Abstract
Purpose
We aimed to study the role of N-acetylcysteine (NAC) in non-acetaminophen-induced acute liver failure (NAI-ALF).
Methods
A total of 47 adult patients were prospectively enrolled with NAI-ALF (group 1 or NAC group) and oral NAC was given. The primary outcome was reduction in mortality with the use of NAC in NAI-ALF. The secondary outcomes were to evaluate safety of NAC and to assess factors predicting mortality. We compared these results with records of NAI-ALF patients admitted in our hospital from 2000 to 2003 (n = 44) who were not given NAC (group 2 or historical controls).
Results
The two groups were comparable for the etiology of ALF, prothrombin time (PT), alanine aminotransferase, creatinine, albumin, etc. The mean age in group 1 was 27.7 ± 11.8 years and in group 2 37.5 ± 18.8 years (P = 0.004). Bilirubin was 20.63 ± 11.03 and 14.36 ± 8.90 mg/dl in groups 1 and 2, respectively (P = 0.004). There were 8 (17%) and 1 (2.3%) pregnant ALF women with acute hepatitis E virus (HEV) infection in groups 1 and 2, respectively (P = 0.031). All patients were given supportive care, including mechanical ventilation. A total of 34 (37.36%) patients survived; 22 (47%) in group 1 (NAC group) and 12 (27%) in group 2 (controls) (P = 0.05). On multivariable regression analysis, patients not given NAC (odds ratio [OR] = 10.3, 95% confidence interval [CI] = 1.6–65.7), along with age older than 40 years (OR = 10.3, 95% CI = 2.0–52.5), PT more than 50 s (OR = 15.4, 95% CI = 3.8–62.2), patients requiring mechanical ventilation (OR = 20.1, 95% CI = 3.1–130.2), and interval between jaundice and hepatic encephalopathy (OR = 5.0, 95% CI = 1.3–19.1) were independent predictors of mortality.
Conclusions
The use of NAC causes reduction in NAI-ALF mortality and its use was safe.
Keywords: Liver failure, Acute, Mortality, Acetylcysteine, Prospective/retrospective studies, Viral, Survival rate
Background
Acute liver failure (ALF) is a rare but severe, life-threatening, multisystemic medical emergency. It is defined as the rapid development of acute liver injury with impaired synthetic function and encephalopathy in a person who previously had a normal liver. O’Grady et al. [1, 2] classified ALF into hyperacute, acute, and subacute liver failure on the basis of encephalopathy less than 7, 8–28, and more than 28 days but less than 26 weeks, respectively, from the onset of jaundice. Hyperacute and acute ALF have better prognosis in terms of survival than that in subacute liver failure.
The role of N-acetylcysteine (NAC), a glutathione precursor, in the treatment of acetaminophen-induced ALF is well established [3]. NAC prevents toxicity by limiting the formation and accumulation of N-acetyl-p-benzoquinone-imine and acts as a glutathione substitute and enhances nontoxic sulfate conjugation [4, 5]. Its anti-inflammatory, antioxidant, inotropic, and vasodilating effects improve microcirculatory blood flow and oxygen delivery to vital organs [6, 7]. NAC has shown to improve cerebral perfusion pressure in randomized controlled trail (RCT) conducted on pigs with ALF [8]. NAC has been used successfully in patients with acetaminophen-induced ALF within 24 h and even when it was administered after 24 h of paracetamol ingestion. Smilkstein et al. [9] and Harrison et al. [10] suggested that in cases when NAC is used after 24 h, it is helpful because of the antioxidant effect rather than its antidote effect. Because of its multiple mechanisms of action, NAC has also been used in small trials in non-acetaminophen-induced ALF (NAI-ALF) with variable results [11, 12]. Liver transplantation has made significant impact on survival of patients with ALF [13, 14]. This option is available only in limited centers of developed countries and better alternatives must be explored in developing and underdeveloped world for the management of ALF where transplant facility is not available. Outcome of ALF in terms of survival is poor in patients with NAI-ALF without liver transplant facility [15]. Recently, in a nonrandomized study, NAC use was reported to be associated with survival benefits in children with NAI-ALF [16].
We started administering NAC in patients with NAI-ALF because of the evidence of obvious efficacy of NAC in paracetamol-induced ALF and with some evidence of its role in NAI-ALF in the absence of liver transplant facility in our hospital. We used NAC in a non-RCT comparing historical controls at the end of 2003 in our hospital because of its multifactorial mode of action and a good safety profile.
The primary outcome of our study was to assess the reduction in ALF-induced mortality with the use of NAC in patients with NAI-ALF. The secondary outcomes were to evaluate the safety of NAC and also finding the factors predicting the survival in patients with NAI-ALF.
Patients and methods
This is a prospective study with historical controls of adult patients with NAI-ALF admitted at Aga Khan University Hospital, Section of Gastroenterology, from 2000 to 2007. Our hospital database for ALF cases was introduced in 2000, and the records of ALF cases were collected from this source for studying the demographic features and outcomes of ALF for historical controls. The cases prior to 2000 were not retrievable because there is no specific ICD code for ALF. We started administering NAC in our patients with NAI-ALF from January 2004.
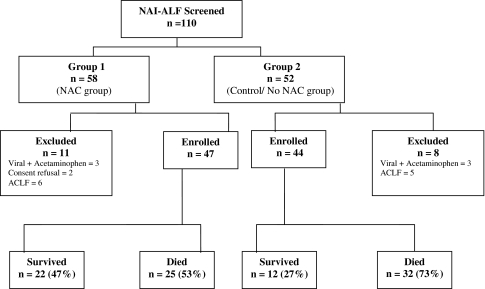
Overall 110 patients were admitted from 2000 to 2007, and 19 were excluded because of different reasons including viral ALF along with history of acetaminophen intake (n = 6) or acute-on-chronic liver disease (n = 11); in two cases, patients’ attendant refused to give consent for NAC administration. The included patients were divided into two study groups: group 1 (2004–2007) included patients prospectively enrolled and treated with NAC (n = 47) and group 2 (2000–2003) included patients not treated with NAC (n = 44). Data were retrospectively collected from the hospital’s ALF database and were double-checked after reviewing the medical records. Figure 1 shows patients enrolment in the two treatment arms. Forty-seven patients with NAI-ALF who were administered NAC were prospectively enrolled from January 2004 till March 2007, whereas the historical control (group 2) included 44 consecutive adult patients with NAI-ALF admitted from January 2000 to December 2003.
Fig. 1.
Study enrolment algorithm. NACN-acetylcysteine, ACLF acute on chronic liver failure; NAI-ALF non-acetaminophen-induced acute liver failure
ALF was defined as the rapid development of acute liver injury with impaired synthetic function and encephalopathy in a person who previously had a normal liver. After informed written consent was obtained from next of kin, the patients in group 1 were administered oral NAC at a dose of 140 mg/kg, followed by 70 mg/kg, for a total of 17 doses, 4 h apart within 6 h of admission. All patients were managed with the standard supportive care treatment, which was similar throughout the study period in both the study groups. The patients received treatment of and prevention for the complications of ALF [17]. The treatment mainly involved continuous intravenous dextrose administration to prevent hypoglycemia; broad-spectrum prophylactic antimicrobials to prevent bacterial infections and antifungals when indicated, and proton pump inhibitors for stress-related ulcers. With the development of advanced hepatic encephalopathy, intensive care was provided with hemodynamic monitoring, fluid and electrolyte balance, ventilatory support, and propofol sedation. Fresh frozen plasma was given in only those cases in which patients had spontaneous bleed or required an invasive procedure, such as a central venous or arterial catheter, etc. Nasogastric tube was inserted for feeding purpose in patients with encephalopathy or for checking upper gastrointestinal (GI) bleed whenever needed [17].
At the time of admission, blood samples of all the patients were taken for the etiological diagnosis of ALF, including hepatitis B surface antigen (HBsAg), hepatitis B core IgM (HBc-IgM), hepatitis A virus IgM (HAV-IgM), and hepatitis E virus IgM (HEV-IgM), and serum samples were also saved for further testing if the etiology was not detected at these initial tests, which included antinuclear antibody, liver kidney microsomal antibody, ceruloplasmin, HSV antibody, and EBV. Hepatitis D antibody was sent for testing if HBsAg or HBc-IgM was positive to detect HDV super- or coinfection as an etiology of ALF. History was also noted for any hepatotoxic drug intake, including homeopathic and herbal medications; pregnancy was checked in female patients of childbearing age with the help of β-HCG level and, later on, an ultrasound was also performed.
Renal impairment was defined as serum creatinine level of more than 2.0 mg/dl. Hemodialysis was performed whenever indicated. Intracranial hypertension was diagnosed clinically in the presence of clinical signs such as abnormal papillary reflexes or hypertonia. The intracranial bolts were not inserted to measure the intracranial pressure (ICP) in all these patients because it was not approved by our hospital ethics review committee (ERC). Intracranial hypertension was treated with mannitol and/or acute short-term hyperventilation, but if the patient was refractory to treatment, mild-moderate hypothermia was used with a cooling blanket [18]. Infection was diagnosed only when blood, urine, or tracheal aspirates were cultured for pathogenic microorganisms.
Primary outcome measure was an overall survival with NAC as compared with standard supportive treatment in the absence of the liver transplantation. Secondary outcome measure was to evaluate the factors related to the survival and safety of NAC in patients with NAI-ALF. A formal approval of this study was taken from our hospital ERC before the collection of data and assigning the patients for receiving NAC.
Statistical analysis
Patients were analyzed in the two groups. Frequency distribution was assessed in terms of means ± 1 SD for quantitative variables and number (percentages) for categorical variables. In univariate analysis, the categorical variables were compared in the two groups by using χ2 test or Fisher exact test where appropriate. For continuous variables, the independent sample t test was used to compare the means in the two groups.
Multiple parameters were compared in survivors and nonsurvivors in a univariate analysis. The factors on univariate analysis with P < 0.25 or any biological significant factors were entered into a stepwise logistic regression analysis to identify independent variables of prognosis. In the multivariable logistic regression analysis, factors with P < 0.05 were taken significant to be in the final model.
All the analyses were performed by the Statistical Package for Social Sciences (SPSS; Release 13, standard version, copyright SPSS; 2004).
The study sample size was calculated considering the mortality in NAI-ALF without liver transplantation ranges from 74% to 88% and hence we took mortality with supportive care as 84% (historical control group). For the NAC group, the mortality was taken as 57% as reported in previous NAI-ALF trial using NAC [11]; with 5% level of significance and 80% study power, the sample size calculated was 44 patients in each arm. All P values were two sided.
Results
There were 47 patients in group 1 (NAC group) and 44 in group 2 (historical control group). The mean age of patients in group 1 was 27.7 ± 11.8 years and in group 2 37.5 ± 18.8 years (P = 0.004). Bilirubin level at the time of admission was 20.6 ± 11.0 mg/dl versus 14.3 ± 8.9 mg/dl (P = 0.004). The other features such as serum albumin, alanine aminotransferase, and creatinine levels, ascites, gender distribution, and the interval between jaundice and development of encephalopathy were similar in the two groups, but there were more pregnant women in group 1 that received NAC than in group 2. The baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of study subjects in the two treatment groups
| Characteristics | N-Acetylcysteine group (group 1, n = 47) | Control group (group 2, n = 44) | P |
|---|---|---|---|
| Male gender | 26 (55.3%) | 24 (54.5%) | 0.941 |
| Age (years) | 27.74 ± 11.79 | 37.52 ± 18.82 | 0.004 |
| Prothrombin time (s) | 59.55 ± 36.07 | 53.05 ± 30.57 | 0.357 |
| Bilirubin (mg/dl) | 20.63 ± 11.03 | 14.36 ± 8.90 | 0.004 |
| Serum albumin (mg/dl) | 2.73 ± 0.55 | 2.70 ± 0.75 | 0.820 |
| Alanine aminotransferase (mg/dl) | 1926 ± 1374.2 | 1457.2 ± 1467.8 | 0.342 |
| Interval between jaundice and hepatitis E (days) | 8.87 ± 9.47 | 13 ± 18.24 | 0.175 |
| Creatinine at admission (mg/dl) | 1.39 ± 0.82 | 1.57 ± 0.90 | 0.312 |
| Presence of ascites | 10 (21.3%) | 16 (36.4%) | 0.111 |
| Pregnancy with acute hepatitis E | 8 (17%) | 1 (2.3%) | 0.031a |
| Hepatic encephalopathy | |||
| Grade I | 6 (12.8%) | 6 (13.6%) | |
| Grade II | 9 (19.1%) | 18 (40.9%) | 0.128 |
| Grade III | 15 (31.9%) | 9 (20.5%) | |
| Grade IV | 17 (36.2%) | 11 (25.0%) | |
aFisher exact test
We categorized the parameters according to the variable used in the King’s College Hospital (KCH) criteria (n = 10) in the two study groups as shown in Table 2. There were 24 (51%) patients with bilirubin levels of more than 17.6 mg/dl in group 1 versus 11 (25%) in group 2 (P = 0.011). Similarly, there were 42 (89.3%) patients in group 1 younger than 40 years as compared with 28 (63.6%) in group 2 (P = 0.004). The interval between jaundice and development of hepatic encephalopathy (≤7 or >7 days) and prothrombin time (PT, in seconds) were similar in the two groups (Table 2).
Table 2.
King’s College Hospital prognostic criteria-related factors in the two groups
| Characteristics | N-Acetylcysteine group (group 1; n = 47) | Control group (group 2; n = 44) | P |
|---|---|---|---|
| Prothrombin time (s) | |||
| <50 | 23 (48.9%) | 25 (56.8%) | |
| >50 | 24 (51.1%) | 19 (43.2%) | 0.452 |
| Bilirubin (mg/dl) | |||
| ≤17.6 | 23 (48.9%) | 33 (75%) | |
| >17.6 | 24 (51.1%) | 11 (25%) | 0.011 |
| Age (years) | |||
| ≤40 | 42 (89.36%) | 28 (63.63%) | |
| >40 | 5 (10.64%) | 16 (36.37%) | 0.004 |
| Interval between jaundice and hepatitis E (days) | |||
| ≤7 | 30 (63.8%) | 24 (54.5%) | |
| >7 | 17 (36.2%) | 20 (45.5%) | 0.368 |
Majority of patients in both the group had ALF due to acute HEV infection; 24 (51%) patients in group 1 and 16 (37%) in group 2 had ALF due to acute HEV infection (P = 0.186). Of these HEV-infected patients, 8 (17%) were pregnant women in group 1 and 1 (2.3%) in group 2 (P = 0.03; Fisher exact test). There were 11 (23.4%) and 14 (31.8%) acute HBV-related ALF cases in groups 1 and 2, respectively. Of these acute HBV (HBc-IgM positive) patients, 3 (6.4%) patients in group 1 and 2 (4.5%) patients in group 2 tested positive for HDV and were categorized as having HDV coinfected ALF. There were no patients with HDV superinfection (defined as HDV antibody-positive patients with HBc-IgM negative and HBsAg positive) in group 1, and there were 6 patients with HDV superinfection in group 2. There were 3 (6.4%) patients in group 1 and 8 (18.2%) in group 2 (P = 0.084) who had ALF induced by antituberculosis treatment (including rifampicin, isoniazid, and/or pyrazinamide). The etiology of ALF in two groups is elaborated in Table 3.
Table 3.
Etiology of ALF in the two treatment groups
| Characteristics | N-Acetylcysteine (group 1; n = 47) | Control group (group 2; n = 44) | P |
|---|---|---|---|
| Acute hepatitis A (HAV-IgM +ve) | 2 (4.3%) | 0 (0%) | 0.495a |
| Acute HBV (HBc-IgM +ve) | 11 (23.4%) | 14 (31.8%) | 0.369 |
| HDV coinfection | 3 (6.4%) | 2 (4.5%) | 1.000a |
| HDV superinfection | 6 (4.5%) | 0 (0%) | 0.026a |
| Acute hepatitis E (HEV-IgM +ve) | 24 (51.1%) | 16 (37.2%) | 0.186 |
| Drug-induced ALF (ATT, but not acetaminophen) | 3 (6.4%) | 8 (18.2%) | 0.084 |
| Acute fatty liver of pregnancy | 0 (0%) | 2 (4.5%) | 0.264 |
| Undetermined etiology | 1 (2.1%) | 4 (9.1%) | 0.193a |
aFisher’s exact test
A total of 56 (61.5%) patients were shifted to intensive care unit; of these, 52 (57.1%) patients required ventilator support, 36 (76.6%) belonged to group 1, and 16 (36.4%) in group 2 (P < 0.001). The mean number of days of admission in hospital in group 1 was 9.4 ± 7.26 versus 8.39 ± 9.55 in group 2 (P = 0.56).
Other supportive treatment given in the two groups is shown in Table 4. Complications noted during hospital course included different infections in 25 (53.2%) patients in group 1 versus 22 (50%) patients in group 2 (P = 0.761). A positive blood culture and sensitivity (C/S) result was reported in 5 (10.6%) patients versus 6 (13.6%) patients (P = 0.209); urine C/S in 9 (20.5%) versus 12 (25.5) in groups 1 and 2, respectively (P = 0.561), and tracheal aspirate C/S grew organisms in 21 (44.7%) in group 1 versus 13 (29.5%) in group 2 (P = 0.136). The renal impairment was observed in 18 (40.9%) patients in group 1 versus 17 (36.2%) patients in group 2 (P = 0.642); signs of raised ICP, that is, dilated or unequal papillary reaction, were observed in 24 (51.1%) and 15 (34%) patients in groups 1 and 2, respectively. Thirty-five (74.5%) patients in group 1 and 14 (31.8%) patients in group 2 were hyperventilated to decrease their intracranial hypertension; 10 (21.3%) patients were subjected to cooling blanket in group 1 versus 4 (9.1%) patients in group 2 (P = 0.107). Mannitol was used for signs of raised ICP at some stage during the hospital course in 43 (91.5%) patients and 24 (54.5%) patients in groups 1 and 2, respectively (P < 0.001); seizures were observed in 27 (57.4%) patients in group 1 versus 10 (22.7%) patients in group 2 (P < 0.001); minor GI bleeding (defined as coffee ground aspirate in nasogastric tube without any hemodynamic change) in 18 (38.3%) patients in group 1 versus 8 (18.2%) patients in group 2 (P = 0.034).
Table 4.
Hospital course of non-acetaminophen-induced ALF in the two groups
| Characteristics | N-Acetylcysteine (group 1; n = 47) | Control group (group 2; n = 44) | P |
|---|---|---|---|
| Renal failure | 9 (19.1%) | 11 (25%) | 0.50 |
| Development of ascites | 10 (21.3%) | 17 (38.6%) | 0.071 |
| Mechanical ventilation | 36 (76.6%) | 16 (36.4%) | <0.001 |
| Infections | 25 (53.2%) | 22 (50%) | 0.761 |
| Features of raised intracranial pressure | |||
| Dilated/unequal pupils | 24 (51.1%) | 15 (34.1%) | 0.102 |
| Seizures activity | 27 (57.4%) | 10 (2.7%) | <0.001 |
| Use of mannitol | 43 (91.5%) | 24 (54.5%) | <0.001 |
| Hyperventilation | 35 (74.5%) | 14 (31.8%) | <0.001 |
| Cooling blanket | 10 (21.3%) | 4 (9.1%) | 0.107 |
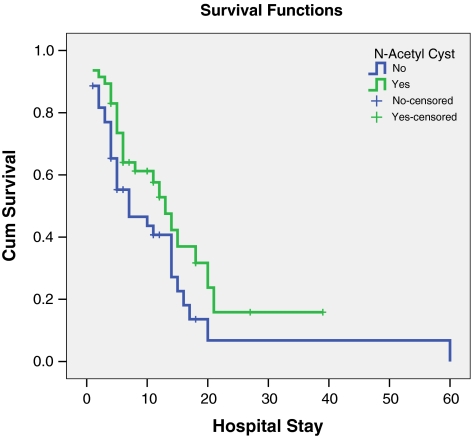
A total of 57 of 91 (62.6%) patients died with ALF complications; 25 (53.2%) patients belonged to group 1 and 32 (72.7%) patients to group 2 (P = 0.05). A comparison of overall survival and length of hospital stay in the two groups is shown in Fig. 2. It suggested a statistically significant survival of patients in the group receiving NAC. The causes of death were sepsis/multiorgan failure in 12 (54.5%) patients versus 22 (75.9%) patients in groups 1 and 2; raised ICP in 7 (31.8%) patients versus 6 (20.7%) patients.
Fig. 2.
Survival and length of hospital stay in the two groups. * P = 0.06 (Log-Rank test)
In the univariate analysis for the mortality in patients with NAI-ALF, age older than 40 years, male gender, GI bleeding during hospital course, development of ascites, PT more than 50 s, infections, use of mannitol, seizures during hospital stay, not using NAC, patients on mechanical ventilator, and the interval between jaundice and onset of hepatic encephalopathy of more than 7 days were significant factors (P < 0.25). These factors were taken for stepwise multiple variable logistic regression analysis to predict the factors responsible for the outcome variables of mortality in the two groups. In the final model, age older than 40 years, PT more than 50 s, not using NAC, patients on mechanical ventilator, and the duration between jaundice and onset of hepatic encephalopathy of more than 7 days were the independent prognostic factors predicting mortality in our study. The odds ratio and 95% confidence interval of the final model are shown in Table 5.
Table 5.
Independent risk factor for mortality in patients with non-acetaminophen-induced ALF
| Factors | Adjusted odds ratio | 95% confidence interval for adjusted odds ratio | Wald P |
|---|---|---|---|
| Age (years) | |||
| ≤40a | |||
| >40 | 10.3 | 2.0–52.3 | 0.005 |
| N-Acetylcysteine | |||
| Givena | |||
| Not given | 10.3 | 1.6–65.7 | 0.014 |
| Type of FHF | |||
| Hyperacute liver failurea | |||
| Acute liver failure | 5.03 | 3.1–130.3 | 0.018 |
| Prothrombin time group (s) | |||
| ≤50a | |||
| >50 | 15.6 | 3.6–67.9 | <0.001 |
| Requirement of ventilator | |||
| Not ventilateda | |||
| Ventilated | 20.1 | 3.1–130.2 | 0.002 |
aReference category
FHF fulminant hepatic failure
Safety of NAC
NAC was administered orally in 47 patients in this study. Adverse effects were noted in 6 (12.7%) patients within 4 h after NAC administration. These were nonspecific maculopapular rash in 2 patients, which resolved without any treatment within 24 h; 1 patient had transient bronchospasm, which responded to salbutamol nebulization; and 4 patients had vomiting, which was attributed to the deteriorating condition due to ALF.
Discussion
There are limited data addressing NAI-ALF and the use of NAC. Moreover, there is no reported RCT at the moment checking the efficacy of NAC against placebo in these patients. In this report of 91 patients with NAI-ALF admitted to a center without the facility of liver transplantation, we found that NAC administration was effective in reducing mortality in 47 patients as compared with 41 historical controls. We also found that the use of NAC was safe in these patients. The two groups were comparable in majority of clinical and biochemical parameters, except that the patients in NAC group, on the one hand, were younger, but, on the other hand, they had worse prognostic factors at their baseline and even then their survival was better. The main etiology of ALF was acute hepatitis E followed by acute hepatitis B, and they were equally distributed in the two groups of patients, although there were more pregnant HEV patients in the NAC group. Acute HEV infection is seen commonly in the third-world country where waterborne infections are common. Khuroo et al. [19] also reported HEV as the most common etiology in patients with ALF from Kashmir, India.
Distribution of different complications of ALF, such as renal impairment, and development of ascites and infections were similar in the two groups. There were significantly more patients with raised ICP in group 1 (NAC group) than in historical control. There were more patients needing mechanical ventilation (78%) in the NAC group due to raised ICP manifested by seizures, variable pupillary reactions, and hyperventilation. There is a possibility that the raised ICP was picked up earlier in the NAC group patients who were admitted from 2004 to 2007 in the hospital because of the improvement in the understanding of the disease.
In ALF, oxygen supply and utilization are impaired by changes in vascular tone at the cellular level. The use of NAC has been shown to increase oxygen utilization in the microcirculation [20]. NAC may act by enhancing the effect of nitric oxide on guanylate cyclase, increasing the formation of cyclic 3′,5′-guanosine monophosphate, and thereby resulting in vasodilatation.
In our study, on the one hand, most patients in the NAC group were younger, but, on the other hand, they had poor prognostic indicators of high bilirubin, HEV with pregnancy [21], and signs of raised ICP needing ventilation, suggesting their critical condition. When we compared our predictors of mortality with KCH criteria for NAI-ALF [22], they were similar except that in our model, high bilirubin level was not a predictor of mortality and not using NAC was a predictor of mortality. The predictors of mortality were similar to those from the study of Khuroo et al. [19] in a setting without the facility of liver transplantation. In our study, the mortality has decreased to 53% with the use of NAC versus 73% in historical control group (P = 0.05); similarly, Khuroo et al. [19] reported mortality without liver transplantation in their study at 72.8%, in which only supportive treatment was offered, which was comparable with our control group, in which only supportive treatment was offered.
One recently published study of NAC use in NAI-ALF in children [16] reported that its use was associated with a shorter length of hospital stay, in which survival with native liver occurred in 22% with supportive care versus 43% with NAC and its use was associated with higher incidence of native liver recovery without transplantation. The results of this study are comparable with our results, in which the survival in the supportive care group was 27% versus 47% in the NAC group.
Strength and limitations of the study
The study has the potential to be clinically relevant because our site has no liver transplant options, so it gives a proper picture of the results of changing/adding a new therapy. In patients with ALF, especially those in a center without access to liver transplantation or for patients who are inappropriate candidates for liver transplantation, mortality is extremely high; therefore, showing a mortality benefit with the use of NAC would be useful, given the relatively low cost and minimal reported adverse effects of this medication. Because NAC has had many proposed effects on liver metabolism, showing efficacy in NAI-ALF may help elucidate its true mechanism of action.
The main shortcoming of our study is its design, that is, prospective study with historical control, which is intrinsically flawed in that the groups being compared are not treated at same time in point. Advances have been made from 2000 to 2007 in the management of acutely ill patients, such as improved strategies for infection control, better use of early goal-directed therapy, and better ventilator management strategies. Patients in the historical control cohort may not have had access to benefit from these advances as the intervention cohort (NAC group), which would leave the historical control cohort (no NAC group) disadvantaged in achieving good outcomes. Although the two groups were not comparable, the data suggest that group 1 had more severe disease than group 2. NAC group has sicker patients as evidenced by the fact that there were more pregnant HEV patients, much higher bilirubin levels, and higher incidence of raised ICP and PT in them. Nevertheless, non-NAC group was considerably older and worse outcomes happen with older patients. Because of these limitations, we tried to control the confounders with multivariable regression analysis.
To our knowledge, two RCTs of NAC in NAI-ALF are underway, one at the University of Pittsburgh and other at KCH, London, and their results will be interesting in the light of our report. It seems that NAC may be beneficial in NAI-ALF, and until the results of the ongoing RCT are available, we may continue to use NAC in patients with NAI-ALF, particularly in centers without the facility of liver transplantation because it was found safe and decreased mortality.
Conclusions
Our results showed that NAC use is associated with a reduction in NAI-ALF mortality and was safe to use. The factors predicting mortality in NAI-ALF without liver transplantation were age older than 40 years, PT more than 50 s, requirement of mechanical ventilator, not using NAC, and the interval between jaundice and development of HEV infection of more than 7 days.
Contributor Information
Khalid Mumtaz, Email: khalid.mumtaz@aku.edu.
Zahid Azam, Email: zahid.azam@aku.edu.
Saeed Hamid, Email: saeed.hamid@aku.edu.
Shahab Abid, Email: shahab.abid@aku.edu.
Sadik Memon, Email: sadik369@hotmail.com.
Hasnain Ali Shah, Email: Hasnain.alishah@aku.edu.
Wasim Jafri, Email: Wasim.jafri@aku.edu.
References
- 1.O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 2.O’Grady JG, Williams R. Classification of acute liver failure. Lancet. 1993;342(8873):743. doi: 10.1016/0140-6736(93)91736-6. [DOI] [PubMed] [Google Scholar]
- 3.Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW, Rumack BH. Acetaminophen overdose: a 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20(10):1058–1063. doi: 10.1016/S0196-0644(05)81352-6. [DOI] [PubMed] [Google Scholar]
- 4.Burgunder JM, Varriale A, Lauterburg BH. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36(2):127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- 5.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60(1):6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison P, Wendon J, Williams R. Evidence of increased guanylate cyclase activation by acetylcysteine in fulminant hepatic failure. Hepatology. 1996;23(5):1067–1072. doi: 10.1002/hep.510230520. [DOI] [PubMed] [Google Scholar]
- 7.Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324(26):1852–1857. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 8.Ytrebo LM, Korvald C, Nedredal GI, Elvenes OP, Nielsen Grymyr OJ, Revhaug A. N-Acetylcysteine increases cerebral perfusion pressure in pigs with fulminant hepatic failure. Crit Care Med. 2001;29(10):1989–1995. doi: 10.1097/00003246-200110000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319(24):1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 10.Harrison PM, Keays R, Bray GP, Alexander GJ, Williams R. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335(8705):1572–1573. doi: 10.1016/0140-6736(90)91388-Q. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Ari Z, Vaknin H, Tur-Kaspa R. N-Acetylcysteine in acute hepatic failure (non-paracetamol-induced) Hepatogastroenterology. 2000;47(33):786–789. [PubMed] [Google Scholar]
- 12.Katoonizadeh A, Decaestecker J, Wilmer A, Aerts R, Verslype C, Vansteenbergen W, et al. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int. 2007;27(3):329–334. doi: 10.1111/j.1478-3231.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23(3):217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 14.Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5(1):29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 15.Escorsell A, Mas A, de la Mata M. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl. 2007;13(10):1389–1395. doi: 10.1002/lt.21119. [DOI] [PubMed] [Google Scholar]
- 16.Kortsalioudaki C, Taylor RM, Cheeseman P, Bansal S, Mieli-Vergani G, Dhawan A. Safety and efficacy of N-acetylcysteine in children with non-acetaminophen-induced acute liver failure. Liver Transpl. 2008;14(1):25–30. doi: 10.1002/lt.21246. [DOI] [PubMed] [Google Scholar]
- 17.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41(5):1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 18.Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology. 2004;127(5):1338–1346. doi: 10.1053/j.gastro.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Khuroo MS, Kamili S. Aetiology and prognostic factors in acute liver failure in India. J Viral Hepat. 2003;10(3):224–231. doi: 10.1046/j.1365-2893.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 20.Ellis A, Wendon J. Circulatory, respiratory, cerebral, and renal derangements in acute liver failure: pathophysiology and management. Semin Liver Dis. 1996;16(4):379–388. doi: 10.1055/s-2007-1007251. [DOI] [PubMed] [Google Scholar]
- 21.Hussaini SH, Skidmore SJ, Richardson P, Sherratt LM, Cooper BT, O’Grady JG. Severe hepatitis E infection during pregnancy. J Viral Hepat. 1997;4(1):51–54. doi: 10.1046/j.1365-2893.1997.00123.x. [DOI] [PubMed] [Google Scholar]
- 22.O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97(2):439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]