Abstract
Effective therapies are needed for the treatment of amyotrophic lateral sclerosis (ALS), a fatal type of motor neuron disease. Morphological, biochemical, molecular genetic, and cell/animal model studies suggest that mitochondria have potentially diverse roles in neurodegenerative disease mechanisms and neuronal cell death. In human ALS, abnormalities have been found in mitochondrial structure, mitochondrial respiratory chain enzymes, and mitochondrial cell death proteins indicative of some non-classical form of programmed cell death. Mouse models of ALS are beginning to reveal possible principles governing the biology of selective neuronal vulnerability that implicate mitochondria. This minireview summarizes work on the how malfunctioning mitochondria might contribute to neuronal death in ALS through the biophysical entity called the mitochondrial permeability pore (mPTP). The major protein components of the mPTP are enriched in mouse motor neurons. Early in the course of disease in ALS mice expressing human mutant superoxide dismutase-1, mitochondria in motor neurons undergo trafficking abnormalities and dramatic remodeling resulting in the formation of mega-mitochondria and coinciding with increased protein carbonyl formation and nitration of mPTP components. The genetic deletion of a major mPTP component, cyclophilin D, has robust effects in ALS mice by delaying disease onset and extending survival. Thus, attention should be directed to the mPTP as rational target for the development of drugs designed to treat ALS.
Keywords: adenine nucleotide translocator, apoptosis, cell death, cyclophilin D, excitotoxicity, mitochondria, motor neuron, ppif, voltage-dependent anion channel
1. Introduction
ALS is a progressive and severely disabling fatal neurological disease in humans characterized by initial muscle weakness, and then muscle atrophy, spasticity, and eventual paralysis and death typically within 3 to 5 years after symptoms begin [1]. The cause of the spasticity, paralysis and death is progressive degeneration and elimination of upper motor neurons (MNs) in cerebral cortex and lower MNs in brainstem and spinal cord [1,2]. Degeneration and loss of spinal and neocortical interneurons has also been found in human ALS [3,4]. More than 5000 people in the United States are diagnosed with ALS each year (ALS Association, www.alsa.org), and, in parts of the United Kingdom, three people die everyday from some form of MN disease (www.uk-mnd-professional-network.com). Other than life support management, no effective treatments exist for ALS [5].
It is still not understood why specific neuronal populations are selectively vulnerable in ALS, such as certain somatic MNs and interneurons [3,4]. The molecular pathogenesis of ALS is understood poorly, contributing to the lack of appropriate target identification and effective mechanism-based therapies to treat this disease. Two forms of ALS exist: idiopathic (sporadic) and heritable (familial). The majority of ALS cases are sporadic with few known genetic contributions, except for missense mutations in TAR-DNA binding protein [6]. Aging is a strong risk factor for ALS because the average age of onset is 55 (ALS Association, www.alsa.org). Familial forms of ALS (fALS) have autosomal dominant or autosomal recessive inheritance patterns and make up ~10% or less of all ALS cases. ALS-linked mutations occur in the genes encoding SOD1 (ALS1), Alsin (ALS2), senataxin (ALS4), vesicle associated membrane protein (VAMP/synaptobrevin)-associated protein B (ALS8), dynactin, TAR-DNA binding protein, and fused in sarcoma (FUS, ALS6) [7,8].
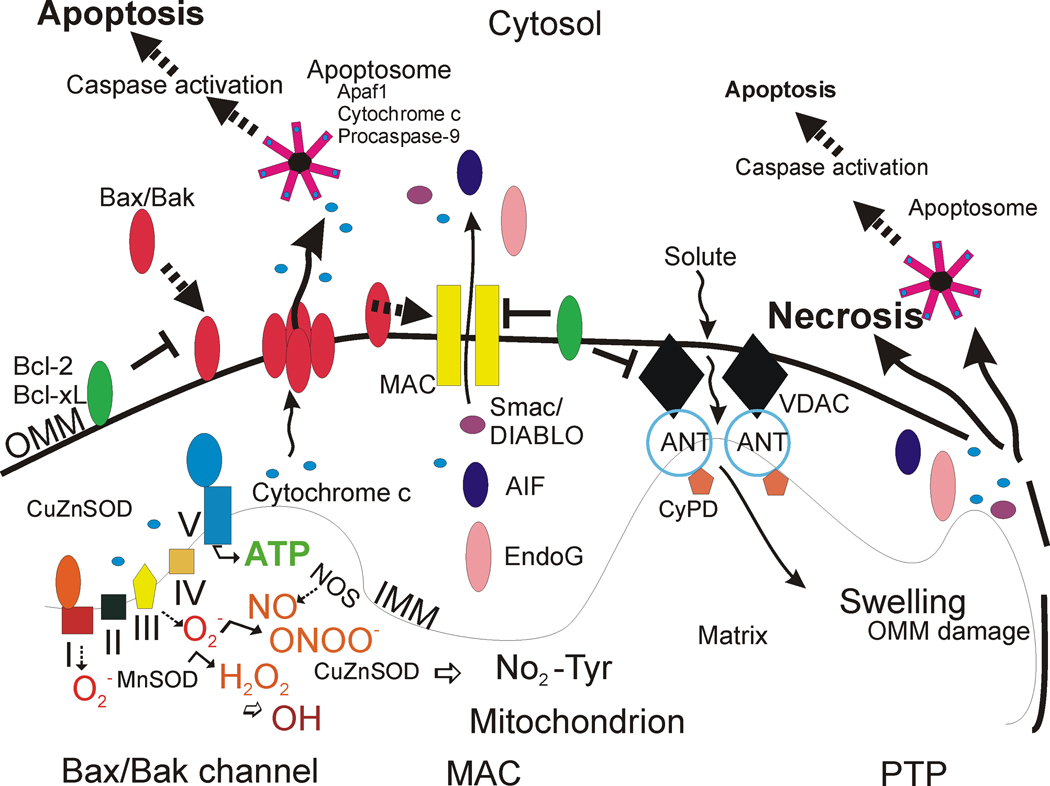
Mitochondrial perturbations have been known for a long time to participate in the mechanisms of neuropathology, particularly disorders involving acute interruptions in O2 and substrate delivery to the brain and bioenergetic failure as seen in tissue ischemia [9,10]. An exciting new understanding of mitochondrial biology has emerged over the past decade that is likely to be very relevant to age-related neurodegenerative disorders [7]. Mitochondria are multi-functional organelles [10]. In addition to their critical role in the production of ATP through the electron transport chain (Fig. 1), these organelles function in intracellular Ca2+ homeostasis, steroid, heme and iron-sulfur cluster synthesis, and programmed cell death [10,11]. They are also sites of formation of reactive oxygen species (ROS), including superoxide anion (O2 •−) and the highly reactive hydroxyl radical (•OH) or its intermediates [10], and reactive nitrogen species such as nitric oxide (•NO) [10]. Thus, mitochondria have functions and properties that might confer an intrinsic susceptibility of subsets of long-lived post-mitotic cells such as neurons to aging and stress. In this regard varying degrees of mitochondrial dysfunction and aberrant mitochondrial maintance and repair could be critical determinants in the regulation of disease and neuronal cell death ranging from necrosis and apoptosis to autophagy [5,12–14].
Figure 1. Mitochrondrial regulation of cell life and death in schematic representation.
The respiratory chain proteins (complex I–IV) establish an electrochemical gradient across the inner mitochondrial membrane (IMM) by extruding protons out of the matrix into the intermembrane space, thereby creating an energy gradient that drives the production on ATP by complex V (lower left). Superoxide (O2 •−) is produced as a by-product in the process of electron transport and is converted to hydrogen peroxide (H2O2) by MnSOD (or CuZnSOD). In pathological settings that can trigger cell aging and death, H2O2 can be converted to hydroxyl radical (OH), or hydroxyl-like intermediates, and mitochondrial nitric oxide synthase (NOS) can produce nitric oxide (NO) that can combine with O2 •− to form peroxynitrite (ONOO−). CuZnSOD can use ONOO− to catalyze the nitriation of mitochondrial protein tyrosine residues (bottom center) such as cyclophilin D (CyPD) and the adenine nucleotide translocator (ANT) [78]. Bcl-2 family members regulate apoptosis by modulating the release of cytochrome c from mitochondria into the cytosol. Two models can account for this process (the Bax/Bak channel model and the mitochondrial apoptosis-induced channel or MAC). In the Bax/Bak1 channel model (left), Bax (Bcl-2-associated X protein) is a pro-apoptotic protein found mostly in the cytosol in healthy mammalian cells but, after specific cell death inducing stumuli, Bax undergoes a conformation shift and translocates to the outer mitochondrial membrane (OMM) where it inserts [55,56]. Bak1 (Bcl-2-antagonist/killer 1) is a similar pro-apoptotic protein localized mostly to the mitochondrial outer membrane. Bax/Bak1 monomers physically interact and form oligomeric or heteromeric channels that are permeable to cytochrome c. The formation of these channels is blocked by Bcl-2 and Bcl-xL at multiple sites. BH3-only members (Bad, Bid, Noxa, Puma) are pro-apoptotic and can modulate the conformation of Bax/Bak1 to sensitize this channel, possibly by exposing its membrane insertion domain (not shown). The MAC could be a channel similar to the Bax/Bak1 channel, but it might also have additional components. Release cytochrome c participates in the formation of the apoptosome in the cytosol that drives the activation of caspase-3 in motor neurons (see Figure 2). Second mitochondria-derived activator of caspases (Smac)/ direct IAP-binding protein with low pI (DIABLO) are released into the cytosol to inactivate the anti-apoptotic actions of inhibitor of apoptosis proteins that inhibit caspases. The DNases AIF and EndoG are released and translocate to the nucleus to stimulate DNA fragmentation. Another model (right) for mitochondrial directed cell death involves the permeability transition pore (PTP). The PPT is a transmembrane channel formed by the interaction of the ANT and the voltage-dependent anion channel (VDAC) at contact sites between the IMM and the OMM [114–120]. CyPD, located in the matrix, can regulate the opening of the PTP by interacting with the ANT. Opening of the PTP induces matrix swelling and OMM rupture leading to release of cytochrome c and other apoptogenic proteins (AIF, EndoG). Certain Bcl-2 family members can modulate the activity of the PTP.
2. Mitochondrial abnormalities in human ALS
2.1. Evidence for mitochondrial abnormalities in human ALS pathogenesis lacks definite causal relationships
Mitochondrial dysfunction has been implicated in the pathogenesis of ALS in humans. Electron microscopy studies have shown mitochondrial morphology abnormalities in skeletal muscle, liver, spinal MNs and cortical upper MN regions of ALS patients [15,16]. A mutation in cytochrome c oxidase subunit I was found in a patient with a MN disease phenotype [17]. Another patient with MN disease had a mutation in a mitochondrial tRNA gene [18]. One type of mitochondrial DNA (mtDNA) mutation, called the common mtDNA deletion (mtDNA4977), is found non-uniformly within different human brain areas; the highest levels are detected in the striatum and substantia nigra [19,20]. However, no significant accumulation of the 5kb common deletion in mtDNA has been found by single-cell analysis of MNs from sporadic ALS cases [21]. Some ALS patients with defects in mitochondrial oxidative phosphorylation in skeletal muscle have a novel SOD1 mutation [22].
2.2. Intracellular Ca2+ abnormalities and excitotoxicity in human ALS pathogenesis: links to mitochondrial dysfunction and oxidative stress
Mitochondria function in the regulation of intracellular Ca2+ levels [11,23]. Regarding ALS, skeletal muscle biopsies of patients with sporadic disease show ultrastructural changes indicative of elevated Ca2+ in MN terminals, with some mitochondria showing an augmented Ca2+ signal [24]. Utilizing specific transport systems mitochondria can move Ca2+ from the cytosol into the matrix by the Ca2+ uniporter and eject Ca2+ via the Na+/Ca2+ exchanger [11] and more catastrophically through the mitochondrial permeability transition pore (mPTP) [25]. Under conditions of elevated cytoplasmic Ca2+, whenever the local free Ca2+ concentration rises above a set-point of ~0.5 µM, mitochondria avidly accumulate Ca2+ to a fixed capacity [11]. The electrical gradient across the mitochondrial inner membrane, the ΔΨm, established by electron transport chain activity (Fig. 1), provides the driving force for the accumulation of Ca2+ into the mitochondrial matrix [23]. Cytosolic Ca2+ concentrations above set-point levels are believed to be achieved during tetanic stimulation and by activation of glutamate receptors on the plasma membrane [11]. In settings of the pathological process called excitotoxicity, resulting from excessive overstimulation of glutamate receptors [26], Ca2+ overload in neurons is significant and cause cell death [27]. When mitochondria become overloaded with Ca2+, they undergo mitochondrial permeability transition (see below) resulting in osmotic swelling and rupture of the outer mitochondrial membrane (Fig. 1). Interestingly, mitochondria within synapses appear to be more susceptible than non-synaptic mitochondria to Ca2+ overload [28].
Exitotoxicity has been implicated in the pathogenesis of ALS for a long time [29] and is another possible mechanism by which MNs can be damaged in ALS [27]. Sporadic ALS patients have reduced levels of synaptosomal high-affinity glutamate uptake [29] and astroglial glutamate transporter EAAT2 (excitatory amino acid transporter 2 or GLT1) in motor cortex and spinal cord [30]. Reductions in levels of activity of EAAT2 in spinal cord could increase the extracellular concentrations of glutamate at synapses on MNs. MNs are sensitive to glutamate excitotoxicity because they have a low proportion of GluR2-edited or under-edited AMPA subtype glutamate receptor on their surfaces, predisposing MNs to risk of excess Ca2+ entry and mitochondrial perturbations [31,32]. Excess glutamate receptor activation in neurons can cause increased intracellular Ca2+, mitochondrial ROS production, bioenergetic failure, and mitochondrial trafficking abnormalities [33]. Ca2+-induced generation of ROS in brain mitochondria is mediated by mitochondrial permeability transition [34]. MNs are particularly affected by inhibition of mitochondrial metabolism which causes elevated cytosolic Ca2+ levels and increased excitability [35].
Mitochondria generate endogenous ROS as by-products of oxidative phosphorylation (Fig. 1) [36]. Because many mitochondrial proteins possess iron-sulfur clusters for oxidation-reduction reactions and because mtDNA lacks protective histones, these macromolecules are particularly vulnerable to ROS attack. Electrons in the electron carriers, such as the unpaired electron of ubisemiquinone bound to coenzyme Q binding sites of complexes I, II, and III, can be donated directly to O2 to generate O2 •− [36]. O2 •− does not easily pass through biological membranes and thus must be inactivated in compartments where it is generated [37]. The mitochondrial matrix enzyme manganese superoxide dismutase (MnSOD or SOD2) or copper/zinc SOD (Cu/ZnSOD or SOD1) in the mitochondrial intermembrane space and cytosol convert O2 •− to hydrogen peroxide (H2O2) in the reaction O2 •− + O2 •− + 2H+→ H2O2 + O2 (Fig. 1) [37]. H2O2 is more stable than O2 •− and can diffuse from mitochondria and into the cytosol and nucleus. H2O2 is detoxified by glutathione peroxidase in mitochondria and in the cytosol and by catalase in peroxisomes. In the presence of reduced transitional metals (Fe2+), H2O2 is catalyzed to •OH [38]. O2 •− can also react with •NO to form the potent nucleophile and oxidant and nitrating agent peroxynitrite (ONOO−) (Fig. 1) [39]. ONOO− or products of ONOO− can damage proteins by nitration [39]. ONOO− is genotoxic directly to neurons by causing single- and double-strand breaks in DNA [40]. NO can be produced in mitochondria [41] and has direct effects in mitochondria. NO at nanomolar concentrations can inhibit rapidly and reversibly respiration [42].
Markers of oxidative stress and ROS damage are elevated in ALS tissues [43]. In human sporadic ALS, protein carbonyls are elevated in motor cortex [44]. Tyrosine nitration is elevated in human ALS nervous tissues [45–47]. Studies of respiratory chain enzyme activities are discrepant. Studies have reported increases in complex I, II, and III activities in vulnerable and non-vulnerable brain regions in patients with mutant SOD1-fALS [48], but other studies have found decreased complex IV activity in spinal cord ventral horn [49] and skeletal muscle [50] of sporadic ALS cases. In sporadic ALS skeletal muscle, reductions in activity of respiratory chain complexes with subunits encoded by the mitochondrial genome are associated with decreased neuronal NO synthase levels [51]. Alterations in skeletal muscle mitochondria are progressive [52] and could be intrinsic to skeletal muscle [53] and not due merely to neurogenic atrophy as assumed commonly.
3. Human ALS and mitochondrial orchestrated programmed cell death (PCD) involving p53
3.1. Degeneration of motor neurons in human ALS has features of PCD
PCD is physiologically regulated cell death [12]. Apoptosis, a form of PCD, is a structurally and biochemically organized, transcriptionally-dependent or –independent, form of cell death. The basic machinery of apoptosis is conserved in yeast, hydra, nematode, fruit fly, zebrafish, mouse, and human [54]. Mitochondria regulate cell death processes (Fig. 1, Fig 2). A variety of mitochondrial proteins function in apoptosis (Table 1) including Bcl-2 family members, cytochrome c, apoptosis inducing factor (AIF), endonuclease G, second mitochondrial activator of caspases (Smac/DIABLO), and Omi/high-temperature requirement protein A2 (HtrA2) inhibitor of the inhibitors of apoptosis proteins (Fig. 1) [55–57]. Other proteins (e.g., humanin, Ku70, 14-3-3 proteins) that are not mitochondrial can modulate mitochondrially translocated cell death proteins by binding and sequestration. For example, Ku70 blocks the translocation of Bax to mitochondria to restrain cell death [58].
Figure 2. PCD mechanisms regulate the degeneration of motor neurons in human amyotrohpic lateral sclerosis.
The levels of multidomain Bcl-2 family members are aberrant in isolated mitochondria from human amyotrohpic lateral sclerosis (ALS) brain and spinal cord [59]. Bcl-2 is depleted from mitochondria by unknown mechanisms (top schematic drawing). Bax and Bak levels are elevated in mitochondria (top schematic drawing). These changes would favor the release of cytochrome c from mitochondria and the accumulation of cytochrome c in the cytoplasm of motor neurons (A). Human ALS motor neurons are rich in mitochondria, as identified by cytochrome c oxidase subunit I (B, brown labeling) and many are positive for cleaved capsase-3 (B, blue-green labeling). These observations are consistent with an accumulation of somatodendritic mitochondria and apoptosome-mediated activation of caspase-3 in ALS motor neurons. These changes appear to be occurring slowly in subsets of motor neurons and could lead to cell death that is a variant phenotype of apoptosis (see Figure 3). In human ALS motor neurons, it is not known yet if alterations occur in the components of the mPTP (top schematic drawing), such as cyclophilin D (CypD), adenine nucleotide translocator (ANT), and voltage-dependent anion channel (VDAC). Scale bars = 6 µm (A); 3 µm (B).
Table 1.
Some Mitochondrial Associated Proteins That Function in Cell Death
| Protein | Function |
|---|---|
| Bcl-2* | Antiapoptotic, blocks Bax/Bak channel formation |
| Bcl-XL | Antiapoptotic, blocks Bax/Bak channel formation |
| Bax* | Proapoptotic, forms pores for cytochrome release |
| Bak* | Proapoptotic, forms pores for cytochrome release |
| Bad | Proapoptotic, decoy for Bcl-2/Bcl-XL promoting Bax/Bak pore formation |
| Bid | Proapoptotic, decoy for Bcl-2/Bcl-XL promoting Bax/Bak pore formation |
| Noxa | Proapoptotic, decoy for Bcl-2/Bcl-XL promoting Bax/Bak pore formation |
| Puma | Proapoptotic, decoy for Bcl-2/Bcl-XL promoting Bax/Bak pore formation |
| p53* | Antagonizes activity of Bcl-2/Bcl-XL, promotes Bax/Bak oligomerization |
| Cytochrome c | Activator of apoptosome |
| Smac/DIABLO | IAP inhibitor |
| AIF | Antioxidant flavoprotein/released from mitochondria to promote nuclear DNA fragmentation |
| Endonuclease G | Released from mitochondria to promote nuclear DNA fragmentation |
| HtrA2/Omi | IAP inhibitor |
| VDAC | mPTP component in outer mitochondrial membrane |
| ANT+ | mPTP component in inner mitochondrial membrane |
| Cyclophilin D+ | mPTP component in mitochondrial matrix |
| TSPO (peripheral benzodiazepine receptor) |
Modulator of mPTP |
| Hexokinase | Modulator of VDAC |
PCD appears to contribute to the selective degeneration of MNs in human sporadic ALS and fALS, albeit seemingly as a non-classical form differing from apoptosis (Fig. 2) [59]. MNs appear to pass through sequential stages of chromatolysis (suggestive of initial axonal injury), somatodendritic attrition without extensive cytoplasmic vacuolation, and then nuclear DNA fragmentation, nuclear condensation, and cell death (Fig. 3) [59]. MNs in individuals dying from sporadic ALS and fALS show the same patterns of degeneration [59]. This cell death in human MNs is defined clearly by genomic DNA fragmentation (determined by DNA agarose gel electrophoresis and in situ DNA nick-end labeling) and cell loss and is associated with accumulation of mitochondria, cytochrome c, and cleaved caspase-3 (Fig. 2, Fig. 3) [60]. However, the morphology of this cell death is distinct from classical apoptosis, despite the nuclear condensation [12,61]. Nevertheless, Bax and Bak1 protein levels are increased in mitochondria-enriched fractions of selectively vulnerable motor regions (spinal cord anterior horn and motor cortex gray matter), but not in regions unaffected by the disease (somatosensory cortex gray matter). In marked contrast, Bcl-2 protein is severely depleted in mitochondria-enriched fractions of affected regions and is sequestered in the cytosol (Fig. 2) [59]. Although these western blot observations lacked direct specificity for MN events [59], subsequent immunohistochemistry (Fig. 2, Fig. 3) [60] and laser capture microdissection of MNs combined with mass spectroscopy-protein profiling have confirmed the presence of intact active caspase-3 in human ALS MNs [62].
Figure 3. Comparison of motor neuron death phenotypes in human and transgenic mouse amyotrohpic lateral sclerosis.
The degeneration of spinal motor neuron (MNs) in human fALS and in G93Ahigh mSOD1 mouse is very different. A–C. Normal anterior horn MNs in human spinal cord are large polygonal multipolar cells as seen with cresyl violet staining (A). In human fALS, the degeneration is typified by shrinkage and progressive condensation of the cytoplasm and nucleus (B,C, * identifies the nucleus). The cell in (c) is identified as a dying MN based the large nucleolus and residual large Nissl bodies. D. These shrunken human MNs usually have large-scale genomic fragmentation of DNA as detected by terminal transferase-mediated biotin-dUTP nick-end labeling (TUNEL). Brown labeling in nucleus (asterisk) reveals the presence of DNA-double strand breaks. E. Degenerating MNs in human ALS are always immunopositive for cleaved caspase-3 (black-dark green labeling) in the somatodendritic attrition stage and accumulate around the nucleus (asterisk) discrete mitochondria (brown-orange labeling, detected with antibody to cytochrome c oxidase subunit I) exhibiting little light microscopic evidence for swelling. F. Human wild-type SOD1 transgenic mice have mostly normal appearing spinal MNs as seen with cresyl violet staining. G–I. In G93Ahigh mSOD1mice (G1 line), the degeneration is typified by progressive cytoplasmic vacuolization and cellular swelling and dissolution (* identifies the nucleus) that begins at about 4–6 week of age develops severely from 8 to 16 weeks of age. This degeneration more closely resembles a form of slowly occurring necrosis. Neither the cytoplasm nor the nucleus undergoes condensation during their degeneration. Scale bars: A (same for B,C) = 8 µm; D (same for E) = 12 µm; F (same for G–I) = 10 µm.
3.2. Degeneration of motor neurons in human ALS is associated with p53 activation
Neuronal apoptosis can be driven by the tumor suppressor p53 [63]. This cell death can be transcriptionally-dependent and transcriptionally-independent [63]. p53 can mediate mitochondrial permeabilization through direct physical interaction with Bcl-2 family members [64]. p53 is activated in MNs in human ALS [65]. p53 levels increase in vulnerable regions in individuals ALS, and p53 accumulates specifically in ALS MN [65]. This p53 is active functionally because it is phosphorylated at serine392 and has increased DNA binding activity [61,65].
These data support the concept of an aberrant re-emergence of a PCD mechanism, involving p53 activation and cytosol-to-mitochondria redistributions of cell death proteins, participating in the pathogenesis of MN degeneration in human ALS [59,95]. The morphological and biochemical changes seen in human ALS are modeled robustly and faithfully at structural and molecular levels in axotomy models of MN degeneration in adult mouse [66] but not in the current commonly used human mutant SOD1 transgenic mouse models (Fig. 3) [60].
4. Mitochondrial pathobiology in cell and mouse models of ALS
A common mutation in human SOD1 that is linked to fALS is the substitution of glycine by alanine at position 93 (G93A) [67,68]. SOD1 is a metalloenzyme of 153 amino acids (~16 kDa) that binds one copper ion and one zinc ion per subunit [37]. This enzyme, functioning as a ~32 kDa non-covalently linked homodimer, is responsible for the detoxification and maintenance of intracellular O2 •− concentration in the low femtomolar range by catalyzing its dismutation [37,69]. SOD1 is ubiquitous (intracellular SOD concentrations are typically ~10–40 µM) in most tissues and possibly greater in neurons [70].
4.1. Evidence for mitochondrial abnormalities in cell models of ALS
Data from cell culture studies argue strongly for mitochondria dysfunction, possibly MN selective, in the presence of mSOD1 [67,71]. Expression of several human SOD1 mutants increases mitochondrial O2 •− levels and causes toxicity in primary MNs [72], neuroblastoma cells [73], and NSC-34 cells (a hybrid cell line with some MN-like characteristics, produced by fusion of MN-enriched embryonic mouse spinal cord cells with mouse neuroblastoma cells) [75]. These responses can be attenuated by over-expression of MnSOD [73]. ALS-mSOD1 variants, compared to wild-type SOD1, associate more with mitochondria in NSC-34 cells and appear to form cross-linked oligomers that shift the mitochondrial GSH/GSSH ratio toward oxidation [71].
4.2. Most evidence for mitochondrial abnormalities in mouse ALS pathogenesis lacks definite causal relationships
Gurney et al were the first to develop transgenic (tg) mice that express the G93A mutant form of human SOD1 [76,77]. Now, these mice are used widely as an animal model of ALS [5,60,67,68]. Human mutant SOD1 (mSOD1) is expressed ubiquitously in these mice by its endogenous promoter in a tissue/cell non-selective pattern against a background of normal mouse SOD1 [76]. Effects of this human mutant gene in mice are profound. Hemizygous tg mice expressing high copy number of the G93A variant of mSOD1 become completely paralyzed and die at ~16–18 weeks of age [76]. G93A-mSOD1 mice with reduced transgene copy number have a much slower disease progression and die at ~8–9 months of age [76,78]. Spinal MNs and interneurons in mice expressing G93Ahigh-mSOD1 undergo prominent degeneration; about 70% of lumbar MNs are eliminated by end-stage disease [79,80]. More work is needed on the cell death and its mechanisms in G93Alow-expressing mice. Subsets of spinal interneurons are lost before MNs G93Ahigh-mSOD1 [79], some of which are the glycinergic Renshaw cells [80]. Unlike the degeneration of MN in human ALS, MNs in these mice do not degenerate with a morphology resembling any form of apoptosis (Fig. 3) [60,79,81]. The MN degeneration seen in G93Ahigh-mSOD1 mice more closely resembles a prolonged necrotic-like cell death process (Fig. 3) [12] involving early-occurring mitochondrial damage, cellular swelling, and dissolution [60,78–80]. Biochemically, the death of MNs is characterized by somal and mitochondrial swelling and formation of DNA single-strand breaks prior to double-strand breaks occurring in nuclear DNA and mitochondrial DNA [79]. The MN death is independent of activation of caspases-1 and 3, and also appears to be independent of capsase-8 and apoptosis-inducing factor activation within MNs [79]. Indeed, caspase-dependent and p53-mediated apoptosis mechanisms might be blocked actively in G93Ahigh-mSOD1 mouse MNs, possibly by upregulation of inhibitors of apoptosis and changes nuclear import of proteins [79].
Mitochondrial pathology has been implicated in the mechanisms of human and mouse ALS [7], but most evidence is circumstantial. In different mSOD1 mouse models of ALS, mitochondria in spinal cord neurons exhibit structural pathology [79,82–86] and some of the mitochondrial degeneration occurs very early in the course of the disease [79,81]. Mitochondrial microvacuolar damage in MNs emerges by 4 weeks of age in G93A mice with high expression [79,81]. It has been argued that mitochondrial damage in G93Ahigh-mSOD1 mice is related to supra-normal levels of SOD1 and might not be related causally to the disease process because transgenic mice expressing high levels of human wildtype SOD1 show some mitochondrial pathology [87], but mitochondrial abnormalities have been found histologically also in G93Alow-mSOD1 mice [88](Martin LJ et al, unpublished observations). Thus, mitochondria could be primary sites of human SOD1 toxicity in transgenic mice irrespective of transgene copy number and expression level of human SOD1, but direct, unequivocal causal relationships have been lacking.
Human SOD1 mutant proteins appear to gain a toxic property or function, rather than having diminished O2 − scavenging activity [89–91], and wild-type SOD1 can gain toxic properties through oxidative modification [92,93]. A gain in aberrant oxidative chemistry could contribute to the mechanisms of mitochondriopathy in G93Ahigh mice [39,94]. G93A-mSOD1 has enhanced free radical-generating capacity compared to wild-type enzyme [91] and can catalyze protein oxidation by hydroxyl-like intermediates and carbonate radical [95]. G93Ahigh mice have increased protein carbonyl formation in total spinal cord tissue extracts at pre-symptomatic disease [96]. Protein carbonyl formation in mitochondrial membrane-enriched fractions of spinal cord is a robust signature of incipient disease [78]. A mass spectroscopy study of G93Ahigh mice identified proteins in total spinal cord tissue extracts with greater than baseline carbonyl modification, including SOD1, translationally controlled tumor protein, and ubiquitin carboxyl-terminal hydrolase-L1 [97]. Nitrated and aggregated cytochrome c oxidase subunit-I and α-synuclein accumulate in G93Ahigh mouse spinal cord [79]. Nitrated MnSOD accumulates also in G93Ahigh mouse spinal cord [79]. Toxic properties of mSOD1 could also be mediated through protein binding or aggregation. Wild-type SOD1 and human mSOD1 associate with mitochondria [85,98]. Human SOD1 mutants associate with spinal cord mitochondria in mSOD1 mice and can bind Bcl-2 [99,100], thus potentially being decoys or dominant negative regulators of cell survival molecules (Fig. 2), but it is not known if this process is occurring specifically in mouse MNs. Bcl-2 is depleted from mitochondria isolated from micropunches of human ALS anterior horn gray matter and accumulates in the cytosol (Fig. 2) [59]. Binding of mSOD1 (and perhaps its low-mobility species) to mitochondria has been reported to be spinal cord selective and age-dependent [101], but this work lacks cellular resolution. A recent biochemical in vitro study has shown that endogenous SOD1 in the mitochondrial intermembrane space controls cytochrome c-catalyzed peroxidation and that G93A-mSOD1 mediates greater ROS production in the intermembrane space compared to wild-type SOD1 [102]. Human SOD1 mutants can also shift mitochondrial redox potential when expressed in cultured cells [71]. Nevertheless, the direct links between the physicochemical changes in mSOD1 and wild-type SOD1 and the mitochondrial functional and structural changes associated with ALS and MN degeneration remain uncertain.
EM studies have shown that the outer mitochondrial membrane (OMM) remains relatively intact to permit formation of mega-mitochondria in MNs cell bodies in G93Ahigh mice [78,79]. Moreover, early in the disease of these mice, mitochondria in dendrites in spinal cord ventral horn undergo extensive cristae and matrix remodeling, while few mitochondria in MN cell bodies show major structural changes [78]. Another interpretation of ultrastructural findings is that the mSOD1 causes mitochondrial degeneration by inducing OMM extension and leakage and intermembrane space expansion [86]. Mechanisms for this damage could be related to mSOD1 gaining access to the mitochondrial intermembrane space [83,102] and the matrix [103] and inducing disturbances in oxidative phosphorylation [104] and antioxidant activity. This mitochondrial conformation seen by EM might favor the formation of the mPTP (Fig. 1); indeed, we found evidence for increased contact sites between the OMM and inner mitochondrial membrane (IMM) in dendritic mitochondria in G93Ahigh mice [78]. Another feature of MNs of young G93Ahigh mice before symptoms is apparent fission of ultrastructurally normal mitochondria in cell bodies and fragmentation of abnormal mitochondria [78]. It is not clear if the cristae and matrix remodeling and the apparent fragmentation and fission mitochondria are related or independent events and if these abnormalities interfere with mitochondrial trafficking; nevertheless, morphological observations enforce the idea that mitochondria are critical to the pathobiology of mSOD1 toxicity to MNs in G93Ahigh mice.
The possibility of changes in mitochondrial trafficking in MNs of mSOD1 mice is mostly unexplored. Some data support the novel idea that mitochondria might act as messengers from distal regions of MNs in mSOD1 mice [7]. G93Ahigh-mSOD1 mouse MNs accumulate mitochondria from the axon terminals and generate higher levels of O2 •−, •NO, and ONOO− than MNs in transgenic mice expressing human wild-type SOD1 [79]. This mitochondrial accumulation occurs at a time when MN cell body volume is increasing, suggestive of ongoing problems with ATP production or plasma membrane Na,K ATPase [79]. G93A-mSOD1 perturbs anterograde axonal transport of mitochondria in cultured primary embryonic MNs [105] making it possible that retrogradely transported mitochondria with toxic properties from the neuromuscular junction fail to be returned to distal processes [7,79]. Mitochondria with enhanced toxic potential from distal axons and terminals could therefore have a “Trojan horse” role in triggering degeneration of MNs in ALS via retrograde transport from diseased skeletal muscle.
MNs in G93Ahigh-mSOD1 mice also accumulate higher levels of intracellular Ca2+ than MNs in transgenic human wild-type SOD1 mice [79]. The intracellular Ca2+ signal in MNs is very compartmental and mitochondrial-like in its appearance [79,106]. Abnormal elevations intracellular Ca2+ in G93Ahigh-mSOD1 mouse MNs have been seen also by different Ca2+ detection methods [107,108]. Brain and spinal cord mitochondria in G93A mice handle Ca2+ abnormally and have impaired Ca2+ uptake capacity [109], but this work lacks cellular resolution. However, recent elegant work using a mouse neuromuscular junction preparation has revealed that, specifically within MN terminals, mitochondrial Ca2+ accumulation is accompanied by greater IMM depolarization in human mutant SOD1 transgenic mice [110].
NO signaling mechanisms in mitochondria of ALS mice have also been implicated in the pathogenesis (Fig. 1). MNs seem to be unique regarding NO production because the express constitutively low levels of inducible NO synthase (iNOS) [106]. G93Ahigh-mSOD1 mouse MNs accumulate nicotinamide adenine dinucleotide phosphate diaphorase and iNOS-like immunoreactivity [79]. iNOS is also up-regulated aberrantly in human sporadic ALS MNs [111]. iNOS gene deletion extends significantly the lifespan of G93Ahigh-mSOD1 mice [79]. Thus, mitochondrial oxidative stress, Ca2+ dysregulation, iNOS activation, protein nitration, and protein aggregation (not necessarily SOD1 though) are all likely intrinsic, cell automonous mechanisms in the process of MN degeneration caused by mSOD1 in mice [79]. The mechanistic basis for the differences between human ALS and mSOD1 mice, regarding cell death phenotype (Fig. 3), is not yet clear but could be related to the supra-normal expression of toxic mSOD1 or to fundamental differences in cell death mechanisms [60] or the tissue inflammatory milieu (Martin LJ, unpublished observations) that drive MNs in mSOD1 transgenic mice to necrotic-like death along the apoptosis-necrosis cell death continuum [12,13,106]. Another contributing factor for this difference between human and mouse MNs is that mitochondria are functionally diverse and have species-specific activities and molecular compositions, including the makeup of the mitochondrial permeability transition pore [112]. These possiblities allow for skepticism regarding the suitability of existing transgenic mSOD1 mouse lines to model human ALS.
5. The mitochondrial permeability transition pore contributes to the causal mechanisms of ALS in mice
Despite the implication of toxic effects of mSOD1 on mitochondria in mouse ALS, cause-effect relationships between abnormal functioning of mitochondria and initiation and progression of disease have been uncertain. These relationships need to be known because this knowledge could provide a rationale for new mechanism-based treatments for ALS. One venue of investigation for mitochondrial damage causality is the mPTP and its possible involvement in ALS.
5.1. The mPTP: definition and molecular composition
Mitochondrial permeability transition is a mitochondrial state in which the proton-motive force is disrupted reversibly or irreversibly [25,76,113–117]. Conditions of mitochondrial Ca2+ overload, excessive oxidative stress, and decreased electrochemical gradient (ΔP), ADP, and ATP can favor mitochondrial permeability transition. This alter state of mitochondria involves the mPTP that functions as a voltage, thiol, and Ca2+ sensor [25,76,113–117]. The mPTP is believed to be a poly-protein transmembrane channel formed at the contact sites between the IMM and the OMM. The collective components of the mPTP are still controversial, but the voltage-gated anion channel (VDAC, or porin) in the OMM, the adenine nucleotide translocator (ANT, or solute carrier family 25) in the IMM, and cyclophilin D (CyPD) in the matrix are believed to be the core components (Table 1) [114–116]. Other components or modulators of the mPTP appear to be hexokinase, creatine kinase, translocator protein 18 kDa (TSPO, or peripheral benzodiazepine receptor), and Bcl-2 family members (Table 1) [113–117].
The VDAC family in human and mouse consists of three proteins of ~31 kDa (VDAC1-3) encoded by three different genes [118]. VDACs are the major transport proteins in the OMM, functioning in ATP rationing, Ca2+ homeostasis, oxidative stress response, and apoptosis [118]. Monomeric VDAC serves as the functional channel, although oligomerization of VDAC into dimers and tetramers can occur and might function in cell death [118]. The VDAC adopts an open conformation at low or zero membrane potentials and a closed conformation at potentials above 30–40 mV making the OMM permeable to most small hydrophilic molecules up to 1.3 kDa for free exchange of respiratory chain substrates [119]. Most data implicating VDAC opening or closing as an important regulator of cell death are based on in vitro conditions, while limited in vivo evidence is available [120]. VDAC1 binds Bak1, hexokinase, gelsolin, and ANT1/ANT2; VDAC2 binds Bak1, hexokinase, cytochrome c, glycerol kinase, and ANT1/ANT2; VDAC3 binds glycerol kinase, CyPD, and ANT1-3 [118]. In human tissues, VDAC1 and VDAC2 isoforms are expressed more abundantly than VDAC3; highest levels are found in kidney, heart, skeletal muscle, and brain [121]. The effects of selective knockout of VDAC isoforms are not equivalent, implying different functions. Mice deficient in either VDAC1 or VDAC3 are viable [122–124], but VDAC2 deficiency causes embryonic lethality [125]. Lack of both VDAC1 and VDAC3 causes growth retardation [124]. VDAC null mouse tissues exhibit deficits in mitochondrial respiration and abnormalities in mitochondrial ultrastructure [122]. Mitochondria without VDAC1 have an intact mitochondrial permeability transition response [126,127]. VDAC2 deletion, but not lack of the more abundant VDAC1, results in enhanced activation the mitochondrial apoptosis pathway and enforced activation of Bak at mitochondria [125], consistent with the idea that VDAC2 is a key inhibitor of Bak-mediated apoptosis [124]. However, other data show that cells lacking individual VDACs or combinations of VDACs have normal death responses to Bax and Bid [127]. New work in yeast has revealed that SOD1 is necessary for proper functioning of VDAC; specifically, SOD1 regulates VDAC channel activity and protein levels in mitochondria [128].
The mitochondrial ANT family in human consists of 3 members (ANT1-3, or solute carrier family 25, members 4, 5, and 6) encoded by three different genes, but in mouse only two isoforms of the ANT are present [128]. The proteins are ~33 kDa and function as homodimers [128]. They are multi-pass membrane proteins, with odd-numbered transmembrane helices having kinks because of proline residues, which mediate exchange of cytosolic ADP for mitochondrial ATP across the inner membrane utilizing the electrochemical gradient [129]. ANT1 binds VDAC1, CyPD, Bax, twinkle (ataxin-8), and cyclophilin-40; ANT2 binds VDAC1-3 and cyclophilin-40; ANT3 binds VDAC1, steroid sulfatase, and translocase of inner mitochondrial membrane 13 and 23 [128]. The ANT isoforms are expressed differentially in tissue- and species-specific patterns [130]. ANT1 is expressed highly in human and mouse heart and skeletal muscle, and human brain has low ANT1 mRNA but high ANT3 mRNA, while mouse brain has high ANT1 mRNA [130]. ANT2 mRNA is very low or not expressed in most adult human and mouse tissues, with kidney having some expression [130]. In tissue mitochondria where more than one ANT is found, it is ANT1 that binds preferentially to CyPD to form the mPTP at contact sites between the inner and outer mitochondrial membranes [131]. It has been proposed that, in the presence of high mitochondrial Ca2+, the binding of CyPD to proline residue 61 (Pro61) in loop 1 of ANT1 results in a conformation that converts the ANT into a non-specific pore [128]. Non-conditional ANT1 null mice are viable and grow normally but develop mitochondrial skeletal myopathy and cardiomyopathy [129]. Ablation both ANT isoforms in mouse liver surprisingly did not change fundamentally mitochondrial permeability transition and cell death in hepatocytes [132], and some ANT ligands induce mitochondrial dysfunction and cytochrome c release independent of mitochondrial permeability transition [133]. Thus, the mechanisms of ANT-induced cell death are not understood fully.
CyPD (also named cyclophilin F, peptidyl prolyl iosmerase F) is encoded by a single gene [114,116,134]. Despite confusing nomenclature, there is only one isoform of CyPD (EC 5.2.1.8, ppif gene product) in mouse and human. The ~20 kDa protein encoded by this gene is a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family. PPIases catalyze the cis-trans isomerization of proline imidic peptide bonds in oligopeptides and accelerate the folding of proteins. CyPD binds ANT1 [129].
During normal mitochondrial function the OMM and the IMM are separated by the intermembrane space, and the VDAC and the ANT do not interact [114]. Permeability transition is activated by the formation of the mPTP; the IMM looses its integrity and the ANT changes its conformation from its native state into a non-selective pore [135]. This process is catalyzed by CyPD that functions in protein cis-trans isomerization and chaperoning [136]. The ANT and CyPD interact directly [137]. The molar concentration of CyPD (in heart mitochondria) is much less (>5%) than ANT; thus, under normal conditions only a minor fraction of the ANT can be in a complex with CyPD [117,138]. When this occurs, small ions and metabolites permeate freely across the IMM and oxidation of metabolites by O2 proceeds with electron flux not coupled to proton pumping, resulting in collapse of ΔP, dissipation of ATP production, production of ROS, equilibration of ions between the matrix and cytosol, matrix volume increases, and mitochondrial swelling [115,119].
5.2. Expression and localization of mPTP components in the CNS
Very few studies have been published on the localizations of mPTP components in the mammalian CNS; thus, details about the cellular expressions in different nervous system cell types are lacking. VDAC expression patterns are complicated by alternative splicing that generates two different VDAC1 mRNAs, three different VDAC2 mRNAs, and two different VDAC3 mRNAs [118]. Studies of nervous tissue have found VDAC in neurons and glial cells [139] and associated with mitochondria, the endoplasmic reticulum, and the plasma membrane [140,141]. Non-mitochondrial localizations of VDAC have been disputed [142]. Information on ANT localizations in nervous tissue is particularly scarce. ANT appears to be expressed in reactive astrocytes [143]. The few existing studies on CyPD localization in mammalian CNS have found it enriched in subsets of neurons in adult rat brain, with some interneurons being positive [144], and relative low levels in astrocytes [145,146].
In mouse spinal cord, the core components of the mPTP (VDAC, ANT, and CyPD) are enriched in MNs as determined by immunohistochemistry [78]. The specific isoforms of ANT and VDAC in MNs have not been determined. CyPD, ANT, and VDAC have mitochondrial and non-mitochondrial localizations in MNs [78]. They are all nuclear-encoded mitochondrial-targeted proteins, thus a possible explanation for their non-mitochondrial localizations is that they are pre-mitochondrial forms. Some cyclophilins are located in the cytoplasm [146], but CyPD immunoreactivity is annulled in ppif−/− mice, demonstrating that the antibody is detecting only CyPD [78]. Spinal cord, brainstem, and forebrain had similar levels of CyPD, as well as similar levels of ANT and VDAC [78]. Thus, differences in the levels of individual mPTP components cannot explain the intrinsic differences in the sensitivity to Ca2+-induced permeability transition seen in spinal cord and brain isolated mitochondria [147, 148]. Not all mitochondria within individual MNs contained CyPD, ANT, and VDAC [78]; this observation supports that idea of that mitochondria in individual cells are not only heterogeneous in shape [149,150] but also in biochemical composition, notably metabolism [151] and genetics [36].
5.3. The mPTP contributes to the pathogenesis of mouse ALS
The mPTP was first implicated in ALS pathogenesis using pharmacological approaches. Cyclopsorine A treatment of G93Ahigh mice, delivered intracerebroventricularly or systemically to mice on a multiple drug resistance type 1a/b background, modestly improved outcome [152–154], but these studies are confounded by the immunosuppressant actions of cyclopsorine A through calcineurin inhibition. Pharmacological studies using CyPD inhibitors devoid of effects on calcineurin need to be done on ALS mice. Another study showed that treatment with cholest-4-en-3-one oxime (TRO19622), a drug that binds VDAC and the 18 kDa translocator protein (TSPO, or peripheral benzodiazepine receptor), improved motor performance, delayed disease onset, and extended survival of G93Ahigh mice [155]. However, another study using a different TSPO ligand (Ro-4864) did not show positive effects with G93Ahigh mice [156].
CyPD and ANT have been identified as targets of nitration in ALS mice [78]. CyPD nitration is elevated in early- to mid-symptomatic stages, but declines to baseline at end-stage disease [78]. ANT nitration is notable particularly because it is found in pre-symptomatic and symptomatic stages but not at end-stage disease or in transgenic mice expressing human wild-type SOD1 [78]. The ANT is important in the context of age-related neurodegenerative disease because it undergoes carbonyl modification during aging in housefly flight muscle [157] and rat brain [158]. In vitro cell-free and cell experiments have shown that NO and ONOO− can act directly on the ANT to induce mitochondrial permeabilization in a cyclosporine A-sensitive manner [159]. Oxidative stress enhances the binding of CyPD to ANT [160]. Some SOD1 mutants are unstable and loose copper [67], and interestingly, copper interactions with ANT and thiol modification of ANT can cause mPTP opening [161–163]. Together these data and future work could reveal that oxidative and nitrative damage to proteins, some of which are core components of the mPTP, in G93Ahigh mice is targeted rather than stochastic and could impinge on the functioning of the mPTP.
The role of CyPD in the process of MN disease has been examined in ALS mice through gene-ablation [78]. G93Ahigh-mSOD1 mice without CyPD show markedly delayed disease onset and lived significantly longer than transgenic mice with CyPD. The effect of CyPD deletion was much more prominent in females than in males [78]. Female mice even showed positive effects with haplo-deletion of CyPD. Ppif gene ablation in transgenic mice with much lower levels of human mSOD1 expression and a slower disease progression (G93Alow-mSOD1 mice) also show significantly delayed disease onset and lived significantly longer than transgenic mice with CyPD [78]. Thus, some form of mitochondrial pathobiology is occurring regardless of whether transgene expression of G93A is high or low.
Nevertheless, G93A-mSOD1 mice without CyPD develop eventually MN disease and die. Other work on CypD null mice has shown that high concentrations of Ca2+ (2 mM) can still lead to mPTP activation without CyPD and that cell deaths caused by Bid, Bax, DNA damage and TNF-α are not affected [164]. The effects of CyPD deficiency on MN cell mechanisms thus need to be examined in more detail, but the cell death phenotype might be switched or converted to another form with the attenuation of mitochondrial swelling. A switch in the cell death morphology and molecular mechanisms in MNs of mSOD1 mice without CyPD is an outcome consistent with the cell death continuum concept [12].
6. Summary and outlook
ALS is the 3rd most common human neurodegenerative disease with an adult onset [1,2]. It is a paralytic disease that destroys MNs and skeletal muscle and cannot yet be cured or treated effectively; thus contracting the disease is fatal [5]. Mitochondria have diverse functions and properties and could be critically important for the development of human ALS [7]. Structural and biochemical data from studies of human ALS and cell/animal models of ALS suggest that mitochondrial dysfunction is a trigger or propagator of neurodegeneration. Mitochondria in subsets of neurons and skeletal muscle cells could be compromised in ALS, rendering these cells intrinsically susceptible to cellular aging and stress. Novel mechanisms for mitochondriopathy and MN degeneration in human and mouse ALS could involve apoptosis, accumulation of intracellular Ca2+, abnormal trafficking of mitochondria with enhanced ROS toxic potential from distal dendrites or terminals at the neuromuscular junction, distal axonopathy and target deprivation, NOS trafficking and localization to MN mitochondria, and ONOO− damage [79]. The mPTP actively participates in the mechanisms of MN death in ALS mice in a gender-dependent pattern. Thus, mPTP activation is a possible triggering event for MN degeneration and MN selective vulnerability in ALS could be related to amount, composition, and trafficking of mitochondria in MNs and the association of MN terminals with skeletal muscle cells. There is precedence for this logic in mouse models of Alzheimer’s disease [165], multiple sclerosis [166], and stroke [167]. Further study of mitochondria in neurons and skeletal myocytes can define new mechanisms of disease in ALS and can lead to the identification of molecular mechanism-based therapies for treating this fatal disease.
ACKNOWLEDGMENTS
The author thanks all of the individuals in his lab, particularly Yan Pan, Ann Price, and Barry Gertz for data generated on human ALS and G93A-mSOD1 mice. This work was supported by grants from the U.S. Public Health Service, NIH-NINDS (NS065895, NS052098) and NIH-NIA (AG016282).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Sathasivam S, Ince PG, Shaw PJ. Apoptosis in amyotrophic lateral sclerosis: a review of the evidence. Neuropathol. Appl. Neurobiol. 2001;27:257–274. doi: 10.1046/j.0305-1846.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 3.Stephens B, Guiloff RJ, Navarrete R, Newman P, Nikhar N, Lewis P. Widespread loss of neuronal populations in spinal ventral horn in sporadic motor neuron disease. A morphometric study. J. Neurol. Sci. 2006;244:41–58. doi: 10.1016/j.jns.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Maekawa S, Al-Sarraj S, Kibble M, Landau S, Parnavelas J, Cotter D, Everall I, Leigh PN. Cortical selective vulnerability in motor neurons disease: a morphometric study. Brain. 2004;127:1237–1251. doi: 10.1093/brain/awh132. [DOI] [PubMed] [Google Scholar]
- 5.Cozzolino M, Ferri A, Carri MT. Amyotrophic lateral sclerosis: from current developments in the laboratory to clinical implications. Antiox. Redox Sig. 2008;10:405–443. doi: 10.1089/ars.2007.1760. [DOI] [PubMed] [Google Scholar]
- 6.Kabashi E, Valdmains PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard J-P, Lacomblez L, Pochigaeva K, Salachas F, Pradat P-F, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 7.Martin LJ. Mitochondriopathy in Parkinson disease and amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2006;65:1103–1110. doi: 10.1097/01.jnen.0000248541.05552.c4. [DOI] [PubMed] [Google Scholar]
- 8.Schymick JC, Talbot K, Traynor GJ. Genetics of amyotrophic lateral sclerosis. Hum. Mol. Genet. 2007;16:R233–R242. doi: 10.1093/hmg/ddm215. [DOI] [PubMed] [Google Scholar]
- 9.Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Internatl. Rev. Cytology. 2002;211:93–148. doi: 10.1016/s0074-7696(02)21011-6. [DOI] [PubMed] [Google Scholar]
- 10.Zorov DB, Isave NK, Yu Plotnikov E, Zorova LD, Stelmashook EV, Vasileva AK, Arkhagelskaya AA, Khrjapenkova TG. The mitochondrion as Janus Bifrons. Biochemistry (Moscow) 2007;72:1115–1126. doi: 10.1134/s0006297907100094. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls DG. Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Intl. J. Biochem. Cell Biol. 2002;34:1372–1381. doi: 10.1016/s1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- 12.Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res. Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 13.Northington FJ, Graham EM, Martin LJ. Apoptosis in perinatal hypoxic-ischemic brain injury: how important is it and should it be inhibited? Brain Res. Rev. 2005;50:244–257. doi: 10.1016/j.brainresrev.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Northington FJ, Zelaya ME, O’Riordan DP, Blomgren K, Flock DL, Hagberg H, Ferriero DM, Martin LJ. Failure to complete apoptosis following neonatal hypoxia-ischemia manifests as “continuum” phenotype of cell death and occurs with multiple manifestations of mitochondrial dysfunction in rodent forebrain. Neuroscience. 2007;149:822–833. doi: 10.1016/j.neuroscience.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki S, Iwata M. Ultrastructural changes of synapses of Betz cell in patients with amyotrophic lateral sclerosis. Neurosci. Lett. 1999;268:29–32. doi: 10.1016/s0304-3940(99)00374-2. [DOI] [PubMed] [Google Scholar]
- 16.Menzies FM, Ince PG, Shaw PJ. Mitochondrial involvement in amyotrophic lateral sclerosis. Neurochem. Intl. 2002;40:543–551. doi: 10.1016/s0197-0186(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 17.Comi GP, Bordoni A, Salani S, et al. Cytochrome c oxidase subunit I microdeletion in a paitent with motor neuron disease. Ann. Neurol. 1998;43:110–116. doi: 10.1002/ana.410430119. [DOI] [PubMed] [Google Scholar]
- 18.Borthwick GM, Taylo RW, Walls TJ, et al. Motor neuron disease in a patient with a mitochondrial tRNAIle mutation. Ann. Neurol. 2006;59:570–574. doi: 10.1002/ana.20758. [DOI] [PubMed] [Google Scholar]
- 19.Soong NW, Hinton DR, Cortopassi G, Arnheim N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat. Genet. 1992;2:318–323. doi: 10.1038/ng1292-318. [DOI] [PubMed] [Google Scholar]
- 20.Corral-Debrinski M, Horton T, Lott MT, et al. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat. Genet. 1992;2:324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 21.Mawrin C, Kirches E, Krause G, et al. Single-cell analysis of mtDNA levels in sporadic amyotrophic lateral sclerosis. NeuroReport. 2004;15:939–943. doi: 10.1097/00001756-200404290-00002. [DOI] [PubMed] [Google Scholar]
- 22.Corti S, Donadonu C, Ronchi D, Bordoni A, Fortunato F, Santoro D, Del Bo R, Lucchini V, Crugnola V, Papadimitriou D, Salani S, Moggio M, Bresolin N, Comi GP. Amyotrophic lateral sclerosis linked to a novel SOD1 mutation with muscle mitochondrial dysfunction. J. Neurol. Sci. 2009;276:170–174. doi: 10.1016/j.jns.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 23.Babcock D, Hille B. Mitochondrial oversight of cellular Ca2+ signaling. Curr. Opin. Neurobiol. 1998;8:398–404. doi: 10.1016/s0959-4388(98)80067-6. [DOI] [PubMed] [Google Scholar]
- 24.Siklos L, Engelhardt J, Harat Y, et al. Ultrastructural evidence for altered calcium in motor nerve terminals in amyotrophic lateral sclerosis. Ann. Neurol. 1996;39:203–216. doi: 10.1002/ana.410390210. [DOI] [PubMed] [Google Scholar]
- 25.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochem. Biophysic. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 26.Olney JW. Brain lesion, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:366–368. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 27.Choi DW. Cellular defences destroyed. Nature. 2005;433:696–698. doi: 10.1038/433696a. [DOI] [PubMed] [Google Scholar]
- 28.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J. Biol. Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 29.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 30.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. (1995) [DOI] [PubMed] [Google Scholar]
- 31.Heath PR, Tomkins J, Ince PG, Shaw PJ. Quantitative assessment of AMPA receptor mRNA in human spinal motor neurons isolated by laser capture microdissection. NeuroReport. 2002;13:1753–1757. doi: 10.1097/00001756-200210070-00012. [DOI] [PubMed] [Google Scholar]
- 32.Kwak S, Kawahara Y. Deficient RNA editing of GluR2 and neuronal death in amyotrophic lateral sclerosis. J. Mol. Med. 2005;83:110–120. doi: 10.1007/s00109-004-0599-z. [DOI] [PubMed] [Google Scholar]
- 33.Chang DTW, Reynolds IJ. Mitochondrial trafficking and motphology in healthy and injured neurons. Prog. Brain Res. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Hansson MJ, Mansson R, Morota S, Uchino H, Kallur T, sumi T, Ishii N, Shimazu M, Keep MF, Jegorov A, Elmer E. Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 2008;45:284–294. doi: 10.1016/j.freeradbiomed.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Bergmann F, Keller BU. Impact of mitochondrial inhinition on excitability and cytosolic Ca2+ levels in brainstem motoneurones. J. Physiol. 2004;555:45–59. doi: 10.1113/jphysiol.2003.053900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn of evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B. Role of free radicals in the neurodegenerative diseases. Drugs & Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 39.Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364:548. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 40.Martin LJ, Liu Z. DNA damage profiling in motor neurons: a single-cell analysis by comet assay. Neurochem. Res. 2002;27:1089–1100. doi: 10.1023/a:1020961006216. [DOI] [PubMed] [Google Scholar]
- 41.Giulini C. Characterization and function of mitochondrial nitric-oxide synthase. Free Radic. Biol. Med. 2003;34:397–408. doi: 10.1016/s0891-5849(02)01298-4. [DOI] [PubMed] [Google Scholar]
- 42.Brown GC, Borutaite V. Nitric oxide, cytochrome c and mitochondriam. Biochem. Soc, Symp. 1999;66:17–25. doi: 10.1042/bss0660017. [DOI] [PubMed] [Google Scholar]
- 43.Beal MF. Oxidatively modified protein in aging and disease. Free Radic. Biol. Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 44.Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Jr, Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 45.Abe K, Pan L-H, Watanabe M, Kato T, Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci. Lett. 1995;199:152–154. doi: 10.1016/0304-3940(95)12039-7. [DOI] [PubMed] [Google Scholar]
- 46.Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki S, Shibata N, Komori T, Iwata M. iNOS and nitrotyrosine immunoreactivity in amyotrophic lateral sclerosis. Neurosci. Lett. 2000;291:44–48. doi: 10.1016/s0304-3940(00)01370-7. [DOI] [PubMed] [Google Scholar]
- 48.Browne SE, Bowling AC, Baik MJ, et al. Metabolic dysfunction in familial, but not sporadic, amyotrophic lateral sclerosis. J. Neurochem. 1998;71:281–287. doi: 10.1046/j.1471-4159.1998.71010281.x. [DOI] [PubMed] [Google Scholar]
- 49.Borthwick GM, Johnson MA, Ince PG, Shaw PJ, Turnbul DM. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: implications for the role of mitochondria in neuronal cell death. Ann. Neurol. 1999;46:787–790. doi: 10.1002/1531-8249(199911)46:5<787::aid-ana17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 50.Vielhaber S, Kunz D, Winkler K, et al. Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain. 2000;123:1339–1348. doi: 10.1093/brain/123.7.1339. [DOI] [PubMed] [Google Scholar]
- 51.Soraru G, Vergani L, Fedrizzi L, D’Ascenzo C, Polo A, Bernazzi B, Angelini C. Activities of mitochondrial complexes correlate with nNOS amount in muscle from ALS patients. Neuropath. Appl. Neurobiol. 2007;33:204–211. doi: 10.1111/j.1365-2990.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 52.Echaniz-Laguna A, Zoll J, Ponsot E, N’Guessan B, Tranchant C, Loeffler J-P, Lampert E. Exp. Neurol. Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the desiese develops; a temporal study in man. Exp. Neurol. 2006;198:25–30. doi: 10.1016/j.expneurol.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 53.Vielhaber S, Winklwer K, Kirches E, Kunz D, Buchner M, Feistner H, Elger CE, Ludolph AC, Riepe MW, Kunz WS. Visualization of defective mitochondrial function in skeletal muscle fibers of patients with sporadic amyotrophic lateral sclerosis. J. Neurol. Sci. 169:133–139. doi: 10.1016/s0022-510x(99)00236-1. (199) [DOI] [PubMed] [Google Scholar]
- 54.Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Diff. 2002;9:367–393. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- 55.Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Ann. Rev. Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- 56.Cory S, Adams JM. The bcl-2 family: regulators of the cellular life-or-death switch. Nat. Rev. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 57.Lorenzo HK, Susin SA. Mitochondrial effectors in caspase-independent cell death. FEBS Lett. 2004;557:14–20. doi: 10.1016/s0014-5793(03)01464-9. [DOI] [PubMed] [Google Scholar]
- 58.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Omahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol. Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 59.Martin LJ. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J. Neuropathol. Exp. Neurol. 1999;58:459–471. doi: 10.1097/00005072-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Martin LJ, Liu Z. Opportunities for neuroprotection in ALS using cell dealth mechanism rationales. Drug Discov. Today. 2004;1:135–143. [Google Scholar]
- 61.Martin LJ. Neuronal cell death in nervous system development, disease, and injury. Intl. J. Mol. Med. 2001;4:455–478. [PubMed] [Google Scholar]
- 62.Ginsberg SD, Hemby SE, Mufson EJ, Martin LJ. Cell and tissue microdissection in combination with genomic and proteomic profiling. In: Zaborszky L, Wouterlood FG, Lanciego JL, editors. Neuroanatomical Tract-Tracing 3. Molecules, Neurons, and Systems. New York: Springer; 2006. pp. 109–141. [Google Scholar]
- 63.Martin LJ, Liu Z, Pipino J, Chestnut B, Landek MA. Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cereb. Cortex. 2009;19:1273–1293. doi: 10.1093/cercor/bhn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 65.Martin LJ. p53 is abnormally elevated and active in the CNS of patients with amyotrophic lateral sclerosis. Neurobiol. Dis. 2000;7:613–622. doi: 10.1006/nbdi.2000.0314. [DOI] [PubMed] [Google Scholar]
- 66.Martin LJ, Liu Z. Injury-induced spinal motor neuron apoptosis is preceded by DNA single-strand breaks and is p53- and Bax-dependent. J. Neurobiol. 2002;50:181–197. doi: 10.1002/neu.10026. [DOI] [PubMed] [Google Scholar]
- 67.Bendotti C, Carrì MT. Lessons from models of SOD1-linked familial ALS. Trends Mol. Med. 2004;10:393–400. doi: 10.1016/j.molmed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog. Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 69.McCord JM, Fridovich I. Superoxide dismutase, an enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 70.Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A. Monomeric Cu, Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic sclerosis. J. Biol. Chem. 2004;279:15499–15504. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- 71.Ferri A, Cozzolino M, Crosio C, Nencini M, Casciati A, Gralla EB, Rotilio G, Valentine JS, Carri MT. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc. Natl. Sci. USA. 2006;103:13860–13865. doi: 10.1073/pnas.0605814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Estévez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 286;1999:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 73.Flanagan SW, Anderson RD, Ross MA, Oberley LW. Overexpression of manganese superoxide dismutase attenuates neuronal death in human cells expressing mutant (G37R) Cu/Zn-superoxide dismutase. J. Neurochem. 2002;81:170–177. doi: 10.1046/j.1471-4159.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 74.Rizzardini M, Mangolini A, Lupi M, Ubezio P, Bendotti C, Cantoni L. Low levels of ALS-linked Cu/Zn superoxide dismutase increase the production of reactive oxygen species and cause mitochondrial damage and death in motor neuron-like cells. J. Neurol. Sci. 323:95–103. doi: 10.1016/j.jns.2005.02.004. (205) [DOI] [PubMed] [Google Scholar]
- 75.Bilsland LG, Nirmalananthan N, Yip J, Greensmith L, Duhcen MR. Expression of mutant SOD1G93A in astrocytes induces functional deficits in motoneuron mitochondria. J. neurochem. 2008;107:1271–1283. doi: 10.1111/j.1471-4159.2008.05699.x. [DOI] [PubMed] [Google Scholar]
- 76.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 77.Dal Canto MC, Gurney ME. Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am. J. Pathol. 1994;145:1271–1279. [PMC free article] [PubMed] [Google Scholar]
- 78.Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin LJ, Liu Z, Chen K, Pan Y, Swaby JA, Golden WC. Motor neuron degeneration in amyotrophoc lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J. Comp. Neurol. 2007;500:20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- 80.Chang Q, Martin LJ. Glycinergic Innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a confocal quantitative analysis. Am. J. Path. 2009;174:574–585. doi: 10.2353/ajpath.2009.080557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bendotti C, Calvaresi N, Chiveri L, Prelle A, Moggio M, Braga M, Silani V, De Biasi S. Early vacuolization and mitochondrial damage in motor neurons of FALS mice are not associated with apoptosis or with changes in cytochrome oxidase histochemical reactivity. J. Neurol. Sci. 2001;191:25–33. doi: 10.1016/s0022-510x(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 82.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 83.Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J. Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaarsma D, Rognoni F, van Duijn W, Verspaget HW, Haasdijk ED, Holstege JC. CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol. 2001;102:293–305. doi: 10.1007/s004010100399. [DOI] [PubMed] [Google Scholar]
- 85.Higgins CMJ, Jung C, Ding H, Xu Z. Mutant Cu, Zn Superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J. Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-06-j0001.2002. RC215:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci. 2003;4:16. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jaarsma D. Swelling and vacuolation or mitochondria in transgenic SOD1-ALS mice: a consequence of supranormal SOD1 expression? Mitochondrion. 2006;6:48–49. doi: 10.1016/j.mito.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Sasaki S, Warita H, Murakami T, Abe K, Iwata M. Ultrastructural study of mitochondria in the spinal cord of transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathol. 2004;107:461–474. doi: 10.1007/s00401-004-0837-z. [DOI] [PubMed] [Google Scholar]
- 89.Deng H-X, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung W-Y, Getzoff ED, Hu P, Herzfeldt B, Roos RP, Warner C, Deng G, Soriano E, Smyth C, Parge HE, Ahmed A, Roses AD, Hallewell RA, Pericak-Vance MA, Siddique T. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 90.Borchelt DR, Lee MK, Slunt HH, Guarnieri M, Xu Z-S, Wong PC, Brown RH, Jr, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. USA. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yim MB, Kang J-H, Yim H-S, Kwak H-S, Chock PB, Stadtman, E.R. ER. A gain-of-function of an amyotrophic lateral sclerosis-associated Cu, Zn-superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc. Natl. Acad. Sci. USA. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kabashi E, Valdmanis PN, Dion P, Rouleau GA. Oxidized/misfolded superoxide dismutase-1: the cause of all amyotrophic lateral sclerosis? Ann. Neurol. 2007;62:553–559. doi: 10.1002/ana.21319. [DOI] [PubMed] [Google Scholar]
- 93.Ezzi SA, Urushitani M, Julien J-P. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J. Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- 94.Liochev SI, Fridovich I. Mutant Cu, Zn superoxide dismutases and familial amyotrophic lateral sclerosis: evaluation of oxidative hypotheses. Free Radic. Biol. Med. 2003;34:1383–1389. doi: 10.1016/s0891-5849(03)00153-9. 2003. [DOI] [PubMed] [Google Scholar]
- 95.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andrus PK, Fleck TJ, Gurney ME, Hall ED. Protein oxidative damage in a transgenic mouse medel of familial amyotrophic lateral sclerosis. J. Neurochem. 1998;71:2041–2048. doi: 10.1046/j.1471-4159.1998.71052041.x. [DOI] [PubMed] [Google Scholar]
- 97.Poon HF, Hensley K, Thongboonkerd V, Merchant ML, Lynn BC, Pierce WM, Klein JB, Calabrese V, Butterfield DA. Redox proteomics analysis of oxidatively modified proteins in G93A-SOD1 transgenic mice- a model of familial amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2005;39:435–462. doi: 10.1016/j.freeradbiomed.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 98.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide (SOD) in rat liver. J. Biol. Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 99.Liu J, Lillo C, Jonsson A, Vande Velde C, Ward CW, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Anderson PM, Brannstrom TM, Gredal O, Wong PC, Williams DS, Cleveland DW. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–15. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 100.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr Amyotrophic lateral sclerosis-associated SOD1 mutant protein bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 101.Vande Velde C, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc. Natl. Acad. Sci. USA. 2008;105:4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldsteins G, Keksa-Goldsteine V, Ahtiniemi T, Jaronen M, Arens E, Akerman K, Chan RH, Koistinaho J. Deleterious role of superoxide dismutase in the mitochondrial intermembrane space. J. Biol. Chem. 2008;283:8446–8452. doi: 10.1074/jbc.M706111200. [DOI] [PubMed] [Google Scholar]
- 103.Vijayverguya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J. Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 105.De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau K-F, Browlees J, Ackerley S, Shaw PJ, McLoughlin DM, Shaw CE, Leigh PN, Miller CCJ, Grierson AJ. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondrial content. Hum. Mol. Genet. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin LJ, Chen K, Liu Z. Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J. Neurosci. 2005;25:6449–6459. doi: 10.1523/JNEUROSCI.0911-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siklos L, Engelhardt JI, Alexianu ME, Gurney ME, Siddique T, Appel SH. Intracellular calcium parallels motoneuron degeneration in SOD-1 mutant mice. J. Neuropath. Exp. Neurol. 1998;57:571–587. doi: 10.1097/00005072-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 108.Jaiswal MK, Keller BU. Cu/Zn superoxide dismutase typical for familial amyotrophic lateral sclerosis increases the vulnerability of mitochondria and perturbs Ca2+ homeostasis in SOD1G93A mice. Mol. Pharmacol. 2009;75:478–489. doi: 10.1124/mol.108.050831. [DOI] [PubMed] [Google Scholar]
- 109.Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, Beal MF, Manfredi G. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J. Neurochem. 2006;96:1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- 110.Nguyen KT, Garcia-Chacon LE, Barrett JN, Barrett EF, David G. The ψm depolarization that accompanies mitochondrial Ca2+ uptake is greater in mutant SOD1 than in wild-type mouse motor terminals. Proc. Natl. Acad. Sci. USA. 2009;106:2007–2011. doi: 10.1073/pnas.0810934106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sasaki S, Warita H, Abe K, Iwata M. Inducible nitric oxide synthase (iNOS) and nitrotyrosine immunoreactivity in the spinal cords of transgenic mice with mutant SOD1 gene. J. Neuropathol. Exp. Neurol. 2001;60:839–846. doi: 10.1093/jnen/60.9.839. [DOI] [PubMed] [Google Scholar]
- 112.Kunz WS. Different metabolic properties of mitochondrial oxidative phosphorylation in different cell types- important implications for mitochondrial cytopathies. Exp. Physiol. 2003;88.1:149–154. doi: 10.1113/eph8802512. [DOI] [PubMed] [Google Scholar]
- 113.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J. Biol. Chem. 1976;251:5069–5077. [PubMed] [Google Scholar]
- 114.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 115.van Gurp M, Festjens N, van Loo G, et al. Mitochondrial intermembrane proteins in cell death. Biochem. Biophys. Res. Comm. 2003;304:487–497. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]
- 116.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blalchy-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 117.Leung AWC, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim. Biophys. Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 118.Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signallin, cell life and cell death. Curr. Pharm. Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 119.Rostovtseva TK, Tan W, Colombini M. On the role of VDAC in apoptosis: fact and fiction. J. Bioenerget. Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- 120.Granville DJ, Gottlieb RA. The mitochondrial voltage-dependent anion channel (VDAC) as a therapeutic target for initiating cell death. Curr. Med. Chem. 2003;10:1527–1533. doi: 10.2174/0929867033457214. [DOI] [PubMed] [Google Scholar]
- 121.Huizing M, Ruitenbeek W, van den Heuvel LP, Dolce V, Iacobazzi V, Smeitink JAM, Palmieri F, Trijbels JMF. Human mitochondrial transmembrane metabolite carriers: tissue distribution and its implication for mitochondrial disorders. J. Bioenerg. Biomembr. 1998;30:277–284. doi: 10.1023/a:1020501021222. [DOI] [PubMed] [Google Scholar]
- 122.Wu S, Sampson MJ, Decker WK, Craigen WJ. Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochem. Biophys. Acta. 1999;1452:68–78. doi: 10.1016/s0167-4889(99)00120-2. [DOI] [PubMed] [Google Scholar]
- 123.Anflous K, Armstrong DD, Craigen WJ. Altered sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J. Biol. Chem. 2006;276:1954–1960. doi: 10.1074/jbc.M006587200. [DOI] [PubMed] [Google Scholar]
- 124.Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, et al. Immotile sperm an infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 2001;276:39206–39212. doi: 10.1074/jbc.M104724200. [DOI] [PubMed] [Google Scholar]
- 125.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsemeyer SJ. VDAC2 inhibits Bak activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 126.Chandra D, Choy G, Daniel PT, Tang DG. Bax-dependent regulation of Bak by voltage-dependent anion channel 2. J. Biol. Chem. 2005;280:19051–19061. doi: 10.1074/jbc.M501391200. [DOI] [PubMed] [Google Scholar]
- 127.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Helestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr. Med. Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 129.Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat. Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 130.Stepien G, Torroni A, Chung AB, Hodge JA, Wallace DC. Differential expression of adenine nucleotide transloactor isoforms in mammalian tissues and during muscle cell differentiation. J. Bio. Chem. 1992;267:14592–14597. [PubMed] [Google Scholar]
- 131.VyssokikH MY, Katz A, Rueck A, Wuensch C, Dorner A, Zorov DB, Brdiczka D. Adenine nucleotide translocator isoforms 1 and 2 are differently distrubited in the mitochondrial inner membrane and have distinct affinities to cyclophilin D. Biochem. J. 2001;358:349–358. doi: 10.1042/0264-6021:3580349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kikoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Machida K, Hayashi Y, Osada H. A novel adenine nucleotide translocase inhibitor, MT-21, induces cytochrome c release through a mitochondrial permeability transition-independent mechanisms. J. Biol. Chem. 2002;277:31243–31248. doi: 10.1074/jbc.M204564200. [DOI] [PubMed] [Google Scholar]
- 134.Baines CP, Kaiser RA, Purcell NH, Blair HS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, II, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 135.Crompton M. Mitochondria and aging: a role for the permeability transition? Aging Cell. 2004;3:3–6. doi: 10.1046/j.1474-9728.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 136.Waldmeier PC, Zimmermann K, Qian T, Tintelnot-Blomley M, Lemasters JJ. Cyclophilin D as a drug target. Curr. Med. Chem. 2003;10:1485–1506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- 137.Woodfield K, Rück A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem. J. 1998;336:287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Johnson N, Khan A, Virji S, Ward JM, Crompton M. Import and processing of heart mitochondrial cyclophilin D. Eur. J. Biochem. 1999;263:353–359. doi: 10.1046/j.1432-1327.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 139.McEnery MW, Dawson TM, Verma A, Gurley D, Colombini M, Snyder SH. Mitochondrial voltage-dependent anion channel. J. Biol. Chem. 1993;268:23289–23296. [PubMed] [Google Scholar]
- 140.Shoshan-Barmatz V, Zalk R, Gincel D, Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochem. Biophys. Acta. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 141.Akanda N, Tofight R, Brask J, Tamm C, Elinder F, Ceccatelli S. Voltage-dependent anion channels (VDAC) in the plasma membrane play a critical role in apoptosis in differentiated hippocampal neurons but not in neural stem cells. Cell Cycle. 2008;7:3225–3234. doi: 10.4161/cc.7.20.6831. [DOI] [PubMed] [Google Scholar]
- 142.Yu WH, Wolfgang W, Forte M. Subcellular localization of human voltage-dependent anion channel isoforms. J. Biol. Chem. 1995;270:13998–14006. doi: 10.1074/jbc.270.23.13998. [DOI] [PubMed] [Google Scholar]
- 143.Buck CR, Jurynec MJ, Upta DE, Law AKT, Bilger J, Wallace DC, McKeon RJ. Increased adenine nucleotide translocator 1 in reactive astrocytes facilitates glutamate transport. Exp. Neurol. 2003;181:149–158. doi: 10.1016/s0014-4886(03)00043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hazelton JL, Petrasheuskaya M, Fiskum G, Kristian T. Cyclophilin D is expressed predominantly in mitochondria of γ-aminobutyric acidergic interneurons. J. Neurosci. Res. 2009;87:1250–1259. doi: 10.1002/jnr.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J. Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bose S, Freedman RB. Peptidyl prolyl cis-trans-isomerase activity associated with the lumen of the endoplasmic reticulum. Biochem. J. 1994;300:865–870. doi: 10.1042/bj3000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sullivan PG, Rabchevsky AG, Keller JN, Lovell M, Sodhi A, Hart RP, Scheff SW. Intrinsic differences in brain and spinal cord mitochondria: implications for therapeutic interventions. J. Comp. Neurol. 2004;474:524–534. doi: 10.1002/cne.20130. [DOI] [PubMed] [Google Scholar]
- 148.Morota S, Hansson MJ, Ishii N, Kudo Y, Elmer E, Uchino H. Spinal cord mitochondria display lower retention capacity compared with brain mitochochondria without inherent differences in sensitivity to cyclophilin D inhibition. J. Neurochem. 2007;103:2066–2076. doi: 10.1111/j.1471-4159.2007.04912.x. [DOI] [PubMed] [Google Scholar]
- 149.Collins TJ, Bootman MD. Mitochondria are morphologically heterogeneous within cells. J. Exp. Biol. 2003;206:1993–2000. doi: 10.1242/jeb.00244. [DOI] [PubMed] [Google Scholar]
- 150.Jensen RE. Control of mitochondrial shape. Curr. Opin. Cell Biol. 2005;17:384–388. doi: 10.1016/j.ceb.2005.06.011. 2005. [DOI] [PubMed] [Google Scholar]
- 151.Hamberger A, Blomstrand C, Lehninger AL. Comparative studies of mitochondria isolated from neuron-enriched and glia-enriched fractions of rabbit and beef brain. J. Cell Biol. 1970;45:221–234. doi: 10.1083/jcb.45.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Keep M, Elmér E, Fong KSK, Csiszar K. Intrathecal cyclosporin prolongs survival of latestage ALS mice. Brain Res. 2001;894:27–331. doi: 10.1016/s0006-8993(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 153.Karlsson J, Fong KS, Hansson MJ, Elmer E, Csiszar K, Keep MF. Life span extension and reduced neuronal death after weekly intraventricular cyclosporine injections in the G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosurg. 2004;101:128–137. doi: 10.3171/jns.2004.101.1.0128. [DOI] [PubMed] [Google Scholar]
- 154.Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. An ALS mouse model with a permeable blood-brain barrier benefits from systemic cyclosporine A treatment. J. Neurochem. 2004;88:821–826. doi: 10.1046/j.1471-4159.2003.02181.x. [DOI] [PubMed] [Google Scholar]
- 155.Bordet T, Buisson B, Michaud M, Drouot C, Galea P, Delaage P, Akentieva NP, Evers AS, Covey DF, Ostuni MA, lacapere JJ-J, Massaad C, Schmacher M, Steidl E-M, Maux D, Delaage M, Henderson CE, Pruss RM. identification and characterization of Cholest-4-en-3-one, oxime ( TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 2007;322:709–720. doi: 10.1124/jpet.107.123000. [DOI] [PubMed] [Google Scholar]
- 156.Mills C, makwana M, Wallace A, Benn S, Schmidt H, Tegeder I, Costigan M, Brown RH, Jr, Raivich G, Woolf C. Ro5-4864 promotes neonatal motor neuron survival and nerve regeneration in adult rats. Eur. J. Neurosci. 2008;27:937–946. doi: 10.1111/j.1460-9568.2008.06065.x. [DOI] [PubMed] [Google Scholar]
- 157.Yan L-J, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. USA. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Prokai L, Yan L-J, Vera-Serrano JL, Stevens SM, Jr, Forster MJ. Mass spectrometry-based survey of age-associated protein carboylation in rat brain mitochondria. J. Mass Spectrom. 2007;42:1583–1589. doi: 10.1002/jms.1345. [DOI] [PubMed] [Google Scholar]
- 159.Vieira HLA, Belzacq A-S, Haouzu D, Bernassola F, Cohen I, Jacotot E, Ferri KF, Hamel CE, Bartle LM, Melino G, Brenner C, Goldmacher V, Kroemer G. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene. 2001;20:4305–4316. doi: 10.1038/sj.onc.1204575. [DOI] [PubMed] [Google Scholar]
- 160.McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. J. 2002;367:541–548. doi: 10.1042/BJ20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Costantini P, Colonna R, Bernardi P. Induction of the mitochondrial permeability transition by N-ethylmaleimide depends on secondary oxidation of critical thiol groups. Potentiation by copper-ortho-phenthroline without dimerization of the adenine nucleotide translocase. Biochim. Biophys. Acta. 1998;1365:385–392. doi: 10.1016/s0005-2728(98)00090-5. [DOI] [PubMed] [Google Scholar]
- 162.Costantini P, Belzacq A-S, Vieira HLA, Larochette N, de Pablo MA, Zamzami N, Susin SA, Brenner C, Kroemer G. Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene. 2000;19:307–314. doi: 10.1038/sj.onc.1203299. [DOI] [PubMed] [Google Scholar]
- 163.García N, Martínez-Abundis E, Pavón N, Correa F, Chávez E. Copper induces permeability transition through its interaction with the adenine nucleotide translocase. Cell Biol Int. 2007;31:893–899. doi: 10.1016/j.cellbi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 164.Grimm S, Brdiczka D. The permeability transition pore in cell death. Apoptosis. 2007;12:841–855. doi: 10.1007/s10495-007-0747-3. [DOI] [PubMed] [Google Scholar]
- 165.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Aranico O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, Yu Z, Fowlkes J, Rahder M, Stern K, Bernardi P, Bourdette D. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. USA. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hertz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]