Abstract
Background
Atrial fibrillation (AF) developing after cardiac surgery is associated with adverse outcomes; however, the mechanism(s) that trigger and maintain AF in these patients are unknown.
Objective
We hypothesized that post-operative AF is maintained by high frequency sources in the left atrium (LA) resulting from ion channel and structural features that differ from the right atrium (RA).
Methods
Forty-four patients with no previous history of AF who underwent cardiac surgery consented to LA and RA biopsies. Histologic sections evaluated fatty infiltration, fibrosis, and iron deposition; quantitative rt-PCR assessed ion channel expression. In a subset of 27 patients, LA and RA unipolar recording leads were also placed. In patients who developed AF, the dominant frequency (DF) for each lead was calculated using Fast Fourier Transform.
Results
DFs during AF were LA: 6.26 ± 0.8 Hz, RA: 4.56±0.7 Hz (p<0.01). rt-PCR revealed LA-to-RA differences in mRNA abundance for Kir2.3 (1.8:1) and Kir3.4 (2.3:1). While LA fibrosis was greater in patients developing AF compared to those remaining in normal sinus rhythm (10.8±11% vs. 3.8±3.5%; p = 0.03), the amount of LA fibrosis inversely correlated with the LA DF.
Conclusion
This is the first demonstration of LA-to-RA frequency differences during post-operative AF, which are associated with left-to-right atrial differences in mRNA levels for potassium channel proteins and LA fibrosis. These results strongly suggest that sources of AF after cardiac surgery are located in the LA and are stabilized by left atrial fibrosis.
Keywords: Fibrillation, Electrophysiology, Fourier analysis, Cardiac Surgery, Fibrosis
Introduction
Atrial fibrillation (AF) develops in 30% of patients following cardiac surgery1 and results in higher morbidity, mortality, and prolonged hospitalization1,2. While several factors likely contribute to post-surgical AF (e.g., stretch, inflammation, pericarditis, ischemia, autonomic influences),1,2 the exact mechanism(s) of initiation and maintenance is(are) unknown.
Analysis of AF in animals and humans has demonstrated regional differences in AF dynamics and maintenance mechanisms. Specifically, AF is maintained by micro-reentrant sources localized in the left atrium (LA)3–6 with fibrillatory conduction toward the right atrium(RA)7. However, despite mapping studies of paroxysmal and chronic AF5.6, post-operative AF frequency dynamics have not been quantified. In addition, human and animal data indicate that alterations in potassium ion channel expression are associated with AF development3,8. Animal models have shown that AF sources localize in the LA, resulting in left-versus-right frequency differences that correlate with inward rectifier potassium channel expression3. However, the molecular determinants of AF frequency in the human heart have predominantly been restricted to the analysis of right atrial tissue9.
Structural remodeling of the atria by fatty infiltration, fibrosis, and iron have been implicated in the pathogenesis of chronic AF10–12, as well as post-surgical AF12,13. Fatty infiltrates and fibrosis form barriers around myocytes creating conditions favorable for reentry12, while atrial iron can lead to oxidative stress resulting in apoptosis and cell death14. Right atrial (RA) biopsies obtained from individuals undergoing cardiac surgery predominate in the literature11,13, and have demonstrated mixed results regarding the importance of pre-operative structural and electrophysiological changes in patients who develop post-operative AF(14).
Here we investigated whether the spatial organization of AF that develops spontaneously after cardiac surgery is associated with stable LA versus RA differences in dominant frequency (DF) that persist over a 1 hour period. In addition, we used quantitative rt-PCR(reverse transcription-polymerase chain reaction) analysis to determine LA-vs-RA differences in mRNA levels for genes coding for membrane ion channels, and quantified fibrosis, fatty infiltration and iron deposition to identify whether pre-operative differences in atrial structure correlate with the development of postoperative AF.
Methods
Patient Recruitment
After Institutional Review Board approval and informed consent, 44 patients scheduled for cardiac surgery were recruited for the study. The exclusion criteria were as follows: <18 years of age, history of AF and/or prior sternotomy.
Intra-Operative Lead Placement
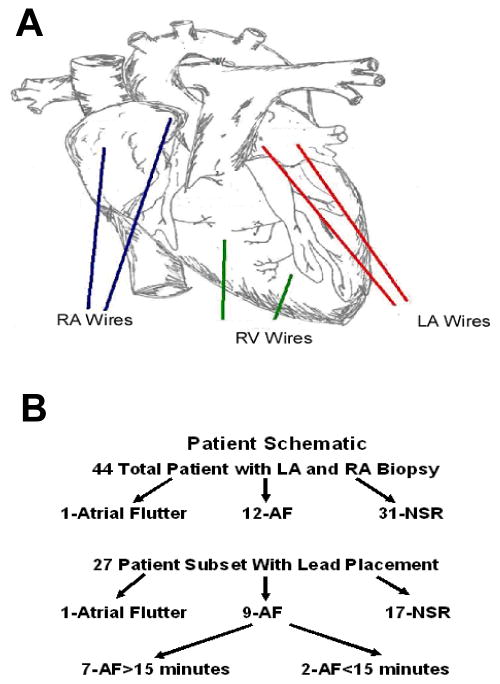
Of 44 patients, a subset of 27 consented to the additional evaluation of LA and RA DFs should they develop AF. Two, 2-0 temporary cardiac pacing wires (Ethicon Inc) were sutured onto the LA surface, and the opposing end brought through the skin. As illustrated in Figure 1A, the first recording wire (LA1) was placed posteriorly at the junction of the LA and LA appendage (LAA). The second (LA2) was placed on the posterior aspect of the LAA approximately 2 centimeters away from LA1. Of note, although ideally leads would be placed on the posterior LA adjacent to the pulmonary vein region, this area is not accessible via an anterior mediastinal surgical approach. Right ventricular (RV) leads were placed in a similar manner on the anterior RV surface, and RA leads were placed on the RA appendage (RAA) and RA free wall (RA1 and RA2, respectively), approximately 3cm apart.
Figure 1.
A: Diagram illustrating the positions of the LA, RA and RV unipolar recording leads. B: Top, heart rhythms occurring post cardiac surgery in all 44 patients after LA and RA biopsies. Bottom, heart rhythms in a subset of 27 patients with lead placement.
Post-Operative Care
Patients were monitored for the development of AF by telemetry. Five days after the operation, the wires were painted with betadine, light tension was applied, and the wires were cut below the level of the skin. All patients were treated following the Society of Thoracic Surgery guidelines after an open heart procedure1.
Signal Acquisition and analysis
In 7 patients who developed spontaneous, new onset post-operative AF (AF was not induced), unipolar recording wires were sampled every 10 minutes for 1 hour using a Bard recording system (Lowell, MA) within 15 minutes after AF initiation. Figure 1 in the Online Supplement shows the data processing procedure whereby a 5-second LA electrogram (a) was band-pass filtered (b), cleared of the QRST activity (c) and subsequently analyzed for its power spectrum (d), which showed a DF at 6.1 Hz.
Atrial Biopsy
Three to five mm biopsies were obtained from both LAA and RAA in all 44 patients. A 3–5 mm biopsy was taken from each atrial appendage, divided, and placed in 10% buffered formalin and RNAlater™ (Ambion).
rt-PCR
The RNA from specimens placed in RNAlater was extracted using a Qiagen RNeasy minikit following the manufacturer’s specifications. Proper conversion of RNA to cDNA required a minimum concentration of 4 ng/μl. Therefore, 2 RA samples from 2 patients who remained in NSR were excluded from analysis. Using an Invitrogen superscript III first strand synthesis kit, 50 ng of RNA was transcribed into cDNA. Primers were selected to cross an intron within the DNA sequence of interest, and run on a 1.5% agarose gel with cDNA ensuring 1 product (see Figure 2 in Online Supplement). Table 1 in the Online Supplement lists the forward and reverse primer sequences for each gene of interest. A 25 μl reaction using SyberGreenER™ (Invitrogen) was run in duplicate on a BioRad iCycler in 96 well plate. The cT (cycle to Threshold) values corresponding to mRNA levels were normalized to GAPDH (details for why GAPDH was chosen are in the Online Supplement).15,16 cT values are based on a log scale and were transformed to delta cT values by subtracting the gene of interest from GAPDH. To compare LA versus RA a differences, the respective data were transformed from cT values to equivalent fold differences as described previously,17 using the following equation: Fold Difference (mean CtLA − mean CtRA) = 2(mean CtLA − mean CtRA). To calculate differences between patients who developed AF vs. those who remained in NSR, the equation was modified to: Fold Difference (mean CtLA_NSR − mean CtLA_AF) = 2(mean CtLA_NSR − mean CtLA_AF). Because the data have been transformed, the standard deviation bars have been omitted as previously described.19
Histology
The 44 LA and RA specimens were sectioned at 6μm and stained with hematoxylin and eosin, picrosirius red, and modified perls as previously described.10,18 Specimens were examined with a Bioquant imaging system to quantify the percentage of fatty infiltration, fibrosis, and iron within the atrial muscle. Images spanning the entire specimen were assembled into a montage and the total sample area and area occupied by either adipocytes, fibrosis, or iron were determined. Epicardial, endocardial, and perivascular fibrosis, iron, or adipocytes were excluded. The percentage of fatty infiltration, fibrosis, or iron from within each sample was calculated. All samples were measured in triplicate by a single investigator.
Statistical Analyses
Variables are reported as mean ± standard deviation. Patient variables and LA vs RA comparisons were evaluated for the equality of variances ensuring normal distribution, and were then compared using a 2-tailed Student’s t-test for statistical differences. Comparisons made between categorical variables were done using Pearson’s Chi squared analysis and Fisher’s exact test. DF comparisons and LA vs RA differences in patients who remained in normal sinus rhythm or developed AF were analyzed using two-way ANOVA. DF versus time was analyzed by averaging the data from the LA leads and comparing them over time to the average data from the RA leads for the identical time point using two-way ANOVA. Linear regression was corrected using the Bonferroni calculation, where p<0.05 was considered significant.
All authors had full access to the data and take responsibility for data integrity. All authors have read and agreed to the manuscript as written.
RESULTS
Of the 44 patients enrolled, 31-remained in NSR, 12-developed new onset AF and 1-developed new onset atrial flutter (Figure 1B). Patient variables (Table 1) demonstrated age as the most important factor in developing AF. Termination of AF was observed in all 12 patients, while one patient developed permanent atrial flutter. Mean AF duration was 5.7±6.0 hours (range 0.25–16 hours). All patients were discharged from the hospital in NSR. The types of cardiac surgery for the total population and for patients with atrial recordings are presented in the Online Supplement (Table 2A and 2B).
Table 1.
Patient population developing Atrial Fibrillation, remaining in Normal Sinus Rhythm (NSR) and patients with atrial recordings (AF-Rec)
| Patient Variable | AF;n=12 | NSR;n=31 | pvalue | AF-Rec n=7 |
|---|---|---|---|---|
| Age (years) | 68±10 | 60±12 | 0.02* | 73±4 |
| Male Gender | 50% | 53% | 0.85 | 75% |
| Left Atrial Diameter (cm) | 5.1±0.6 | 4.8±1.5 | 0.38 | 5.0±0.6 |
| Right Atrial Diameter (cm) | 4.6±0.6 | 4.8±1.5 | 0.42 | 4.6±0.5 |
| Ejection Fraction (%) | 57±11 | 53±10 | 0.26 | 56±12 |
| Diastolic Dysfunction (%) | 71 | 41 | 0.05* | 75% |
| Creatine Kinase MB (ng/ml) | 42±51 | 25±25 | 0.24 | 42±64 |
| Central Venous Pressure | 10.3±3 | 10.7±4 | 0.6 | 9.5±2 |
| Pulmonary Wedge Pressure | 17±2 | 16±6 | 0.2 | 14±4 |
| Congestive Heart Failure Class I | 3 | 25 | 0.003* | 2 |
| Congestive Heart Failure Class II | 4 | 6 | 0.53 | 4 |
| Congestive Heart Failure Class III | 4 | 1 | 0.17 | 0 |
| Coronary Artery Bypass Grafts | 4 | 21 | 0.0006* | 3 |
| Valve Repair/Replacement | 6 | 7 | 0.76 | 4 |
| Combined Coronary Artery Bypass | 2 | 3 | 0.70 | 1 |
| Grafts and Valve Repair/Replacement | ||||
| Day of Discharge (days) | 12.7±7 | 6.4±4 | 0.009 * | 11.3±7 |
Denotes statistical significance
Atrial Electrogram Recordings
Forty-seven atrial electrograms were recorded simultaneously from the LA and RA in 7 AF patients. We were unable to obtain recordings in 2 patients with AF episodes that persisted for less than 15 minutes, and 3 patients were not part of the DF analysis subset.
Panel A of Figure 2 illustrates representative examples of continuous 5-sec electrograms (upper tracings) and their respective spectra (lower tracings) obtained from the LA and RA during AF. Visual inspection reveals that the individual interbeat intervals are shorter in the LA than RA, which clearly correlate with the different DFs, resulting in the corresponding LA-to-RA DF difference.
Figure 2.
DF differences in post-operative AF. A. Representative 5-second electrograms (top) and spectra after Fast Fourier Transformation (bottom) from the LA and RA. B: Mean DFs recorded from LA1, LA2, RA1 and RA2 during AF. C. Time course of LA and RA DF changes during 1 hour of continuous recording. Asterisks denote statistical significance.
Panel B demonstrates the average DF values. LA DF was significantly greater than RA DF in all AF patients regardless of the individual LA or RA leads considered: LA1: 6.29±0.6, LA2: 6.23±0.6 RA-1: 4.58±0.7 RA2: 4.55±0.63 Hz. (p < 0.01). Panel C depicts the time course of mean DF values at 10 minute intervals from the LA and RA in the 5 patients who remained in AF for >1 hour. The LA-to-RA DF difference was present throughout the entire time course despite a slight deceleration in the LA DF over the 60 minute period.
LA versus RA Ion Channel Transcripts
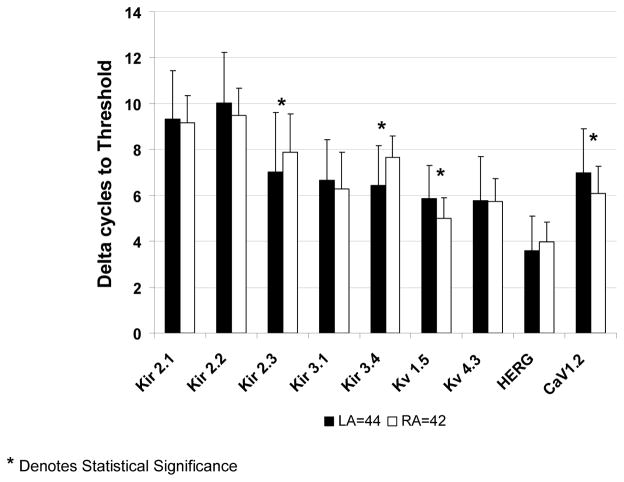
In Figure 3, the normalized cT (delta cT) values of relevant ion channel transcripts were lower for Kir2.3 and Kir3.4 in LA samples reflecting higher mRNA expression in LA than RA. Delta cT values in LA were higher for Kv1.5 and Cav1.2 reflecting lower expression in LA than RA. To gain insight into the importance of such differences, the data were transformed to fold differences (see Methods) after statistical differences between groups were established. As demonstrated in panel A of Figure 4, mRNA levels of Kir3.4 were 2.3 higher in the LA, which corresponded to the strongest left vs. right atrial difference. While the delta cT values for Kir2.3, Kv1.5, and CaV1.2 were significantly different between LA and RA samples, this translated to less than a two-fold difference in all circumstances. As shown in Panel B of Figure 4 (see also Table 3 in Online Supplement), no significant differences were observed in mRNA levels between patients who developed AF versus those who remained in NSR.
Figure 3.
Comparison of normalized cT values of mRNA levels from 44 left and 42 right atrial tissue samples for Kir2.1, Kir2.2, Kir2.3, Kir3.1, Kir3.4, Kv1.5, Kv4.3, HERG and CaV1.2 by quantitative rt-PCR. Asterisks indicate statistical significance. Delta cT is the cycle to threshold of the gene of interest subtracted from the cycle to threshold of GAPDH.
Figure 4.
Fold differences in ion channel transcripts. A: Same results as in Figure 3 expressed as the ratio of LA to RA mRNA levels. B: Comparison of LA ion channel mRNA levels from 31 patients in normal sinus rhythm relative to 12 patients who developed post-operative AF. Error bars are omitted in both panels due to transformation of the data and the subsequent loss of meaning. Data were transformed from cT values to equivalent fold differences, e.g., Fold Difference (mean CtLA − mean CtRA) = 2(mean CtLA − mean CtRA)17.
Evidence for Structural Remodeling
Examples presented in Figure 5(A – D) demonstrate the range of fibrosis seen in our samples. There was no significant difference between the LA and RA biopsies in the area of tissue available for analysis (Online Supplement Table 4). However, the percent area that was occupied by fibrosis was higher in the left atrium of patients that developed AF. Figure 6A demonstrates a statistically significant difference in LA fibrosis between those patients who developed AF and those who remained in NSR. In contrast, there was no difference in RA fibrosis.
Figure 5.
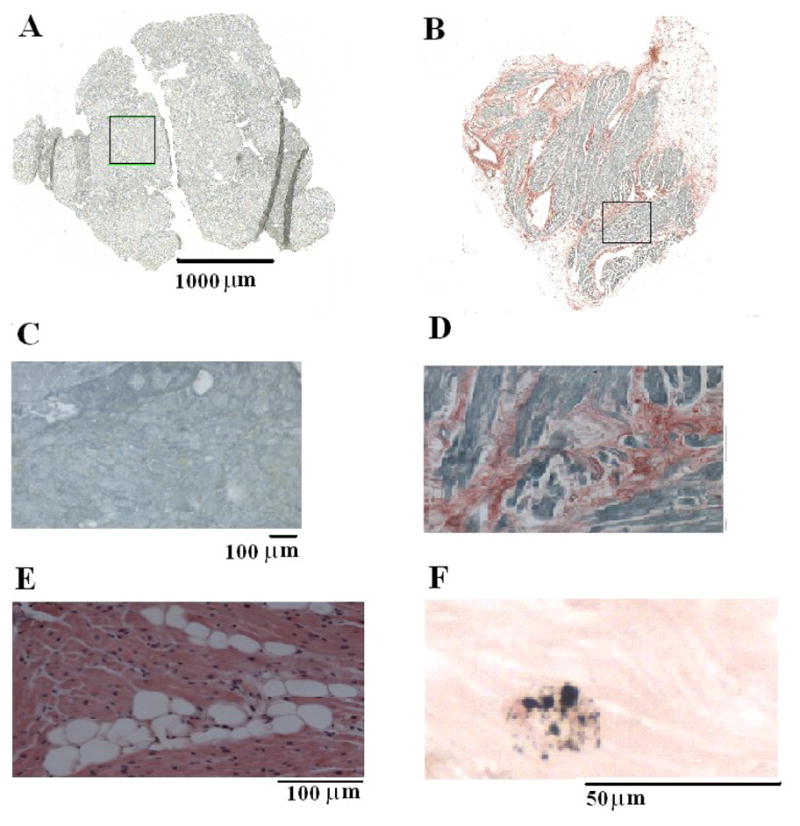
LA fibrosis, fatty deposits and iron deposits in patients undergoing cardiac surgery. A and B, montage images of atrial tissue after staining with picrosirius red (fibrosis-red, muscle-green). A: LA biopsy from a 30-year old patient who remained in normal sinus rhythm shows no evidence of fibrosis. B: LA biopsy from a 74 year old patient who developed atrial fibrillation shows 16% atrial fibrosis. C: Enlarged (10X) boxed region in A, demonstrates a lack of fibrosis. D: Enlarged (10x) boxed region in B demonstrates the highest amount of fibrosis. E: 20X left atrial sample stained with hematoxylin and eosin demonstrating fatty infiltration (white circles) within the muscle section. F: 40X left atrial sample stained with modified Perls demonstrating atrial muscle (pink) and iron deposition (blue).
Figure 6.
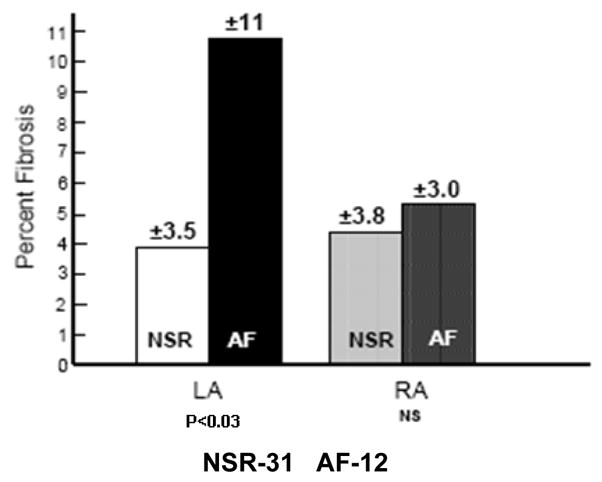
Different levels of fibrosis in left and right atrial tissue of patients who developed post-operative atrial fibrillation (AF) versus those who remained in normal sinus rhythm (NSR).
Fatty infiltration was observed in 7/44 patients, 3 of whom developed postoperative AF. Figure 5E represents an example of fatty infiltration in left atrial muscle. There were no differences in the amount of fatty infiltration between the left and right atria, or in those patients who developed AF (Table 4 in Online Supplement). However, patients with evidence of fatty infiltration were significantly older (73.8 ± 6 vs 60.1 ± 11 years, p < 0.001) than those patients without fatty infiltration.
Iron deposition was present in all patients. Figure 5F demonstrates an example of iron deposition. There was a significantly higher amount of iron in the left atrium in all patients (Table 4 in Online Supplement); however, there was no difference in those patients who developed AF.
DF to Fibrosis Correlation
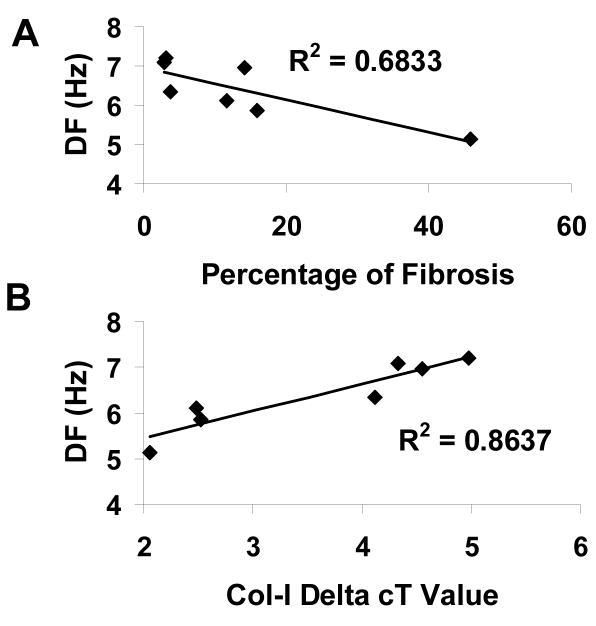
Collagen-I expression was similar in patients who developed AF and those who remained in NSR (Table 3 in Online Supplement). However, in Figure 7A, examination of the impact of atrial fibrosis and collagen-I expression on the AF dominant frequency in the 7 patients who developed AF and had intracardiac recordings, demonstrated that an increasing percentage of LA fibrosis resulted in slower LA frequencies. This resulted in a R2 value of 0.68 (p = 0.02). However, this did not remain significant after Bonferroni correction. Nevertheless, as Figure 7B demonstrates, when examining collagen-I expression in left atrial tissue there was a strong correlation between collagen-I expression and LA DF (lower delta cT values represent increased collagen-I expression) where the R2 value was 0.86 (p = 0.002) which remained significant after Bonferroni correction.
Figure 7.
A. Relationship between LA fibrosis and LA dominant frequency in 7 postoperative AF patients. B: Relationship between LA collagen-I expression (where lower delta cT values represent increased collagen I expression) and LA DF in 7 patients who developed post-operative AF.
Discussion
The main findings of this study are as follows: 1. AF developing spontaneously after cardiac surgery is characterized by a significant left-to-right atrial excitation frequency difference, which is evident within 15 minutes of AF onset and remains stable for over 1 hour of continuous recording. 2. mRNA levels of Kir2.3 and Kir3.4 are greater in the LA than in the RA, while Kv1.5 and Cav1.2 are lower in the LA than RA, 3. The presence of pre-operative left atrial fibrosis in the hearts of patients is associated with the development of AF in the post-operative phase of cardiac surgery while fatty infiltration and iron deposition are less critical. 4. Increasing amounts of LA fibrosis, particularly from collagen-I are associated with a lower LA dominant frequency during AF.
LA vs RA Dominant Frequencies
In accordance with previous DF measurements in patients with paroxysmal AF,5,6 our data clearly demonstrate that LA DFs are higher than RA DFs. This is in agreement with multiple studies supporting a specific role for the pulmonary veins-posterior left atrial area in maintaining AF.5,6,19 Observations in experimental models and in patients established that higher DFs were found in the LA while other atrial areas were activated more slowly.3–7 It is noteworthy that DF analyses performed previously in patients with paroxysmal AF were done days after AF initiation, and for only seconds to minutes.5,6 In this report, we demonstrate that frequency differences were manifest very early (<15 minutes) after the onset of AF and lasted up to one hour. These differences in LA and RA DF are likely the result of LA-to-RA differences in effective refractory period (ERP) during AF.20–22 While we did not attempt to confirm these ERP differences using programmed stimulation, the higher DF in the LA during AF is clearly indicative of a shorter ERP in that chamber. Moreover, the higher LA DF is likely reflecting the increased potassium channel expression and shorter action potential durations than in the RA.20–22
Our results demonstrate a decrease in LA excitation frequency during AF with increasing amounts of fibrosis (Figure 5C and 5D), which is consistent with data in a sheep heart model of AF in the setting of heart failure that specifically demonstrated reduced LA DFs and maintained LA-to-RA difference in the presence of fibrosis.10 That report10 demonstrated that increasing fibrosis resulted in increased interbeat variability, complexity of propagation and breakthroughs emerging at the junctions between the posterior wall and the PVs region, where fibrosis was greatest, supporting the idea that the LA is the source of the AF in that model.
LA-RA differences in K+ Channel Transcripts
LA-to-RA differences in excitation frequency during AF likely involve, at least in part, differences in ion channel gene expression,8 an idea supported by our finding of different mRNA levels in the LA and RA of three major K+ channels and one calcium channel (Figures 3 and 4). Reductions in LA CaV1.2 expression may contribute to AF maintenance in the left atrium by reducing the refractory period, similar to ion channel remodeling during chronic AF23 whereas LA reductions in K1.5 expression may act to oppose that effect. Kir2.3 is a crucial determinant of functional reentry in both atria24 and ventricles,25 and animal studies have demonstrated a greater abundance of Kir3.4 channels, and higher IK,ACh density in LA than RA myocytes. As such, the 1.8-fold and 2.3-fold difference, respectively, in Kir2.3 and Kir3.4 mRNA levels found in our patients, suggest that if functionally expressed, higher LA IK1 and IK,ACh should contribute to a shorter effective refractory period as well as to higher frequency and stability of reentrant sources in the LA than the RA.
The mRNA levels of K+ channels were similar in patients who developed AF and those who remained in NSR, which agrees with previous studies comparing RA ion current levels in cardiac surgery patients.9,26 Altogether, we conclude that chamber specific differences in ion channel protein expression may be important in establishing left-to-right DF differences and wave propagation dynamics but are not essential in the spontaneous development of post-operative AF.
Structural Differences
Previous observations describing RA fibrosis produced mixed results.11,13,14 The RA appendage can be biopsied easily with minimal risk, making it an ideal tissue to sample. However, to date, a preponderance of evidence suggests that the RA is a bystander during AF maintenance.5,6 In contrast, as demonstrated nearly 10 years ago,21 the LA is typically the location of high-frequency sources in both paroxysmal and persistent AF. Therefore, it is reasonable to speculate that LA fibrosis may have a greater impact on AF initiation and maintenance than RA fibrosis.
The accumulation of fibrosis has been found to modify the electrophysiologic properties of the atrium by reducing the velocity of the impulse and providing a substrate for reentry and AF initiation.10,27,28 Fibrosis accumulates in response to hypertension and/or diastolic dysfunction, co-morbid conditions that can be found in most, if not all, cardiac surgical patients. It has been well documented that advanced age and diastolic dysfunction, variables identified within our patient population, are both associated with post-operative AF1 and fibrosis.10 Fatty infiltration was observed in only 7 patients, and therefore it is difficult to draw conclusions on a limited sample size. However, our data do suggest that fatty infiltration increases with increased age accounting for its association with AF. Iron deposition was significantly greater within the left atrium; however, it was not associated with AF development. Therefore, on the basis of our results, we postulate that the combined presence of diastolic dysfunction and fibrosis in patients undergoing cardiac surgery establishes the adequate substrate and sets the stage for spontaneous AF elicited by electrical derangements secondary to such potential intra-operative triggers as stretch, inflammation, pericarditis, ischemia and autonomic outpouring.1,2
Study Limitations
Obvious ethical considerations prohibited us from conducting electrogram recordings during post-operative AF at higher spatial resolution, and did not enable us to precisely localize the AF source(s). Further, LA and RA biopsies were of limited area in comparison to the overall atrial dimensions. However, these patients were undergoing a major operation, and there are limits in the amount of tissue that could be taken. Unfortunately, the same limitation obviated immunochemical or patch clamp analyses. Therefore, the existence of chamber-specific differences in functional expression of sarcolemmal channel proteins as the molecular basis for LA-to-RA DF differences awaits confirmation. The interpretation of PCR data depends on the investigator’s choice for normalization, further details can be found within the Online Supplement. Last, figure 7 demonstrated a correlation between LA DF and LA fibrosis from within the lead placement subset. Although there was greater LA fibrosis in patients who developed post-operative AF in this subset (12.2±14% vs. 3.6±3.4%), it did not reach statistical significance using this smaller number of patients.
Conclusion
Comparison of the events surrounding post-operative AF development suggests that sources of AF are located in the left atrium and are predicated by the presence of left atrial fibrosis which may help initiate and maintain the arrhythmia. The main findings of this work, a stable and consistent left-to-right DF difference in post-operative AF patients and the association of left atrial fibrosis with the development of post-operative AF, may facilitate designing novel interventional or pharmacological treatments to prevent or terminate AF by specifically targeting the LA. Ongoing research on possible molecular factors that may predispose the atria to fibrillate after cardiac surgery, and that may be responsible for the observed LA-to-RA frequency difference in the majority of patients, should provide important insight into the molecular mechanisms of the AF organization demonstrated here.
Supplementary Material
Acknowledgments
Supported by NHLBI Grants P01-HL039707, P01-HL087226 and R01-HL080159. (JJ).
List of Abbreviations
- AF
Atrial fibrillation
- NSR
Normal Sinus Rhythm
- LA
Left Atrial
- RA
Right Atrial
- LAA
Left atrial appendage
- RAA
Right atrial appendage
- FFT
Fast Fourier Transform
- DF
Dominant Frequency
- RV
Right Ventricle
- cT
Cycle to Threshold
- rt-PCR
reverse transcription polymerase chain reaction
- ERP
Effective refractory period
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mckeown PP. Executive Summary: American college of chest physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:6–8. doi: 10.1378/chest.128.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 2.Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. JAMA. 1996;276:300–306. [PubMed] [Google Scholar]
- 3.Sarmast F, Kolli A, Zaitsev A, et al. Cholinergic atrial fibrillation IK,ACh gradients determine unequal left/right frequencies and rotor dynamics. Cardiovasc Res. 2003;59:863–873. doi: 10.1016/s0008-6363(03)00540-6. [DOI] [PubMed] [Google Scholar]
- 4.Berenfeld O, Mandapati R, Dixit S, et al. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the Langendorff-perfused sheep heart. J Cardiovasc Electrophysiol. 2000;11:869–879. doi: 10.1111/j.1540-8167.2000.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 6.Atienza F, Almendral J, Moreno J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 7.Berenfeld O, Zaitsev AV, Mironov SF, et al. Frequency dependant breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circulation Res. 2002;90:1173–1180. doi: 10.1161/01.res.0000022854.95998.5c. [DOI] [PubMed] [Google Scholar]
- 8.Nattel S, Maguy A, Le Bouler S, et al. Arrhythmogenic ion channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 9.Workman AJ, Pau D, Redpath CJ, et al. Postoperative atrial fibrillation is influenced by beta blocker therapy but not by pre-operative atrial cell electrophysiology. J Cardiovasc Electrophysiol. 2006;17:1230–1238. doi: 10.1111/j.1540-8167.2006.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka K, Zlochivier S, Vikstrom K, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 11.Cosgrave J, Foley JB, Flavin R, et al. Preoperative atrial histological changes are not associated with postoperative atrial fibrillation. Cardiovasc Path. 2006;15:213–217. doi: 10.1016/j.carpath.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 13.Nakai T, Chandy J, Nakai K, et al. Histologic assessment of right atrial appendage myocardium in patients with atrial fibrillation after coronary artery bypass graft surgery. Cardiology. 2007;108:90–96. doi: 10.1159/000095936. [DOI] [PubMed] [Google Scholar]
- 14.Workman AJ, Kane KA, Rankin AC. Cellular basis for human atrial fibrillation. Heart Rhythm. 2008;5:S1–S6. doi: 10.1016/j.hrthm.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki T, Higgins PJ, Crawford DR. Control selection for RNA quantitation. Biotechniques. 2000;29:332–337. doi: 10.2144/00292rv02. [DOI] [PubMed] [Google Scholar]
- 16.Zicha S, Moss I, Allen B, et al. Molecular basis of species-specific expression of repolarizing K currents in the heart. Am J Physiol Heart Circ Physiol. 2003;285:1641–1649. doi: 10.1152/ajpheart.00346.2003. [DOI] [PubMed] [Google Scholar]
- 17.Epperson LE, Martin SL. Quantitative assessment of ground squirrel mRNA levels in multiple stages of hibernation. Physiol Genomics. 2002;10:93–102. doi: 10.1152/physiolgenomics.00004.2002. [DOI] [PubMed] [Google Scholar]
- 18.Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Tox App Pharm. 2005;202:199–211. doi: 10.1016/j.taap.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 20.Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation time course and mechanisms. Circulation. 1996;94:2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]
- 21.Wiiffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;7:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 22.Wijffels MC, Kirchhof CJ, Dorland R, et al. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation. 1997;96:3710–3720. doi: 10.1161/01.cir.96.10.3710. [DOI] [PubMed] [Google Scholar]
- 23.Yue L, Melnyk P, Gaspo R, et al. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 24.Pandit SV, Berenfeld O, Anumonwo JM, et al. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noujaim SF, Pandit SV, Berenfeld O, et al. Up-regulation of the inward rectifier K+ current (IK1) in the mouse heart accelerates and stabilizes rotors. Physiol. 2007;578:315–326. doi: 10.1113/jphysiol.2006.121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrev D, Wettwer E, Kortner A. Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovasc Res. 2002;54:397–404. doi: 10.1016/s0008-6363(01)00555-7. [DOI] [PubMed] [Google Scholar]
- 27.Weber KT, Pick R, Jalil JE, et al. Patterns of myocardial fibrosis. J Mol Cell Cardiol. 1989;21:121–31. doi: 10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- 28.Anyukhovsky EP, Sosunov EA, Plotnikov A, et al. Cellular electrophysiologic properties of old canine atria provides a substrate for arrhythmogenesis. Cardiovasc Res. 2002;54:462–69. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.