Abstract
A 71-year-old man presented to our hospital with 3-week history of fever in the background of loss of both weight and appetite over the past 3 months. He was found to have a large 10-cm mass in the right lobe of the liver on a triple-phase computed tomographic scan. The tumor showed a distinct fatty component, with areas of arterial enhancement and venous washout suggestive of hepatocellular carcinoma (HCC), another component showing progressive and late enhancement suggestive of cholangiocarcinoma (CC), and a third component showing persistent hypoenhancement relative to the liver parenchyma. He underwent surgical resection. This was histopathologically a biphasic tumor composed of areas showing hepatocytic differentiation, in contiguity with areas showing infiltrative glands set within fibrous stroma in keeping with combined hepatocellular and cholangiocarcinoma (cHCC-CC). A third component of pleomorphic spindle and epithelioid appearance in keeping with sarcomatous transformation was also found intimately related to the CC component. The patient developed extensive thoracic and abdominal metastases 2 months after surgery and died shortly after.
Keywords: Liver neoplasms, Combined hepatocellular and cholangiocarcinoma, Sarcomatoid transformation, Computed tomography (CT)
Introduction
Combined hepatocellular and cholangiocarcinoma (cHCC-CC) with sarcomatoid transformation is an extremely rare primary liver cancer. The diagnosis carries a very poor prognosis with high risk of recurrence and metastases. There are a few published case reports, but direct radiologic–pathologic correlation has not been well described. We describe a case of a patient showing the various components of this tumor on a multiphasic computed tomographic (CT) scan, correlating with the histopathologic features.
Case report
A 71-year-old Chinese man presented to our hospital with 3-week history of fever in the background of loss of both weight and appetite over the past 3 months. Physical examination was unremarkable except for low-grade fever of 37.8°C. Complete blood cell count on admission showed a raised total white blood cell count of 18 × 109/dl, and there was a mild elevation in the liver enzymes (serum alanine transaminase, 88 U/l; aspartate transaminase, 55 U/l; and alkaline phosphatase, 278 U/l). The patient’s serology was negative for viral hepatitis B or C carrier status and serum alpha-fetoprotein level (1.6 μg/l) was within normal limits.
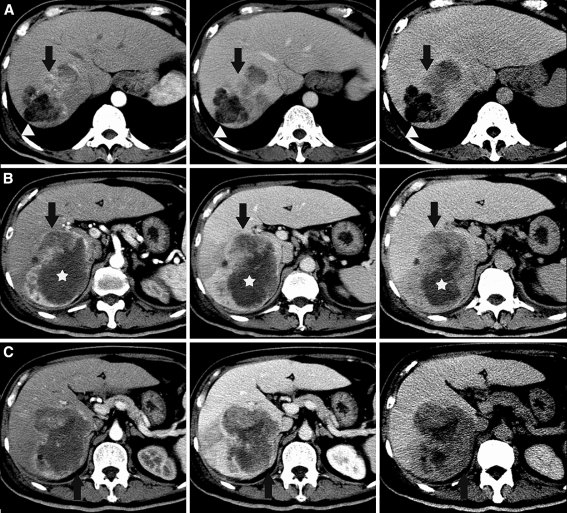
A triple-phase contrast-enhanced CT scan of the liver in the arterial, portal venous, and equilibrium phases was performed (Fig. 1A–C). This showed a lobulated multinodular tumor with several different density and enhancement patterns. At the superior portion of the tumor was a distinctly marked, hypodense fatty component measuring about −31 Hounsfield units (arrowheads, row A), with adjacent areas of arterial phase enhancement and venous and delayed phase washout (arrows, row A) suggestive of HCC. A few satellite nodules showed similar fatty density. More inferiorly, a mid-anterior component showed progressive and late enhancement (arrows, row B) suggestive of CC and a central component showed persistent hypodensity with little or no enhancement (stars, row B) in keeping with tumor necrosis. In addition, a nonspecific solid component showing persistent and slight hypoenhancement relative to the liver parenchyma (arrows, row C) was present in the subcapsular region.
Fig. 1.
Triple phase contrast-enhanced CT of the liver in the arterial, portal venous, and equilibrium phases shows a lobulated multinodular tumor with several different density and enhancement patterns. At the superior portion of the tumor was distinctly markedly hypodense fatty component (arrowheads, row A) with adjacent areas of arterial phase enhancement, and venous and delayed phase washout (arrows, row A) suspicious for hepatocellular carcinoma. A few satellite nodules showed similar fatty density. More inferiorly, a mid-anterior component showed progressive and late enhancement (arrows, row B) suggestive of cholangiocarcinoma, and a central component showed persistent hypodensity with little or no enhancement (stars, row B) in keeping with tumor necrosis. Additionally, in the subcapsular region was a non-specific solid component showing persistent and slight hypoenhancement relative to the liver parenchyma (arrows, row C)
The patient subsequently underwent successful right hemihepatectomy and cholecystectomy and was discharged well.
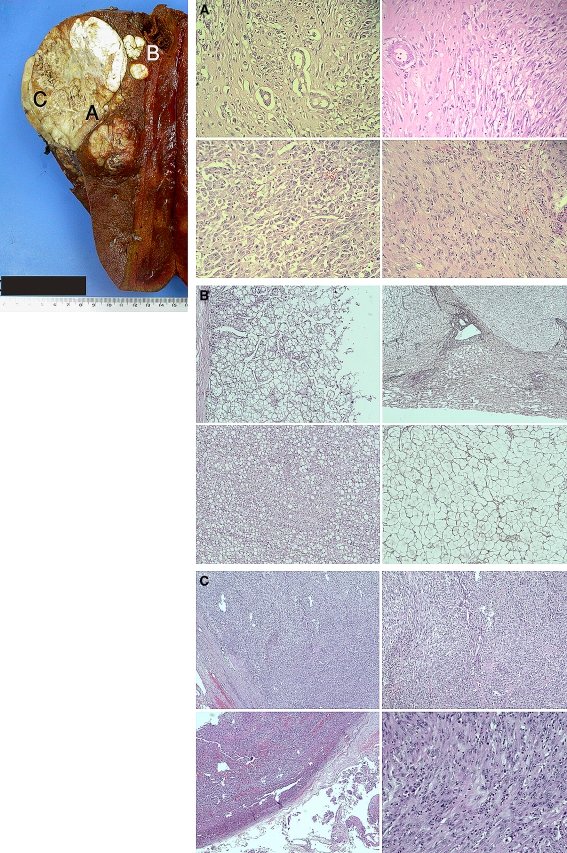
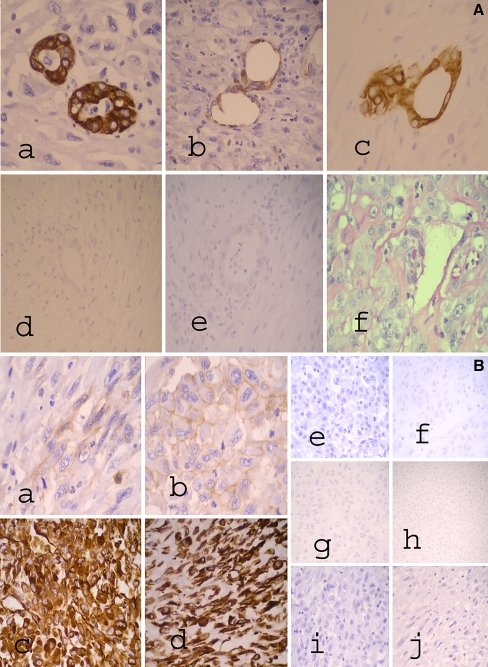
Gross and microscopic histopathologic analyses of the resected liver specimen revealed a solid, partially (50%) necrotic, biphasic tumor within a noncirrhotic liver composed of areas showing hepatocytic differentiation, in contiguity with, but independent from, areas showing infiltrative glands set within fibrous stroma in keeping with cHCC-CC (Fig. 2a, b). In particular, at the superior portion of the tumor were satellite nodules of well-differentiated Edmondson-Steiner grade II HCC elements, with cells containing fat vacuolation of the cytoplasm, loss of reticulin fibers, and focal areas with some invasion into the portal tracts (Fig. 2c). The CC component was well differentiated. A third subcapsular component of pleomorphic spindle and epithelioid cells with mitoses and necrosis in keeping with high-grade sarcomatoid transformation was also found intimately related to the CC component (Fig. 2b, d). The malignant glandular CC was focally positive for mucicarmine displaying cytoplasmic globules (Fig. 3A). On immunohistochemistry, the CC component stained positive for antikeratin monoclonal antibodies AE1/3, CAM 5.2, CK7, CK19, and CD56 (CC markers) (Fig. 3A). The CC component was negative for Glypican-3, Hepar-1 (hepatocytic markers), and CD117 (c-kit) (Fig. 3A). The dedifferentiated tumor comprising both epithelioid and spindle-cell components stained negative for epithelial markers, Hepar-1, Glypican-3, CK7, CK19, CD31, CD34, S100, smooth muscle actin, anaplastic lymphoma kinase (ALK-1), and CD117 (c-kit). The epithelioid component stained positive for CD56 and vimentin. The pleomorphic spindle-cell component was weakly positive for CD56 and strongly positive for vimentin (Fig. 3B). The relative proportions of the tumor consisted of 30% HCC, 30% CC, and 40% sarcomatoid component, the latter was composed of 30% epithelioid component and 70% pleomorphic spindle-cell component. The location of the various tumor components correlated with the CT findings.
Fig. 2.
(Top left) Gross-cut specimen of the tumor showing a large 12 cm solid and partially necrotic tan mass. Microscopic analysis of parts A, B (satellite nodules), and C of the tumor are presented in the slides (right). A Hematoxylin and eosin (H & E) stain showing malignant glands of the cholangiocarcinoma closely associated with spindle cells of the sarcomatous component (top right), epithelioid morphology (bottom left) and spindle and pleomorphic (bottom right) sarcomatous components. B H & E (left side) and Reticulin stains (right side) of the satellite nodules showing well-differentiated HCC with acinar (left, top) and trabecular (left, bottom) patterns. There is loss of reticulin and the trabeculae are more than 3 cells thick. Focal portal tract invasion was also present (not shown). C Subcapsular part of tumor which shows highly cellular fibrosarcoma-like areas and pleomorphic nuclei with mitoses. Areas of spindle cells (top right) and epithelioid cells (bottom right) are present
Fig. 3.
A Cholangiocarcinoma component with immunohistochemical and Mucicarmine stains showing (a) CK7, (b) CD56, and (c) CK19 positivity, (d) Hepar-1, and (e) Glypican-3 negativity, and (f) Mucicarmine stain showing cytoplasmic globules. B Sarcomatous component with selected immunohistochemical stains for epithelioid and spindle-cell elements. In (a), (b) CD56 shows focal weak positivity in both (a) spindle and (b) epithelioid cell elements. In (c), (d) Vimentin is strongly positive in both (c) epithelioid and (d) spindle cell elements. For epithelioid cell elements, (e) CK7 and (f) CK19 are negative, (g) Hepar-1 and (h) Glypican-3 are also negative, and (i) CD117 is negative. For spindle-cell elements, (j) CD117 is negative
The patient subsequently presented to the emergency department 6 weeks after surgery with progressively worsening shortness of breath. CT scan of the thorax and abdomen showed extensive pleural and pulmonary metastases with bilateral pleural effusions. There was also tumor recurrence at the surgical bed with extensive metastatic retroperitoneal (para-aortic, retroaortic, and aortocaval) lymphadenopathy. The patient died shortly after.
Discussion
Combined hepatocellular and cholangiocarcinoma is an uncommon form of primary liver tumor, accounting for 1–6.9% [1] of all primary liver tumors. It is defined by the presence of histologic evidence of both hepatocellular and cholangiocellular differentiation. The coexistence of hepatocellular and cholangiocellular cells as well as cells intermediate between HCC and CC suggests a common stem cell origin. Morphologically, the 2 cell types can exist in 1 of 3 ways, namely, separately (i.e., double cancer), contiguously but independently, or intermingled within a mass. Sarcomatoid transformation of cHCC-CC is extremely rare and is defined in histopathology as areas showing the proliferation of spindle-shaped cells and pleomorphic malignant cells with bizarre nuclei and abundant mitoses [1–6].
The close association of the CC component with the sarcomatoid areas and the focal CD56 positivity in the spindle and epithelioid sarcomatoid as well as CC components suggest a possible derivation of the sarcomatoid component from dedifferentiation of the CC component. CD56 positivity has been previously reported in CC and sarcomatoid HCC [7]. CD56 has been reported in 2 cases of carcinosarcomas of the biliary tract [8, 9]. Other neuroendocrine markers such as synaptophysin and chromogranin have also been reported to be positive in liver carcinosarcomas. We believe that CD56 positivity has not been reported in cHCC-CC with sarcomatoid change in the liver before. Whether CD56 positivity truly represents a cholangiocytic line of differentiation or possible stem cell derivation remains to be further studied.
It has been suggested that tumor size or number is not a good biologic indicator for cHCC-CCs and that histologic subgrouping based on the presence of a sarcomatoid component and the extent of the CC component reflects more accurately the biologic behavior of cHCC-CC [1]. Furthermore, it has been shown that sarcomatoid transformation is associated with extensive metastases [1, 3] and this was illustrated in our patient.
The use of multiphasic CT in the diagnosis of HCC and mass-forming CC is well-documented, and differentiation by evaluating the temporal relation of contrast enhancement is now routinely practiced. Similarly, preoperative diagnosis of cHCC-CC based on the identification of the enhancement characteristics of the individual tumor components is possible [10–12]. The HCC component typically with avid hepatic arterial supply often shows early phase (arterial) enhancement and low attenuation (“washout”) in the later venous and equilibrium phases. If other features such as fatty metamorphosis are demonstrated, the diagnosis of HCC component is strengthened. The CC component conversely shows classic low attenuation in the arterial phase, with increased and persistent enhancement in the venous to late phase due to the abundance of fibrous stroma from desmoplastic reaction.
To the best of our knowledge, the enhancement characteristics of the sarcomatoid areas within cHCC-CC have not been previously described. In our patient, an area of nonspecific hypovascular contrast enhancement was seen in the subcapsular region correlating in location to the histolopathologic finding of sarcomatoid area. This pattern of enhancement follows the usual pattern typically seen in hypovascular liver metastastic lesions more commonly encountered in daily radiologic practice. This is likely due to poor contrast uptake by the sarcomatoid tissue in any phase compared with both the normal liver and the HCC or CC component. However, this pattern of enhancement is not specific for sarcomatous change. The relative proportions of HCC and CC components and the presence of intermediate cells may also cause nonclassic enhancement patterns, even in cases of cHCC-CC without sarcomatous change, for which various types of enhancement pattern have been reported [12].
Conclusion
Sarcomatoid transformation in cHCC-CC is very rare and bears a poor prognosis. We herein describe the radiologic and histopathologic features of a case of a patient with the various tumor components showing good correlation especially for the HCC and CC components, whereas other areas showing enhancement or features atypical for HCC or CC components, while nonspecific, may raise the possibility of sarcomatous transformation. The histopathology shows clear-cut cHCC-CC features, with close association of the CC and the sarcomatous areas. Furthermore, the expression of CD56 in both the CC and sarcomatoid components suggests that sarcomatoid change arose from the CC component.
Contributor Information
Uei Pua, Email: druei@yahoo.com.
Su-Chong Low, Email: albert.low.s.c@sgh.com.sg.
Yu-Meng Tan, Email: tan.yu.meng@sgh.com.sg.
Kiat-Hon Lim, Email: lim.kiat.hon@sgh.com.sg.
References
- 1.Aishima S, Kuroda Y, Asayama Y, et al. Prognostic impact of cholangiocellular and sarcomatous components in combined hepatocellular and cholangiocarcinoma. Hum Pathol. 2006;37:283–291. doi: 10.1016/j.humpath.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Papotti M, Sambataro D, Marchesa P, et al. A combined hepatocellular/cholangiocellular carcinoma with sarcomatoid features. Liver. 1997;17:47–52. doi: 10.1111/j.1600-0676.1997.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 3.Jeong BJ, Hyun DH, Lee KW, et al. A case of sarcomatoid combined hepatocellular-cholangiocarcinoma. Korean J Gastroenterol. 2004;43:56–60. [PubMed] [Google Scholar]
- 4.Kim JH, Lee YG, Lee J. A case of combined hepatocellular-cholangiocarcinoma with sarcomatoid transformation and second primary colon cancer. Korean J Hepatol. 2004;10:142–147. [PubMed] [Google Scholar]
- 5.Nakajima T, Kubosawa H, Kondo Y, Konno A, Iwama S. Combined hepatocellular-cholangiocarcinoma with variable sarcomatous transformation. Am J Clin Pathol. 1988;90:309–312. doi: 10.1093/ajcp/90.3.309. [DOI] [PubMed] [Google Scholar]
- 6.Boonsakan P, Thangnapakorn O, Tapaneeyakorn J, Kositchaiwat S, Bunyaratvej S. Case report combined hepatocellular and cholangiocarcinoma with sarcomatous transformation. J Med Assoc Thai. 2007;90(3):574–580. [PubMed] [Google Scholar]
- 7.Cho MS, Lee SN, Sung SH, Han WS. Sarcomatoid hepatocellular carcinoma with hepatoblastoma-like features in an adult. Pathol Int. 2004;54:446–450. doi: 10.1111/j.1440-1827.2004.01640.x. [DOI] [PubMed] [Google Scholar]
- 8.She R, Szakacs J. Carcinosarcoma of the liver: a case report and review of the literature. Arch Pathol Lab Med. 2005;129(6):790–793. doi: 10.5858/2005-129-790-COTLAC. [DOI] [PubMed] [Google Scholar]
- 9.Sodergren MH, Silva MA, Read-Jones SL, Hubscher SG, Mirza DF. Carcinosarcoma of the biliary tract: two case reports and a review of the literature. Eur J Gastroenterol Hepatol. 2005;17(6):683–685. doi: 10.1097/00042737-200506000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Nishie A, Yoshimitsu K, Asayama Y, et al. Detection of combined hepatocellular and cholangiocarcinomas on enhanced CT: comparison with histologic findings. Am J Roentgenol. 2005;184:1157–1162. doi: 10.2214/ajr.184.4.01841157. [DOI] [PubMed] [Google Scholar]
- 11.Aoki K, Takayasu K, Kawano T, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology. 1993;18:1090–1095. doi: 10.1002/hep.1840180512. [DOI] [PubMed] [Google Scholar]
- 12.Fukukura Y, Taguchi J, Nakashima O, et al. Combined hepatocellular and cholangiocarcinoma: correlation between CT findings and clinicopathological features. J Comput Assist Tomogr. 1997;21:52–58. doi: 10.1097/00004728-199701000-00011. [DOI] [PubMed] [Google Scholar]