Abstract
Background and aims
Ultrasonography is the most frequently used modality in surveillance for HCC among patients with chronic hepatitis C. However, the optimal surveillance interval is still controversial and the usefulness of supplementary tumor marker determination has not been confirmed.
Methods
A total of 243 cases of naive HCC were detected among 1,431 patients with chronic hepatitis C under outpatient-based surveillance. The mode of HCC detection, including ultrasound surveillance interval, was retrospectively examined and the relation between the interval and detected tumor size was analyzed. Tumor volume doubling time was estimated from exponential increase in serum tumor marker levels when applicable.
Results
HCC was first detected by ultrasonography in 221 patients. Ultrasound surveillance interval, ranging between 2 and 8 months, was not correlated with the size of tumor at detection. Patients with cirrhosis were likely to be surveyed at shorter intervals. The size of tumor exceeded 30 mm only in three (1.4%) cases. They were all positive for a biomarker and the estimated tumor doubling time was short. In 14 cases, HCC was first detected by CT indicated by abnormal rise in tumor marker levels despite negative ultrasound findings. In the remaining eight cases, ultrasonography had been replaced by CT as surveillance modality because of excessive obesity or coarseness of liver parenchyma.
Conclusions
Ultrasound surveillance at 6-month intervals was appropriate in general for the detection of HCC at a size smaller than 30 mm. However, in patient with established cirrhosis, more frequent screening would be needed to detect tumors of the same size.
Keywords: Hepatocellular carcinoma, Hepatitis C, Surveillance, Ultrasound, Tumor marker, Doubling time, Size of tumor, Surveillance interval
Introduction
HCC is one of the most common cancers worldwide [1–4]. Most HCC patients have a chronic liver disease in the background liver, among which chronic viral hepatitis due to hepatitis C virus (HCV) or hepatitis B virus (HBV) is very common [5–7]. Surveillance for HCC is now a part of standard clinical practice for patients with chronic viral hepatitis [8]. Considering recent advances in HCC treatments, surveillance is required to detect HCC while tumors are small enough for the indication of curative treatments such as surgical resection and radiofrequency ablation [9–13].
Ultrasonography is usually used as the modality of HCC surveillance because of its cost-effectiveness and non-invasiveness. Contrast-enhanced CT or MRI is usually reserved for the confirmatory diagnosis of HCC [14]. The efficacy of ultrasound surveillance on HCC detection depends on both ultrasound resolution and surveillance interval. Although ultrasound resolution has been much improved technologically, the optimal interval of surveillance may be still controversial. In Japan, an official guideline, the Clinical Practice Guidelines for Hepatocellular Carcinoma 2005, recommends ultrasound surveillance with an interval of 6 months for patients at a risk of HCC and with a shorter interval, 3–4 months, for extremely high-risk patients [15]. On the other hand, the guideline of American Association for the Study of Liver Diseases has proposed that ultrasound surveillance should be preformed at a fixed interval of 6 months for patients at a risk of HCC, regardless of its magnitude [8]. The latter guideline explicitly indicates that the surveillance interval should depend on expected tumor doubling time.
Once HCC occurs, the nodule will grow at a speed that is independent of the former probability of HCC development. Thus, surveillance with a fixed interval, disregarding the magnitude of the risk of HCC, seems relevant if the aim of surveillance is to detect any HCC nodules that are satisfactorily small. However, there remain a couple of issues to be addressed. First, it is to be confirmed whether the 6-month interval is short enough for the detection of HCC while tumors are small enough for curative treatments. Second, the sensitivity of ultrasonography should be substantially high. A failure to detect HCC tumors will result in delayed detection of oversized tumors.
The Japanese guideline, which recommends distinct surveillance intervals according to the magnitude of the risk of HCC, has been generally, although not strictly, observed in the clinical practice in Japan. At the authors’ institution, the interval of ultrasound surveillance on patients with chronic hepatitis C with suspected cirrhosis was usually shorter than 6 months. This provided us with the chance to investigate the relationship between ultrasound surveillance intervals and the tumor size at detection. Although one can expect that shorter surveillance intervals lead to the detection of smaller tumors, it is of interest to know whether the magnitude of difference is large enough to be clinically relevant. In the present study, we also sought to evaluate the role of HCC-specific tumor marker determination, which is recommended by the Japanese guideline but sometimes discouraged elsewhere.
Patients and methods
Patients
A total of 1,431 patients with chronic hepatitis C, excluding those also positive for HBsAg and those with a present or past history of HCC, were followed up at the authors’ institution between 1994 and 2004. HCC developed in 340 of them during the follow-up period of 6.1 years on average [16]. Among these naive HCC patients, 97 had undergone HCC surveillance exclusively or alternatively at other institutions and were excluded from the present analysis. In the remaining 243 cases, we analyzed the relationship between the interval of ultrasound surveillance and the size of tumor at detection.
Abdominal ultrasound
Abdominal ultrasonography was performed on outpatient basis with ultrasound devices SSA-250A or SSA-370A (Toshiba Medical Systems Corporation, Tokyo, Japan) or SSD-2000 (Aloka Co., Tokyo, Japan). The examination was performed after fasting of at least 6 h. The examiners, all highly experienced in abdominal ultrasonography, were aware of patients’ clinical data including previous ultrasound reports. The ultrasound surveillance interval of each patient was determined by the outpatient clinic physician in charge. Although there were no rigid protocols for the surveillance interval, patients with more advanced liver disease were likely to undergo ultrasonography at a shorter interval. HCC-specific serum tumor markers, AFP and DCP, were determined every 1–3 months.
Diagnosis of HCC
When an intrahepatic nodule suggestive of HCC was detected on ultrasonography, contrast-enhanced dynamic CT or MRI was ordered. On CT/MRI, a nodule was considered to be HCC when both hyperattenuation in the arterial phase and hypoattenuation in the late phase were evidenced [8, 17, 18]. When CT/MRI findings were not conclusive, the nodule was followed up with ultrasonography within 3 months. Ultrasound-guided tumor biopsy was performed when there was a definite increase in size of the nodule while CT/MRI remained inconclusive. CT/MRI was also performed when there was an abnormal elevation in an HCC-specific tumor marker while ultrasound findings were negative. In most patients, ultrasound-guided liver biopsy was also performed and histology was evaluated according to the classification of Desmet et al. [19].
Calculation of surveillance interval
The surveillance interval was defined as the interval between the ultrasound surveillance that first detected a nodule, which was subsequently diagnosed to be HCC by CT/MRI, and the immediately previous surveillance with negative findings. Sometimes, an intrahepatic nodule detected by ultrasonography was not diagnosed as HCC on the subsequent CT/MRI, followed up with ultrasonography, and later diagnosed to be HCC. The interval of subsequent follow-up ultrasonography in such cases was usually short and biased. Thus, we defined the surveillance interval as the interval between the ultrasonography that first detected the nodule and the immediately previous one. Sometimes, HCC was detected on CT/MRI that was ordered because of abnormal elevation in tumor marker levels. The ultrasound surveillance interval was not definable in such cases and they were analyzed separately.
Estimation of tumor doubling time
There was usually an interval of 1–2 months between the detection of a tumor on ultrasonography and the treatment. The increase in tumor size as measured by ultrasonography was usually subtle, but there was a measurable increase in tumor marker levels, AFP or DCP, when they were positive. In such cases, we estimated tumor doubling time assuming exponential increase in tumor marker levels as follows: C2 = C1 × 2t/DT, where C1 and C2 are the first and second tumor marker levels, respectively, t is the interval of determination, and DT is the estimated doubling time [20].
Statistical analysis
Data were expressed as the mean ± standard deviation unless otherwise indicated. Continuous variables were compared with unpaired Student’s t test (parametric) and Mann–Whitney test (non-parametric). A trend in the tumor size at detection over the surveillance interval was assessed by Spearman’s rank correlation coefficient and Jonckheere-Terpstra trend test. A P value less than 0.05 on 2-tailed test was considered significant. Data were processed and analyzed by using the S-PLUS 2000 (MathSoft, Inc., Seattle, WA).
Results
Surveillance interval and tumor size at detection
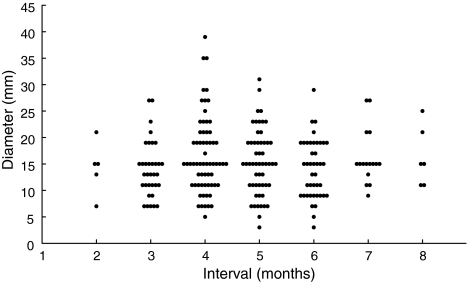
The intrahepatic tumor was first detected by surveillance ultrasonography in 221 patients. In the remaining 22 patients, HCC was first detected by CT (see the following text). The relationship between the ultrasound surveillance interval in months, rounded off to whole numbers, and the size of tumor on the ultrasonography among these 221 patients is shown in Fig. 1. There was no significant trend in the tumor size over the surveillance interval: Spearman’s rank correlation coefficient was 0.0283 (P = 0.6753) and Jonckheere-Terpstra trend test revealed no significant trend in tumor size (P = 0.7072). Note that most of the small nodules were not diagnosed as HCC immediately after the detection on ultrasonography but confirmed later in follow-up, usually after showing an increase in size The tumor size at detection was not different between the patients who had received surveillance at an interval shorter than 6 months (N = 157) and those at an interval of 6 months or longer, up to 8 months (N = 64) (P = 0.50 by Kruskal–Wallis test). Tumor was larger than 30 mm in diameter at detection in three patients in the former group and none in the latter (P = 0.56). Taken together, the tumor size at detection exceeded 30 mm in three of 221 (1.4%) patients.
Fig. 1.
Size of tumors at detection. The mean size of tumors at detection was 16.5 ± 6.4 mm when the interval was shorter than 6 months and 15.8 ± 5.5 mm when the interval was 6–8 months (P = 0.469). Tumor was larger than 30 mm in diameter at detection in three patients, whose surveillance interval each was 4 months
Baseline characteristics of patients are shown in Table 1. Reflecting the characteristics of patients with chronic hepatitis C in Japan currently, the mean age reached 67 years. Those patients who had received surveillance at a shorter interval had significantly lower platelet count and albumin concentration and higher bilirubin concentration, suggesting that shorter surveillance interval had been applied to patients with more advanced liver disease. Background liver biopsy was performed at the time of HCC treatment in 174 (78.7%) patients. Cirrhosis was diagnosed in 89 of 123 (72.4%) patients who had been surveyed with a shorter interval and 26 of 51 (51.0%) patients with a longer interval (P < 0.001).
Table 1.
Characteristics of patients
| Factors | Surveillance intervala | P | |
|---|---|---|---|
| <6 months (N = 157) | 6–8 months (N = 64) | ||
| Ageb (year) | 66.9 ± 7.2 | 67.1 ± 8.4 | 0.86 |
| Male, n (%) | 93 (59.2) | 46 (71.9) | 0.092 |
| Aspartate aminotransferasec (IU/l) | 69.0 (51.0–98.0) | 66.5 (47.8–92.0) | 0.31 |
| Alanine aminotransferasec (IU/l) | 64.0 (44.0–90.0) | 62.0 (45.3–92.0) | 0.86 |
| Albuminb (g/dl) | 3.4 ± 0.5 | 3.6 ± 0.5 | 0.028 |
| Bilirubinc (mg/dl) | 0.9 (0.7–1.2) | 0.8 (0.6–1.0) | 0.017 |
| Prothrombin timec (%) | 71.0 (62.9–80.5) | 75.7 (67.4–84.5) | 0.050 |
| Platelet countc (×103/μl) | 79 (62–110) | 117 (81–151) | <0.001 |
| Ascitesd, n (%) | 26 (16.6) | 11 (17.2) | 1.00 |
| Size of tumorsb (mm) | 16.5 ± 6.4 | 15.8 ± 5.5 | 0.47 |
| Number of tumors, n (%) | |||
| 1 | 90 (57.3) | 40 (62.5) | 0.41 |
| 2–3 | 54 (34.4) | 21 (32.8) | |
| >3 | 13 (8.3) | 3 (4.7) | |
| Vascular invasion, present | 1 (0.6) | 0 (0) | 1.00 |
| Background livere, n (%) | |||
| Cirrhosis | 98/123 (79.7) | 26/51 (51.0) | <0.001 |
aExact trend test
bExpressed as mean ± SD
cExpressed as median (25th–75th percentiles)
dThose controlled by diuretics were included
eLiver biopsy was performed on 123 patients in less than 6-month interval group and 51 patients in 6- to 8-month interval group
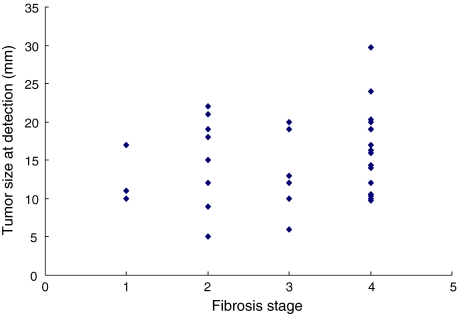
Thirty-two patients among them had had the ultrasound surveillance interval of exactly 6 months prior to HCC detection. Fibrosis stage was judged to be F1 in three, F2 in eight, F3 in six, and F4 in 15 patients. Tumor size was 14.1 ± 5.3 mm in non-cirrhotic (F1–F3) patients and 16.2 ± 5.7 mm in cirrhotic (F4) patients (P = 0.279 by Student’s t test; Fig. 2).
Fig. 2.
Relationship between the fibrosis stages and the tumor size at detection among patients who received ultrasonography at 6-month intervals and also underwent liver biopsy. Liver biopsy was performed in 124 patients. Thirty-two patients among them had had the ultrasound surveillance interval of 6 months prior to HCC detection. Fibrosis stage was judged to be F1 in three, F2 in eight, F3 in six, and F4 in 15 patients. Tumor size was 14.1 ± 5.3 mm in non-cirrhotic (F1–F3) patients and 16.2 ± 5.7 mm in cirrhotic (F4) patients (P = 0.279 by Student’s t test)
Estimated doubling time
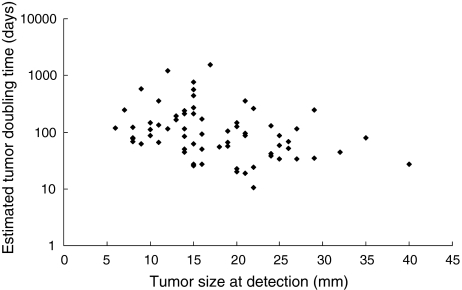
Tumor doubling time was estimated only in patients who were positive for AFP (≥100 ng/ml) or DCP (≥80 mAU/ml). Among 80 such patients, sequential data were available in 67 of 221 (30.3%) patients. This is a biased subset because tumor marker is more likely to be positive when tumor is larger (Table 2). All the three cases in which tumor was larger than 30 mm in diameter at detection were positive for tumor markers. The association between the estimated tumor doubling time and tumor size at detection is shown in Fig. 3. Median tumor doubling time was 87.0 days, and 25th and 75th percentiles were 50.3 and 167.8 days, respectively. The estimated doubling time varied widely when tumor was relatively small at detection, whereas the doubling time was short for tumors that were detected at a large size. The three tumors larger than 30 mm in diameter at detection had a doubling time of 26.9, 44.8, and 80.1 days.
Table 2.
Size at detection and tumor marker positivity
| ≤20 mm (N = 171) | 21–30 mm (N = 47) | >30 mm (N = 3) | Total (N = 221) | |
|---|---|---|---|---|
| AFP (≥100 ng/ml) | 35 (20.5%) | 16 (34.0%) | 2 (66.7%) | 53 (24.0%) |
| DCP (≥80 mAU/ml) | 21 (12.3%) | 12 (25.5%) | 2 (66.7%) | 35 (15.8%) |
| At least one was positive | 54 (31.6%) | 23 (48.9%) | 3 (100%) | 80 (36.2%) |
Fig. 3.
Association between tumor doubling time, estimated by exponential increase in tumor marker level, and tumor size at detection. Median tumor doubling time was 87.0 days; 25th percentile, 50.3 days, and 75th percentile, 167.8 days. The estimated doubling time varied widely in each case, but those tumors that were of large size at detection showed short doubling time. The three nodules that were larger than 30 mm in diameter at detection had a doubling time of 26.9, 44.8, and 80.1 days
Exceptional cases
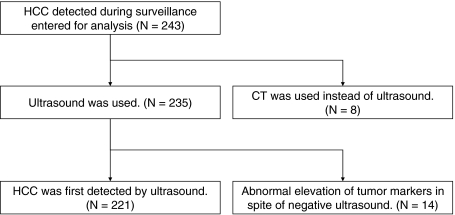
Among the 243 patients studied, HCC was first detected not on ultrasonography but on CT in 22 (9.1%) patients. CT was used in surveillance instead of ultrasonography in eight (3.3%) patients because of extremely coarse liver parenchyma or excessive obesity. In the remaining 14 of 243 (5.8%) patients, CT was ordered because of abnormal elevation of tumor markers (AFP in nine, DCP in three, and AFP + DCP in two), although immediately previous ultrasonography had given negative findings (Fig. 4). Among these 14 patients, the size of tumor at detection was 12–43 mm (>30 mm in two patients), which was significantly larger than the size of tumor detected by ultrasound surveillance (P = 0.0003 by Mann–Whitney test).
Fig. 4.
Schematic description of studied patients. HCC hepatocellular carcinoma, CT computed tomography
Discussion
This retrospective analysis indicated that ultrasound surveillance at 6-month intervals was generally acceptable as a means for detecting small-sized HCC among patients with chronic hepatitis C. Most HCC nodules were not exceeding 30 mm in diameter at detection. Such cases were suitable for receiving curative treatment such as surgical resection or radiofrequency ablation, although the actual indication depended also on the liver function reservoir of each patient [21, 22]. Shorter surveillance intervals did not provide further advantages in terms of tumor size (15.8 ± 5.5 mm with 6-month interval and 16.5 ± 6.4 mm with shorter interval).
In theory, a shorter surveillance interval will lead to detection at a smaller size. The reason why we found no difference is not clear. However, shorter surveillance interval was preferably assigned to patients with more advanced liver fibrosis (Table 1). Liver parenchyma of such patients might have coarse echogenicity, resulting in a larger threshold for tumor detection on ultrasonography. When compared among patients under 6-month surveillance interval, HCC nodules were detected at larger size in cirrhotic patients than in non-cirrhotic ones, although the difference was not statistically significant. Although not provable in this retrospective study, it is likely that those patients assigned to a shorter surveillance interval, among whom cirrhosis was dominant, would have had still larger tumors if surveyed within 6-month interval. This may bear greater importance when the quality of ultrasound devices or the skill of examiners has some shortcomings. Even under better external conditions, resolution of ultrasonography may be seriously impaired by patients’ conditions such as extreme obesity. In the current study, ultrasonography had been replaced by CT in surveillance of such patients (8/243, 3.3%).
The current study suggested that tumor growth speed is an important factor affecting the tumor size at detection. Those tumors that were of large size at detection were positive for HCC-specific tumor markers and the deduced tumor growth speed was rapid. Although very few nodules (3/221, 1.4%) were larger than 30 mm in diameter at detection on ultrasound surveillance, other 14 cases were detected on CT that was ordered not because of ultrasound findings, which had been negative, but because of an abnormal rise in tumor markers. Tumor size was larger than 30 mm in two of these cases. For the remaining 12 patients, it is not known whether the tumor would have been smaller than 30 mm at detection without tumor marker determination. The ultrasound-based surveillance at the authors’ institution failed to detect tumors while they were smaller than 30 mm in diameter in at least 2.1% (5/235) patients.
A simple solution to reduce the proportion of oversized detection would be to adopt shorter surveillance interval [23, 24]. However, the present study showed that the 4-month interval was not short enough, and it may not be practical to further shorten the surveillance interval in terms of cost-effectiveness. It should also be noted that the prognosis of patients with rapid growing tumor may be poor even after curative hepatectomy [25, 26]. Sheu et al. [23], by comparing tumor sizes on two ultrasound examinations at an interval of 36–860 days, reported a median tumor doubling time of HCC as 117 days (range = 29–398 days). Although the investigators proposed an optimal ultrasound interval of 4–5 months, some tumors may not be small enough at detection with this interval if the doubling time is at the shorter side of the range.
Although AFP has been known as an HCC-specific biomarker for more than 30 years, the usefulness of AFP in HCC surveillance has been questioned [27]. Serum AFP level is negative in more than half cases of HCC, and it may become positive in hepatitis or cirrhosis without HCC [28]. Thus, the evaluation of AFP based on a single-point value is of limited use. However, sequential elevation in serum AFP levels is more specific for HCC [29, 30]. Larger tumors, which are likely to have shorter doubling time, have higher positivity for AFP [31]. Thus, the sequential determination of AFP can be complementary to ultrasound surveillance. The determination of DCP, which is more specific to HCC than is AFP, may serve in a similar manner. These tumor markers should be measured at an interval shorter than that of ultrasound surveillance if the measurement is to supplement ultrasonography.
Ultrasonography had not been used as the mode of surveillance in eight patients because the resolution was judged not satisfactory. Contrast-enhanced CT was used instead and successful in finding HCC nodules at acceptable sizes. In the surveillance of patients with coarse liver parenchyma, modalities other than conventional ultrasonography are preferable. However, the cost-effectiveness and invasiveness of contrast-enhanced CT should be considered if its indication is to be broadened. Recently developed contrast-enhanced ultrasonography may be promising but is yet to be evaluated in future studies.
In conclusion, patients with chronic hepatitis C without cirrhosis could be appropriately screened at 6-month intervals, as recommended by various guidelines, and such a protocol would be able to detect small tumors in most cases. However, in patients with established cirrhosis, more frequent screening would be needed to detect tumors of the same size and the 6-monthly recommendations would result in the detection of larger tumors than that in patients without cirrhosis.
Abbreviations
- AFP
α-Fetoprotein
- CT
Computed tomography
- DCP
Des-γ-carboxy prothrombin
- HBsAg
HBV surface antigen
- HCC
Hepatocellular carcinoma
- MRI
Magnetic resonance imaging
References
- 1.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. Lyon (France): IARC Scientific Publications; 2002. [Google Scholar]
- 2.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Capocaccia R, Sant M, Berrino F, Simonetti A, Santi V, Trevisani F. Hepatocellular carcinoma: trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol. 2007;102:1661–1670. doi: 10.1111/j.1572-0241.2007.01337.x. [DOI] [PubMed] [Google Scholar]
- 4.Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17–S26. doi: 10.1053/j.gastro.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, IHIT Study Group et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 6.Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, Okudaira T, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C-viral infection in Japan. Hepatology. 1995;22:1027–1033. doi: 10.1002/hep.1840220403. [DOI] [PubMed] [Google Scholar]
- 7.Sun CA, Wu DM, Lin CC, Lu SN, You SL, Wang LY, et al. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol. 2003;157:674–682. doi: 10.1093/aje/kwg041. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 9.Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 10.Solmi L, Primerano AM, Gandolfi L. Ultrasound follow-up of patients at risk for hepatocellular carcinoma: results of a prospective study on 360 cases. Am J Gastroenterol. 1996;91:1189–1194. [PubMed] [Google Scholar]
- 11.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 12.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Chan AC, Poon RT, Ng KK, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg. 2008;247:666–673. doi: 10.1097/SLA.0b013e31816a747a. [DOI] [PubMed] [Google Scholar]
- 14.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohki T, Tateishi R, Sato T, Masuzaki R, Imamura J, Goto T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:459–464. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 18.Nino-Murcia M, Olcott EW, Jeffrey RB, Jr, Lamm RL, Beaulieu CF, Jain KA. Focal liver lesions: pattern-based classification scheme for enhancement at arterial phase CT. Radiology. 2000;215:746–751. doi: 10.1148/radiology.215.3.r00jn03746. [DOI] [PubMed] [Google Scholar]
- 19.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. doi: 10.1002/hep.1840190629. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez ML, Nelson EC, Devere White RW, Lara PN, Jr, Evans CP. Current applications for prostate-specific antigen doubling time. Eur Urol. 2008;54:291–300. doi: 10.1016/j.eururo.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, Lim HK, Rhim H, Lee WJ, Joh JW, Park CK. Recurrence of hepatocellular carcinoma after liver transplantation: patterns and prognostic factors based on clinical and radiologic features. AJR Am J Roentgenol. 2007;189:352–358. doi: 10.2214/AJR.07.2088. [DOI] [PubMed] [Google Scholar]
- 23.Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259–266. doi: 10.1016/0016-5085(85)90324-5. [DOI] [PubMed] [Google Scholar]
- 24.Ebara M, Ohto M, Shinagawa T, Sugiura N, Kimura K, Matsutani S, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. A study in 22 patients. Gastroenterology. 1986;90:289–298. doi: 10.1016/0016-5085(86)90923-6. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki N, Yoshino M, Yoshida T, Suzuki M, Moriyama N, Takayasu K, et al. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time. A preliminary report. Cancer. 1989;63:2207–2210. doi: 10.1002/1097-0142(19890601)63:11<2207::AID-CNCR2820631124>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Cucchetti A, Vivarelli M, Piscaglia F, Nardo B, Montalti R, Grazi GL, et al. Tumor doubling time predicts recurrence after surgery and describes the histological pattern of hepatocellular carcinoma on cirrhosis. J Hepatol. 2005;43:310–316. doi: 10.1016/j.jhep.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Sherman M. Alphafetoprotein: an obituary. J Hepatol. 2001;34:603–605. doi: 10.1016/S0168-8278(01)00025-3. [DOI] [PubMed] [Google Scholar]
- 28.Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–575. doi: 10.1016/S0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 29.Kuwahara T, Sakai T, Majima Y, Hirai K, Tanikawa K. Serial changes in serum alpha-fetoprotein prior to detection of hepatocellular carcinoma in liver cirrhosis. Hepatogastroenterology. 1993;40:347–351. [PubMed] [Google Scholar]
- 30.Arrieta O, Cacho B, Morales-Espinosa D, Ruelas-Villavicencio A, Flores-Estrada D, Hernandez-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer. 2007;7:28. doi: 10.1186/1471-2407-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farinati F, Marino D, Giorgio M, Baldan A, Cantarini M, Cursaro C, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]