevention needs. International Journal of STD & AIDS. 2000;11(2):71–75. doi: 10.1177/095646240001100201. [DOI] [PubMed] [Google Scholar]
- Karapetyan AF, Sokolovsky YV, Araviyskaya ER, Zvartau EE, Ostrovsky DV, Hagan H. Syphilis among intravenous drug-using population: Epidemiological situation in St Petersburg, Russia. International Journal of STD & AIDS. 2002;13(9):618–623. doi: 10.1258/09564620260216326. [DOI] [PubMed] [Google Scholar]
- Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edwin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357(9266):1397–1401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- Kurbanov F, Kondo M, Tanaka Y, Zalalieva M, Giasova G, Shima T, Jounai N, Yuldasheva N, Ruzibakiev R, Mizokami M, Imai M. Human immunodeficiency virus in Uzbekistan: Epidemiological and genetic analyses. AIDS Research and Human Retroviruses. 2003;19(9):731–738. doi: 10.1089/088922203769232520. [DOI] [PubMed] [Google Scholar]
- Liu H, Grusky O, Li X, Ma E. Drug users: A potentially important bridge population in the transmission of sexually transmitted diseases, including AIDS, in China. Sexually Transmitted Diseases. 2006;33(2):111–117. doi: 10.1097/01.olq.0000199762.28231.ee. [DOI] [PubMed] [Google Scholar]
- Lopez-Zetina J. Predictors of syphilis seroreactivity and prevalence of HIV among street recruited injection drug users on Los Angeles County. Sexually Transmitted Infections. 2000;76(6):462–469. doi: 10.1136/sti.76.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles CM, Kay LS, Crepaz N, Herbst JH, Passin WF, Kim AS, Rama SM, Thadiparthi S, DeLuca JB, Mullins MM. Best-evidence interventions: Findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000–2004. American Journal of Public Health. 2007;97(1):133–143. doi: 10.2105/AJPH.2005.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell GL, Douglas RG, Bennett JE, Dolin R. Infectious Diseases and Their Etiological Agents. In: Mandell GL, Dolin R, Bennett JE, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 5th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. pp. 2778–2780. [Google Scholar]
- Mathers CD, Stein C, Ma Fat D, Rao C, Inoue M, Tomijima N, Bernard C, Lopez AD, Murray CJL. Global Programme on Evidence for Health Policy Discussion Paper No. 50. Geneva: World Health Organization; 2002. Global Burden of Disease 2000: Version 2 methods and results. [Google Scholar]
- Nelson KE, Vlahov D, Cohn S, Odunmbaku M, Lindsay A, Antohony JC, Hook EW., 3rd Sexually transmitted diseases in a population of intravenous drug users: association with seropositivity to the Human Immunodeficiency Virus (HIV) Journal of Infectious Diseases. 1991;164(3):457–463. doi: 10.1093/infdis/164.3.457. [DOI] [PubMed] [Google Scholar]
- Ostrovskii DV. The clean syringe and HIV infection. A clean syringe can be obtained by any drug addict in a bus stationed in places of active drug trading. Zh Mikrobiol Epidemiol Immunobiol. 1999;Jan-Feb(1):118–119. Russian. [PubMed] [Google Scholar]
- Palacios R, Jiménez-Oñate F, Aguilar M, Galindo MJ, Rivas P, Ocampo A, Berenguer J, Arranz JA, Ríos MJ, Knobel H, Moreno F, Ena J, Santos J. Impact of syphilis infection on HIV viral load and CD4 cell counts in HIV infected patients. Journal of Acquired Immune Deficiency Syndromes. 2007;44(3):356–359. doi: 10.1097/QAI.0b013e31802ea4c6. [DOI] [PubMed] [Google Scholar]
- Panda S, Chatterjee A, Bhattacharjee S, Ray B, Saha MK, Bhattacharya SK. HIV, hepatitis B and sexual practices in the street-recruited injection drug users of Calcuta: Risk perception versus observed risks. International Journal of STD & AIDS. 1998;9(4):214–218. doi: 10.1258/0956462981922061. [DOI] [PubMed] [Google Scholar]
- Panda S, Chatterjee A, Sarkar S, Jalan KN, Maitra T, Mukherjee S, Mukherjee B, Deb BC, Abdul-Quader AS. Injection drug use in Calcutta: A potential focus for an explosive HIV epidemic. Drug and Alcohol Review. 1997;16(1):17–23. doi: 10.1080/09595239700186291. [DOI] [PubMed] [Google Scholar]
- Panda S, Kumar MS, Saravanamurthy PS, Mahalingam P, Vijaylakshmi A, Balakrishnan P, Cantes B, Tamby PA, Jabbar S, Rangaiyan G, Flessenkaemper S, Grosskurth H, Gupte MD. Sexually transmitted infections and sexual practices in injection drug users and their regular partners in Chennai, India. Sexually Transmitted Diseases. 2007;34(4):250–253. doi: 10.1097/01.olq.0000258485.23066.a6. [DOI] [PubMed] [Google Scholar]
- Panda S, Saha U, Pahari S, Nathan M, Poddar S, Neogi D, Sarkar M, Pal NK, Mahalanabis D. Drug use among the urban poor in Kolkata: Behaviour and environment correlates of low HIV infection. National Medical Journal of India. 2002;15(3):128–134. [PubMed] [Google Scholar]
- Peak A, Rana S, Maharjan SH, Jolley D, Crofts N. Declining risk of HIV among injecting drug users in Kathmandu, Nepal: The impact of a harm-reduction programme. AIDS. 1995;9(9):1067–1070. doi: 10.1097/00002030-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Phipps W, Stanley H, Kohn R, Stansell J, Klausner JD. Syphilis, Chlamydia, and Gonorrhea Screening in HIV-Infected Patients in Primary Care, San Francisco, California, 2003. AIDS Patient Care and STDs. 2005;19(8):495–498. doi: 10.1089/apc.2005.19.495. [DOI] [PubMed] [Google Scholar]
- Platt L, Rhodes T, Judd A, Judd A, Koshkina E, Maksimova S, Latishevskaya N, Renton A, McDonald T, Parry JV. Effects of sex work on the prevalence of syphilis among injection drug users in 3 Russian cities. American Journal of Public Health. 2007;97(3):478–485. doi: 10.2105/AJPH.2005.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt L, Rhodes T, Judo A, Koshkina E, Maksimova S, Latishevskaya N, Renton A, McDonald T, Parry JV. Effects of sex work on the prevalence of syphilis among injection drug users in 3 Russian cities. American Journal of Public Health. 2007;97(3):478–485. doi: 10.2105/AJPH.2005.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2008. R: A language and environment for statistical computing. ISBN 3-900051-07-0, Retrieved online May 16, 2008 at http://www.R-project.org. [Google Scholar]
- Rhodes T, Platt L, Maximova S, Koshkina E, Latishevskaya N, Hickman M, Renton A, Bobrova N, McDonald T, Parry JV. Prevalence of HIV, hepatitis C and syphilis among injection drug users in Russia: A multi-city study. Addiction. 2006;101(2):252–266. doi: 10.1111/j.1360-0443.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- Ross MW, Hwang LY, Zack C, Bull L, Williams ML. Sexual risk behaviours and STIs in drug abuse treatment populations whose drug of choice is crack cocaine. International Journal of STD & AIDS. 2002;13(11):769–774. doi: 10.1258/095646202320753736. [DOI] [PubMed] [Google Scholar]
- Ruan Y, Cao X, Qian H, Zhang L, Qin G, Jiang Z, Song B, Hu W, Liang S, Chen K, Yang Y, Li X, Wang J, Chen X, Hao C, Song Y, Xing H, Wang N, Shao Y. Syphilis among female sex workers in southwest China: Potencial for HIV transmission. Sexually Transmitted Diseases. 2006;33(12):719–723. doi: 10.1097/01.olq.0000218881.01437.99. [DOI] [PubMed] [Google Scholar]
- Ruan Y, Chen K, Hong K, He Y, Liu S, Zhou F, Qin G, Chen J, Xing H, Shao Y. Community-based survey of HIV transmission modes among intravenous drug users in Sicuani, China. Sexually Transmitted Diseases. 2004;31:623–627. doi: 10.1097/01.olq.0000140018.24262.4a. [DOI] [PubMed] [Google Scholar]
- Rumi MAK, Siddiqui MA, Salam MA, Iqbal MR, Azam MG, Chowdhury AK, Habibullah Khan AYM, Hasan KN, Hassan MS. Prevalence of infectious diseases and drug abuse among Bangladeshi workers. Southeast Asian Journal of Tropical Medicine & Public Health. 2000;31(3):571–574. [PubMed] [Google Scholar]
- Scheinmann R, Hagan H, Lelutiu-Weinberger C, Stern R, Des Jarlais DC, Flom PL, Strauss S. Non-injection drug use and hepatitis C virus: A systematic review. Drug and Alcohol Dependence. 2007;89:1–12. doi: 10.1016/j.drugalcdep.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer G. meta: Meta-analysis. R package version 0.8–2. 2007 Retrieved online May 16, 2008 at http://cran.at.r-project.org/web/packages/meta/index.html.
- Steinbrook R. HIV in India—a complex epidemic. New England Journal of Medicine. 2007;356(11):1089–1093. doi: 10.1056/NEJMp078009. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Philbin MM, Semple SJ, Pu M, Orozovich P, Martinez G, Lozada R, Fraga M, de la Torre A, Staines H, Magis-Rodriguez C, Patterson TL. Correlates of injection Drug Use among female sex workers in two Mexico-U.S. border cities. Drug and Alcohol Dependence. 2008;92:132–140. doi: 10.1016/j.drugalcdep.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Bank. Data and Statistics: Country Groups. 2007 Retrieved online October 18, 2007 at http://go.worldbank.org/D7SN0B8YU0.
- Wai BH, Singh S, Varma SL. HIV infection in females dependent on drugs. Addiction. 1996;91:435–438. doi: 10.1046/j.1360-0443.1996.91343513.x. [DOI] [PubMed] [Google Scholar]
- Watters JK, Estilo MJ, Kral AH, Lorvick JJ. HIV infection among female injection-drug users recruited in community settings. Sexually Transmitted Diseases. 1994;21:321–328. doi: 10.1097/00007435-199411000-00005. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections Overview and Estimates. 2001 Retrieved online September 20, 2007 at http://www.who.int/docstore/hiv/GRSTI/index.htm.
- Zetola N, Klausner J. Syphilis and HIV Infection: An update. Clinical Infectious Diseases. 2007;44:1222–1228. doi: 10.1086/513427. [DOI] [PubMed] [Google Scholar]