Abstract
Purpose
The proportion of B-HCC cases in Taiwan has progressively decreased over the last 20 years. It was not really due to an overall decrease in B-HCC but due to an increase in HCV-related HCC. The identification of potential HCV endemic areas in Taiwan has consequently become important.
Methods
Data were collected retrospectively from eight Taiwan medical centers from 1981 to 2001, the geographical variations of male C-HCC townships in Taiwan were illustrated on maps. Goodness of fit was used to compare the anti-HCV prevalence in townships and cities, with the mean anti-HCV prevalence for Taiwan as a whole. Township-, city-, and county-specific prevalence of anti-HCV was presented as the median, ranges, and SMRs.
Results
Geographic variation can be analyzed in only 263 townships and cities. The maps were designed on the basis of different SMRs. The mean anti-HCV prevalence for male HCC patients in Taiwan was 31.9% (95% confidence interval: 30.7–33.0). Twenty-five townships distributed throughout central-western and south-western Taiwan have significantly higher prevalence (P < 0.05) (12 townships SMR ≥ 2; 13 townships 1.5 ≤ SMR < 2). Twenty-two townships have significantly lower prevalence (P < 0.05) (6 townships 0.5 ≤ SMR<1; 16 townships SMR < 0.5). Four different patterns of geographic variation in different counties were also noted and demonstrated.
Conclusion
We successfully highlighted some potential high HCV endemic townships in Taiwan.
Keywords: Hepatocellular carcinoma, Hepatitis B virus, Hepatitis C virus, Geographic variation Taiwan
Introduction
Chronic HBV and HCV infections are the two major etiologies of HCC in Taiwan [1].To control HBV infection, a mass immunization program in Taiwan was launched on 1 July 1984, dramatically decreasing HBV carrier rates and childhood B-HCCs [2, 3]. The average annual incidence of HCC in children 6–14 years of age declined from 0.70 per 100,000 children between 1981 and 1986 to 0.57 between 1986 and 1990 and to 0.36 between 1990 and 1994 [3]. A further 80–85% decrease in HCC among Taiwanese adults can be expected within the next 3–4 decades [4]. There is still no effective vaccine to control HCV infection, but interferon-based combined treatment may cure the infection and thereby prevent later complications of HCCs [5]. We recently reported an increase in C-HCC in Taiwan over the last 20 years [6]. To gain better control of HCC in Taiwan, it is important to know the trends and gain a more detailed understanding of possible high HCV endemic areas to implement a cost-effective strategy to eradicate HCV infection. The major viral etiology in male Taiwanese HCC patients is HBV [1, 6]. We have shown in an earlier study that HBsAg prevalence is more closely correlated with HCC mortality than anti-HCV prevalence among male subjects [7]. Hence, those areas having higher HCV-related male HCC patients could potentially be HCV endemic areas. Our previous report focused only on variations between different counties [6], but this variation was noted even among different townships within the same county. Using the same cohort, we further report on these townships.
Materials and methods
Patients and data collection
This is a retrospective multicentric study. The methods of patient enrollment and items recorded have been reported elsewhere [6, 7]. Eight medical centers participated in this study. Patient medical records from 1994 to 2001, with a diagnosis of International Classification of Diseases, Ninth Revision, code 155, were retrieved from the computer database of each hospital. Hepatologists and experienced, well-trained specialized nurses in each hospital then reviewed the medical records. Only patients diagnosed with HCC were enrolled. Each patient’s name, medical record number, national citizen identification number, birthday, sex, residency, ZIP code, diagnostic criteria for HCC, definitive diagnosis date, HBsAg status, and anti-HCV status were recorded. We included patients from 1994 to 2001 for analysis because anti-HCV data were not universally available in Taiwan before 1994. Because patients might have made multiple visits to different hospitals, we used their national citizen identification numbers to eliminate any potentially duplicated records.
Diagnosis of HCC
We arbitrarily divided the diagnostic criteria of HCC from 1 to 4. Criterion 1 applied to a diagnosis of HCC verified by either pathology or cytology. Criterion 2 involved a diagnosis of HCC based on α-fetoprotein levels higher than 400 ng/ml, plus at least 1 imaging study that showed a typical HCC image. Criterion 3 was used for the diagnosis of HCC that did not fit criteria 1 or 2 initially but fit either criteria 1 or 2 during the follow-up period. Criterion 4 was used when the diagnosis of HCC was based on typical imaging studies but did not fit criteria 1–3. HCC patients with diagnostic criteria 1–3 were grouped in the definite diagnosis group. A total of 6,654 patients were enrolled for further analysis.
Data analysis
Foxpro® (Version 5.0) was used to create the database and SPSS® (Version 10.0) for statistical analysis. We used goodness of fit to compare the anti-HCV prevalence in townships and cities, with the mean anti-HCV prevalence for Taiwan as a whole. Statistical significance was defined as P < 0.05.
There are 16 counties in Taiwan’s main island and in total 356 townships and cities. Geographic variation could be analyzed in only 263 townships and cities; 93 other townships and cities could not be analyzed because of the number of patients enrolled was fewer than 5. The major cause of inadequate enrollment was due to the small population of these townships. Anti-HCV prevalence and 95% confidence intervals were calculated. Township-, city-, and county-specific prevalence of anti-HCV was presented as median, ranges, and SMRs. SMR (herein represented as relative proportion of HCV-associated HCC) is defined as anti-HCV prevalence of HCC patients of a certain city or county divided by anti-HCV prevalence of HCC patients in Taiwan as a whole.
Results
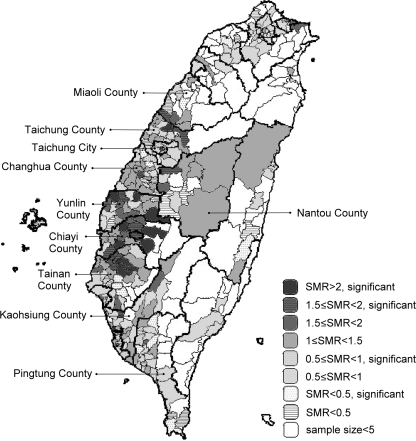
The mean anti-HCV prevalence for male HCC patients in Taiwan was 31.9% (95% confidence interval: 30.7–33.0). Figure 1 presents the geographic variation of different townships in Taiwan as a whole. Twenty-five townships located in different counties throughout central-western Taiwan and southwestern Taiwan have significantly higher prevalence rates (Table 1). SMR ≥2 was noted in 12 townships, Lucao (Chiayi), Mailiao (Yunlin), Guantian (Tainan), Lioujia (Tainan), Zihguan (Kaohsiung), Jhuci (Chiayi), Siluo (Yunlin), Jhongpu (Chiayi), Liouying (Tainan), Gukeng (Yunlin), Singang (Chiayi), and Houli (Taichung), whereas 1.5 ≤ SMR < 2 was noted in 13 townships, Siaying (Tainan), Yuanli (Miaoli), Dongshih (Taichung), Yijhu (Chiayi), Lunbei (Yunlin), Minsyong (Chiayi), Sinying (Tainan), Chiayi City, Yanshuei (Tainan), Alian (Kaohsiung), West district (Taichung City), Caotun (Nantou), and Sihu (Changhua). Another 11 townships, Fangshan (Pingtung), Sanyi (Miaoli), Jiasian (Kaohsiung), Dapi (Yunlin), Baojhong (Yunlin), Sikou (Chiayi), Yuanchang (Yunlin), Sinyi (Keelung City), Dalin (Chiayi), Taibao (Chiayi), and Taisi (Yunlin), have anti-HCV prevalence rates of more than 50%, but there are no statistically significant differences. Twenty-two townships have significant lower rates of prevalence, including 6 townships Changhua (Changhua), Siaogang (Kaohsiung City), Wanhua (Taipei City), Situn (Taichung City), Pingtung (Pingtung), and Banciao (Taipei) with 0.5 ≤ SMR<1. Sixteen townships, Jhonghe (Taipei), Wandan (Pingtung), Wurih (Taichung), Neipu (Pingtung), Jhongli (Taoyuan), Huatan (Changhua), Lujhou (Taipei), Hemei (Changhua), Shanhua (Tainan), Sinhua (Tainan), Sinshih (Tainan), Yuli (Hualien), Hengchun (Pingtung), Gaoshu (Pingtung), Gongguan (Miaoli), and Nuannuan (Keelung City), were found to have SMR of less than 0.5.
Fig. 1.
Different relative proportion of HCV associated HCC (SMR) among male HCC patients in different townships of Taiwan
Table 1.
Different townships and counties stratified according to HCV SMR
| SMR | Test results (township number) | Townships (sorting by prevalence of anti-HCV in descending order) |
|---|---|---|
| ≧2 (12 townships) | Significantly higher (12) | Lucao (Chiayi),a Mailiao (Yunlin),a Guantian (Tainan),a Lioujia (Tainan),a Zihguan (Kaohsiung),a Jhuci (Chiayi),a Siluo (Yunlin),a Jhongpu (Chiayi),a Liouying (Tainan),a Gukeng (Yunlin),a Singang (Chiayi),a Houli (Taichung)a |
| Not significantly different (0) | None | |
| 1.5≦SMR<2 (30 townships) | Significantly higher (13) | Siaying (Tainan),a Yuanli (Miaoli),a Dongshih (Taichung),a Yijhu (Chiayi),a Lunbei (Yunlin),a Minsyong (Chiayi),a Sinying (Tainan),a Chiayi City,a Yanshuei (Tainan),a Alian (Kaohsiung),a West district (Taichung City), Caotun (Nantou), Sihu (Changhua) |
| Not significantly different (17) | Fangshan (Pingtong),a Sanyi (Miaoli),a Jiasian (Kaohsiung),a Dapi (Yunlin),a Baojhong (Yunlin),a Sikou (Chiayi), Yuanchang (Yunlin),a Sinyi (Keelung City),a Dalin (Chiayi),a Taibao (Chiayi),a Taisi (Yunlin),a Rueifang (Taipei), Anping (Tainan City), Jiangjyun (Tainan), Gueiren (Tainan), Mingjian (Nantou), Linluo (Pingtung) | |
| 1≦SMR<1.5 (69 townships) | Significantly higher (0) | None |
| Not significantly different (69) | Not listed | |
| 0.5≦SMR<1 (114 townships) | Significantly lower (6) | Changhua (Changhua), Siaogang (Kaohsiung City), Wanhua (Taipei City), Situn (Taichung City), Pingtung (Pingtung), Banciao (Taipei) |
| Not significantly different (108) | Not listed | |
| <0.5 (38 townships) | Significantly lower (16) | Jhonghe (Taipei), Wandan (Pingtung), Wurih (Taichung), Neipu (Pingtung), Jhongli (Taoyuan), Huatan (Changhua), Lujhou (Taipei), Hemei (Changhua), Shanhua (Tainan),b Sinhua (Tainan),b Sinshih (Tainan),b Yuli (Hualien),b Hengchun (Pingtung),b Gaoshu (Pingtung),b Gongguan (Miaoli),b Nuannuan (Keelung City)b |
| Not significantly different (22) | Not listed | |
| Sample size <5 | 93 townships | Not listed |
aPrevalence of anti-HCV in 33 townships was more than 50%
bPrevalence of anti-HCV was 0%
There were four different patterns (Fig. 1) regarding the geographic variations of different townships in each county. The first pattern included Yunlin, Chiayi, and Tainan counties. In each county, there were at least four more significantly high prevalence townships with anti-HCV prevalences for male HCC patients. The second pattern included Miaoli, Taichung, and Nantou counties. There was only a limited number of high to significantly high prevalence townships that seemed to be neighbors, although located in different counties. The third pattern included Taichung City as well as Changhua and Kaohsiung counties. Only one significantly high prevalence township was identified in each county and city. The fourth pattern included other counties except those mentioned above (e.g., Pingtung County). There were either no significantly high prevalence townships or else the information was inadequately enrolled there.
Discussion
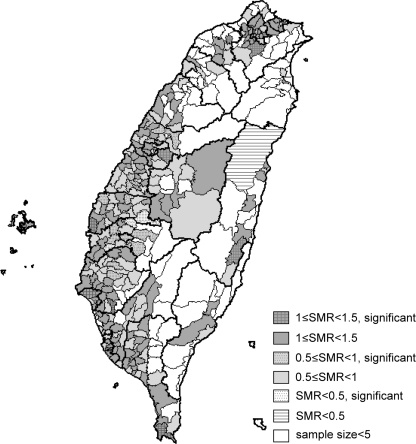
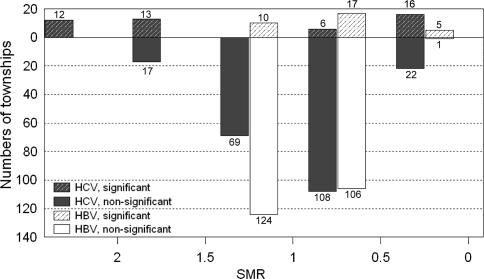
It has been observed that a recent increase in HCC in Western countries was related to HCV infections acquired 2–4 decades ago and that C-HCC incidents are likely to continue increasing over the next decade [8, 9].The same trend has also been observed in Taiwan, as reported in recent study [6], as well as noted in Japan [10]. HBV and HCV are the two major viral etiologies of HCC patients in Taiwan [6]. Our previous case-control study found that chronic HCV infection was the major cause of excess mortality from HCC in an HBV-HCV endemic area [11]. The same finding was shown later in a large-scale community-based survey [7]. The predominant etiology of male HCC was HBV. The male-to-female ratio in B-HCC was 6.4, whereas it was 1.7 in the C-HCC [6]. Hence, those areas having higher HCV-related male HCC patients could potentially be HCV endemic areas. Further analysis has revealed geographic variations among different townships with regard to anti-HCV prevalence of male HCC patients. These variations exist both among different counties and among different townships within the same county. Four different patterns were identified in different parts of Taiwan. In contrast to the fourth pattern, the first pattern indicated the areas with the greatest HCV prevalence, that is, central-western to southwestern Taiwan, including Chiayi, Yunlin, and Tainan counties. This finding correlated well with several high-incidence HCV infection areas in Taiwan reported in previous smaller studies. This was especially true in southern Taiwan [7, 11, 12]. It is interesting to note that some significantly low prevalence townships still exist in these high-incidence HCV prevalent counties. Together with the second and the third patterns, the geographical distribution strongly suggests common shared factors for those patients living in these areas. Previously identified risk factors associated with high HCV infection might include iatrogenic routes of infection. For example, local customs and medical treatment seeking behaviors, such as frequent intravenous injections for minor illnesses and incomplete disinfection of medical equipment in the past, may have contributed to the high prevalence of HCV [13, 14]. In view of the high HCV rates among male HCC patients, especially in central-western and southwestern Taiwan counties, further field investigation to search for other risk factors responsible for the transmission of this disease may still be warranted. We also tried to see whether this geographic variation existed in B-HCC patients. As shown in Fig. 2, SMR for geographic variation of B-HCC patients mostly ranged between 0.5 and 1.5. In comparison with C-HCC patients, it was much less significant (P < 0.001) (Fig. 3).
Fig. 2.
Different relative proportion of HBV associated HCC (SMR) among male HCC patients in different townships of Taiwan
Fig. 3.
Comparison of SMR among male HCC patients in different townships of Taiwan using relative proportion of HCV and HBV associated HCC
Several potential biases do exist in this study, such as the study being based on hospital cases only. Different HCV prevalence among different townships may be varied because of different transmission routes. We failed to address this issue due to lack of data of risk factors in these patients. For townships with particular high HCV prevalence, further investigation of the risk factors in these areas may still be justified to see whether there are active, common, shared risk factors that we seek to prevent. Also, the HCC patients of the clinical diagnosis group were not included in the analyses of geographic variations and exclusion of such patients might have created a potential bias. The population of patients residing in mountainous areas was usually relative small and led to inadequate enrollment and patient information. However, this integrated population is likely to represent the largest target population of male HCC patients ever reported in Taiwan. Our observations correlated well with the recently published results of the community-based screening program carried out by the Liver Disease Prevention and Treatment Research Foundation in Taiwan [15] [a wide variation in crude HCV seroprevalence was noted, ranging from 0.4 to 10.5%. The analysis of age-adjusted HCV seroprevalence revealed that Miaoli County (7.6%) had the highest HCV prevalence, followed by Chiayi County (6.1%), Chiayi City (6.0%), Yunlin County (4.8%), Tainan County (4.0%)], and provided more detailed data regarding townships’ HCV prevalence rates revealed by the larger survey. In addition to the nationwide community survey, we also conducted a massive screening of about 60,000 adult residents in Tainan County and reported the township- and village-specific prevalence [16], which proved the correlation between township HCV-prevalence and HCC mortality rate [7]. This study may therefore be worth using as a guide for further investigations of the true prevalence rates of these endemic areas.
HCV infection takes 2–4 decades to lead to HCC [8, 9]; thus, the geographic variations of male C-HCC patients do not represent the current state of infection but rather the past state of infection in those areas. This may be why we failed to demonstrate any geographical variation in metropolitan areas such as Taipei and Kaohsiung cities, because a large proportion of people living in these places come from different parts of the country and have had relatively shorter periods of residency.
Furthermore, because this study enrolled only HCC patients, we may have missed some endemic areas where HCV is a newly introduced infection. In this regard, active screening methods are still warranted to identify HCV-infected endemic areas, especially in those areas where some high-risk behaviors still exist.
Interferon-based antiviral treatment has achieved success in eradicating HCV infection in Taiwan [5] and other countries [17]. However, many people with chronic HCV infection may not realize that they carry this infection unless screening blood tests are undertaken. It is well known that the yield from targeted screening, particularly among intravenous drug users, would be substantially higher than for the general population [18]. Identifying these potential high HCV endemic townships has made these areas ideal targets for a more cost-effective screening strategy. This large cohort study may provide a guide for identifying the possible high HCV endemic areas in Taiwan as a whole and aid in further implementation of cost-effective strategy in carrying out further surveillance. Because the cost of disease surveillance and prevention is always high and financial resources are always limited, a high priority for doing survey work in these endemic areas may be more cost-effective and help the government to save money.
In conclusion, our study has designed maps to illustrate some potentially high HCV endemic townships in Taiwan. Prevention of new HCV infections and treatment of chronic hepatitis C are now major public health issues. A nationwide, multicenter study in Taiwan using interferon or interferon/ribavirin to treat chronic HCV infection had been proven to reduce HCC and improve survival rates. Pegylated interferon-based antiviral treatment is now well known to be better and more cost-effective [19] than traditional interferon therapy. Such treatment should lead to further decreases in HCC incidence in the near future, but further efforts should be made to further validate our observations.
Acknowledgments
We are indebted to our colleagues at the Department of Medical Records for their excellent work in the cancer registry system and to the physicians for their care of the patients. We also thank Chih-Yun Lin for the tedious secretarial work and the drawing of the maps.
Abbreviations
- B + C-HCC
HBV-HCV-related HCC
- B-HCC
HBV-related HCC
- C-HCC
HCV-related HCC
- HBeAg
Hepatitis B e antigen
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- NBNC-HCC
Non-HBV-non-HCV-related HCC
- SMR
Standardized mortality ratio
Footnotes
This study was supported by grants from the Department of Health, Taiwan (DOH90-TDB-03, DOH90-HP-1001, DOH90-HP-1002, and DOH90-HP-1003).
References
- 1.Raza SA, Clifford GM, Franceschi S. Worldwide variation in the relative importance of hepatitis B and hepatitis C viruses in hepatocellular carcinoma: a systematic review. Br J Cancer. 2007;96:1127–1134. doi: 10.1038/sj.bjc.6603649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni YH, Chang MH, Huang LM, et al. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med. 2001;135:796–800. doi: 10.7326/0003-4819-135-9-200111060-00009. [DOI] [PubMed] [Google Scholar]
- 3.Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children, Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 4.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395–403. doi: 10.1016/S1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 5.Yu ML, Lin SM, Chuang WL, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985–994. [PubMed] [Google Scholar]
- 6.Lu SN, Su WW, Yang SS, et al. Secular trends and geographic variations of hepatitis B virus and hepatitis C virus-associated hepatocellular carcinoma in Taiwan. Int J Cancer. 2006;119:1946–1952. doi: 10.1002/ijc.22045. [DOI] [PubMed] [Google Scholar]
- 7.Tsai MC, Kee KM, Chen YD, et al. Excess mortality of hepatocellular carcinoma and morbidity of liver cirrhosis and hepatitis in HCV-endemic areas in an HBV-endemic country: geographic variations among 502 villages in southern Taiwan. J Gastroenterol Hepatol. 2007;22:92–98. doi: 10.1111/j.1440-1746.2006.04489.x. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Taura N, Hamasaki K, Nakao K, et al. Aging of patients with hepatitis C virus-associated hepatocellular carcinoma: long-term trends in Japan. Oncol Rep. 2006;16:837–843. [PubMed] [Google Scholar]
- 11.Lu SN, Lee CM, Changchien CS, et al. Excess mortality from hepatocellular carcinoma in an HCV-endemic township of an HBV-endemic country (Taiwan) Trans R Soc Trop Med Hyg. 1999;93:600–602. doi: 10.1016/S0035-9203(99)90063-9. [DOI] [PubMed] [Google Scholar]
- 12.Lu SN, Chue PY, Chen HC, et al. Different viral aetiology of hepatocellular carcinoma between two hepatitis B and C endemic townships in Taiwan. J Gastroenterol Hepatol. 1997;12:547–550. doi: 10.1111/j.1440-1746.1997.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang CS, Chang TT, Chou P. Differences in risk factors for being either a hepatitis B carrier or anti-hepatitis C+ in a hepatoma-hyperendemic area in rural Taiwan. J Clin Epidemiol. 1998;51:733–738. doi: 10.1016/S0895-4356(98)00060-2. [DOI] [PubMed] [Google Scholar]
- 14.Sun CA, Chen HC, Lu SN, et al. Persistent hyperendemicity of hepatitis C virus infection in Taiwan: the important role of iatrogenic risk factors. J Med Virol. 2001;65:30–34. doi: 10.1002/jmv.1097. [DOI] [PubMed] [Google Scholar]
- 15.Chen CH, Yang PM, Huang GT, et al. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc. 2007;106:148–155. doi: 10.1016/S0929-6646(09)60231-X. [DOI] [PubMed] [Google Scholar]
- 16.Chen PF, Kee KM, Chen YD, et al. Village distribution and geographic variations of the prevalence of chronic hepatitis B, C and hypertransaminemia: an analysis of adult health examinations in 520 villages of Tainan County, Taiwan. J Intern Med Taiwan. 2006;17:276–290. [Google Scholar]
- 17.Brok J, Gluud LL, Gluud C. Effects of adding ribavirin to interferon to treat chronic hepatitis C infection: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2005;165:2206–2212. doi: 10.1001/archinte.165.19.2206. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Clark EC, Helfand M, et al. Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:465–479. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd J, Brodin H, Cave C, et al. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2004;8:iii–iv, 1–125 [DOI] [PubMed]